Abstract
An accelerated protocol for large-scale propagation of Dendrocalamus asper, an edible bamboo, has been described. Seven axillary shoots were induced in vitro from each excised tender node (15–20 mm in length) containing single axillary bud when nodal segments were inoculated in semisolid Murashige and Skoog (MS) medium fortified with 5 mg/l 6-benzylaminopurine (BAP). Maximum multiple shoot formation (14) was observed when in vitro generated axillary shoots were transferred to liquid MS medium containing 5 mg/l BAP and 40 mg/l adenine sulphate. A maximum of 93.33 % shoots were effectively rooted when transferred to liquid MS medium supplemented with 1 mg/l indole-3-biutyric acid (IBA). A simple acclimatization procedure of 55 days, primarily in cocopeat for 20 days and finally in a blend of sand, soil and farm yard manure (1:1:1 v/v), ensured a very high survival rate within next 35 days. After acclimatization, rooted plantlets were further multiplied by splitting of rhizomes, formed in vivo within 90 days of growth. After 90 and 180 days of acclimatization, plants were successfully transferred to the field and maintained in an unirrigated condition with the initial supplementation of farm yard manure @ 10 kg/pit; where around 85 % survivability with 25 culms per bush attaining an average height of 4.5 m was recorded up to four years.
Keywords: Acclimatization, Dendrocalamus asper, Field performance, Micropropagation, Macropropagation, Nodal segments
Introduction
Dendrocalamus asper, commonly known as Sweet Bamboo, is a multipurpose tropical clumping bamboo of economic value. People consume tender shoots of D. asper as an excellent scrumptious food (Anon 1995). In the year 2000, USA alone has imported US$ 16.8 million of edible bamboo shoots from China, Indonesia, Japan, Taiwan and Thailand (United State Department of Agriculture online service, September 07, 2001). Moreover, the mature culms are utilized in the construction, handicraft, outriggers on fishing boats and these are suitable for pulp and paper also. Indian Council of Forestry Research and Education, Dehradun, India has successfully introduced this exotic bamboo to India in 1994 (Singh et al. 2004). The production of seed is irregular in D. asper due to its monocarpic flowering which occurs in 100 years old plant culms (Nadgir et al. 1984). Availability of seeds is limited and available seeds are of short-lived viability. Hence, this species is chiefly propagated by rhizome or culm cuttings in its native land. Nevertheless, cuttings are bulky, tricky to handle and have been found to grow very slowly (Hassan 1980; Rao et al. 1985). Further year round, vegetative propagation is complicated due to seasonal specificity of material (Saxena and Bhojwani 1993). Consequently, the species, being exotic to India, authentic source material is scarce. Thus, it becomes imperative to take up alternative approaches for its fast multiplication and tissue culture techniques offer a potent tool for this purpose. With the view of its importance and irregular availability of the source materials, recent researchers are emphasizing on micropropagation of D. aper using different explants. Seeds (Arya et al. 1999) and rhizomes (Nirmala et al. 2011) were successfully used for mass propagation of this species in vitro. However, both seeds and rhizome is scarce; on the other hand, to date, no such report is available which combines both in vitro and in vivo multiplication of D. asper to enhance the rate of multiplication and evaluate the performance of the offsprings in the field. In accordance to that, the objective of the present study was to optimize a protocol for mass multiplication of D. asper using nodal segment explants in vitro followed by their further multiplication in vivo. To accomplish this purpose, a field assessment has been carried out to observe the performance of the plants, thus raised.
Materials and methods
Explant source
Dendrocalamus asper plantlets were brought from Indian Council of Forestry Research and Education, Ranchi, India. The young, actively growing first three nodal segments were collected in different seasons and washed thoroughly under running tap water and washed again with a few drops of Teepol (Glaxo India Ltd, Mumbai, India) for 5 min and then dipped for 30 min in 1 % Bavistin solution. The explants were then washed three times with sterile water before the treatment in 0.1 % (w/v) HgCl2 with continuous shaking for 6–8 min depending on the size of the explant. The explants were again rinsed were rinsed vigorously three times in sterile water and both the ends were trimmed to 15–20 mm. The whole process of surface sterilization was performed in a laminar air flow chamber.
Culture condition
Murashige and Skoog (MS) (1962) basal medium (consisting of salts, vitamins and 3 % sucrose) was used after solidifying with 0.7 % (w/v) agar. Different plant growth regulators (PGRs) like 6-benzylaminopurine (BAP), 6-furfurylaminopurine (Kn), indole-3-butyric acid (IBA), and indole-3-acetic acid (IAA) were added at various concentrations to MS medium before the pH of the medium was adjusted to 5.7. Media were autoclaved at 1.06 kgcm−2 and 121 °C for 15 min. Cultures at all growth stages were incubated under artificial conditions: 25 ± 2 °C, 60 % RH and a 16-h photoperiod (using white fluorescent tubes) under a photosynthetic photon flux density of 40 μmolm−2 s−1.
Axillary bud initiation and multiple shoot proliferation
For axillary bud initiation, liquid and semisolid MS medium were used. Initiated axillary buds were separated and cultured for multiple shoot proliferation. Ten different levels (1–10 mg/l for each) of BAP or Kn were used in MS medium for this purpose, whereas MS medium without any PGR served as a control. To enhance the shoot multiplication frequency, five varying levels BAP (1–10 mg/l) were further supplemented with 40 mg/l adenine sulphate (ADS) whereas MS devoid of PGR was a control. During shoot multiplication and proliferation, the cultures were incubated for 21 days as a single cycle. The performances of media were evaluated in terms of response, number, and length of shoots.
Root induction and elongation
The micro-shoots grown in vitro, were advanced for root induction and elongation. Liquid MS basal medium was supplemented with six diverse concentrations (1–3 mg/l for each) of IAA or IBA in which only MS medium (exclusive of PGR) acted as a control. The efficiency of IAA and IBA was compared in terms of response to root induction, days to root induction, number and length of roots. The plantlets with well-developed rhizosphere were advanced for acclimatization.
Acclimatization
A simple acclimatization process was established to make the most of the survival rate in a competent approach. Prior to the core part of acclimatization, the well-rooted plantlets were transferred into net pots, kept in portrays, packed with sterile cocopeat (processed coconut husks) and allowed to grow for 20 days. The whole setup was covered with a transparent polythene sheet, with good aeration facility to ensure the retention of high humidity. Then the partially acclimatized plantlets were shifted into the poly buckets (10 cm diameter) having an optimum pot mixtures of sand, soil and farm yard manure (1:1:1 v/v) for better plant establishment. The plants were allowed to grow for 35 days in this condition.
Multiplication in vivo
During the acclimatization process, the in vivo formation of rhizomes was observed after about 90 days of growth during which the tissue culture raised saplings were maintained in poly-buckets (10 cm diameter). Each plantlet was again multiplied into two/ three by splitting of de novo formed rhizomes and then grown in a balanced blend of sand and soil and FYM used earlier, with ensured water supply, devoid of greenhouse condition.
Field transfer
The well acclimatized plants, 90 and 180 days of age were then transferred to the field provided by the Department of Forest, Govt. of West Bengal at Arabarri, West Midnapore, West Bengal, India in the end of August. Before field transfer, pits (0.5 × 0.5 × 0.5 m) were dug and filled with 10 kg FYM per pit initially. Later, at 4 month field growth stage N:P:K (10:20:16) was supplemented for enhancement of ex vitro growth which was actually the only fertilizer source used. To avoid the infection from soil-borne pathogen, Furadan 3 G @ 10 g/plant was applied at early stage. The plant population was maintained in a complete unirrigated condition in the field. Starting from 1 month growth stage, the mortality rate was assessed up to 6 months. The field growth performance was observed after each year and data accumulation on plant height, and number of culms / bush continued till fourth year.
Experimental design and statistical analysis
The data were recorded according to a complete randomized design. Each explant was considered as an experimental unit. All the aforementioned experiments were repeated thrice using 20 explants in each replication. The recorded numerical information aided in determining the standard error. The survival percentage was calculated from the number of plants recovered, subjected to field growth. Observations on cultures were carried out on every alternate day. The collected data were subjected to the analysis of variance, and significant differences among the treatments were tested by Duncan’s multiple range test (Duncan 1955) at 5 % level using SPSS (Version 11, SPSS Inc. Chicago, USA) software package (Nirmala et al. 2011).
Results and discussion
Mass multiplication in vitro: axillary bud, multiple shoot and root growth
The present experiment exposed some interesting outcome from in vitro culture to the field performance of D. asper. Presence of latent infection proved to be the major constraints to achieve contamination-free D. asper culture in vitro. Bacterial contamination was sustained even up to second cycle of multiple shoot culture which indicates that they are probably endemic in nature. An impact of season for explant collection was observed on establishment and response of culture. Pre-monsoon (May-June) proved to be the best season in terms of response of the explant (95.43 %, Table 1) recorded in vitro but the rate of contamination was higher (30.57 %) (Table 1). Pre-summer (March-April) and post- monsoon (September-October) resulted in moderate response of 81.33 % and 69.67 % respectively along with comparatively less contamination (23.21 % and 13.35 %, respectively). This supports the earlier observation of Das and Pal (2005) in Bambusa balcooa, and later, Devi and Sharma (2009) in another edible bamboo (Arundinaria callosa Munro). However, pre-summer appeared to be the best in terms of least phenol production together with frequency of response. Though, the least contamination (4.87 %) was recorded during winter (December-February), but this collection season proved to be unsuitable due to maximum phenol production resulting lowest response (53.33 %) of explants.
Table 1.
Effect of Dendrocalamus asper explant collection season on establishment of culture
| Season | Percent response | Percent of culture contaminated |
|---|---|---|
| Pre-summer | 69.67 ± 4.41c | 13.35 ± 0.58c |
| Post-monsoon | 81.33 ± 1.67b | 23.21 ± 1.33b |
| Pre-monsoon | 95.43 ± 3.33a | 30.57 ± 1.58a |
| Winter | 53.33 ± 4.41d | 4.87 ± 1.27d |
Data represent mean ± SE of 20 replicates per treatment in three repeated experiments
Means within columns separated by Duncan’s multiple range test P = 0.05; Duncan 1955
For the establishment of in vitro culture, two sets of PGR compositions were utilized. Five levels (1–10 mg/l) of BAP were tested against five similar levels (1–10 mg/l) of Kn. A relatively high level of BAP (5 mg/l) promoted earliest axillary shoot initiation, within 7 days after inoculation (Fig. 1a). A maximum of 95.77 % explants responded in this media formulation where 7 (~7.33) axillary shoots with 5.66 cm length were observed per inoculated nodal segment (Table 2). BAP proved to be more efficient than Kn as the cytokinin source to induce axillary shoots in D. asper which supports the earlier report of Bisht et al. (2010) on black bamboo (Gigantochloa atroviolaceae). The higher concentration of BAP, in the present study, resulted in reduction in the rate of multiplication of shoot gradually where it dropped down from 7.33 to 2.33 with the increased BAP levels (from 5 mg/l to 10 mg/l). Semisolid MS media performed better than liquid MS for establishment of culture (data not presented) probably due to less aeration. However, the latter was successful during the other stages of growth including multiplication, growth and rooting of the micro-shoots. In course of shoot multiplication and proliferation, excised and separated axillary shoots were inoculated on both liquid and semisolid MS medium fortified with BAP and ADS. Liquid MS medium with 5 mg/l BAP and 40 mg/l ADS proved best for this purpose in terms of response of the axillary buds, number and length of the multiple shoots. After 60 days of axillary bud inoculation, around 14 well developed multiple shoots per inoculum were observed (Fig. 1b). At that time, average shoot length was 6.77 cm (Table 3). Supplementation of ADS with BAP was unable to alter / improve the frequency of response significantly, but enhanced the number of shoots and their proliferation successfully. In a sustained subculture method, on the other hand, the rate of multiplication was simultaneously increased 30 fold with the passage up to 15 cycles (subcultures) which proved to be better than the previous report of Arya et al. (1999).
Fig. 1.
Micropropagation of Dendrocalamus asper. a Axillary shoots initiation from nodal explants in semi-solid MS medium supplemented with 5 mg/l BAP, b Shoot multiplication and proliferation in liquid MS medium with the supplementation of 5 mg/l BAP and 40 mg/l ADS, c Plantlet with healthy and stout in vitro roots, successfully achieved in liquid MS medium plus 1 mg/l IBA
Table 2.
Effect of BAP and Kn on shoot multiplication of Dendrocalamus asper
| BAP (mg/l) | Kn (mg/l) | Percent response | No. of shoots | Shoot length (cm) |
|---|---|---|---|---|
| 0 | 0 | 2.33 ± 0.33i | 1.00 ± 0.00e | 0.89 ± 0.27h |
| 0 | 1 | 9.47 ± 0.61h | 1.00 ± 0.00e | 1.17 ± 0.20g |
| 0 | 3 | 10.90 ± 0.98h | 4.33 ± 0.33b | 2.60 ± 0.36def |
| 0 | 5 | 18.87 ± 0.88g | 2.00 ± 0.00d | 2.17 ± 0.15ef |
| 0 | 7.5 | 40.20 ± 0.23e | 1.67 ± 0.33de | 2.10 ± 0.12ef |
| 0 | 10 | 36.47 ± 1.92f | 1.67 ± 0.33de | 2.00 ± 0.21f |
| 1 | 0 | 73.17 ± 1.66d | 2.33 ± 0.33d | 3.73 ± 0.27c |
| 3 | 0 | 85.87 ± 1.00b | 3.33 ± 0.33c | 4.50 ± 0.31b |
| 5 | 0 | 95.77 ± 0.99a | 7.33 ± 0.33a | 5.67 ± 0.26a |
| 7.5 | 0 | 83.73 ± 1.23b | 3.33 ± 0.33c | 3.10 ± 0.10d |
| 10 | 0 | 77.80 ± 2.16c | 2.33 ± 0.33d | 2.73 ± 0.08de |
Data represent mean ± SE of 20 replicates per treatment in three repeated experiments
Means within columns separated by Duncan’s multiple range test P = 0.05; Duncan 1955
Table 3.
Effect of ADS in combination with BAP on shoot multiplication of Dendrocalamus asper
| BAP (mg/l) | ADS (mg/l) | Percent response | No. of shoots | Shoot length (cm) |
|---|---|---|---|---|
| 0 | 0 | 4.33 ± 0.33f | 1.00 ± 0.00e | 1.22 ± 0.31f |
| 0 | 40 | 45.37 ± 2.44e | 1.33 ± 0.33d | 2.20 ± 0.17e |
| 1 | 40 | 78.17 ± 0.84d | 5.33 ± 0.33c | 5.13 ± 0.49bc |
| 3 | 40 | 89.87 ± 1.17b | 8.33 ± 0.88b | 5.93 ± 0.42ab |
| 5 | 40 | 98.07 ± 0.81a | 14.00 ± 1.00a | 6.77 ± 0.38a |
| 7.5 | 40 | 88.07 ± 0.75b | 5.00 ± 0.58c | 4.30 ± 0.26cd |
| 10 | 40 | 82.37 ± 1.45c | 4.33 ± 0.33c | 3.70 ± 0.15d |
Data represent mean ± SE of 20 replicates per treatment in three repeated experiments
Means within columns separated by Duncan’s multiple range test P = 0.05; Duncan 1955
The results demonstrated that cytokinin fruitfully promoted multiplication of shoots in an enhanced mode, in the presence of ADS, where ADS devoid of PGRs could not achieve significant result. According to Gantait and Mandal (2010), ADS acts as an elicitor or enhancer of growth in combination or synergism with endogenous or exogenously supplemented PGRs. In the present study, semisolid MS medium with appropriate growth supplement for initial culture establishment followed by liquid medium for shoot multiplication and rooting was most suitable to optimize the in vitro mass propagation protocol, successfully. This method, particularly the alteration of semi solid to liquid MS medium, appears to be the most effective method of micropropagation of D. asper and the first complete report of micropropagation from the nodal explants, as, the other report describes use of seeds (Arya et al. 1999) or rhizomes (Nirmala et al. 2011) as explants. But, the species being exotic to India, the seeds are not available in this country while the plants are reported to set seed only after 100 years (Arya et al. 1999).
After 60–65 days of establishment culture at least 14–15 micro-shoots were observed per explant; the regenerated clumps were separated, each with 4–5 shoots, and transferred to the rooting media. Earliest root initiation was observed within 10 days approximately, and maximum response (93.33 %) was obtained on liquid MS medium supplemented with 1 mg/l IBA. More than 7 roots per inoculated shoot were scored after 20 days of culture (Fig. 1c). During this stage of growth, the average root length was 6.43 cm (Table 4). Though, spontaneous rooting was observed during multiple shoot proliferation in course of maturity of the plantlets in vitro but application of suitable PGR reduced the time period required for rooting. Similar result on stimulatory effect of IBA over in vitro rooting of D. asper was reported earlier by Arya et al. (1999). The performance of IAA and IBA was tested where IBA proved to be better than IAA. This is in support to the earlier report of Saxena and Bhojwani (1993) in Dendrocalamus longispathus. Notwithstanding the fact, IAA was proved to be inactive in D. aspar root initiation and elongation.
Table 4.
Effect of IBA and IAA on in vitro rooting of Dendrocalamus asper
| IBA (mg/l) | IAA (mg/l) | Percent response | Days to root | No. of roots | Root length (cm) |
|---|---|---|---|---|---|
| 0 | 0 | 71.67 ± 4.41b | 28.00 ± 1.00a | 2.00 ± 0.00d | 1.33 ± 0.15e |
| 0 | 1 | 46.67 ± 1.67c | 26.67 ± 0.89a | 2.00 ± 0.00d | 1.63 ± 0.18e |
| 0 | 2 | 58.33 ± 6.01c | 23.00 ± 1.00b | 2.33 ± 0.33d | 2.40 ± 0.25d |
| 0 | 3 | 53.33 ± 4.41c | 17.67 ± 0.33c | 3.33 ± 0.33c | 3.87 ± 0.15c |
| 1 | 0 | 93.33 ± 1.67a | 10.33 ± 0.89e | 7.33 ± 0.33a | 6.43 ± 0.18a |
| 2 | 0 | 88.33 ± 3.33a | 13.67 ± 0.67d | 5.67 ± 0.33b | 5.80 ± 0.32a |
| 3 | 0 | 85.00 ± 2.89a | 15.33 ± 0.89cd | 5.00 ± 0.00b | 4.70 ± 0.25b |
Data represent mean ± SE of 20 replicates per treatment in three repeated experiments
Means within columns separated by Duncan’s multiple range test P = 0.05; Duncan 1955
Acclimatization
In the first phase of acclimatization, the sterile cocopeat provided optimum anchorage to the plantlets and facilitated induction of new roots indicating the completion of primary acclimatization (Fig. 2a) in 20 days. Spraying of water with coverage of transparent polyethylene ensured high humidity and increase in temperature which evidently encouraged the rapid acclimatization process. Transferring the plantlets to a balanced mixture of sand, soil and farm yard manure (1:1:1 v/v) for the next 35 days, resulted 98 % well acclimatized plantlets (Fig. 2b). Chiefly, retention of high humidity was the basis of this successful acclimatization. Excepting the utilization of the intermittent water spraying, along with the covering with transparent polyethylene sheet (Thomas and Ravindra 1997) during first phase acclimatization, the use of farm yard manure for final acclimatization also played a significant part in retaining the moisture (Gantait et al. 2010a). This technique proficiently produced a high frequency of plantlets within a short span of time.
Fig. 2.
Acclimatization and macropropagation of Dendrocalamus asper. a Plantlets after primary acclimatization of 20 days in cocopeat, b Plantlets after final acclimatization of 35 days in sand, soil and farm yard manure (1:1:1 v/v), cIn vivo propagation through splitting of ex vitro generated rhizomes (inset) during acclimatization: macropropagation
Multiplication in vivo
A conventional multiplication approach was successfully integrated with the in vitro propagation method. Utilizing the in vivo formed rhizomes during acclimatization of the saplings in poly-buckets (10 cm diameter), and splitting them into pieces, a macropropagation method has been refined (Fig. 2c). Earlier, Arya et al. (1999) also reported the in vivo rhizome formation during acclimatization of D. asper at two months growth stage. Micropropagation followed by macropropagation proved to be the novel approach of the present study as the multiplication was 2.3-2.7 fold higher again in vivo. Multiplication of D. asper using in vitro rhizome formation was reported earlier by Nirmala et al. (2011) which was not suitable for commercial application. Contrarily, the present report describes an easy to adopt protocol, most suitable for commercial application, reported so far.
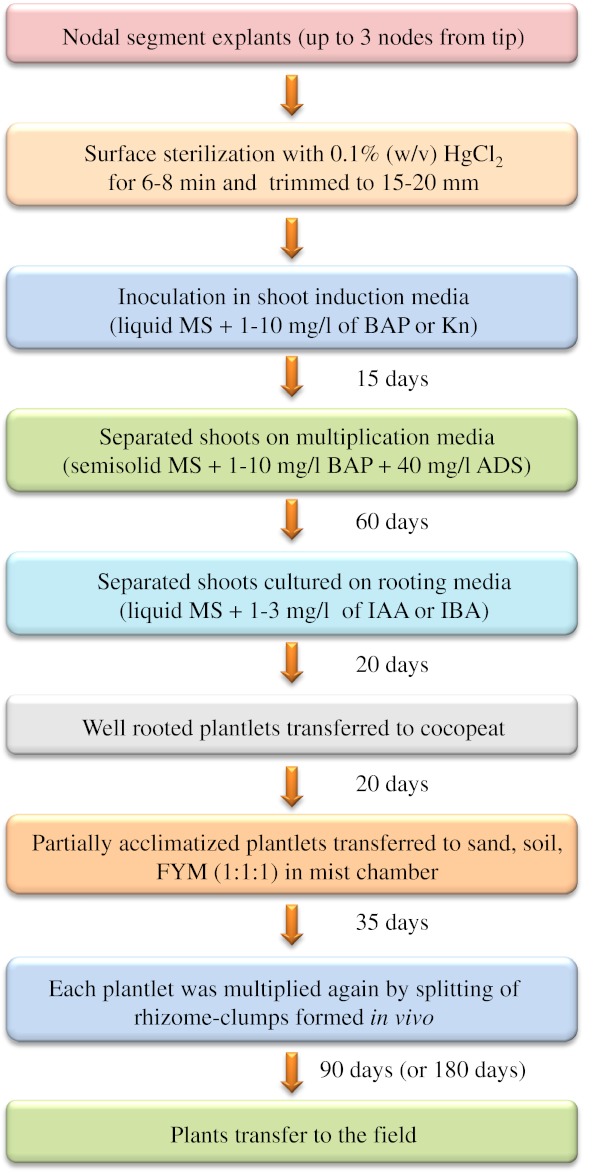
An illustrative diagram (Fig. 3) has been presented to describe the entire activities adopted during in vitro propagation, acclimatization, macropropagation in vivo and the field performance.
Fig. 3.
Diagram, illustrating entire activity on in vitro propagation, acclimatization, consecutive macropropagation in vivo and field performance of Dendrocalamus asper
Field performance
Well acclimatized plantlets of 90 days and 180 days old (Fig. 4a) were successfully transferred to the field. After the assessment of survival rate initially, the morphological parameters such as plant height, no of culms/ per bush were assessed. Survival percentage was as high as 93 % in first month and decreased up to 91 % after 6 months of planting. Mortality was higher (around 25 %) among younger (90 days old) plants and almost nil among the 180 days old plants. Successful plantlets in the field produced profuse multiple shoots and grew luxuriantly, became bushy in appearance and gained a plant height of 1.5-2 m, in first year. At that time the average number of culms per bush was 11 (Fig. 4b) (data not presented). Although no thorough care was taken, after four years the plants triumphed over the initial mortality and were well grown with 25 culms per bush attaining an average height of 4.5 m showing 85 % survival (Fig. 4c). It is clear from our study, in vitro-generated D. asper performed very promisingly. The probable reason of quality performance of in vitro-generated plants would be the complete expression of morphogenetic potential from the disease-free, favorable environment in which they were exposed during in vitro propagation (Gantait et al. 2010b) and during their cultivation in vivo.
Fig. 4.
Demonstration on propagation of Dendrocalamus asper. a Plantlets ready to field transfer at 90 days of growth following acclimatization, b Growing avenue of plants in the field at first year, c Well-grown plants in field at 4 years of growth stage
Conclusion
The present protocol is efficient and rapid method which can be adopted commercially for mass multiplication of edible bamboo, D. asper. Also the technology developed may be used to get sustained supply of bamboo, D. asper, which requires raising them on a mass scale for plantations and forestation purposes. The significance of this two step multiplication via micropropagation followed by macropropagation lies in the accelerated rate of multiplication and simplicity of the protocol, both in vitro and in vivo. Commercial exploitation of this protocol for multiplication of this economically important species is possible as demand for fresh explant can be met easily.
Acknowledgement
Authors are indebted to Department of Biotechnology, Govt. of India, for the financial support to carryout the present experiment.
Abbreviations
- ADS
Adenine sulphate
- BAP
6-benzylaminopurine
- IAA
Indole-3-acetic acid
- IBA
Indole-3-butyric acid
- Kn
6-furfurylaminopurine
- MS
Murashige and Skoog (1962)
- PGR
Plant growth regulator
References
- Plant resources of South East Asia No. 7. Bamboos. Leiden: Backhuys Publication; 1995. [Google Scholar]
- Arya S, Sharma S, Kaur R, Arya ID. Micropropagation of Dendrocalamus asper by shoot proliferation using seeds. Plant Cell Rep. 1999;18:879–882. doi: 10.1007/s002990050678. [DOI] [Google Scholar]
- Bisht P, Pant M, Kant A. In vitro propagation of Gigantochloa atroviolaceae Widjaja through nodal explants. J Am Sci. 2010;6:1019–1025. [Google Scholar]
- Das M, Pal A. In vitro regeneration of Bambusa balcooa Roxb.: factors affecting changes of morphogenetic competence in the axillary buds. Plant Cell Tiss Org Cult. 2005;81:109–112. doi: 10.1007/s11240-004-3017-x. [DOI] [Google Scholar]
- Devi WS, Sharma GJ. In vitro propagation of Arundinaria callosa Munro- an edible bamboo from nodal explants of mature plants. The Open Plant Sci J. 2009;3:35–39. [Google Scholar]
- Duncan DB. Multiple range and multiple F test. Biometrics. 1955;11:1–42. doi: 10.2307/3001478. [DOI] [Google Scholar]
- Gantait S, Mandal N. Tissue culture of Anthurium anderanum: a significant review and future prospective. Int J Bot. 2010;6:207–219. doi: 10.3923/ijb.2010.207.219. [DOI] [Google Scholar]
- Gantait S, Mandal N, Bhattacharyya S, Das PK. Sustainable in vitro propagation and clonal fidelity in strawberry. Int J Plant Dev Biol. 2010;4:19–25. [Google Scholar]
- Gantait S, Mandal N, Bhattacharyya S, Das PK. An elite protocol for accelerated quality-cloning in Gerbera jamesonii Bolus cv. Sciella. In Vitro Cell Dev Biol-Plant. 2010;46:537–548. doi: 10.1007/s11627-010-9319-2. [DOI] [Google Scholar]
- Hassan SM. Studies on the structures and growth of bamboo buds in the light of their probable use in tissue culture. Bano Bigyan Patrika. 1980;9:7–16. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Nadgir AL, Phadke CH, Gupta PK, Parsharmi VA, Nair S, Marscarenhas AF. Rapid multiplication of bamboo by tissue culture. Silvae Genet. 1984;33:219–223. [Google Scholar]
- Nirmala C, Ali AH, Badal T. De novo organogenesis in the form of rhizome in Dendrocalamus asper and D. membranaceus. Curr Sci. 2011;100:468–470. [Google Scholar]
- Rao IU, Rao IVR, Narang V. Somatic embryogenesis and regeneration of plants in the bamboo Dendrocalamus strictus. Plant Cell Rep. 1985;4:191–194. doi: 10.1007/BF00269286. [DOI] [PubMed] [Google Scholar]
- Saxena S, Bhojwani SS. In vitro clonal multiplication of 4-year old plants of the bamboo Dendrocalamus longispathus Kurz. In Vitro Cell Dev Biol-Plant. 1993;29:135–142. [Google Scholar]
- Singh S, Kumar P, Ansari SA. A simple method for large-scale propagation of Dendrocalamus asper. Sci Hort. 2004;100:251–255. doi: 10.1016/j.scienta.2003.08.006. [DOI] [Google Scholar]
- Thomas P, Ravindra MB. Effect of pruning or removal of in vitro formed roots on ex vitro root regeneration and growth in micropropagated grapes. Plant Cell Tissue Organ Cult. 1997;51:177–180. doi: 10.1023/A:1005928615179. [DOI] [Google Scholar]