Abstract
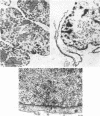
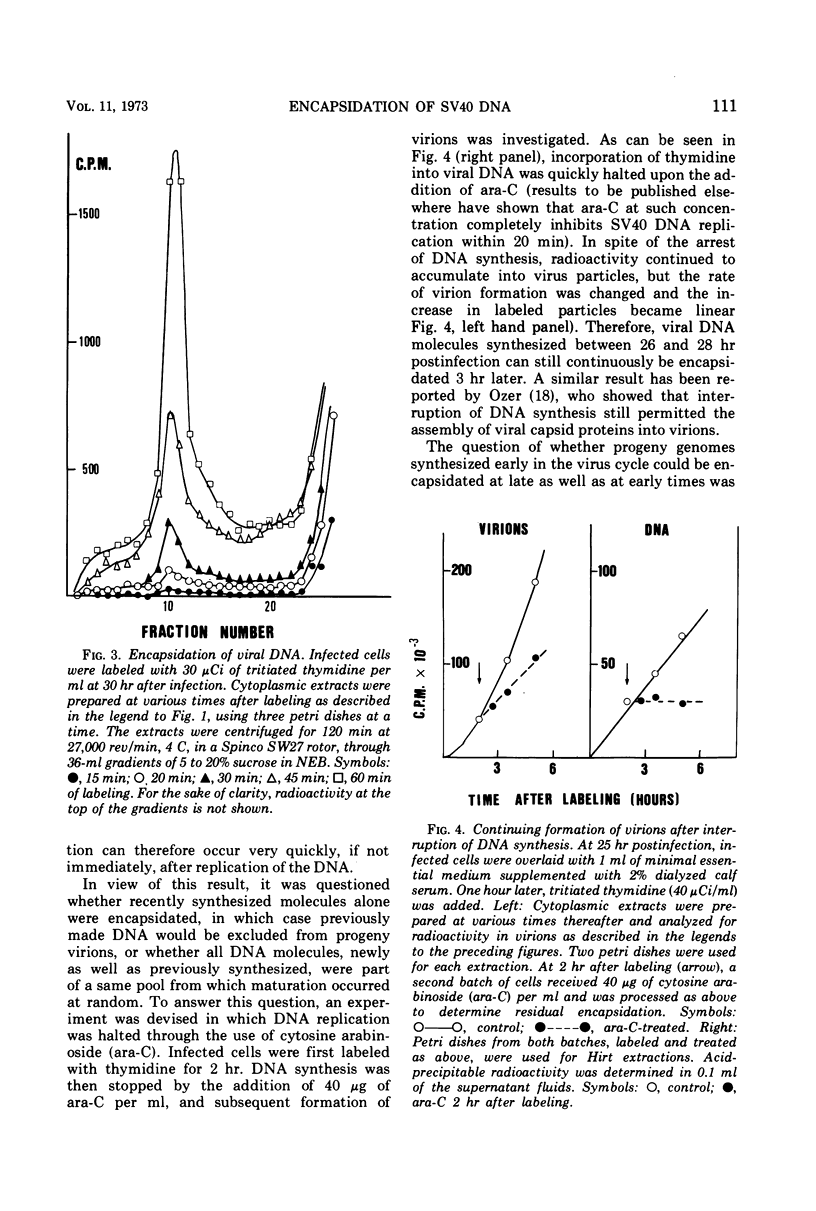
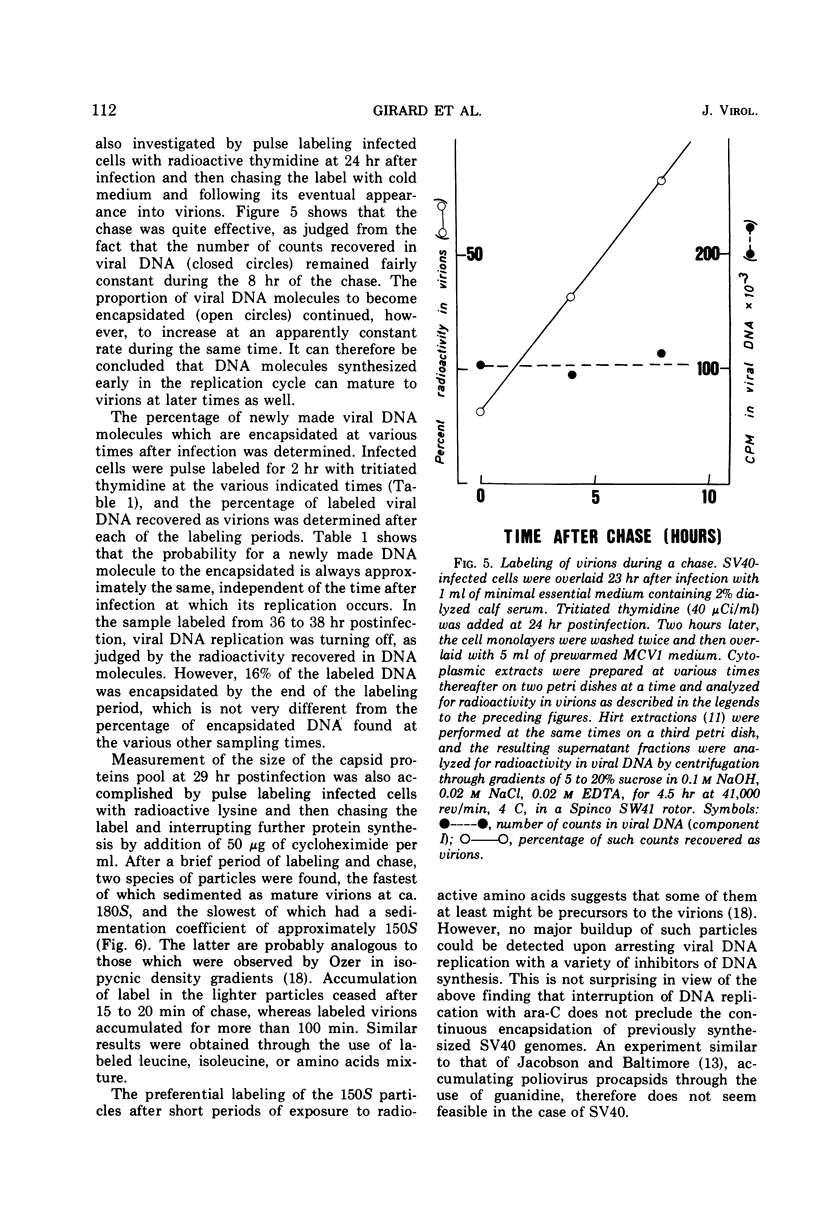
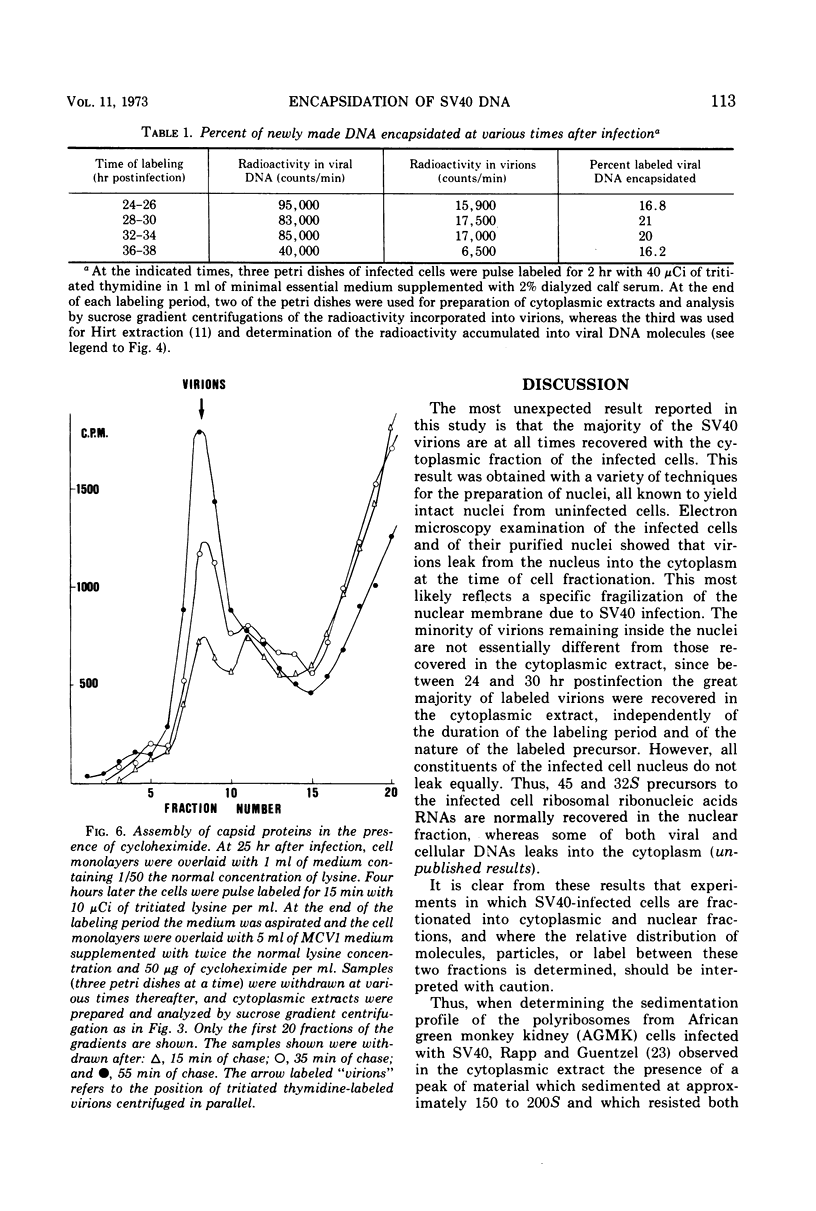
Growing subcloned CV1-cells were infected with simian virus 40, and the time course of virus formation was determined. When infected cells were fractionated into cytoplasmic and nuclear fractions, most of the progeny virus particles were recovered in the cytoplasmic extract and not in the nuclei. This result was independent of the technique used for the preparation of nuclei and of the time after infection at which the extracts were prepared. Leakage of the virions from the nucleus occurred during the course of cell fractionation, suggesting that the nuclear membrane of the infected cells is damaged. Virions were found to accumulate in a nonlinear fashion, at the time when the number of viral deoxyribonucleic acid (DNA) molecules increases linearly with time after infection. This suggests that the size of the intracellular pool of capsid proteins increases constantly during the late phase of virus replication. Progeny viral DNA to become encapsidated is withdrawn at random from the pool of replicated DNA molecules.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Winocour E., Sachs L. Characterization of the simian virus 40-specific RNA in virus-yielding and transformed cells. J Mol Biol. 1968 Feb 14;31(3):415–429. doi: 10.1016/0022-2836(68)90418-x. [DOI] [PubMed] [Google Scholar]
- BERNHARD W., FEBVRE H. L., CRAMER R. [Electron microscopic demonstration of a virus in cells infected in vitro by the polyoma agent]. C R Hebd Seances Acad Sci. 1959 Jul 20;249:483–485. [PubMed] [Google Scholar]
- Barbanti-Brodano G., Swetly P., Koprowski H. Early events in the infection of permissive cells with simian virus 40: adsorption, penetration, and uncoating. J Virol. 1970 Jul;6(1):78–86. doi: 10.1128/jvi.6.1.78-86.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borun T. W., Scharff M. D., Robbins E. Preparation of mammalian polyribosomes with the detergent Nonidet P-40. Biochim Biophys Acta. 1967 Nov 21;149(1):302–304. doi: 10.1016/0005-2787(67)90715-0. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Huang E. S., Pagano J. S. Structural polypeptides of simian virus 40. J Virol. 1971 May;7(5):635–641. doi: 10.1128/jvi.7.5.635-641.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H., Sauer G. Identification of virus-induced proteins in cells productively infected with simian virus 40. J Virol. 1972 Jan;9(1):1–9. doi: 10.1128/jvi.9.1.1-9.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frearson P. M., Crawford L. V. Polyoma virus basic proteins. J Gen Virol. 1972 Feb;14(2):141–155. doi: 10.1099/0022-1317-14-2-141. [DOI] [PubMed] [Google Scholar]
- GILDEN R. V., CARP R. I., TAGUCHI F., DEFEND V. THE NATURE AND LOCALIZATION OF THE SV 40-INDUCED COMPLEMENT-FIXING ANTIGEN. Proc Natl Acad Sci U S A. 1965 Mar;53:684–692. doi: 10.1073/pnas.53.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANBOULAN N., TOURNIER P., WICKER R., BERNHARD W. An electron microscope study of the development of SV40 virus. J Cell Biol. 1963 May;17:423–441. doi: 10.1083/jcb.17.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard M., Marty L., Suarez F. Capsid proteins of Simian virus 40. Biochem Biophys Res Commun. 1970 Jul 13;40(1):97–102. doi: 10.1016/0006-291x(70)91051-x. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Morphogenesis of poliovirus. I. Association of the viral RNA with coat protein. J Mol Biol. 1968 Apr 28;33(2):369–378. doi: 10.1016/0022-2836(68)90195-2. [DOI] [PubMed] [Google Scholar]
- Kit S., Dubbs D. R., Frearson P. M., Melnick J. L. Enzyme induction in SV40-infected green monkey kidney cultures. Virology. 1966 May;29(1):69–83. doi: 10.1016/0042-6822(66)90197-8. [DOI] [PubMed] [Google Scholar]
- Manteuil S., Pages J., Stehelin D., Girard M. Replication of simian virus 40 deoxyribonucleic acid: analysis of the one-step growth cycle. J Virol. 1973 Jan;11(1):98–106. doi: 10.1128/jvi.11.1.98-106.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda K., Dulbecco R. Regulation of transcription of the SV40 DNA in productively infected and in transformed cells. Proc Natl Acad Sci U S A. 1968 Jun;60(2):525–532. doi: 10.1073/pnas.60.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer H. L. Synthesis and assembly of simian virus 40. I. Differential synthesis of intact virions and empty shells. J Virol. 1972 Jan;9(1):41–51. doi: 10.1128/jvi.9.1.41-51.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer H. L., Tegtmeyer P. Synthesis and assembly of simian virus 40. II. Synthesis of the major capsid protein and its incorporation into viral particles. J Virol. 1972 Jan;9(1):52–60. doi: 10.1128/jvi.9.1.52-60.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S., Scherrer K., Becker Y., Darnell J. E. POLYRIBOSOMES IN NORMAL AND POLIOVIRUS-INFECTED HELA CELLS AND THEIR RELATIONSHIP TO MESSENGER-RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):654–662. doi: 10.1073/pnas.49.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pétursson G., Weil R. A study on the mechanism of polyoma-induced activation of the cellular DNA-synthesizing apparatus. Synchronization by FUdR of virus-induced DNA synthesis. Arch Gesamte Virusforsch. 1968;24(1):1–29. doi: 10.1007/BF01242898. [DOI] [PubMed] [Google Scholar]
- RAPP F., BUTEL J. S., FELDMAN L. A., KITAHARA T., MELNICK J. L. DIFFERENTIAL EFFECTS OF INHIBITORS ON THE STEPS LEADING TO THE FORMATION OF SV40 TUMOR AND VIRUS ANTIGENS. J Exp Med. 1965 Jun 1;121:935–944. doi: 10.1084/jem.121.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPP F., MELNICK J. L., KITAHARA T. TUMOR AND VIRUS ANTIGENS OF SIMIAN VIRUS 40: DIFFERENTIAL INHIBITION OF SYNTHESIS BY CYTOSINE ARABINOSIDE. Science. 1965 Feb 5;147(3658):625–627. doi: 10.1126/science.147.3658.625. [DOI] [PubMed] [Google Scholar]
- Rapp F., Guentzel M. J. Polyribosomes of cells abortively or productively infected with adenovirus, papovavirus, or their hybrid. Arch Gesamte Virusforsch. 1969;28(3):255–268. doi: 10.1007/BF01240941. [DOI] [PubMed] [Google Scholar]
- Sauer G. Apparent differences in transcriptional control in cells productively infected and transformed by SV40. Nat New Biol. 1971 Jun 2;231(22):135–138. doi: 10.1038/newbio231135a0. [DOI] [PubMed] [Google Scholar]
- Walter G., Roblin R., Dulbecco R. Protein synthesis in Simian virus 40-infected monkey cells. Proc Natl Acad Sci U S A. 1972 Apr;69(4):921–924. doi: 10.1073/pnas.69.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Stubblefield E. A new method for the rapid isolation of chromosomes, mitotic apparatus, or nuclei from mammalian fibroblasts at near neutral pH. Exp Cell Res. 1970 Mar;59(3):469–478. doi: 10.1016/0014-4827(70)90656-7. [DOI] [PubMed] [Google Scholar]