Abstract
Phytic acid and raffinose series oligosaccharides (RFOs) have anti-nutritional properties where phytic acid chelates minerals and reduces their bioavailability to humans and other animals, and RFOs cause flatulence. Both phytic acid and RFOs cannot be digested by monogastric animals and are released as pollutant-wastes. Efforts are being made to reduce the contents of these factors without affecting the viability of seeds. This will require a thorough understanding of their metabolism in different crops. Biosynthetic pathways of both metabolites though are interlinked but not well described. This study was made on metabolism of these two contents in developing chickpea (Cicer arietinum L cv GL 769) seeds. In this study, deposition of RFOs was found to occur before deposition of phytic acid. A decline in inorganic phosphorus and increase in phospholipid phosphorus and phytic acid was observed in seeds during development. Acid phosphatase was the major phosphatase in seed as well as podwall and its activity was highest at early stage of development, thereafter it decreased. Partitioning of 14 C label from 14 C-glucose and 14 C-sucrose into RFOs and phytic acid was studied in seeds in presence of inositol, galactose and iositol and galactose, which favored the view that galactinol synthase is not the key enzyme in RFOs synthesis.
Keywords: Phytic acid, Raffinose, Stachyose, Chickpea, Phosphate
Introduction
Two pathways, lipid independent and lipid dependent, are suggested for biosynthesis of phytic acid in higher plants. Under lipid independent pathway, phytic acid is synthesized from glucose-6-phosphate (Glu-6-P) (Loewus and Murthy 2000) where Glu-6-P isomerizes to 1 L-myo-inositol-1-phosphate (MI-1-P) by enzyme myo-inositol-1-phosphate synthase (MIPS) (Fig. 1a). MI-1-P can also be synthesized from myo-inositol by its phosphorylation by myo-inositol kinase (MIK) and reversible reaction can also occur which is catalyzed by myo-inositol-1-phosphate phosphatase (MIP). Further steps in the biosynthesis of phytic acid are the step-wise phosphorylations of MI-1-P by phosphoinositol kinases and are not well described. Lipid dependent pathway for phytic acid biosynthesis starts from free myo-inositol through phosphatidyl inositol phosphorylations occurring at the membrane bound intermediate levels (Stevenson-Paulik et al.2002). Mostly, lipid independent pathway has been suggested to be functional in higher plants (Sun et al.2000) where MIPS is considered to be catalyzing the first committed step for this biosynthesis and MIK facilitates a salvage mechanism for free myo-inositol to be recycled into a pool of MI-1-P (Loewus and Murthy 2000). Action of MIK allows the synthesis of phytic acid from free myo-inositol, though little is known about the regulation, localization and role of MIK (Loewus and Murthy 2000).
Fig. 1.

a. Probable biosynthetic pathways for phytic acid, raffinose and stachyose in higher plants where Glu, glucose; Glu-6-P, glucose-6-phosphate; F-6-P, fructose-6-phosphate; F, fructose; MI-1-P, myo-inositol-1-phosphate; Suc, sucrose; MI, myo-inositol; SS, sucrose synthase; INV, invertase; SPS, sucrose-phosphate synthase, MIPS, myo-inositol-1-phosphate synthase; MK, myo-inositol kinase; MIP, myo-inositol-1-phosphate phosphatase; RS, raffinose synthase; StS, stachyose synthase; GolS, galactinol synthase. b. Biomass (mg/seed) and c. % moisture content of seed and podwall during pod development, where DAF represents days after flowering. Vertical bars show the deviations from mean of three replicates of 10 seeds each. Vertical bars are not shown where SD was smaller than the symbol
The raffinose series oligosaccharides (RFOs) are the major soluble carbohydrates in seeds. They protect seeds from damage and desiccation. Biosynthesis of RFOs proceeds by the reversible addition of galactose units from galactinol (O-α-D-galactopyranosyl-(1 → 1)-L-myo-inositol) to sucrose where myo-inositol is recycled to galactinol. Chain elongation is catalyzed by raffinose synthase (RS) and stachyose synthase (StS) (Peterbauer and Richter 2001). Key enzyme of the pathway is believed to be galactinol synthase (GolS) which reversibly synthesizes galactinol from UDP-Galactose and myo-inositol (Keller and Pharr 1996). This model was originally based on a correlation of GolS activity with RFO content which implies that the concentration of galactinol is the critical factor. However, recent reports are contrary to these reports which say that there is not tight correlation between GolS and RFO content (Peterbauer et al.2001; Downie et al.2003; Karner et al.2004).
Biosynthesis of phytic acid and RFOs share at least one common intermediate myo-inositol-1-phosphate and/or myo-inositol as decreased synthesis of myo-inositol-1-phosphate by creating mutation in MIPS enzyme led to decreased content of not only phytic acid but RFOs also in soybean seeds (Hitz et al.2002). Our study was aimed to understand raffinose and phytic acid anabolism in developing chickpea seeds with motive to elucidate these pathways and to figure out interactions among them during seed development.
Materials and methods
Materials
Chickpea (Cicer arietinum L. cv. GL-769) crop was sown in the fourth week of October. Developing flowers were tagged for 6–7 days and then developing pods were collected at 14, 21, 28, 35 and 42 days after flowering (DAF). Pods and seeds were separated on ice and brought under ice to the laboratory. Enzymes were extracted immediately form fresh tissues and activities were determined on the same day.
Extraction of enzymes
All extractions were performed at 4 °C. Acid and alkaline phosphatases were extracted with 0.1 M sodium acetate buffer (pH 5.0) and 0.1 M Tris HCl buffer (pH 8.0) respectively (Kaur et al.1999). Acid and alkaline invertases were extracted with 0.02 M sodium phosphate buffer (pH 7.0) (Chopra et al.2003). Inorganic pyrophosphatase was extracted with 0.1 M Tris HCl buffer (pH 7.3) (Heppel 1955) and 3-phosphoglycerate phosphatase was extracted with 25 mM MES-KOH buffer (pH 6.0) (Villareal and Juliano 1977). Acid and alkaline phytases were extracted with 0.1 M sodium acetate buffer (pH 5.0) and 0.1 M Tris HCl buffer (pH 8.0) respectively containing 1 mM CaCl2 and 0.1 % triton-X-100 (Kaur et al.1999). In all these procedures, required tissue (0.5–1 g) crushed in chilled pestle and mortar was extracted with 3 ml of their respective extraction buffers. The extract was passed through cheese cloth and centrifuged at 10,000 g for 15 min at 4 °C. The supernatant was passed through sephadex G-25 column to remove low molecular weight components from crude extract before estimating enzyme activities. α-galactosidase was extracted by using 0.1 M sodium acetate buffer of pH 5 (McClearly and Matheson 1974).
Assays of enzymes
All enzymes were assayed in duplicates at 37 °C. Conditions of the assay system for each enzyme were standardized to give linear rates with time with respect to substrate concentrations.
Acid and alkaline phosphatases were assayed by using 2.2 mM p-nitrophenylphosphate in 0.05 M sodium acetate buffer pH 5.0 for acid phosphatase/0.1 M Tris HCl buffer pH 8.0 for alkaline phosphatase and enzyme extract of 0.1 ml in a total volume of 3.1 ml. After 20 min of incubation, reaction was stopped by adding 2 ml of 0.2 M NaOH and p-nitrophenol released after the enzymatic reaction was read at 420 nm (Kaur et al.1999). Inorganic pyrophosphatases and 3-phosphoglycerate phosphatases were assayed using sodium pyrophosphate and 3-phosphoglyceric acid respectively as their substrates (Heppel 1955; Villareal and Juliano 1977). Pi produced after the reaction was measured (Kaur et al.1999). Activities of acid and alkaline invertases were determined by incubating 0.1 M sucrose in 0.1 M sodium acetate buffer pH 5.0 for acid invertase and 0.1 M sodium phosphate buffer pH 8.0 for alkaline invertase with 0.1 ml of enzyme extract in a total volume of 1 ml. Reducing sugars formed after sucrose hydrolysis were estimated (Chopra et al. 2003). Acid and alkaline phytases were assayed using 2.2 mM sodium phytate in 0.05 M sodium acetate buffer pH 5.0 containing 1 mM CaCl2 for acid phytase and in 0.1 M Tris HCl buffer pH 8.0 containing 1 mM CaCl2 for alkaline phytase and enzyme extract of 0.1 ml in a total volume of 3.1 ml. Reaction was stopped by adding 2 ml of 10 % perchloric acid and Pi released after the enzymatic reaction was estimated (Kaur et al.1999). Activity of α-galactosidase was measured using 0.1 ml of 12.5 mM p-nitro-phenyl-α-D-galactopyranoside as its substrate in 0.05 M sodium acetate buffer pH 5.0 and 0.1 ml of enzyme preparation (McClearly and Matheson 1974). Reaction was terminated by addition of 2.8 ml of 2 % sodium carbonate. Absorbance of p-nitrophenol released was read at 420 nm.
Protein content was measured by using folin-phenol reagent (Lowry et al.1951).
Extraction and estimation of water soluble inorganic and organic phosphorus and phospholipid phosphorus
Water soluble inorganic and organic phosphorus and phospholipids phosphorus were extracted and estimated as described previously (Kaur et al.1999).
Extraction and estimation of sugars and starch
Free sugars were quantitatively extracted twice with 80 % ethanol, then twice with 70 % ethanol and contents of free fructose, sucrose, glucose were determined as described previously (Chopra et al.2003). Sucrose was determined by an enzymatic hydrolysis of the extract with acid invertase (Sigma) and then determining the glucose using glucose oxidase and peroxidase reaction (Chopra et al.2003). Raffinose and stachyose were separated on TLC silica gel G glass plates without fluorescent indicator (Eastman Kodak Company) by spotting known volume of carbohydrate extract using solvent system (butanol: isopropanol: water in 30: 120: 40 v/v/v). The standard sugars and one lane of sample were sprayed by urea-orthophosphoric acid spray reagent and fructose containing sugars were detected by heating TLC plates at 85 °C for 5 min. The portion corresponding to raffinose and stachyose from unsprayed TLC plates was cut and put into 5 ml of distilled water. Equivalent portion of blank TLC plate was also cut and put in 5 ml of distilled water. The sugars were extracted and fructose content of these oligosaccharides was determined by using resorcinol-HCl reagent. The starch was estimated from sugar free residue obtained after ethanol extraction as described previously (Chopra et al.2003).
Extraction and estimation of phytic acid
Phytic acid was extracted with 1.2 % HCl at room temperature and then precipitated in a boiling water bath with 0.4 % FeCl3.6H2O in 0.07 N HCl. The precipitated ferric phytate salt was isolated via centrifugation and dissolved in nitric acid. Organic phosphorus was estimated as described previously (Kaur et al.1999).
Separation of phytic acid by ion exchange chromatography
Phytic acid from the previous extraction was separated on Dowex-1-X8 (anion exchanger in chloride form) using increasing gradient of HCl. Phytic acid was eluted in 3 N HCl.
Uptake of uniformly labeled 14 C sucrose and 14 C glucose and their partitioning into phytic acid, starch and raffinose oligosaccharides
To 1 ml of respective radiolabeled sugars (1 μCi) containing either glucose or galactose or inositol, three uniformly developed seeds of 30-35 DAF stage were dipped. The mixture was incubated at 300 C for 6 h. The reaction was stopped with 0.3 N HCl when 14 C incorporation in phytic acid or with 80 % ethanol when radioactivity incorporation into starch and soluble sugars was to be studied. After stoppage of the metabolic reactions, the seeds were taken out, washed rapidly (15–30 s) with chilled water to remove any adhering radioactivity. The seeds were then plunged into 0.3 N HCl for phytic acid analysis or in 80 % ethanol for sugar analysis. Samples were stored at −20 °C till used for further analysis.
Phytic acid was extracted with 0.3 N HCl and separated by ion exchange chromatography using Dowex-1-X8 anion exchanger. The radioactivity infractions containing phytic acid was measured using Bray’s scintillation fluid using Beckmann LS 6500 scintillation counter.
Soluble sugars were extracted from seeds stored in 80 % ethanol and residue left form this extraction was used for the extraction of starch. The soluble sugars were resolved on TLC plate. Strips corresponding to raffinose and stachyose from the unsprayed plate were cut and put in scintillation vial and 10 ml of scintillation fluid (4 g PPO and 200 mg POPOP in one liter of toluene) to each vial was added. Radioactivity was determined. After extraction of starch from insoluble tissue, known amount of pooled supernatant was dried in scintillation vial in an oven at 50 °C and 10 ml of toluene based scintillation fluid was added and radioactivity was measured.
Statistical analysis
Samples were extracted in triplicates and estimated in duplicates. Mean ± S. D. was calculated. Values were evaluated for significance of differences by Tukey’s test (p < 0.05).
Results
Rapid accumulation of biomass in seeds was observed between 21 DAF to 35 DAF (Fig. 1b). There is continuous decline in moisture content in seeds, however loss of water content in pod wall was rapid between 28 DAF to 35 DAF whereas seeds loose water rapidly during the last phase of development (Fig. 1c).
In developing seeds, sucrose was found to be prominent sugar where its maximum content was found at 21 DAF, thereafter it decreased. Free fructose was detected only after 28 DAF (Fig. 2a), however a low level but almost constant amount of glucose was detected at all developing stages. Maximum accumulation of raffinose and stachyose occurred during 21–28 DAF thereafter their contents increased slightly till maturity (42 DAF). Acid invertase, alkaline invertase and alpha-galactosidase enzymes involved in RFO metabolism were studied (Fig. 2c) where alpha-galactosidase activity was found at higher levels during early stages (14–28 DAF) and thereafter its activity decreased. Acid invertase was present only at 14 DAF and alkaline invertase was found to have activity till 28 DAF (Fig. 2c).
Fig. 2.

Changes in the contents of sugars and starch in seed (a) and podwall (b) and the activities of acid invertase, alkaline invertase and alpha-galactosidase in seeds (c) and podwall (d) during pod development. Suc, sucrose; Glc, glucose; Fru, fructose; Raf, raffinose; Stc, stachyose; ACI, acid invertase; AKI, alkaline invertase, AG, alpha-galactosidase, DAF, days after flowering. Values are the mean ± S. D. of three replicates. Values marked with different letter (s) indicate significant differences at different DAF as according to Tukey’s test (p < 0.05)
In podwall of developing pods, sucrose was the prominent sugar but its content decreased rapidly between 14 and 21 DAF (Fig. 2b). Acid invertase was present in podwall during whole development phase whereas alkaline invertase could not be detected after 28 DAF (Fig. 2d). Similar to sucrose, starch content was highest at 14 DAF (Fig. 2b), thereafter decreased. Low levels of raffinose and stachyose were also observed in podwall during late stages of pod development (35 and 42 DAF) along with a low level of α-galactosidase.
Increasing trends of phospholipid-P and phytic acid were observed where maximum accumulation of phospholipid-P occurred between 14 and 21 DAF and phytic acid occurred between 28 and 42 DAF in seeds (Fig. 3a). Decreasing trend of inorganic-P was observed, however water soluble organic-P did not follow any trend, first it increased till 21 DAF then decreased till 35 DAF, then again increased slightly on 42 DAF in seeds. Very high activities of acid phosphatase (ACP) and 3-PGA phosphatase (3-PGAP) were observed in seeds at 14 DAF in comparison to pyrophosphate phosphatase (PPP), alkaline phosphatase (AKP), acid phytase (APHY) (Fig. 3c). These activities decreased rapidly between 14 and 28 DAF. Acid phytase was found to be at very low level and alkaline phytase could not be detected in seeds. In podwall, phytic acid could not be detected. Total phosphorus contents (inorganic-P, water soluble organic-P and phospholipids-P) decreased rapidly between 14 and 28 DAF in podwall during development (Fig. 3b). Similar to seeds, very high activities of acid phosphatase and 3-PGA phosphatase were found in podwall, these were highest at 14 DAF, thereafter it decreased. A low level of alkaline phosphatase was observed and phytase (acid and alkaline) could not be detected (Fig. 3d).
Fig. 3.
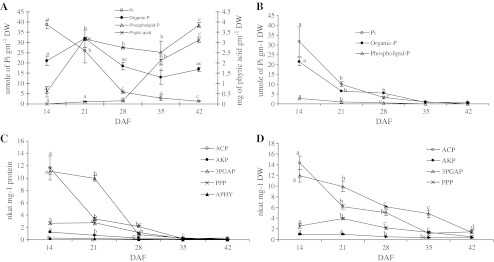
Changes in contents of different phosphorus reserves in seed (a) and podwall (b) and the activities of different phosphatases in seeds (c) and podwall (d) during pod development where Pi, water soluble inorganic phosphorus; Organic-P, water soluble organic phosphorus, phospholipid-P, phospholipid phosphorus fraction, ACP, acid phosphatase; AKP, alkaline phosphatase; 3PGAP, 3-phosphoglycerate phosphatase, PPP, pyrophosphate phosphatase; APHY, acid phytase; DAF, days after flowering. Phytic acid content is given in mg of phytic acid gm-1 DW while other phosphorus contents are given in umole of Pi gm-1 DW. Values are the mean ± S. D. of three replicates. Values marked with different letter (s) indicate significant differences at different DAF as according to Tukey’s test (p < 0.05)
Incorporation from exogenous uniformly labeled glucose (14 C-glucose) and sucrose (14 C-sucrose) into phytic acid and raffinose oligosaccharides was studied in the presence of inositol, galactose and both inositol and galactose in developing seeds of 35 DAF (Table 1). A stage of 35 DAF was selected as metabolites like phytic acid and RFOs were being accumulated during this phase (Figs. 2 and 3). 14 C incorporation into phytic acid from 14 C-sucrose was found to be significantly lower from that of exogenous 14 C-glucose. 14 C incorporation into raffinose and stachyose was also comparatively lower from 14 C-sucrose than from 14 C-glucose, but amount of reduction in incorporation was not to that extent as observed in phytic acid. Addition of inositol to exogenous glucose led to 25 % and 32 % reduction in 14 C incorporation into phytic acid from 14 C-glucose and 14 C-sucrose respectively. Addition of galactose to exogenous glucose led to increased 14 C incorporation into raffinose and decreased 14 C incorporation into phytic acid from 14 C-glucose. Addition of inositol and galactose together to exogenous glucose led to decreased 14 C-incorporation into phytic acid from14C-glucose and increased 14 C-incorporation into raffinose series oligosaccharides from 14 C-sucrose (Table 1).
Table 1.
Incorporation of label as CPM (counts per minute) from exogenously uniformly labeled glucose (14 C-glucose) and sucrose (14 C-sucrose) into phytic acid, raffinose and stachyose in the presence of various metabolites (Glc, glucose; I, inositol; Gal, galactose as studied in chickpea seeds (at 35 DAF). Values are mean of three replicates ± S. D. Data followed by different letters in a column are significantly different at p < 0.05 according to Tukey’s test
| CPM in Phytic acid | CPM in Raffinose | CPM in Stachyose | ||||
|---|---|---|---|---|---|---|
| 14 C-Glc | 14 C-Suc | 14 C-Glc | 14 C-Suc | 14 C-Glc | 14 C-Suc | |
| Glc | 4,355 ± 115a | 857 ± 46a | 5,970 ± 105ab | 2,500 ± 486a | 4,905 ± 120a | 715 ± 66a |
| Glc+I | 3,245 ± 15b | 585 ± 45b | 6,705 ± 1644a | 2,700 ± 810a | 4,625 ± 625a | 1,440 ± 45b |
| Glc+Gal | 3,890 ± 40c | 775 ± 25a | 1,0530 ± 1110c | 3,490 ± 654a | 6,345 ± 1380a | 940 ± 214a |
| Glc+Gal+I | 1,180 ± 99d | 805 ± 105a | 3,908 ± 1072b | 5,303 ± 443b | 4,905 ± 212a | 1,995 ± 75c |
Discussion
Rapid decrease in sucrose, water soluble organic-P and phospholipids-P in podwall from 14 to 21 DAF period accompanied by rapid increase of these contents in seeds (Figs. 2 and 3) indicates their probable transport from podwall to developing seed. The rapid decline of sucrose from 21 to 28 DAF with increase in RFOs in seeds without increase in glucose content as well as invertase activity (Fig. 2) indicates the partitioning of sucrose into RFOs during this time of development. This data is supported by the similar trend of sucrose, raffinose and stachyose during seed development in pea, lupin, mungbean and lentil seeds (Chopra et al.2000; Peterbauer et al. 2001; Chopra et al.2003; Pinheiro et al. 2005). Rapid accumulation of phytic acid occurred from 28 to 35 DAF in seeds after RFOs accumulation though it remained depositing till 42 DAF (Fig. 3a).
Phosphate assimilation, storage and metabolism are of critical importance for plant growth and development. Efficient utilization of phosphorus requires a class of enzymes known as phosphatases which function to hydrolyze Pi from orthophosphate monoesters in a thermodynamically favorable process. Phosphatases are important in the production, transport and recycling of Pi. Acid phosphatases was found to be the dominating enzyme in seeds as well as in podwall during pod development thereafter it decreased (Figs. 3c and d). Similar trend was found for alkaline phosphatase though activity of alkaline phosphatase was low as compared to that of acid phosphatase at all developmental stages of pod. Acid phosphatases were reported to be more active than alkaline phosphatases in different tissues of plants (Gupta et al.1998; Kaur et al.1999). Decreasing trend of phosphatases during development favors the deposition of phosphorus reserves which are supported by the increasing content of phytic acid from 28 to 42 DAF, phospholipids-P and water soluble organic-P from 35 to 42 DAF (Fig. 3). There was a positive correlation between phosphatase activity and water soluble inorganic phosphorus (Pi) content and a negative correlation between phosphatase activity and phosphorus reserves (phytic acid, phospholipids-P, water soluble organic-P)) in developing seeds. Alkaline pyrophosphatase and 3-PGA phosphatase activities were determined with the motive to relate these activities with starch synthesis as 3-PGA is a potent activator of starch synthesis hence low activity of 3-PGA phosphatase should be correlated with starch synthesis. But as similar to phosphatases, a decreasing trend of both 3-PGA phosphatase and alkaline pyrophosphatase was found during development which indicates these activities might be the reflection of broad specificity of non-specific phosphatases.
14 C incorporation from 14 C-sucrose into phytic acid and raffinose oligosaccharides of developing seeds at 35 DAF (Table 1) was found to be significantly lower from that of exogenous 14 C-glucose. Radiolabelled sugars were accompanied with 0.1 % cold glucose which might reduce the uptake of 14 C-sucrose from exogenous medium. Secondly phytic acid is synthesized from glucose where sucrose has to cleave first into glucose and fructose and conversion of fructose to glucose may be the slower process and thereby leading to reduction in the availability of glucose for its conversion into phytic acid. 14 C incorporation into raffinose was comparatively lower from 14 C-sucrose than from 14 C-glucose, but amount of reduction in incorporation was not to that extent as observed in phytic acid. This can be due to the fact that 14 C-sucrose could directly serve as substrate for raffinose synthase whereas 14 C-glucose transfers its label to RFOs through 14 C-galactose moiety of galactinol mostly.
Addition of inositol to exogenous glucose led to reduction of 14 C incorporation into phytic acid. As myo-inositol was reported as suppressor of myo-inositol-1-phosphate synthase (MIPS) gene expression (Mitsuhashi et al.2005) and MIPS was supposed to be catalyzing the first committed step in phytic acid biosynthesis (Loewus and Murthy 2000), so reduction of 14 C incorporation into phytic acid was expected in the presence of exogenous inositol. Addition of galactose to exogenous glucose led to increased 14 C incorporation into RFOs from 14 C-glucose. Addition of galactose may somehow increase the availability of galactinol leading to increased synthesis of RFOs. Addition of inositol and galactose together to exogenous glucose led to decreased 14 C-incorporation into phytic acid from 14 C-glucose and increased 14 C-incorporation into RFOs from 14 C-sucrose. As both inositol and galactose could increase the amount of galactinol, hence divert the label to other routes than going to phytic acid. Increased 14 C-incorporation into RFOs from 14 C-sucrose can possibly be explained on the basis of direct entry of 14 C-sucrose as being a substrate for raffinose synthase however 14 C- glucose has the most probable route of incorporating its label through 14 C-galactosyl moiety of galactinol. Though galactinol accumulation increases RFOs synthesis but it may also be diverted to other pathways. This data is supported by the fact that RFOs contents were positively correlated to the contents of inositol and sucrose but not the contents of galactinol (Karner et al.2004). Cyclic alpha-galactosides (Gal-C) along with RFOs are reported to be synthesized in developing seeds by the same route and same set of enzymes (raffinose synthase and stachyose synthase) (Lahuta et al.2005). So all these reports indicate galactinol synthase is probably not the only key enzyme which regulates the partitioning of carbon into RFO pool as previously thought. Either galactinol has other functions or other metabolic fates in plant cells or other factors besides galactinol synthase activity are affecting RFO accumulation.
Acknowledgement
Zhawar VK is thankful to the Council of Scientific and Industrial Research, New Delhi for the award of Junior Research Fellowship.
Abbreviations
- Glu-6-P
Glucose-6-Phosphate
- MI-1-P
1L-Myo-Inositol-1-Phosphate
- MIPS
Myo-Inositol-1-Phosphate Synthase
- MIK
Myo-Inositol Kinase
- MIP
Myo-Inositol-1-Phosphate Phosphatase
- RFOs
Raffinose Series Oligosaccharides
- RS
Raffinose Synthase
- StS
Stachyose Synthase
- GolS
Galactinol Synthase
- DAF
Days After Flowering
- 3-PGA
3-Phosphoglyceric Acid
- Gal-C
Cyclitol α-Galactosides
References
- Chopra J, Kaur N, Gupta AK. Ontogenic changes in enzymes of carbon metabolism in relation to carbohydrate status in developing mungbean reproductive structures. Phytochem. 2000;53:539–548. doi: 10.1016/S0031-9422(99)00545-2. [DOI] [PubMed] [Google Scholar]
- Chopra J, Kaur N, Gupta AK. Changes in the activities of carbon metabolizing enzymes with pod development in lentil (Lens culinaris L.) Acta Physiol Plant. 2003;25:185–191. doi: 10.1007/s11738-003-0052-x. [DOI] [Google Scholar]
- Downie B, Gurusinghe S, Dahal P, Thacker RR, Snyder JC, Nonogaki H, Yim K, Fukanaga K, Alvarado V, Bradford KJ. Expression of a galactinol synthase gene in tomato seeds is up-regulated before maturation desiccation and again after imbition whenever radicle protrusion is prevented. Plant Physiol. 2003;131:1347–1359. doi: 10.1104/pp.016386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AK, Kaur V, Kaur N. Appearance of different phosphatase forms and phosphorus partitioning in nodules of chickpea (Cicer arietinum L.) during development. Acta Physiol Plant. 1998;20:369–374. doi: 10.1007/s11738-998-0022-4. [DOI] [Google Scholar]
- Heppel LA. Inorganic pyrophosphatase from yeast. Methods Enzymol. 1955;2:570–576. doi: 10.1016/S0076-6879(55)02255-6. [DOI] [Google Scholar]
- Hitz WD, Carlson TJ, Kerr PS, Sebastian SA. Biochemical and molecular characterization of a mutation that confers a decreased raffinosaccharide and phytic acid phenotype on soybean seeds. Plant Physiol. 2002;128:650–660. doi: 10.1104/pp.010585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karner U, Peterbauer T, Raboy V, Jones DA, Hedley CL, Richter A. Myo-Inositol and sucrose concentrations affect the accumulation of raffinose family oligosaccharides in seeds. J. Exp. Bot. 2004;55:1981–1987. doi: 10.1093/jxb/erh216. [DOI] [PubMed] [Google Scholar]
- Kaur V, Kaur N, Gupta AK. Phosphatase activity and phosphorus partitioning in nodules of developing mungbean. Acta Physiol. Plant. 1999;21:215–220. doi: 10.1007/s11738-999-0035-7. [DOI] [Google Scholar]
- Keller F, Pharr DM. Metabolism of carbohydrates in sinks and sources: galactosyl-sucrose oligosacchrides. In: Zamski E, Schaffer AA, editors. Photoassimilate distribution in plants and crops. New York: Marcel Dekker; 1996. pp. 115–184. [Google Scholar]
- Lahuta LB, Horbowicz M, Gojlo E, Goszczynska J, Goreck RJ. Exogenously applied D-pinnitol and D-chiro-inositol modifies the accumulation of α-D-galactosides in developing tiny vetch (Vicia hirsute (L.) S. F. gray) seeds. Acta Societatis Botanicorum Poloniae. 2005;74:287–296. [Google Scholar]
- Loewus FA, Murthy PPN. Myo-Inositol metabolism in plants. Plant Sci. 2000;150:1–19. doi: 10.1016/S0168-9452(99)00150-8. [DOI] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J. Biol. Chem. 1951;193:265–268. [PubMed] [Google Scholar]
- McClearly BV, Matheson NK. α-D-galactosidase activity and galactomannan and galactosyl sucrose oligosaccharide depletion in germinating legume seeds. Phytochem. 1974;13:1747–1757. doi: 10.1016/0031-9422(74)85084-3. [DOI] [Google Scholar]
- Mitsuhashi N, Ohnishi M, Sekiguchi Y, Kwon YU, Chang YT, Chung SK, Inoue Y, Reid RJ, Yagisawa H, Mimura T. Phytic acid synthesis and vacuolar accumulation in suspension-cultured cells of Catharanthus roseus induced by high concentration of inorganic phosphate and cations. Plant Physiol. 2005;138:1607–1614. doi: 10.1104/pp.105.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterbauer T, Richter A. Biochemistry and physiology of raffinose family oligosaccharides and galactosyl cyclitols in seeds. Seed Sci Res. 2001;11:185–191. [Google Scholar]
- Peterbauer T, Lahuta LB, Blochl A, Mucha J, Jones DA, Hedley CL, Gorecki RJ, Richter A. Analysis of the raffinose family oligosaccharide pathway in pea seeds with contrasting carbohydrate composition. Plant Physiol. 2001;127:1764–1772. doi: 10.1104/pp.010534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro C, Rodrigues AP, de Carvalho IS, Chaves MM, Ricardo CP. Sugar metabolism in developing lupin seeds is affected by a short-term water deficit. J. Exp. Bot. 2005;56:2705–2712. doi: 10.1093/jxb/eri263. [DOI] [PubMed] [Google Scholar]
- Stevenson-Paulik J, Odom AR, York JD. Molecular and biochemical characterization of two plant inositol polyphosphate 6-/3-/5-kinases. J. Bio. Chem. 2002;45:42711–42718. doi: 10.1074/jbc.M209112200. [DOI] [PubMed] [Google Scholar]
- Sun Y, Thompson M, Lin G, Butler H, Gao Z, Thornburgh S, Yau K, Smith DA, Shukla VK. Inositol 1, 3, 4, 5, 6-pentakisphosphate 2-kinase from maize: Molecular and biochemical characterization. Plant Physiol. 2000;144:1278–1291. doi: 10.1104/pp.107.095455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villareal RM, Juliano BO. Some properties of 3-phosphoglycerate phosphatase from developing rice grain. Plant Physiol. 1977;59:134–138. doi: 10.1104/pp.59.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]


