Abstract
In vitro regeneration of pigeon pea through organogenesis and somatic embryogenesis was demonstrated with pigeon pea cv. JKR105. Embryonic axes explants of pigeon pea showed greater regeneration of shoot buds on 2.5 mg L−1 6-benzylaminopurine (BAP) in the medium, followed by further elongation at lower concentrations. Rooting of shoots was observed on half-strength Murashige and Skoog (MS) medium with 2 % sucrose and 0.5 mg L−1 3-indolebutyric acid (IBA). On the other hand, the regeneration of globular embryos from cotyledon explant was faster and greater with thidiazuron (TDZ) than BAP with sucrose as carbohydrate source. These globular embryos were maturated on MS medium with abscisic acid (ABA) and finally germinated on half-strength MS medium at lower concentrations of BAP. Comparison of regeneration pathways in pigeon pea cv. JKR105 showed that the turnover of successful establishment of plants achieved through organogenesis was more compared to somatic embryogenesis, despite the production of more embryos than shoot buds.
Keywords: Pigeon pea, Organogenesis, Somatic embryogenesis, Cytokinins, Auxins
Introduction
Pigeon pea [Cajanus cajan (L.) Millsp.] (Family: Fabaceae) popularly known as red gram is a grain legume grown in the semi-arid tropics. Its production and productivity, however, are constrained by several abiotic and biotic stresses. Although wild species of pigeon pea may provide genetic diversity for tolerance traits to these stresses, not present in cultivated species, but often are associated with undesirable agronomic traits that are difficult to overcome in conventional plant breeding programs (Muehlbauer 1993). This has necessitated to explore in vitro regeneration protocols for exploitation of plant cell totipotent capacity to micropropagate elite plant clones from differentiated tissues through organogenesis or/and somatic embryogenesis (Davey et al. 1994). The early history of pigeon pea tissue culture has been comprehensively reviewed by Krishna et al. (2010).
In pigeon pea, the regeneration of plants by organogenesis has been reported via pre-existing meristems like apical meristem (Cheema and Bawa 1991), undifferentiated callus (Kumar et al. 1983; George and Eapen 1994), differentiated non-meristematic leaf tissue (Eapen and George 1993; Eapen et al. 1998; Geetha et al. 1998; Singh et al. 2002; Dayal et al. 2003; Villiers et al. 2008); and seedling tissues such as hypocotyl (Cheema and Bawa 1991; Geetha et al. 1998), cotyledon (George and Eapen 1994; Geetha et al. 1998; Mohan and Krishnamurthy 1998; Chandra et al. 2003), cotyledonary node (Mehta and Mohan Ram 1980; Kumar et al. 1983, 1984; Naidu et al. 1995; Shiva Prakash et al. 1994; Geetha et al. 1998; Singh et al. 2003), epicotyl (Kumar et al. 1984; Naidu et al. 1995; Geetha et al. 1998), and embryonal axes (Sarangi and Gleba 1991; George and Eapen 1994; Naidu et al. 1995; Franklin et al. 2000).
Similarly, the somatic embryogenesis has been reported through in vitro cultures from different tissue sources of various pigeon pea cultivars of Asian origin (Nalini et al. 1996; Anbazhagan and Ganapathi 1999; Mohan and Krishnamurthy 2002; Chandra et al. 2003; Singh et al. 2003). Plant regeneration has been achieved via callus (Nalini et al. 1996; Sreenivasu et al. 1998), suspension cultures from leaf-derived callus (Anbazhagan and Ganapathi 1999), and cotyledonary node (Singh et al. 2003). Despite several reports available on organogenesis and somatic embryogenesis using cultivars of Asian and African origin, highly reproducible protocols in the same genotype are quite few. Thus, the present investigation is an attempt to focus the regeneration response by organogenesis and somatic embryogenesis in pigeon pea cv. JKR105.
Materials and methods
Healthy seeds of pigeon pea cv. JKR105 (JK Agri Genetics Ltd, Hyderabad) were surface sterilized by agitating in Erlenmeyer flask containing mercuric chloride (HgCl2), and Triton X-100 (10 μl) for 20 min, followed by 8–10 washes in sterile double distilled water. Subsequently, the seeds were soaked in sterile distilled water for 18 h at 26 ± 2 °C in the dark, drained aseptically, and washed twice in sterile double distilled water. Pre-soaked seeds were inoculated aseptically on cotton wet-bed with MS liquid medium containing 2.5 mg L−1 BAP (6-benzylaminopurine) for germination. Three-day old germinated de-coated seeds were used to excise embryonic axes and cotyledons for studies in organogenesis and somatic embryogenesis, respectively. All the combinations tested for the organogenesis and somatic embryogenesis have been repeated thrice.
Organogenesis
In order to regenerate shoot buds through organogenesis, the embryonic axes explants were cultured on MS basal medium supplemented either alone with BAP, KIN (kinetin), and TDZ (thidiazuron) or in combination of BAP with NAA and IBA (3-indolebutyric acid)]. Scoring was recorded on the explant response, after 4 weeks, for shoot bud induction (Fig. 1). The regenerated shoot buds were cultured for elongation on MS medium by addition of different concentrations of BAP alone (0.1, 0.2, 0.5, 0.7, 1.0, 1.5, 2.0, 2.5, and 5.0 mg L−1), BAP (1.0, 2.0, and 5.0 mg L−1) combined with IAA (indole-3-acetic acid) alone (0.2 and 0.5 mg L−1) or with GA3 (gibberellic acid-3) (2.0 mg L−1) (Table 1). The elongated shoots were transferred later on to the full- and half-strength MS medium, combined with IBA alone (0.25, 0.5, and 2.0 mg L−1) and sucrose. The rooted shoots were finally transferred to plastic bags for hardening and grown to maturity in the greenhouse.
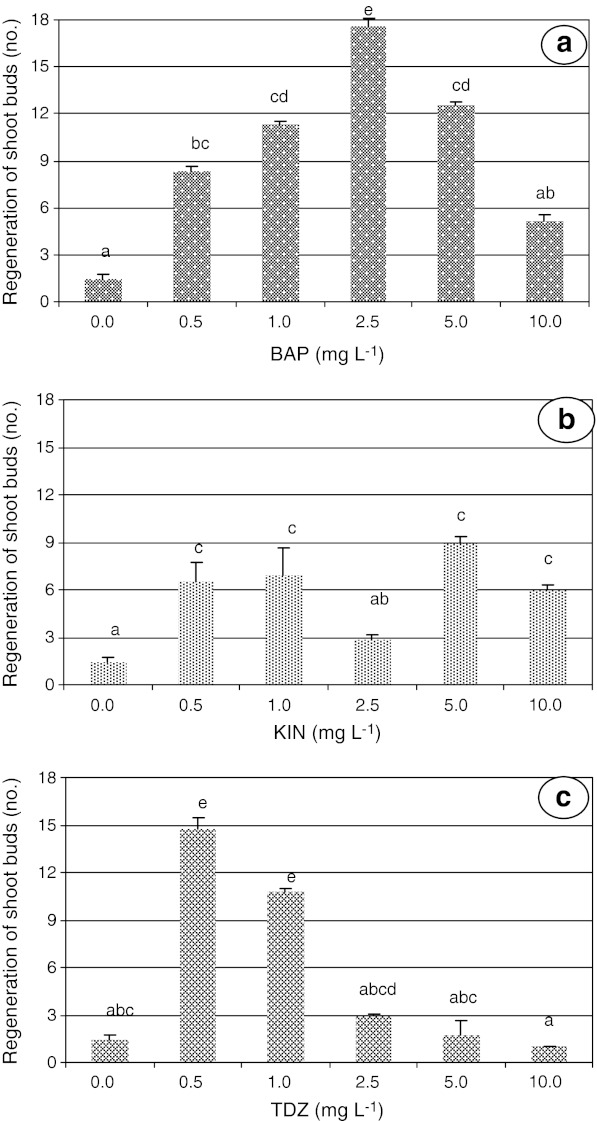
Fig. 1.
Effect of BAP, KIN, and TDZ supplemented to MS medium, at various concentrations, on shoot bud regeneration in pigeon pea
Table 1.
Effect of elongation medium on the regeneration response of shoot buds in pigeon pea
| Elongation medium [Plant growth regulators (mg L−1)] | Shoot length (cm) (Mean + SE) | Elongation (%) | Effect |
|---|---|---|---|
| BAP (0.1) | 2.8 ± 0.20de | 64.12 | No leaf falling and no yellowing of shoot and leaves |
| BAP (0.2) | 3.2 ± 0.20cd | 68.10 | No leaf falling and no yellowing of shoot and leaves |
| BAP (0.5) | 5.5 ± 0.23a | 88.60 | No leaf falling and no yellowing of shoot and leaves |
| BAP (0.7) | 4.5 ± 0.35b | 71.20 | Leaf falling |
| BAP (1.0) | 2.1 ± 0.03fgh | 55.81 | Leaf falling |
| BAP (1.5) | 2.1 ± 0.08fgh | 37.17 | Leaf falling |
| BAP (2.0) | 2.2 ± 0.29fg | 23.00 | Leaf falling |
| BAP (2.5) | 2.2 ± 0.20fg | 21.80 | Leaf falling |
| BAP (5.0) | 1.5 ± 0.14ij | 18.50 | Leaf falling |
| BAP (1.0) + IAA (0.2) | 2.2 ± 0.05fg | 48.30 | Yellowing of shoot and leaves |
| BAP (1.0) + IAA (0.2) + GA3 (2.0) | 2.6 ± 0.11ef | 42.10 | Leaf falling and yellowing of shoot and leaves |
| BAP (1.0) + IAA (0.5) | 2.8 ± 0.05de | 35.20 | Yellowing of shoot and leaves |
| BAP (1.0) + IAA (0.5) + GA3 (2.0) | 3.7 ± 0.08c | 31.20 | Leaf falling and yellowing of shoot and leaves |
| BAP (2.0) + IAA (0.2) | 2.1 ± 0.03fgh | 40.07 | Leaf falling and yellowing of shoot and leaves |
| BAP (2.0) + IAA (0.2) + GA3 (2.0) | 2.2 ± 0.10fg | 21.03 | Leaf falling and yellowing of shoot and leaves |
| BAP (2.0) + IAA (0.5) | 1.6 ± 0.25fghi | 33.33 | No leaf falling and no yellowing of shoot and leaves |
| BAP (2.0) + IAA (0.5) + GA3 (2.0) | 2.2 ± 0.32fg | 21.02 | Leaf falling and yellowing of shoot and leaves |
| BAP (5.0) + IAA (0.2) | 1.3 ± 0.12ijk | 29.17 | Yellowing of shoot and leaves |
| BAP (5.0) + IAA (0.2) + GA3 (2.0) | 1.5 ± 0.08ij | 23.86 | Leaf falling and yellowing of shoot and leaves |
| BAP (5.0) + IAA (0.5) | 0.9 ± 0.08k | 31.11 | No leaf falling and no yellowing of shoot and leaves |
| BAP (0.5) + IAA (0.5) + GA3 (2.0) | 0.8 ± 0.20k | 29.09 | Leaf falling and yellowing of shoot and leaves |
Means followed by the same letter in the column are not significantly different (P < 0.05; LSD)
Somatic embryogenesis
Cotyledon explants were cultured on MS medium supplemented with different concentrations of NAA (0.1, 0.9, 1.8, 3.7, 7.4, 11.1, and 14.8 mg L−1), 2,4-D (0.2, 1.1, 2.2, 4.4, 8.8, 13.2, and 17.6 mg L−1), TDZ (0.2, 0.4, 0.6, 0.8, and 1.0 mg L−1), and BAP (0.2, 1.1, 2.2, 3.3, 4.4, 6.6, and 11.2 mg L−1); and in combination of either BAP (0.1 or 1.0 mg L−1) with 2,4-D (0.2, 1.1, 2.2, 4.4, 8.8, 13.2, and 17.6 mg L−1) or NAA (0.1, 0.9, 1.8, 3.7, 7.4, 11.1, and 14.8 mg L−1). All the media concentrations were additionally mixed with 3.0 % sucrose/glucose. After 45 days of incubation period, the smooth nodular outgrowths evident on the adaxial surface of cotyledon explants enlarged into distinct globular pro-embryoids. The globular embryos derived from TDZ and BAP were transferred and incubated for 30 days on the same medium, hormone-free MS basal, and MS basal media with 1.8 mg L−1 ABA for maturation. Subsequently, the matured embryos were transferred to the hormone-free half-strength MS basal, and 0.5 or 0.1 mg L−1 BAP supplemented to half-strength MS basal media for germination over a period of 15 days. In vitro-germinated somatic embryo plantlets transferred from the culture bottles were gently washed under tap-water to remove the adhered agar and medium. Later, the emblings were transferred to the plastic cups containing autoclaved soils for hardening. They were grown till maturity for acclimatization, under greenhouse conditions.
Statistical analysis
The data was subjected to analysis of variance, and the treatment means were separated using least significant difference (LSD) test at P > 0.05 (Gomez and Gomez 1984).
Results and discussion
Organogenesis
Successful germination was achieved on sterilized cotton-bed containing MS liquid medium with 2.5 mg L−1 BAP rather than MS liquid medium alone (Fig. 4a). This may be due to the triggering action of cytokinins not only for the growth of leaves and lateral buds but also for the development of secondary xylem. Similar results were obtained in pea (Kuraishi and Okumura 1956; Sugiura 2004) and tobacco (Medford et al. 1989). Additionally, the incubation under darkness may also be a key factor in realizing good germination frequency. Such a phenomenon has been attributed to involvement of PhyB noticed in Arabidopsis (Shinomura et al. 1994). In contrast, the initial induction of cells with morphogenetic potential requires dark incubation, while plantlet formation resulted under light conditions in pigeon pea (Franklin et al. 2000). Present results are not in agreement with Romero et al. (2005), who reported high germination frequency in Ehninaceae sp. under 16–24 h light conditions.
Fig. 4.
Regeneration in pigeon pea through organogenesis: a-Seed germination on cotton bed with cytokinin; b and c-Multiple shoot regeneration from embryonic axes explants; d-Regenerated shoot on rooting medium; e-Plantlet during hardening
Shoot bud regeneration
Explant incubation of embryonic axes showed significantly higher regeneration of shoot buds (17.5) with 2.5 mg L−1 BAP (Fig. 1 and Fig. 4b, c). But the regeneration responses of explants at other concentrations of BAP are significantly not different from 1.0 mg L−1. Mehta and Mohan Ram (1980) reported that higher concentration of BAP induced direct shoot regeneration from the cotyledonary surface. While low concentrations of BAP favored the development of shoot buds from the pre-existing meristems (Shiva Prakash et al. 1994; Ignacimuthu et al. 1997). However, higher number of viable plants were regenerated on KIN medium in several cereal crops (Bhaskaran and Smith 1990). In contrast, the present studies demonstrated that 5.0 mg L−1 KIN had low shoot bud regeneration compared to 2.5 mg L−1 BAP and 0.5 mg L−1 TDZ (Fig. 1a, b, c). Similar results were obtained with TDZ compared to 2,4-D, KIN, and NAA in Sugarcane (Saccharum spp.) (Gallo-Meagher et al. 2000), beans (Phaseolus vulgaris) (Malik and Saxena 1992a), chickpea (Cicer arietinum) (George and Eapen 1997), and peanut (Arachis hypogaea) (Kanyand et al. 1994). In pigeon pea, higher regeneration of shoot buds (14.7) from the embryonic axes explants was observed on 0.5 mg L−1 TDZ. Eapen et al. (1998) achieved similar results of high regeneration frequency of shoots from the leaf explants with TDZ. Comparison of regeneration response between 2.5 mg L−1 BAP and 0.5 mg L−1 TDZ showed that the former enhanced greater number of shoots (17.5) than the latter with hyper-hydration. These results are in consonance with those reported in Alfalfa sp. and Coleus sp. with TDZ (Malathi et al. 2006). Further, this phenomenon was attributed to explant inability to degrade and leading to accumulation of TDZ (Mok and Mok 2001).
Better performance of BAP over auxins for shoot regeneration has been reported in pigeon pea (Franklin et al. 2000). Present study also showed that NAA and 2,4-D reduced the regeneration capacity of shoots. Despite higher number of shoots (17.5) retrieved by 2.5 mg L−1 BAP alone, but in combination with 0.5 mg L−1 NAA and 5.0 mg L−1 IBA resulted in poor regeneration by 5.60 and 1.67 shoot buds, respectively (Figs. 2 a–d and 3 a–d). These findings strongly support the multiple shoot regeneration under depleted auxin supplementation in legumes (Ignacimuthu et al. 1997; Franklin et al. 1998). In addition, it may be due to integration of BAP with endogenous auxin concentrations promoted calli regeneration but resulted in subsidized shoot bud formation.
Fig. 2.
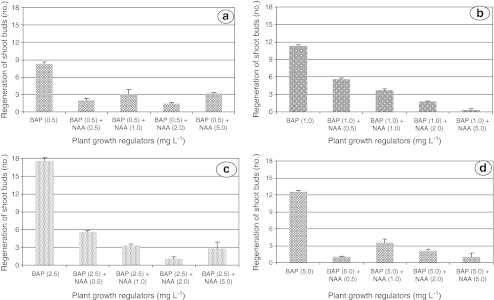
Effect of BAP in combination with NAA supplemented to MS medium at various concentrations on shoot bud regeneration in pigeon pea
Fig. 3.
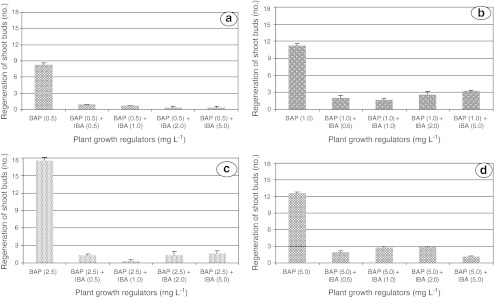
Effect of BAP in combination with IBA supplemented to MS medium at various concentrations on shoot bud regeneration in pigeon pea
Shoot bud elongation
Explants with a mass of shoot bud initials derived from 2.5 mg L−1 BAP sub-cultured on MS basal medium supplemented with BAP alone or combined with IAA and/or GA3, enhanced their elongation. Over an incubation period of 3 weeks, it was observed that 0.5 and 0.7 mg L−1 BAP responded better with a shoot length of 5.5 and 4.5 cm, respectively (Table 1). On the other hand, the explants cultured on <0.5 mg L−1 and >0.5 mg L−1 BAP showed significantly low shoot bud elongation. Furthermore, alteration in media composition (Malik and Saxena 1992b; Shiva Prakash et al. 1994), substitution of plant growth regulators (Mohamed et al. 1991; Nagi et al. 1997), and changes in light conditions had a significant effect on shoot elongation. Franklin et al. (2000) observed that the incubation of embryonal axes of pigeon pea, under light–dark conditions, though favored the shoot bud formation, was less efficient for elongation. IAA alone and combined with GA3 supplemented to 5.0 mg L−1 BAP showed significant reduction in shoot elongation, and conversely had greater elongation on the same combination(s) supplemented with 1.0 mg L−1 BAP. Katsumi and Kazama (1978) reported enhanced auxin biosynthesis by supplementation of GA3, which may become occasionally toxic for growth and shoot elongation (Soetikno 1981). Eapen and George (1993) obtained regeneration of calli from the leaf discs of pigeon pea and peanut, and indicated that BAP + IAA or IAA + aspartic acid are essential for shoot bud elongation. In addition, Kumar et al. (1983) reported regeneration from the leaf callus of pigeon pea on Blaydes (1966) medium with 0.5 mg L−1 BAP, 0.1 mg L−1 NAA, and 0.01 mg L−1 GA3.
Elongated shoot rooting
Elongated shoots derived on BAP showed that all the combinations were ineffective for rooting, due to falling and yellowing of leaves, except half-strength MS supplemented by 0.5 mg L−1 IBA and 2 % sucrose (Table 2). Some pigeon pea genotypes (VBN1, VBN2, SA1, and CO5), however, have prolific rooting on the same medium with 3 % sucrose (Franklin et al. 2000). This genotypic variation may be due to carbon source concentration. Interestingly, in the present investigation, the nodal part embedded in the medium also displayed complete rooting of elongated shoots (Fig. 4d). Whereas the rooting medium supplemented with 3 % sucrose showed vigorous callus growth at the shoot base. However, lower concentrations of sucrose (<1.5 %) in the medium caused leaf yellowing due to carbon deficiency. MS medium combined with 1.0 mg L−1 NAA resulted 90 % rooting (Eapen et al. 1998), and 1 mg L−1 IBA had 80–85 % rooting (Mohan and Krishnamurthy 1998). Finally the rooted shoots were transferred to the autoclaved soil for hardening and grown till maturity (Fig. 4e).
Table 2.
Effect of rooting medium on the regeneration response of shoot explants in pigeon pea
| Rooting medium | Root length (cm) (Mean ± SE) | Symptoms | ||
|---|---|---|---|---|
| MS medium | IBA (mg L−1) | Sucrose (%) | ||
| MS | 0.25 | 3.0 | No response | Callus at shoot base |
| MS | 2.00 | 3.0 | No response | Callus at shoot base |
| half-strength MS | 0.50 | 3.0 | No response | Callus at shoot base |
| MS | 0.25 | 2.0 | No response | Leaf falling from shoot |
| MS | 2.00 | 2.0 | No response | Leaf falling from shoot |
| half-strength MS | 0.50 | 2.0 | 5.9 ± 0.3 | Rooting |
| MS | 0.25 | 1.5 | No response | Leaf yellowing |
| MS | 2.00 | 1.5 | No response | Leaf yellowing |
| half-strength MS | 0.50 | 1.5 | No response | Leaf yellowing |
Somatic embryogenesis
Since embryonic calli exhibit prolific multiplication and somatic embryos originate from single cells, the regeneration approach through embryogenesis was considered as the most preferred pathway for genetic transformation (Hansen and Wright 1999), for which a genotype-independent protocol was standardized by using pigeon pea cv. JKR105. Induction of somatic embryogenesis in pigeon pea has been reported on EC6 basal (Patel et al. 1994; Mohan and Krishnamurthy 2002) and MS basal media (Nalini et al. 1996; Sreenivasu et al. 1998; Singh et al. 2003) for various Asian and African cultivars of pigeon pea.
In the present study, the cotyledon explants produced smooth nodular outgrowths on the adaxial surface were enlarged into distinct globular pro-embryoids. After 15-day incubation period, they began showing swelling in all combinations of the media (Fig. 5a). The induction of globular embryos was observed after 45-day incubation on MS basal medium supplemented with 0.2, 0.4, and 0.6 mg L−1 TDZ; and 0.2, 1.1, 2.2, 3.3, and 4.4 mg L−1 BAP, including addition of sucrose/glucose as a carbon source (Table 3). Present studies demonstrated that the globular embryos regenerated on the surface of cotyledon explants although higher (30.8) on the medium supplemented with 0.6 mg L−1 TDZ were significantly not different from 0.4 and 0.2 mg L−1 TDZ (Fig. 5b). Similarly, 1.1 mg L−1 BAP yielded 13.9 globular embryos, but did not vary irrespective of lower and higher concentrations (0.2, 2.2, 3.3, and 4.4 mg L−1). TDZ has been used to elicit multiple shoot formation in a broad range of plant species (Malik and Saxena 1992a,b), and somatic embryogenesis in a few crop species (Gill and Saxena 1993; Gill et al. 1993; Lu 1993). While other studies indicated successful utilization of cytokinins for somatic embryogenesis not only in pigeon pea (Patel et al. 1994; Sreenivasu et al. 1998; Mohan and Krishnamurthy 2002; Chandra et al. 2003; Singh et al. 2003) but also other leguminous crops (Maheswaran and Williams 1984; Gill and Saxena 1992; Malik and Saxena 1992b; Murthy et al. 1996).
Fig. 5.
Regeneration in pigeon pea through somatic embryogenesis: a-Cotyledon explants on TDZ medium; b-Somatic embryo regeneration on the surface of cotyledons; c-Somatic embryo on ABA maturation medium; d-Somatic embryo germination; e-Embling during hardening
Table 3.
Combined effect of plant growth regulators and carbohydrates in MS medium on the response of somatic embryogenesis and globular embryos in pigeon pea
| Plant growth regulator | Sucrose | Glucose | |||
|---|---|---|---|---|---|
| Plant growth regulator | Concentration (mg L−1) | Type of response | Globular embryos explant−1 (no.) (Mean ± SE) | Type of response | Globular embryos explant−1 (no.) (Mean ± SE) |
| 2,4-D | 0.2 | Callus | ….. | Callus | ….. |
| 2,4-D | 1.1 | Callus | ….. | Callus | ….. |
| 2,4-D | 2.2 | Callus | ….. | Callus | ….. |
| 2,4-D | 4.4 | Callus | ….. | Callus | ….. |
| 2,4-D | 8.8 | Callus | ….. | Callus | ….. |
| 2,4-D | 13.2 | Callus | ….. | Callus | ….. |
| 2,4-D | 17.6 | Callus | ….. | Callus | ….. |
| BAP | 0.2 | Somatic embryo | 10.6 ± 1.0d | Somatic embryo | 9.8 ± 0.4de |
| BAP | 1.1 | Somatic embryo | 13.9 ± 0.7d | Somatic embryo | 12.8 ± 0.6d |
| BAP | 2.2 | Somatic embryo | 12.8 ± 0.8d | Somatic embryo | 11.7 ± 0.5de |
| BAP | 3.3 | Somatic embryo | 7.9 ± 0.6d | Somatic embryo | 6.6 ± 0.7e |
| BAP | 4.4 | Somatic embryo | 8.6 ± 0.5d | Somatic embryo | 7.9 ± 0.3de |
| BAP | 6.6 | – | – | ….. | |
| BAP | 11.2 | – | – | ….. | |
| NAA | 0.1 | Callus | ….. | Callus | ….. |
| NAA | 0.9 | Callus | ….. | Callus | ….. |
| NAA | 1.8 | Callus | ….. | Callus | ….. |
| NAA | 3.7 | Callus | ….. | Callus | ….. |
| NAA | 7.4 | Callus | ….. | Callus | ….. |
| NAA | 11.1 | Callus | ….. | Callus | ….. |
| NAA | 14.8 | Callus | ….. | Callus | ….. |
| TDZ | 0.2 | Somatic embryo | 26.4 ± 0.7ab | Somatic embryo | 19.8 ± 0.2abc |
| TDZ | 0.4 | Somatic embryo | 25.0 ± 1.4abc | Somatic embryo | 21.1 ± 1.1ab |
| TDZ | 0.6 | Somatic embryo | 30.8 ± 0.7a | Somatic embryo | 23.1 ± 0.7a |
| TDZ | 0.8 | Callus | ….. | Callus | ….. |
| TDZ | 1.0 | Callus | ….. | Callus | ….. |
Means followed by the same letter in the column are not significantly different (P < 0.05; LSD)
The MS medium comprising TDZ and BAP was found less effective for globular embryo formation with glucose than sucrose. This was evident when glucose supplemented to the MS medium by regenerating 19.8, 21.1, and 23.1 globular embryos with 0.2, 0.4, and 0.6 mg L−1 TDZ; and 12.8, 11.7, 6.6, and 7.9 embryos on 1.1, 2.2, 3.3, and 4.4 mg L−1 BAP (Table 3). Although it has been reported that among the carbohydrates, sucrose was highly effective for the induction of somatic embryogenesis (Anbazhagan and Ganapathi 1999), higher concentrations of cytokinins also promoted somatic embryogenesis in pigeon pea (Patel et al. 1994). In contrast, the present results demonstrated that higher concentrations of cytokinins did not favor embryo induction, but resulted in more callusing. On the other hand, somatic embryogenesis was effective with glucose in Phaseolus coccineus (Genga and Allavena 1991); maltose in Alfalfa sp. (Strickland et al. 1987; Denchev et al. 1991) and Glycine max (Finer and McMullen 1991); and the culture maintenance in darkness for many crop species (Thorpe 1988). In contrast, somatic embryogenesis was predominantly seen when incubated at 16 L: 8D.
The combination of auxins (2,4-D and NAA) and cytokinin (BAP), at different concentrations, supplemented to the MS medium displayed the formation of white/green colored calli. In contrast, somatic embryogenesis has been reported on medium containing NAA and BAP (Nalini et al. 1996), and also in combination of BAP, KIN, and Ads (adenine sulphate) (Patel et al. 1994). This type of genotypic variability in response to induction of somatic embryos was reported in pigeon pea (Patel et al. 1994; Sreenivasu et al. 1998; Mohan and Krishnamurthy 2002; Singh et al. 2003), soybean (Barwale et al. 1986; Komatsuda and Ohyama 1988; Parrott et al. 1989; Bailey et al. 1993), and peanut (Sellars et al. 1990; Ozias-Akins et al. 1992; George and Eapen 1993; Baker et al. 1995; McKently 1995; Chengalrayan et al. 1998).
In pigeon pea, the somatic embryo formation has been observed on calli derived from cotyledon and leaf explants (Sreenivasu et al. 1998), and from suspension culture of leaf explants (Anbazhagan and Ganapathi 1999). Interestingly, the direct appearance of globular embryos on cotyledon explants has been noticed without callus formation. Normal embryos without plantlets were achieved from immature cotyledon and embryonal axes explants on auxin supplemented medium (George and Eapen 1994).
Cotyledon structure development
The globular embryos did not develop further when the cotyledon explants with globular embryos transferred to the same, hormone-free, and MS basal media. These observations suggest that TDZ may not be ideal for further development of globular embryos and considered as lethal for embryo development. Similar results were reported in Geranium sp. (Visser-Tenyenhuis et al. 1994). In contrast, Sreenivasu et al. (1998) observed the induction of somatic embryos via callus from the leaf explants on TDZ. Our results showed that the development of globular embryos to cotyledon structures was evident on the MS medium supplemented with 1.8 mg L−1 ABA (Fig. 5c). The maturation of normal cotyledonary embryos was 20 %, while the remaining embryos either formed callus or turned necrotic. The maturation of somatic embryos on ABA medium was reported for pigeon pea (Mohan and Krishnamurthy 2002), Phaseolus sp (Malik and Saxena 1992a), and chickpea (Suhasini et al. 1994).
Somatic embryo germination
Culturing of mature cotyledonary somatic embryos on MS basal medium supplemented with 1.8 mg L−1 ABA yielded good response for germination on half-strength MS medium augmented with 0.1 mg L−1 BAP (Fig. 5d). The development of shoots and roots was extremely poor on hormone free half-strength MS medium. Whereas Singh et al. (2003) observed good germination of somatic embryos on the same medium. While the addition of 0.5 mg L−1 BAP to half-strength MS medium has promoted the shoot formation and cotyledon swelling without root development; and 0.1 mg L−1 BAP produced both shoot and root systems with 20 % frequency (Fig. 5d). These results are in consonance with Mohan and Krishnamurthy (2002), who reported 22 % germination of somatic embryos on the same medium in pigeon pea cv. T-15-15, GAUT-82-90 and GAUT-82-99. Finally the embling with sufficient root and shoot system were transferred to the autoclaved soil for hardening (Fig. 5e).
Comparison of regeneration frequencies on embryo formation and shoot buds, respectively from cotyledon and embryonic axes explants of pigeon pea cv. JKR105 indicated that the former pathway was highly efficient (Fig. 1, Table 3). But subsequent in vitro development of embryos caused low yield in final emblings due to initial realization of 20 % cotyledonary stage embryos on ABA medium, followed by 20 % germination frequency. These studies suggest using any of these regeneration pathways with pigeon pea cv. JKR105, for further improvement through genetic transformation by using Bt gene(s) for resistance to the pod borer, Helicoverpa armigera.
Acknowledgements
Authors express their indebtedness to Shri PS Dravid (President, JK Agri Genetics Ltd., Hyderabad, India), Shri SK Gupta (COO, JK Agri Genetics Ltd., Hyderabad, India) for providing necessary facilities. The author GK is thankful to Dr BU Singh (Advisor, JK Agri Genetics Ltd., Hyderabad, India) for guidance, valuable suggestions, continuous encouragement, and help extended in the manuscript preparation; and Dr Manish K. Patel (Associate Breeder, JKAL, Hyderabad, India) for providing the seed of pigeon pea cv. JKR105.
References
- Anbazhagan VR, Ganapathi A. Somatic embryogenesis in cell suspension cultures of pigeon pea (Cajanus cajan L. Millsp.) Plant Cell Tiss Org Cult. 1999;56:179–184. doi: 10.1023/A:1006258911079. [DOI] [Google Scholar]
- Bailey MA, Parrott WA, Boerma HR. Genotype effects on proliferative embryogenesis and plant regeneration of soybean. In Vitro Cell Dev Biol-Plant. 1993;29:102–108. doi: 10.1007/BF02632279. [DOI] [Google Scholar]
- Baker CM, Durham RE, Burns JA, Parrott WA, Wetzstein HY. High frequency of somatic embryogenesis in peanut (Arachis hypogaea L.) using mature dry seed. Plant Cell Rep. 1995;15:38–42. doi: 10.1007/BF01690250. [DOI] [PubMed] [Google Scholar]
- Barwale UB, Kerns HR, Widholm JM. Plant regeneration from callus cultures of several soyabean genotypes via embryogenesis and organogenesis. Planta. 1986;167:473–481. doi: 10.1007/BF00391223. [DOI] [PubMed] [Google Scholar]
- Bhaskaran S, Smith RH. Regeneration in cereal tissue culture: A review. Crop Sci. 1990;30:1328–1336. doi: 10.2135/cropsci1990.0011183X003000060034x. [DOI] [Google Scholar]
- Blaydes DF. Interaction of kinetin and various inhibitors in the growth of soybean tissue. Physiol Planta. 1966;19:748–753. doi: 10.1111/j.1399-3054.1966.tb07060.x. [DOI] [Google Scholar]
- Chandra A, Gupta V, Burma P, Pental D. Patterns of morphogenesis from cotyledon explants of pigeon pea. In Vitro Cell Dev Biol-Plant. 2003;39:514–519. doi: 10.1079/IVP2003437. [DOI] [Google Scholar]
- Cheema HK, Bawa J. Clonal multiplication via multiple shoots in some legumes (Vigna unguiculata and Cajanus Cajan) Acta Hort. 1991;289:93–94. [Google Scholar]
- Chengalrayan K, Mhaske VB, Hazra S. Genotypic control of peanut somatic embryogenesis. Plant Cell Rep. 1998;17:522–525. doi: 10.1007/s002990050435. [DOI] [PubMed] [Google Scholar]
- Davey MR, Kumar V, Hammatt N. In vitro methods for legumes. In: Vasil IK, Thorpe TA, editors. Plant cell and tissue culture. Dordrecht: Kluwer; 1994. pp. 313–329. [Google Scholar]
- Dayal S, Lavanya M, Devi P, Sharma KK. An efficient protocol for shoot regeneration and genetic transformation of pigeon pea (Cajanus cajan (L.) Millsp.) by using leaf explants. Plant Cell Rep. 2003;21:1072–1079. doi: 10.1007/s00299-003-0620-y. [DOI] [PubMed] [Google Scholar]
- Denchev P, Velcheva M, Atanassov A. A new approach to direct somatic embryogenesis in Medicago. Plant Cell Rep. 1991;10:338–341. doi: 10.1007/BF00193154. [DOI] [PubMed] [Google Scholar]
- Eapen S, George L. Plant regeneration from leaf discs of peanut and pigeon pea: Influence of benzyladenine, indole acetic acid and indoleacetic acid-amino acid conjugates. Plant Cell Tiss Org Cult. 1993;35:223–227. doi: 10.1007/BF00037274. [DOI] [Google Scholar]
- Eapen S, Tivarekar S, George L. Thidiazuron-induced shoot regeneration in pigeon pea (Cajanus cajan L.) Plant Cell Tiss Org Cult. 1998;53:217–220. doi: 10.1023/A:1006060318752. [DOI] [Google Scholar]
- Finer JJ, McMullen MD. Transformation of soybean via particle bombardment of embryogenic suspension culture tissue. In Vitro Cell Dev Biol-Plant. 1991;27:175–182. doi: 10.1007/BF02632213. [DOI] [Google Scholar]
- Franklin G, Jeyachandran R, Melchias G, Ignacimuthu S. Multiple shoot induction and regeneration of pigeon pea (Cajanus cajan (L.) Millsp.) cv. Vamban: from apical and axillary meristem. Curr Sci. 1998;74:936–937. [Google Scholar]
- Franklin G, Jeyachandran R, Ignicimuthu S. Factors affecting regeneration of pigeon pea (Cajanus cajan L. Millsp) from mature embryonal axes. Plant Grow Reg. 2000;30:31–36. doi: 10.1023/A:1006394402210. [DOI] [Google Scholar]
- Gallo-Meagher M, English RG, Abouzid A. Thidiazuron promotes shoot regeneration of sugarcane embryogenic callus. In Vitro Cell Dev Biol-Plant. 2000;36:37–40. doi: 10.1007/s11627-000-0009-3. [DOI] [Google Scholar]
- Geetha N, Venkatachalam P, Prakash V, Lakshmi Sita G. High frequency induction of multiple shoots and plant regeneration from seedling explants of pigeon pea (Cajanus cajan L.) Curr Sci. 1998;75:1036–1041. [Google Scholar]
- Genga A, Allavena A. Factors affecting morphogenesis from immature cotyledon of Phaseolus coccineus L. Plant Cell Tiss Org Cult. 1991;27:189–196. doi: 10.1007/BF00041289. [DOI] [Google Scholar]
- George L, Eapen S. Influence of genotype and explant source on somatic embryogenesis in peanut. Oleagineux. 1993;48:361–363. [Google Scholar]
- George L, Eapen S. Organogenesis and embryogenesis from diverse explants in pigeon pea (Cajanus cajan L.) Plant Cell Rep. 1994;13:417–420. doi: 10.1007/BF00234150. [DOI] [PubMed] [Google Scholar]
- George L, Eapen S. Influence of cytokinins and explant type on plant regeneration in chickpea (Cicer arietinum L.) Physiol Mol Biol Plants. 1997;3:129–134. [Google Scholar]
- Gill R, Saxena PK. Direct somatic embryogenesis and regeneration of plants from seedlings explants of peanut (Arachis hypogaea L.): promotive role of thidiazuron. Can J Bot. 1992;70:1186–1192. doi: 10.1139/b92-147. [DOI] [Google Scholar]
- Gill R, Saxena PK. Somatic embryogenesis in Nicotiana tabacum L: Induction by thidiazuron of direct embryo differentiation from cultured leaf discs. Plant Cell Rep. 1993;12:154–159. doi: 10.1007/BF00239097. [DOI] [PubMed] [Google Scholar]
- Gill R, Gerrath JM, Saxena PK. High frequency direct somatic embryogenesis in thin layer cultures of hybrid seed geranium (Pelargonium hortorum) Can J Bot. 1993;71:408–413. doi: 10.1139/b93-045. [DOI] [Google Scholar]
- Gomez KA, Gomez AA. Statistical procedures for agricultural research. 2. New York: John Wiley and Sons; 1984. [Google Scholar]
- Hansen G, Wright MS. Recent advances in the transformation of plants. Trends Plant Sci. 1999;4:226–231. doi: 10.1016/S1360-1385(99)01412-0. [DOI] [PubMed] [Google Scholar]
- Ignacimuthu S, Franklin G, Melchias G. Multiple shoot formation and in vitro fruiting from cotyledonary nodes of Vigna mungo L. Hepper. Curr Sci. 1997;73:733–735. [Google Scholar]
- Kanyand M, Desai AP, Prakash CS. Thidiazuron promotes high frequency regeneration of peanut (Arachis hypogaea) plants in vitro. Plant Cell Rep. 1994;14:1–5. doi: 10.1007/BF00233288. [DOI] [PubMed] [Google Scholar]
- Katsumi M, Kazama H. Gibberellin control of cell elongation in cucumber hypocotyl sections. Bot Mag (Tokyo) Special Issue. 1978;1:141–158. [Google Scholar]
- Komatsuda T, Ohyama K. Genotypes of high competence for somatic embryogenesis and plant regeneration in soybean Glycine max. Theor App Gen. 1988;75:695–700. [Google Scholar]
- Krishna G, Reddy PS, Ramteke PW, Bhattacharya PS. Progress of tissue culture and genetic transformation research in pigeon pea [Cajanus cajan (L.) Millsp.] Plant Cell Rep. 2010;29:1079–1095. doi: 10.1007/s00299-010-0899-4. [DOI] [PubMed] [Google Scholar]
- Kumar AS, Reddy TP, Reddy GM. Plantlet regeneration from different callus cultures of pigeon pea (Cajanus cajan L.) Plant Sci Lett. 1983;32:271–278. doi: 10.1016/0304-4211(83)90032-9. [DOI] [Google Scholar]
- Kumar AS, Reddy TP, Reddy GM. Multiple shoots from cultured explants of pigeon pea and Atylasia species. SABRAO J. 1984;16:101–105. [Google Scholar]
- Kuraishi S, Okumura FS. The effect of kinetin on leaf growth. Bot Mag (Tokyo) 1956;69:300–306. [Google Scholar]
- Lu CY. The use of thidiazuron in tissue culture. In Vitro Cell Dev Biol-Plant. 1993;29:92–96. doi: 10.1007/BF02632259. [DOI] [Google Scholar]
- Maheswaran G, Williams EG. Direct somatic embryoid formation on immature embryos of Trifolium repens, T. pratense and Medicago sativa and rapid clonal propogation of T. repens. Ann Bot. 1984;54:201–211. [Google Scholar]
- Malathi S, Vasanthi N, Rajasekharan R. Potential application of urea-derived herbicides as cytokinins in plant tissue culture. J Biol Sci. 2006;31:599–605. doi: 10.1007/BF02708412. [DOI] [PubMed] [Google Scholar]
- Malik KA, Saxena PK. Regeneration in Phaseolus vulgaris: high frequency induction of direct shoot formation in intact seedlings by N6 benzylaminopurine and thidiazuron. Planta. 1992;186:384–389. doi: 10.1007/BF00195319. [DOI] [PubMed] [Google Scholar]
- Malik KA, Saxena PK. Thidiazuron induces high frequency shoot regeneration in intact seedlings of pea (Pisum sativum), chickpea (Cicer arietinum) and lentil (Lens culinaris) Aust J Plant Physiol. 1992;19:731–740. doi: 10.1071/PP9920731. [DOI] [Google Scholar]
- McKently AH. Effect of genotype on somatic embryogenesis from axes of mature peanut embryos. Plant Cell Tiss Org Cult. 1995;42:251–254. doi: 10.1007/BF00029995. [DOI] [Google Scholar]
- Medford JI, Horgan R, El-Sawi Z, Klee HJ. Alterations of endogenous cytokinins in transgenic plants using a chimeric isopentenyl transferase gene. Plant Cell. 1989;1:403–413. doi: 10.1105/tpc.1.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta U, Mohan Ram HY. Regeneration of plantlets from the cotyledons of Cajanus cajan L. Ind J Exp Biol. 1980;18:800–802. [Google Scholar]
- Mohamed MF, Read PE, Coyne DP. Plant regeneration in vitro from the embryonic axes of common and tepary beans. Bean Improv Coop Bull. 1991;34:149–153. [Google Scholar]
- Mohan ML, Krishnamurthy KV. Plant regeneration in pigeon pea (Cajanus cajan (L.) Millsp.) by organogenesis. Plant Cell Rep. 1998;17:705–710. doi: 10.1007/s002990050469. [DOI] [PubMed] [Google Scholar]
- Mohan ML, Krishnamurthy KV. Somatic embryogenesis and plant regeneration in pigeon pea. Biol Planta. 2002;45:19–25. doi: 10.1023/A:1015134725621. [DOI] [Google Scholar]
- Mok DWS, Mok MC. Cytokinin metabolism and action. Ann Rev Plant Physiol Mol Bio. 2001;2:89–118. doi: 10.1146/annurev.arplant.52.1.89. [DOI] [PubMed] [Google Scholar]
- Muehlbauer FJ. Use of wild species as a source of resistance in cool-season food legumes. In: Singh KB, Saxena MC, editors. Breeding for Stress Tolerance in Cool Season Food Legumes, International Center for Agricultural Research in the Dry Areas (ICARDA) Chichester: John Wiley and Sons; 1993. pp. 359–372. [Google Scholar]
- Murthy BNS, Victor J, Singh RP, Fletcher RA, Saxena PK. In vitro regeneration of chickpea (Cicer arietinum L.): Stimulation of direct organogenesis and somatic embryogenesis by thidiazuron. Plant Grow Reg. 1996;19:233–240. doi: 10.1007/BF00037796. [DOI] [Google Scholar]
- Nagi W, Ignacimuthu S, Becker J. Genetic engineering and regeneration of Phaseolus and Vigna. State the art and new attempts. J Plant Physiol. 1997;150:625–644. [Google Scholar]
- Naidu RB, Kulkarni DD, Krishnamurthy KV. Genotype dependent morphogenic potentiality of various explants of a food legume, the pigeon pea (Cajanus cajan L.) In Vitro Cell Dev Biol- Plant. 1995;31:26–30. doi: 10.1007/BF02632222. [DOI] [Google Scholar]
- Nalini M, Reena MJT, Sastri DC, Moss JP. Somatic embryogenesis in pigeon pea (Cajanus cajan L. Millsp.) Ind J Exp Biol. 1996;34:282–284. [Google Scholar]
- Ozias-Akins P, Anderson WF, Holbrook CC. Somatic embryogenesis in Arachis hypogaea L: genotype comparison. Plant Sci. 1992;83:103–111. doi: 10.1016/0168-9452(92)90067-V. [DOI] [Google Scholar]
- Parrott WA, Hoffman LM, Hildebrandt DF, Williams EG, Collins GB. Recovery of primary transformants of soybean. Plant Cell Rep. 1989;7:615–617. doi: 10.1007/BF00272042. [DOI] [PubMed] [Google Scholar]
- Patel DB, Barve DM, Nagar N, Mehta AR. Regeneration of pigeon pea (Cajanus cajan) somatic embryogenesis. Ind. J Exp Biol. 1994;32:740–744. [Google Scholar]
- Romero FR, Delate K, Hannapel DJ. The effect of seed source, light during germination, and cold-moist stratification on seed germination in three species of Echinacea for organic production. Hortic Sci. 2005;40:1751–1754. [PMC free article] [PubMed] [Google Scholar]
- Sarangi BK, Gleba YY. Direct multiple regeneration in Cajanus cajan (L.) Millsp. Acta Hortic. 1991;289:149–150. [Google Scholar]
- Sellars RM, Southward GM, Phillips GC. Adventitious somatic embryogenesis from cultured immature zygotic embryos of peanut using soybean as a model system. Crop Sci. 1990;30:408–414. doi: 10.2135/cropsci1990.0011183X003000020035x. [DOI] [Google Scholar]
- Shinomura T, Nagatani A, Chory J, Furuya M. The induction of seed germination in Arabidopsis thaliana is regulated principally by phytochrome B and secondarily by phytochrome A1. Plant Physiol. 1994;104:363–371. doi: 10.1104/pp.104.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiva Prakash N, Pental D, Bhalla-Sarin N. Regeneration of pigeon pea (Cajanus cajan) from cotyledonary node via multiple shoot formation. Plant Cell Rep. 1994;13:623–627. doi: 10.1007/BF00232933. [DOI] [PubMed] [Google Scholar]
- Singh ND, Sahoo L, Sonia JPK. In vitro shoot organogenesis and plant regeneration from cotyledonary node and leaf explants of pigeon pea (Cajanus cajan L. Millsp.) Physiol Mol Biol-Plants. 2002;8:133–140. [Google Scholar]
- Singh ND, Sahoo L, Neera BS, Jaiwal PK. The effect of TDZ on organogenesis and somatic embryogenesis in pigeon pea (Cajanus cajan L. Millsp) Plant Sci. 2003;164:341–347. doi: 10.1016/S0168-9452(02)00418-1. [DOI] [Google Scholar]
- Soetikno SS. Germination of turions in potamogeton berchtold II. Bot Gaz. 1981;142:454–460. doi: 10.1086/337246. [DOI] [Google Scholar]
- Sreenivasu K, Malik SK, Ananda KP, Sharma RP. Plant regeneration via somatic embryogenesis in pigeon pea (Cajanus cajan (L.) Millsp.) Plant Cell Rep. 1998;17:294–297. doi: 10.1007/s002990050395. [DOI] [PubMed] [Google Scholar]
- Strickland SG, Nichol JW, McCall CM, Stuart DA. Effects of carbohydrate source on alfalfa somatic embryogenesis. Plant Sci. 1987;48:113–121. doi: 10.1016/0168-9452(87)90138-5. [DOI] [Google Scholar]
- Sugiura H. Effects of 6-benzylaminopurine and ethephon applications on flowering and morphology in summer-to-autumn-flowering chrysanthemum under open field conditions. J Pestic Sci. 2004;29:308–312. doi: 10.1584/jpestics.29.308. [DOI] [Google Scholar]
- Suhasini K, Sagare AP, Krishnamurthy KV. Direct somatic embryogenesis from mature axes in chickpea. Plant Sci. 1994;102:189–194. doi: 10.1016/0168-9452(94)90037-X. [DOI] [Google Scholar]
- Thorpe TA. In vitro somatic embryogenesis. ISI Atlas of Sci-Animal and Plant Sci. 1988;1:81–88. [Google Scholar]
- Villiers SD, Emongor Q, Njeri R, Gwata E, Hoisington D, Njagi I, Silim S, Sharma KK. Evaluation of the shoot regeneration response in tissue culture of pigeon pea (Cajanus cajan [L.] Millsp.) varieties adapted to eastern and southern Africa. Afr J Biotechnol. 2008;7:587–590. [Google Scholar]
- Visser-Tenyenhuis C, Murthy BNS, Odumeru J, Saxena PK. Modulation of somatic embryogenesis in hypocotyl-derived culture of geranium (Pelargonium x hortorum Bailey) cv. Ringo Rose by a bacterium. In vitro Cell Dev Biol-Plant. 1994;30:140–143. doi: 10.1007/BF02632203. [DOI] [Google Scholar]