Abstract
The study presents the impact of drought stress on five finger millet varieties (PR202, VL146, VL315, PES400 and VR708), representing contrasting areas of Indian sub-continent. Drought stress induced increase in the activity of superoxide dismutase, ascorbate peroxidase and glutathione reductase was higher in PR202 and VL315, while the activity was lower in the varieties PES400 and VR708. Ascorbate peroxidase : superoxide dismutase ratio, which is a crucial factor in alleviating drought stress, was higher in varieties PR202 and VL315, whilst the varieties PES400 and VR708 exhibited a lower ratio under stress. The variety PES400 recorded maximum stress induced damage, as indicated by higher accumulation of malondialdehyde and hydrogen peroxide; whereas the variety PR202 recorded least stress induced cytotoxic damage. The results clearly indicate that better drought tolerance of the variety PR202 is positively related to the capacity of its antioxidant system to scavenge reactive oxygen species, resulting in a reduced incidence of oxidative damage. Ascorbate peroxidase : superoxide dismutase ratio is found to be a critical factor governing the stress tolerance potential of different varieties. Therefore, varieties PR202 and VL315 were found to be tolerant while PES400 was susceptible to drought stress.
Keywords: Oxidative stress, Drought, APX: SOD, Reactive oxygen species, Finger millet
Introduction
Drought, a situation of severe water deficit, recurrently occurs in various parts of the world, often with devastating effects on crop productivity. Incidentally, the arid and semi-arid zones that are primarily affected by water deficit, have traditionally contributed around 40 per cent of the total production of all categories of food grains (Thakurta 2010). The drought-induced stomatal closure increases the oxidative load on the plant tissues, causing perturbations in biochemical pathways leading to the accumulation of excessive reactive oxygen species (ROS). This oxidative stress results in lipid peroxidation and damage to other important bio-molecules (Sairam and Tyagi 2004). Apart from morphological adaptations to drought stress, plants have evolved a variety of physiological and biochemical processes, which act as components of defense against stress.
Plants protect themselves from drought induced oxidative damage, through an array of anti-oxidative enzymes like superoxide dismutase (SOD), catalase (CAT), guaiacol peroxidase (GPX), ascorbate peroxidase (APX) etc., which act synergistically to limit the levels of reactive oxygen species. Several reports underline a direct relationship between enhanced antioxidant enzyme activities and increased tolerance to environmental stresses (Liu et al. 2011; Sayfzadeh and Rashidi 2011).
Due to increasing incidence of drought, the time is opportune for identifying such crops that are able to tolerate extreme water deficit stress, without having a significant effect on their growth and development. Finger millet (Eleusine coracana) is a staple crop in Africa as well as in India. In India it is cultivated mainly in the tarai regions of Himalayas and the southern peninsula. Finger millet grains are rich in methionine and tryptophan, which is lacking in the diets of poor people living on starchy foods like cassava, plantain, polished rice, and maize meal. Wheat and rice provide food security, but crops like finger millet promise nutritional security for the world. Being a hardy crop, it is relatively easy to grow finger millet under stressful regimes, without hampering the net productivity. Tolerance to drought stress may be attributed to an efficient antioxidant potential and increased signal perception (Puranik et al. 2011). However, there exists a huge variation in the degree of adaptability to drought, among different varieties of finger millet.
As no literature is available which characterizes the antioxidant potential of finger millet, therefore the present experiments were conducted to screen and identify a drought tolerant variety, amongst five different finger millet varieties. The varieties used in the experiments, belong to distinct geographical locations in northern and southern India. PR202 and VR708 are mainly used in the southern peninsula and were developed in Peddapuram, (Andhra Pradesh) and Vijay nagar (Karnataka), respectively. PES400 (Pantnagar variety), VL146 and VL315 (both developed by VPKAS, Almora) are developed for the hilly regions of Uttarakhand. The identified tolerant variety could prove its worth for varietal improvement through molecular breeding and as a source for isolating potent allelic variants of genes implicated in drought tolerance.
Material and methods
Plant material
Growth conditions
Seeds of five varieties of finger millet (PR202, VL146, VL315, PES400 and VR708), were obtained from VPKAS Almorah, Uttarakhand, India. Seeds were washed for 5 min with a mild detergent (Tween-20) and were then surface sterilized for 1 min with 0.5 % sodium hypochlorite. Seeds were germinated in pots containing sand, soil and vermi-compost in 1:2:1 ratio. Seedlings were grown in a poly-house under controlled conditions (at 28 °C with light intensity of 40 μE m−2 s−1) for 45 days. Water deficit stress was imposed on 45-day-old plants, by withholding irrigation, until the soil water content declined progressively to 45 % of the soil water holding capacity, which occurred after 6-days of withholding water. Control plants were irrigated on alternate days and maintained at 75–80 % of soil water holding capacity. Sampling was done after 6 days of imposition of stress, from the top fully emerged young leaves from control and stressed plants, for quantifying various parameters. The stressed plants were re-watered again after collecting samples and then maintained at the soil water conditions between 75 and 80 % of water holding capacity.
Analytical methods
Stress marker analysis
Free proline was determined by the method of Bates et al. (1973). Leaf tissue was homogenized in 3 % sulfosalicylic acid and the chromophore was extracted in toluene. The absorbance of the chromophore was measured at 520 nm. Concentration of proline in the samples was computed from a standard curve of L-proline.
Hydrogen peroxide was measured by the method of Alexieva et al. (2001). Leaf tissue was homogenized in 10 ml of 0.1 % (w/v) aqueous tri-chloro-acetic acid (TCA) and centrifuged at 10,000xg for 30 min at 4 °C. The reaction mixture containing the supernatant, potassium phosphate buffer and KI reagent was incubated for 1 h in dark and subsequently the absorbance was measured at 390 nm. The concentration of H2O2 was calculated using a standard curve of H2O2.
Procedure of Heath and Packer (1968) was followed for measuring the malondialdehyde (MDA) content. Leaf tissue was homogenized in 10 ml of 0.25 % TBA (w/v) prepared in 10 % TCA. The homogenate was heated at 95 °C for 30 min and centrifuged at 10,000xg for 30 min. Absorbance of the supernatant was measured at 532 nm and 600 nm. Absorbance at 600 nm was subtracted from the absorbance at 532 nm for non-specific absorbance. The concentration of MDA was calculated by using an extinction coefficient of 155 mM−1 cm−1.
Electrolyte leakage was measured by immersing 1 g of uniform sized leaf discs in 15 mL of deionized water for 60 min. Percent electrolyte leakage of the sample was estimated by measuring the electrical conductivity (EC) of the water after 60 min (EC1) and after disrupting the cell membrane, by heating the samples at 100 °C for 30 min (EC2). Membrane stability is presented as percent electrolyte leakage = [(EC1/EC2) × 100].
Photosynthesis related parameters
Chlorophyll contents in the leaves of treated plants was measured by the method of Hiscox and Israelstam (1979) and chlorophyll stability index was calculated by dividing the chlorophyll content of stressed plants by chlorophyll content of control plants, and expressed in percentage (Deshmukh et al. 1991).
Enzyme assays
Ascorbate peroxidase activity was determined as described by Chen and Asada (1989). All Enzyme activities were expressed as enzyme units per milligram of protein. One unit of APX activity was defined as the amount of enzyme required to reduce 1 μmol of H2O2 min−1, under assay conditions.
Guaiacol peroxidase activity was determined as described by Urbanek et al. (1991). The increase in absorbance was recorded at 470 nm. The enzyme activity was quantified using a molar extinction coefficient of 26.6 mM−1 cm−1.
Catalase activity was measured according to Beers and Sizer (1952). The decrease in absorbance was recorded at 240 nm. The enzyme activity was calculated by using the H2O2 molar extinction coefficient of 36 M−1 cm−1.
Superoxide dismutase activity was assayed by monitoring the inhibition of photochemical reduction of nitro blue tetrazolium salt (NBT), as described by Beyer and Fridovich (1987). One unit of SOD activity was defined as the amount of enzyme required to cause 50 % inhibition in the reduction of NBT, monitored at 560 nm, under assay conditions.
Glutathione reductase activity was determined by the method of Halliwell and Foyer (1978). Enzyme assay was monitored by recording the decrease in absorbance at 340 nm. Enzyme activity was determined using the molar extinction coefficient for NADPH as 6.2 mM−1 cm−1.
Protein content in all the samples was measured using Bradford dye binding method (Bradford 1976).
Statistical analysis
The data presented are mean values ± SE. Measurements were performed on three replicates for each treatment (n = 3). The data were submitted to factorial analysis of variance (ANOVA), with treatment (control and drought) used for analyzing varieties and the differences between the means were compared using least significant differences at p < 0.05. Different letters denote significant differences among five varieties in control plants (lowercase letters) and under drought stressed conditions (uppercase letters).
Results and discussion
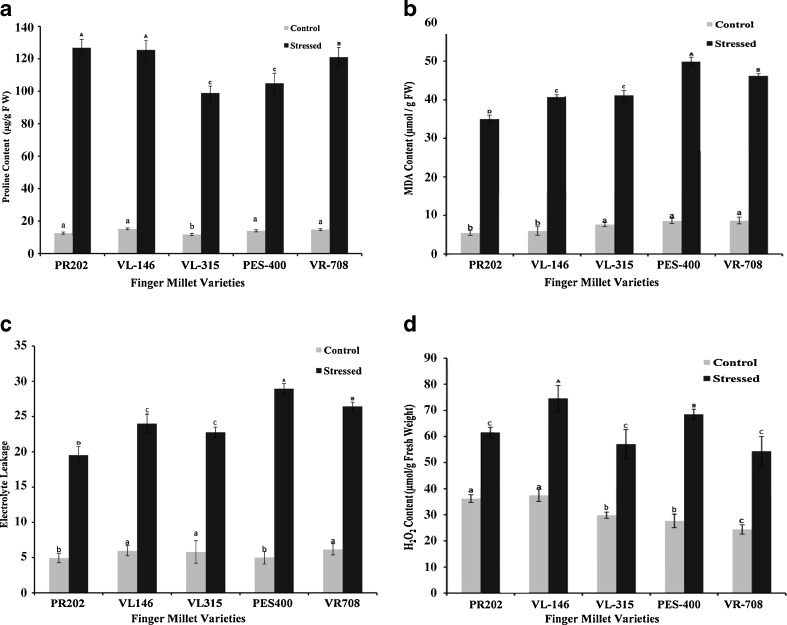
Exposure to drought stress caused a droopy appearance of the shoots, and the leaves starting turning inwards from the outside edges. The extent of these drought induced symptoms varied among different varieties. A significant increase in proline content was recorded in the leaves of stressed plants in all the varieties tested (Fig. 1a) in the present experiments. A maximum increase of 10 fold in proline content was recorded in the variety PR202, followed by VL315 and VL146 which recorded 8.44 and 8.26 fold increase in proline contents, respectively. Increased accumulation of proline under drought stress confers an ability to lower osmotic potential, as well as to protect DNA, enzymes and cellular membranes from oxidative damage. (Liu et al. 2009; Gomes et al. 2010). Thus a higher proline accumulation in PR202, under stress, can be correlated with better stress tolerance characteristics. The variety PES400 recorded the lowest increase of 7.53 fold in proline level under stress, over that of control plants. Varietal differences in stress induced proline were clearly evident in finger millet, indicating a direct correlation between proline accumulation and differential oxidative stress tolerance response among different varieties of finger millet tested (Cao et al. 2011; Liu et al. 2011). Varietal differences in proline accumulation were also observed in wheat seedlings subjected to drought (Yadav et al. 2004).
Fig. 1.
Proline content (a), MDA (b), Electrolyte leakage (c) and H2O2 (d), in different finger millet varieties exposed to drought stress. Different letters denote significant differences (P < 0.05) among five varieties in control plants (lower-case letters) and in drought stressed plants (upper-case letters). Line above bars represents Mean ± standard error
Lipid peroxidation, as reflected by MDA (malondialdehyde) accumulation and electrolyte leakage, is related to the extent of free radical generation in plants. Peroxidation of membrane lipids, especially the unsaturated lipids, is one of the main causes for loss of membrane integrity leading to increased electrolyte leakage. Maximum peroxidative damage under stress was recorded in PES400 (49.87 %), while PR202 recorded minimum damage (35.01 %) under stress (Fig. 1b). The values for lipid peroxidation in VL146, VL315 & VR708 were 40.69, 41.15 and 46.17 respectively. No significant varietal differences in malondialdehyde contents were observed under control conditions. In our study, a lower production of drought induced MDA in PR202 suggests greater protection from oxidative damage, as compared to other varieties like PES400 which recorded extensive stress induced damage. El-Tayeb (2006) has also cited that MDA content was lower in the leaves of drought-tolerant Phaseolus acutifolius than in drought-sensitive Phaseolus vulgaris. Reduced electrolyte leakage under drought stress reflects better membrane integrity and tolerance towards oxidative stress (Liu et al. 2011). Maximum drought induced damage to membrane integrity was recorded in the variety PES400 (Fig. 1c), that showed a 480 % increase in electrolyte leakage under stress. The variety PR202 and VL315 recorded minimum damage to membrane integrity, with a 295 % increase in electrolyte leakage under stress. A lower electrolyte leakage in PR202 and VL315 indicates that these varieties can withstand stress better than the other varieties tested.
Chlorophyll stability index (CSI) is an important parameter that reflects the ability of the affected plant to sustain photosynthesis under stress (Sayed 1999). CSI values were highest for the variety PR202 (86.95 %) followed by VL146 and VL315 at 80.67 % and 74.89 % respectively. A higher CSI value of PR202 indicates that the imposed stress did not have a major detrimental effect on chlorophyll content of the affected plant and translates to sustained photosynthetic machinery. Lowest CSI was recorded for the varieties PES400 (67.65 %) and VR708 (70.07 %), which indicates a susceptible character to drought.
H2O2 is an important signaling molecule and its levels are kept under check by a battery of H2O2 metabolizing enzymes (Kaushal et al. 2011). Any increase in H2O2 has severe consequences for the affected cell. In our experiments, variety PR202 recorded a 70 % increase in H2O2 levels under stress, over the control values, while PES400 recorded a 147 % increase under similar conditions (Fig. 1d), indicating that PES400 is more susceptible to oxidative damage. In terms of absolute H2O2 contents, varieties VL146 and PES400 recorded highest H2O2 accumulation. A higher accumulation of H2O2 under stress signifies sub-optimal functioning of H2O2 metabolizing enzymes. Stress induced H2O2 can also react with superoxide radicals to form highly reactive hydroxyl radicals (Prousek 2007), in metal catalyzed fenton reaction. These hydroxyl radicals initiate self- propagating reactions leading to peroxidation of membrane lipids and destruction of proteins, finally leading to cell death (Jaw and Ching 1998).
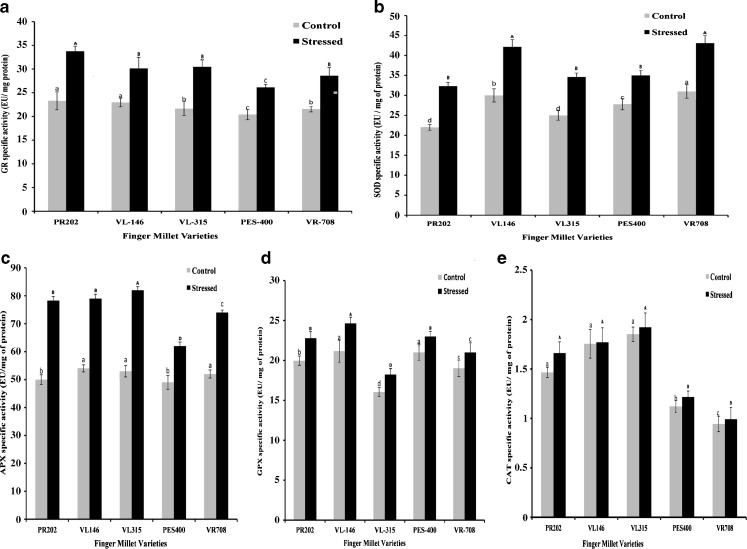
A significant increase in the stress induced activity of GR was recorded in all the varieties tested (Fig. 2a). PR202 and VL315 recorded maximum increase in GR activity under stress. Increase in stress induced GR activity helps to maintain a pool of reduced glutathione, which is one of the main non-enzymatic antioxidant in the cell. The induction of GR specific activity ranged from 28 % in PES400 to 45 % in PR202. Under stress, GR activity increased by 40, 32, and 31 % in the varieties VL315, VR708 and VL146. Stress induced changes in the level of H2O2 have also been reported to alter GR activity (Nahakpam and Shah 2011), that possibly helps in restoring the cellular redox balance. Further, increase in GR activity results in increased availability of NADP+ that can accept electrons from ferrodoxin, thereby minimizing the chances of super oxide formation under stress (Akcay et al. 2010). Thus an increase in GR activity under drought stress resulted in improved protection against oxidative damage.
Fig. 2.
Specific activity of Glutathione reductase (a), Superoxide dismutase (b), Ascorbate peroxidase (c), Guaiacol peroxidase (D) and Catalase (e), in different finger millet varieties exposed to drought stress. Different letters denote significant differences (P < 0.05) among five varieties in control plants (lower-case letters) and in drought stressed plants (upper-case letters). Line above bars represents Mean ± standard error
In such a scenario, an increase in stress induced SOD activity could help to reduce superoxide radicals produced under oxidative stress. An increased SOD activity should positively correlates with a lower risk of cellular damage and increased stress tolerance capabilities. However, H2O2 is a by-product of SOD activity. At physiological pH, H2O2 exists in a neutral form (pKa = 11.8) and hence can easily penetrate through biological membranes (Joshi et al. 2011). Therefore, excessive increase in SOD activity could also prove counter- productive for the system. A significant increase in superoxide dismutase specific activity was recorded under stress, in all the varieties tested (Fig. 2b). A 47 % increase in stress induced SOD activity, over the control values, was recorded in PR202, followed by VL146, VL315 and VR708 which recorded a 40, 39 and 39 % increase, respectively. PES400 recorded a minimal increase of 26 % in SOD activity under stress, as compared to the control. Our results suggest that maximal stress induced SOD activity does not directly correlates with increased stress tolerance. Varieties PR202 and PES400, which showed contrasting characters with respect to their drought tolerance potential, have no statistically significant differences in their SOD activity. In fact PR202 recorded lesser SOD activity than VL146 and VR708 (Fig. 2b). Therefore, an increase in SOD activity could possibly cause an increase in reactive oxygen species, unless matched by an increase in the activity of H2O2 metabolizing enzymes like APX or GPX. A higher activity of H2O2 metabolizing enzymes could efficiently get rid of the excess H2O2 produced (Sayfzadeh and Rashidi 2011).
A significant increase in APX specific activity was recorded in drought stressed plants as compared to the control plants (Fig. 2c), in all the varieties tested. A maximum increase of 56 % in the APX activity was observed in PR202 followed by VL315 which recorded a 54 % increase. The varieties VR708 and PES400 showed an increase of only 42 % and 26 %, respectively under stress. No significant difference in the stress induced APX activity was found among the varieties PR202 and VL146. Increase in specific activity of APX under drought stress is in agreement with the results obtained by Sairam and Srivastava (2000), who have reported a positive correlation between increased H2O2 metabolizing enzyme activities and decreased lipid per-oxidation in drought tolerant plant varieties.
Present experiments indicate that rather than absolute SOD or APX specific activities, APX: SOD activity ratio is of critical importance in determining the stress tolerance potential of different varieties. In case of VR708, PES400 and VL146, the increase in stress induced SOD activity is not matched by a compensatory increase in the activity of the H2O2 metabolizing enzyme i.e. APX. The optimum free radical scavenging activity, as measured through APX: SOD was found to be maximum for the varieties PR202 and VL315 (2.4 each), while PES400 and VR708 recorded a lower APX: SOD ratio of 1.77. The above observations are also corroborated by the results obtained for other important antioxidant parameters, evaluated in the present study.
Drought stress also increased specific activity of GPX in all the varieties tested (Fig. 2d). Increase in GPX activity varied from 9 % in PES400 to a maximum of 16 % in VL146. Varieties VR708 and PES400 recorded minimal increase in GPX activities (10 % and 9 % respectively), under stress. In terms of absolute specific activity values, VL315 recorded lowest GPX activity and VL146 & PR202 recorded maximal GPX activity. It was also observed that the total specific activity of GPX was significantly lower than the APX activity, indicating that the contribution of APX in detoxifying H2O2 is greater than GPX. Similarly, the contribution of CAT in H2O2 catabolism, under drought stress, was found to be inconsequential as compared to APX. No significant change in CAT activity was recorded under stress as compared to the control treatments except in the variety PR202 which recorded a 13 % increase in CAT activity under stress (Fig. 2e). Increase in stress induced CAT activity in other varieties varied from 1 % in VL146 to 8.5 % in PES400. Similar results have also been reported by Akcay et al. (2010) and Joshi et al. (2011), wherein they have shown that APX has a major role in H2O2 metabolism under stress, as compared to CAT and GPX.
The present findings indicate that finger millet plants responded to water deficit stress by enhancing their anti-oxidative capacity. APX: SOD activity ratio and differential accumulation of various osmolytes in the tested varieties suggests that these play an important role in the overall oxidative stress tolerance potential in finger millet. Comparatively higher specific activity of APX under drought stress, as compared to GPX and CAT, further substantiate the role of APX to be of critical importance for the detoxification of stress induced H2O2. Our experiments for the first time clearly indicate that the APX: SOD ratio is of critical importance in determining the level of oxidative stress tolerance in plants.
It is reported that the extent and magnitude of growth recovery after re-watering, depends on the intensity and duration of drought, as prolonged dry spells may cause irreversibly injury to tissues (Xu et al. 2010). In the present experiments on restoring the stressed plants back to 75–80 % of the water holding capacity of the soil mixture, leaves in all the genotypes again turned green over a period of time. PR202 regained near normal phenotype after 5 to 6 days of re-watering; while PES400 took 9–10 days to restore normal phenotype. Although 95 % of the PR202 plants were resurrected, however in case of PES400 only 70 % of the plants could be revived and reached maturity.
Therefore, it can be concluded that finger millet variety PR202 has maximal tolerance towards drought stress, while PES400 was found to be the most susceptible variety. These observations are also supported by the fact that PR202 is a variety that is most prevalent in southern India and is mainly grown in rain-fed conditions under water deficit like conditions. On the other hand, PES400 is recommended for the tarai region of Himalayas, where the conditions are less harsh, in terms of water availability as the area receives sufficient annual rainfall of more than 1,350 mm.
Acknowledgements
The authors (SA and DB) are thankful to the Department of Biotechnology, Govt. of India, for financial support under the research project “Program mode support for agricultural biotechnology”. Help rendered by Dr. B. Saini, Professor of English language, in improving the manuscript is thankfully acknowledged.
References
- Akcay UC, Ercan O, kavas M, Yildiz L, Yilmaz, Oktem A, Yucel M (2010) Drought-induced oxidative damage and antioxidant responses in peanut (Arachis hypogaea L.) seedlings. Plant Growth Regul 61(1):21–28
- Alexieva V, Sergiev I, Mapelli S, Karanov E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001;24:1337–1344. doi: 10.1046/j.1365-3040.2001.00778.x. [DOI] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water–stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Beers RF, Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–140. [PubMed] [Google Scholar]
- Beyer WF, Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in condition. Anal Biochem. 1987;161:559–566. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cao HX, Sun CX, Shao HB, Lei XT. Effects of low temperature and drought on the physiological and growth changes in oil palm seedlings. Afr J Biotechnol. 2011;10(14):2630–2637. [Google Scholar]
- Chen GX, Asada K. Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol. 1989;30:987–998. [Google Scholar]
- Deshmukh PS, Sairam RK, Shukla DS. Measurement of ion leakage as a screening technique for drought resistance in wheat genotypes. Ind J Plant Physiol. 1991;34:89–91. [Google Scholar]
- El-Tayeb MA (2006) Differential responses of pigments, lipid per-oxidation, organic solutes, catalase and per-oxidase activity in the leaves of two Vicia faba L. cultivars to drought. Intern J Agri Biol 8(1):116–122
- Gomes FP, Oliva MA, Mielke MS, Almeida AAF, Aquino LA. Osmotic adjustment, proline accumulation and cell membrane stability in leaves of Cocos nucifera submitted to drought stress. Sci Hortic. 2010;126:379–384. doi: 10.1016/j.scienta.2010.07.036. [DOI] [Google Scholar]
- Halliwell B, Foyer CH. Properties and physiological function of a glutathione reductase purified from spinach leaves by affinity chromatography. Planta. 1978;139:9–17. doi: 10.1007/BF00390803. [DOI] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hiscox JD, Israelstam GF. A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot. 1979;59:1332–1334. doi: 10.1139/b79-163. [DOI] [Google Scholar]
- Jaw NL, Ching HK. Effect of oxidative stress caused by Hydrogen peroxide on senescence of rice leaves. Bot Bull Acad Sin. 1998;39:161–165. [Google Scholar]
- Joshi PK, Saxena SC, Arora S. Characterization of Brassica juncea antioxidant potential under salinity stress. Acta Physiol Plant. 2011;33(3):811–822. doi: 10.1007/s11738-010-0606-7. [DOI] [Google Scholar]
- Kaushal N, Gupta K, Bhandhari K, Kumar S, Thakur P, Nayyar H (2011) Proline induces heat tolerance in chickpea (Cicer arietinum L.) plants by protecting vital enzymes of carbon and antioxidative metabolism. Physiol Mol Bio Plants. doi:10.1007/s12298-011-0078-2 [DOI] [PMC free article] [PubMed]
- Liu ZJ, Zhang XL, Bai JG, Suo BX, Xu PL, Wang L. Exogenous paraquat changes antioxidant enzyme activities and lipid peroxidation in drought-stressed cucumber leaves. Sci Hortic. 2009;121:138–143. doi: 10.1016/j.scienta.2009.01.032. [DOI] [Google Scholar]
- Liu C, Liu Y, Guo K, Dayong Fan D, Li G, Zheng Y, Yu L, Yang R. Effect of drought on pigments, osmotic djustment and antioxidant enzymes in six woody plant species in karst habitats of south western China. Environ Exp Bot. 2011;71:174–183. doi: 10.1016/j.envexpbot.2010.11.012. [DOI] [Google Scholar]
- Nahakpam S, Shah K (2011) Expression of key antioxidant enzymes under combined effect of heat and cadmium toxicity in growing rice seedlings. Plant Growth Regul 63(1):23–35
- Prousek J. Fenton chemistry in biology and medicine. Pure Appl Chem. 2007;79(12):2325–2338. doi: 10.1351/pac200779122325. [DOI] [Google Scholar]
- Puranik S, Jha S, Srivastava PS, Sreenivasulu N, Prasad M. Comparative transcriptome analysis of contrasting foxtail millet cultivars in response to short-term salinity stress. J Plant Physiol. 2011;168:280–287. doi: 10.1016/j.jplph.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Sairam RK, Srivastava GC. Induction of oxidative stress and antioxidant activity by hydrogen peroxide treatment in tolerant and susceptible genotype. Biol Plant. 2000;43(3):381–386. doi: 10.1023/A:1026730008917. [DOI] [Google Scholar]
- Sairam RK, Tyagi A. Physiology and molecular biology of salinity stress tolerance in plants. Curr Sci. 2004;86:407–421. [Google Scholar]
- Sayed AS. Effects of lead and kinetin on the growth, and some physiological components of safflower. Plant Growth Regul. 1999;29:167–174. doi: 10.1023/A:1006216630915. [DOI] [Google Scholar]
- Sayfzadeh S, Rashidi M. Response of antioxidant enzymes activities of sugar beet to drought stress. J Agr Biol Sci. 2011;6:27–33. [Google Scholar]
- Thakurta PG. The magic of millets in drought prone country. Asian Age. 2010;30:1–2. [Google Scholar]
- Urbanek H, Gebarowska K, Herka K. Elicitation of defense responses in bean leaves by Botrytis cinerea polygalacturonase. Acta Phys Plant. 1991;13:43–50. [Google Scholar]
- Xu Z, Zhou G, Shimizu H. Plant responses to drought and rewatering. Plant Signal Behav. 2010;5:649–654. doi: 10.4161/psb.5.6.11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav RS, Sharma RK, Yadav RM. Effect of simulated drought on free proline accumulation in some wheat (Triticum aestivum L.) genotypes. Indian J Agric Biochem. 2004;17:43–44. [Google Scholar]