Abstract
Availability of Zn to plant is hampered by its immobile nature and adverse soil conditions. Thus, Zn deficiency is observed even though high amount is available in soil. Root-shoot barrier, a major controller of zinc transport in plant is highly affected by changes in the anatomical structure of conducting tissue and adverse soil conditions like pH, clay content, calcium carbonate content, etc. Zn deficiency results in severe yield losses and in acute cases plant death. Zn deficiency in edible plant parts results in micronutrient malnutrition leading to stunted growth and improper sexual development in humans. To overcome this problem several strategies have been used to enrich Zn availability in edible plant parts, including nutrient management, biotechnological tools, and classical and molecular breeding approaches.
Keywords: Zinc deficiency, Biofortification, Zinc efficiency, Micronutrient malnutrition
Introduction
Polished rice grains contain only about 20 % of the daily requirement of zinc and a small amount of iron. Not surprisingly, therefore, in countries where rice is a major staple, Fe and Zn deficiencies are most prevalent with almost over three billion people affected worldwide (Welch and Graham 2004), most of this population residing in developing countries.
Micronutrient malnutrition has been recognized as a gigantic and rapidly growing public health problem not only amongst the poor but also across the whole spectrum of people living on an unbalanced diet dominated by a single staple grain such as rice (Virk and Barry 2009). Rice (Oryza sativa L.) is a “global grain” cultivated widely across the world and feeds millions of mankind. It is one of the most important staple food crops and is almost exclusively consumed by humans, with 90 % of rice grown and consumed in south and southeast Asia, where the normal consumption of rice range from 300 to around 800 g per day per person. In some parts of the world rice consumption is as high as 990 g per day per person (Virk and Barry 2009). Considering its importance for human food, it is one of the most important crop plants on earth (Lucca et al. 2002). However, as with many other staple food crops rice contains low levels of important micronutrients especially Fe and Zn (Virk and Barry 2009; Bouis and Welch 2010). Micronutrient malnutrition has affected lives of billions, with about 5 billion suffering from iron and 2.7 billion suffering from zinc deficiency all over the world (Anonymous 2004). A striking figure of 27 % of total population in India is affected by Zn deficiency related disorders such as poor immune system, diarrhoea, poor physical and mental growth (World Health Organization 2007). Zn deficiency claims about 4.4 % of the total child deaths in the world (Black 2003).
Rice is an indispensable staple food for half of the world’s population providing 50–85 % of daily energy source and is consumed in large amounts. Therefore, even a small increase in the nutritive value of rice can be highly significant for human nutrition (Zeng et al. 2010; Chandel et al. 2010). For this biofortification has emerged as one possible solution to alleviate malnutrition (Zimmermann and Hurrell 2002; Chandel et al. 2010; Bouis and Welch 2010; Waters and Sankaran 2011).
Among the micronutrients, Zn is the most limiting nutrient whose deficiency is a wide spread nutritional disorder of wetland rice (Neue and Lantin 1994). It is most common in flooded rice soils and has become increasingly important during the past decades. Zinc deficiency in rice appears right from seedling stage in nursery and 3 weeks after transplanting in transplanted rice plots. It was reported that zinc supply in form of fertilizer enhances rice yield (Sudhalakshmi et al. 2007; Jiang et al. 2008); but movement of zinc from plant parts to grains under Zn fertilizer application was not observed (Jiang et al. 2008).
Why zinc?
Unfortunately, populations residing both in developed and developing countries consume cereals as primary food components. Cereals are inherently low in Zn contents with lesser bioavailability. Poor grain nutritive value of cereals is an important reason for widespread micronutrient malnutrition among populations eating rice as staple food (Chandel et al. 2010). The micronutrient zinc is essential for all organisms (Andreini et al. 2006; Broadley et al. 2007). Zn deficiency in humans is widespread and is estimated to affect more than 25 % of the world’s population (Maret and Sandstead 2006). According to a WHO report (World Health Organization 2002), Zn deficiency ranks fifth amongst the most important health risk factors in developing countries and eleventh worldwide. Zn deficiency in humans is a serious threat not only to the health of individuals but also to the economy of developing nations. Overcoming malnutrition related disorders has been identified as a top priority by a panel of distinguished economists (Chandel et al. 2010).
According to the Copenhagen Consensus food fortification is one of the most cost-effective long-term strategies for mineral nutrition (Horton 2006) and ranks third in terms of cost-benefit balance. Fortification of dairy products such as bread and milk with different minerals (and vitamins) has been successful in industrialized countries (Underwood and Smitasiri 1999). Fortification takes place during food processing and increases the product price. These factors make fortified products unaffordable to the most impoverished people living in remote rural areas. Zinc fortification has been implemented in the industrial world but rarely in developing countries. One exception is Zn-fortified wheat and maize flours in Mexico, which are used to make bread and tortillas, the two principal staples (IZINCG 2007). Organizations such as the Zinc Task Force (ZTF) and the International Zinc Nutrition Consultative Group (IZINCG) are fighting against Zn malnutrition by promoting diverse strategies to eliminate it.
Importance of zinc for humans
Zn is required in small but critical amounts by both plant and animals (including humans). Zn is the only metal to be involved in all six classes of enzymes: oxido-reductases, transferases, hydrolases, lyases, isomerases and ligases (Barak and Helmke 1993). In higher animals and humans it is estimated that approximately 3,000 proteins contain Zn prosthetic groups (Tapeiro and Tew 2003). Zn ions are also neurotransmitters, and cells in the salivary glands, prostate, immune system and intestine use Zn signalling (Herschfinkel et al. 2007).
Zn plays a key role in physical growth and development, functioning of immune system, reproductive health, sensory function and neurobehavioural development (Hotz and Brown 2004). Recommended daily intakes range between 3 and 16 mg Zn day−1, depending on age, gender, type of diet and other factors. It has been estimated that around 33 % of the world’s human population has diets deficient in Zn, but this ranges between 4 and 73 % in different countries (Hotz and Brown 2004). In humans and higher animals Zn is a ‘Type 2’ nutrient, which means that its concentration in blood does not decrease in proportion to the degree of deficiency. As a result, physical growth slows down and excretion is reduced to conserve Zn. Most children suffering from Zn deficiency have stunted linear growth (Graham 2008).
Zn deficiency in human body reduces serum testosterone level, is linked to oligospermia, a severe immune dysfunction mainly affecting T helper cells, hyperammonemia, neurosensory disorders and decrease in lean body mass (Prasad 2008). To overcome this situation past decade has witnessed growing interest in developing varieties of staple grain crops with enhanced concentrations of Zn to improve the nutritional quality of grain for human consumption (White and Broadley 2005; Cakmak 2008; McDonald et al. 2008; Wissuwa et al. 2008). On the basis of recommended daily allowances (RDA) and bioavailability values, 22 μg/g grain Zn contents in polished rice grains have been decided (HarvestPlus 2005), but current status of consumable rice shows that it has only around 20 % of this value. Thus, Zn enriched cereal grains would potentially generate major health benefits. Moreover, adequate Zn content is known to enhance crop productivity (Cakmak 2008).
Bioavailability refers to the portion of an ingested nutrient that can be absorbed in the human gut. The bioavailability of minerals can be reduced or enhanced by the consumption of food rich in antinutrients (inhibitors of absorption) or nutritional enhancers, respectively. People in developing countries, whose diet is primarily cereal-based, are disproportionately affected by mineral deficiencies because cereals not only have low levels of minerals, but also high levels of phytate, which chelates mineral ions and inhibits their absorption (White and Broadley 2005). A varied diet comprising fresh fruit, vegetables, fish and meat provides sufficient nutrients and enhancers to promote adequate mineral absorption in the gut, hence, a nutrient rich diet should contain all these components (Gomez-Galera et al. 2010).
Importance of zinc for plants
Zinc (Zn) ions have both beneficial and toxic effects on plant cells. It is inimitable in several plant metabolic processes such as enzyme activation like RNA polymerases, superoxide dismutase, alcohol dehydrogenase, carbonic anhydrase, protein synthesis and metabolism of carbohydrate, lipid and nucleic acid (Cakmak 2000; Palmer and Guerinot 2009). Also Zn ions are integral parts of Zn finger family of transcription factors controlling cell proliferation and differentiation (Valle and Falchuk 1993; Lin et al. 2005; Palmer and Guerinot 2009). Besides these, Zn plays major role in chloroplast development and function, of which most important are the Zn-dependent activity of SPP peptidase and the repair process of photo system II by turning over photodamaged D1 protein (Hansch and Mendel 2009). Thus cells need mechanisms for maintaining zinc homeostasis when available supplies decreases (Eide 2009). In plants, Zn deficiency reduces growth, tolerance to stress and chlorophyll synthesis (Kawachi et al. 2009; Lee et al. 2010).
Zinc deficiency
Singh et al. 2005 stated “One of the widest ranging abiotic stresses in world agriculture arises from low zinc availability in calcareous soils, particularly in cereals.” Among all the micronutrients, Zn deficiency is the most widespread micronutrient disorder among different crops (Naik and Das 2008).
In India, zinc deficiency was first reported by Nene (1966) on paddy fields. Zinc deficiency was identified as khaira in India, Akagare type II in Japan (Yoshida and Tanaka 1969), Taya-Taya and Apulapaya in Philippines (Yoshida et al. 1973), Hadda in Pakistan (Yoshida and Tanaka 1969) and the suffocating disease in Taiwan (Yoshida et al. 1973). Form then onwards, zinc deficiency was realized as plant nutritional problem throughout rice growing countries such as Japan, USA, Brazil, and Phillipines (Deb 1992). Rice, one of the world’s most important cereal crops is affected by Zn deficiency. At least 70 % of the rice crop is produced in flooded conditions resulting in increment in phosphorus and bicarbonate concentration which reduces soil Zn availability to the crop. About 50 % paddy soils are Zn deficient with 35 % in Asia alone (Cakmak 2008). In an Indian scenario around 49 % soils from all the main agricultural areas are deficient in Zn (Naik and Das 2008).
There are many factors that have led to the present state of Zn deficiency in agricultural soils. The main soil factors responsible for causing Zn deficiency in staple food crops, such as rice are low total contents of Zn, high pH, high contents of calcite, high concentrations of bicarbonate ions and salts and high levels of available phosphorus (Alloway 2009). Zinc deficiency has increased with the introduction of modern varieties, crop intensification and increased Zn removal (Slaton et al. 2001). Besides these factors multiple cropping coupled with the use of high analysis Zn-free fertilizers has depleted the soil zinc source (Singh et al. 1999).
The physiological stress caused by Zn deficiency results in development of abnormalities in plants. In case of severe ‘acute’ Zn deficiency, visible symptoms include stunted growth, chlorosis of leaves, small leaves and spikelet sterility. The quality of plant products is also adversely affected and plants have increased susceptibility to injury by high light intensity and temperature and to infection of certain fungal diseases (Cakmak 2000). On marginally Zn deficient soils, yields can be reduced and quality affected without the appearance of obvious symptoms; this is called ‘hidden’ (or ‘latent’) deficiency (Alloway 2009).
Zinc in soil
The total Zn content in soil depends upon the parent rock, weathering, organic matter, texture and pH. The most quoted range of total Zn in normal soils in 10–300 mg kg−1 (Swaine 1955; White 1993) with a mean value of 50 mg kg−1 (Vinogradov 1959). Total Zn (mg kg−1) in some Indian soils was 47 in entisols, 60 inceptisols, 61 aridisols, 63 in vertisols, 44 in alfisols, 43 in altisols, 30 in mollisols and 72 in oxisols (Katyal and Sharma 1991). Soils formed from basic rocks such as basalt are richer in Zn then those from acid rocks such as granite and gneisses (Vinogradov 1959). Total Zn content in soils is generally lower in lighter soils and higher in heavier soils (Frank et al. 1976).
Zn is known to occur in soil in a number of discrete chemical forms differing in their solubility and availability to plants (Deb 1992). Zn exists as five distinct pools in soils viz., water soluble, exchangeable, adsorbed, chelated or complexed Zn. These pools differ in strength (or reversibility) and therefore in their susceptibility to plant uptake, leaching and extractability. The equilibrium among different pools is influenced by pH, concentration of Zn and other cations, particularly iron and manganese (Mandal et al. 1993). The Zn that is available to plants is that present in the soil solution, or is adsorbed in a labile form. The soil factors affecting the availability of Zn to the plants are those which control the amount of Zn in the soil solution and its sorption–desorption from/into the soil solution. These factors include: the total Zn content, clay content, calcium carbonate content, redox conditions, and microbial activity in the rhizosphere, soil moisture status, concentration of other trace elements, concentration of macro-nutrients, especially phosphorus and climate (Alloway 2008). With more or less contribution of all of these factors combined, results in reduced availability of Zn in soils for plant absorption which leads to deficiency of Zn in plants, and when these plants and their parts are consumed by humans and animals Zn deficiency occurs in them, giving rise to severe problem of micronutrient malnutrition. To overcome this problem cultivars having better Zn uptake potential or improved partitioning of Zn to edible parts or a combination of these two approaches is necessary. For this a number of strategies have been used and proposed which have been enlisted and discussed in this review.
Zinc enrichment
During the push of the green revolution towards food security through increasing the yield of staples, little thought was given to human health and the nutritional value of diets (Graham et al. 2000). However, most of the research on plant breeding over the past few decades has concentrated on increasing resistance to environmental stresses, pests and pathogens (Borlaug 2000). Genetic engineering has even been used to improve the sensory appeal of agricultural products, such as tomatoes (Lewinsohn et al. 2001). However, the recent application of plant biotechnology to improve the nutritional content of staple food crops has perhaps the greatest potential to benefit global health (Graham et al. 1999; Frossard et al. 2000; Ye et al. 2000; Lucca et al. 2001; Waters and Sankaran 2011). Because poverty limits food access for much of the developing world’s population, it is important that affordable staple foods be as nutritious as possible (Zimmermann and Hurrell 2002).
A number of strategies have been used for enrichment of Zn in edible parts of cereals which include fertilizer management, cultural management practices, genetic engineering, strategy to pull micronutrients to grains, biofortification etc. Factors such as soil organic matter content, pH, internal Fe/Zn level and fertilization affected the concentration of mineral ions in soil as well as their availability in soil solution (Machado et al. 2004; Cakmak 2009). Irrigation and fertilizer management have been reported to increase the accumulation of Zn in grains of rice and wheat (Hao et al. 2007). Judicious application of fertilizer Zn helps increase crop production as well as it helps enrichment of Zn in plant organs including grains (Khan et al. 2002; Sudhalakshmi et al. 2007; Jiang et al. 2008; Phattarakul et al. 2012).
Besides nutrient management, a strategy to “pull” micronutrients into the plants by increasing sink strength could be used (Vasconcelos et al. 2003). In this scenario, homeostatic mechanisms would allow the plant to adjust uptake to meet the additional demand. The main “pull” strategies that have been described utilize overexpression of soybean Fe storage protein ferritin in rice grains (Goto et al. 1999). This strategy resulted in variable results between transgenic events and segregating lines, with no increase in some, or others with increase upto threefold of Fe concentration (Goto et al. 1999). This strategy also resulted in increase in Fe concentration in polished rice grains (Vasconcelos et al. 2003). Although, besides using inter generic transgenic strategy, another strategy involving the overexpression of plant’s phytochelatin synthases can also contribute to “pull” micronutrients in roots and shoots of the plants (Clemens and Persoh 2009).
Genetic engineering can also be applied for micronutrient enrichment of cereals. For successful increase of seed micrunutrient concentrations, genes to be expressed differently should be targeted to the appropriate localization, at the appropriate developmental stage, and the substrates for transporters (micronutrients) should be present. Constitutive expression of transporters may lead to an unwanted accumulation of micronutrients at undesirable places, this uncontrolled accumulation of micronutrients may have other unintended consequences (Waters and Sankaran 2011). Constitutively over expressing a ZIP transporter in barley resulted in higher short-term Zn uptake, and plants with higher concentrations of Fe and Zn (Ramesh et al. 2004).
For seeds and grains, phloem sap loading, translocation and unloading rates within the reproductive organs are important characteristics that must be considered when the aim is to increase micronutrient accumulation in edible parts of the seeds and grains (Welch and Graham 2004).
Recently, food based approach ‘biofortification’ has been recognized as an efficient means to reduce micronutrient and protein malnutrition. It involves the development of functional staple food crops that are selectively bred to enhance specific nutritional qualities, such as levels of biologically available iron and zinc (Chandel et al. 2010). Biofortification strategies demand that essential micronutrients leave the root and are targeted to the edible parts of the plants, such as cereal grains (Palmgren et al. 2008).
Biofortification
Plant derived foods provide an important source of dietary minerals. This is especially true for developing countries where plant foods are a predominant portion of the diet. The concentration of some minerals, especially Zn is inherently low in plants as opposed to animal derived foods, resulting in severe problem of micronutrient malnutrition (Cakmak 2008; White and Broadley 2009). Biofortification is a recent approach aimed at increasing the bio available nutrients such as Fe and Zn, in these staple crops (Pfeiffer and McClafferty 2007) rather than using fortificants or supplements. The quantities of minerals in seeds depends on uptake from the rhizosphere into the roots, translocation to the transpiring shoots in the xylem, transfer into leaves or other tissues, and finally, translocation into the seeds in the phloem. A major challenge of biofortification is an incomplete understanding of the pathways and the rate limiting steps involved in translocating minerals to the seeds (Waters and Sankaran 2011).
Biofortification, which aims to increase micronutrient concentrations in the edible parts of plants through breeding or the use of biotechnology, is considered to be a cost-effective way to alleviate micronutrient malnutrition in the rural populations in developing countries where the problem is most prevalent (Mayer et al. 2008). Biofortification may also include other approaches, such as the use of micronutrient fertilizers (agronomic biofortification) or enhancement of micronutrient bioavailability by manipulating the levels of pronutrient or antinutrient components in foods (Zhao and McGrath 2009). For, Zn breeding for higher concentration in grain is possible as there is sufficient genotypic variation in germplasms of major cereal crops (White and Broadley 2009). Also for Zn, agronomic biofortification may also be necessary on soils with low zinc availability, which represent nearly half of the cereal grown areas of the world (Cakmak 2008). For biofortification, transgenic approaches have also been pursued. An example is the endosperm specific expression of the recombinant human lactoferrin (rHLF) gene in rice grain, resulting in production of the HLF protein up to 0.5 % of the grain weight and twofold increase in the grain Fe concentration (Nandi et al. 2002).
Although biofortification may seem to be an interesting approach, however there are some constraints that limit the enrichment of zinc in plants. These include factors related to both soil and plants. Majority of the soils cropped to cereals in the world have many adverse soil chemical properties which has been reported for the countries like Turkey, India, China, Australia, Pakistan leading to impaired Zn nutrition of cereals. Hence, the genetic capacity of newly developed (biofortified) cultivars to absorb sufficient amount of Zn from soils and accumulate it in the grain to achieve the nutritional benefit may not be expressed to the full extent (Cakmak 2008). A breeding approach to produce nutritionally improved cereals relies on genetic diversity in natural populations that can be cross bred to introduce traits/genes from one variety or line into a new genetic background. There is limited documentation for the nutritional diversity in grain Zn content of cultivated wheat or rice varieties (White and Broadley 2005; Cakmak 2008). A transgenic approach to increase the Zn content of cereal grains might involve the manipulation of transporters involved in Zn translocation. With respect to Zn uptake, translocation and deposition, a predominant role seems to be played by the members of the ZIP family (Ramesh et al. 2004). Furthermore, with respect to root-shoot allocation of Zn, the Zn pump HMA 4 seems to be a major player, and it remains to be tested whether HMA 4 might be used for transgenic biofortification approaches in cereals (Palmgren et al. 2008). But public acceptability of transgenic crops still remains a major concern, therefore a more conventional approach would be to select the Zn efficient genotypes through conventional or molecular breeding based on physiological traits to overcome the problem of Zn deficiency or toxicity.
Also there are certain bottle-necks in plant biofortification in relation to the plants. The first and most important barrier resides at the root soil interface (Welch 2001). To increase the micronutrient metal uptake by roots, the available level of micronutrient in the root-soil interface must be increased to allow its more absorption by plant root cells. This could be done by stimulating certain root cell processes that modify micronutrient solubility and movement to root surfaces, such metal complexing compounds and reductants (Welch and Graham 2004). Second, absorption mechanism (i.e. transporters and ion channels) located in the root cell plasma membrane, must be sufficiently active and specific enough to allow for the accumulation of micronutrient metals once they enter the root cells (Sinclair and Kramer 2012). Third, once taken up by root cells, the micronutrients must be efficiently translocated to and accumulated in edible plant organs. For this purpose anatomical change in xylem orientation at the root shoot junction must be considered which makes it difficult for micronutrients to cross the root-shoot junction (Yamaguchi et al. 2012). Finally, to be effective, the micronutrient metal species accumulated in edible portions must be bioavailable to people that eat the plant material in a meal (Waters and Sankaran 2011). These factors in combination with climatic constrains make biofortification of cereals difficult to achieve (Palmgren et al. 2008).
Moreover, as plant food contain substances that influence the bioavailability of these nutrients to humans (Table 1) it is necessary to demonstrate the efficacy of micronutrient enrichment of plant foods towards improving the nutritional health of targeted populations (Welch and Graham 2004). This requires that the bioavailability of Fe, Zn and other micronutrients enriched in genotypes of staple plant foods be demonstrated, in order to assure a human health impact before advancing genotypes for breeding programme (Graham et al. 2001).
Table 1.
Antinutrients in plant foods that reduces Fe and Zn bioavailability and food source in which present (from Graham et al. 2001)
| Antinutrients | Major dietary source |
|---|---|
| Phytic acid/phytin | Legume seeds and cereal grains |
| Fibre | Whole cereal grain products |
| Certain tannins and other polyphenolics | Tea, Coffee, Beans, Sorghum |
| Oxalic acid | Spinach leaves |
| Haemagglutinins (eg lectins) | Most legumes and wheat |
| Goitrogens | Brassica and Alliums |
| Heavy metals (Cd, Hg, Pb, etc.) | Contaminated leafy vegetables and roots |
Zinc acquisition
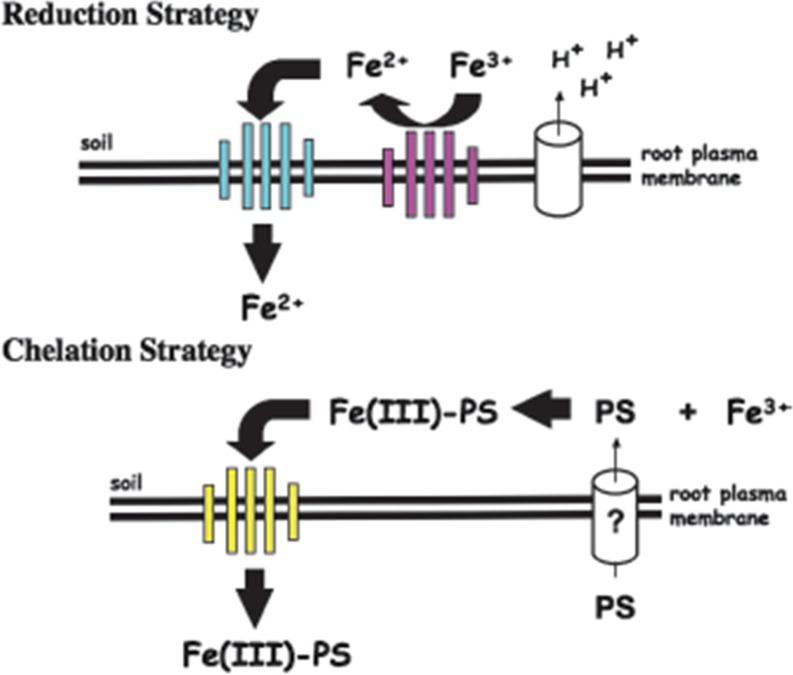
As sessile organisms, plants have developed strategies to obtain essential metal micronutrients from soils of varying compositions. Not surprisingly, they use a variety of mechanisms to assimilate metals while preventing toxicity, which involve the regulation of transport, chelation, and sequestration (Colangelo and Guerinot 2006). Plant cells utilize two separate mechanisms for acquiring metals. Reduction based strategy I operates mainly in dicotyledonous and non-graminaceous plants, where higher oxidation states of metal is reduced to lower oxidation state before being transported into the cell by its specific transporter present on the root cell membrane. Grasses such as rice, corn and wheat use the chelation based strategy II (Fig. 1).
Fig. 1.
A diagrammatic representation showing reduction based strategy I and chelation based strategy II for metal ion uptake in plants. (Source: Guerinot 2007)
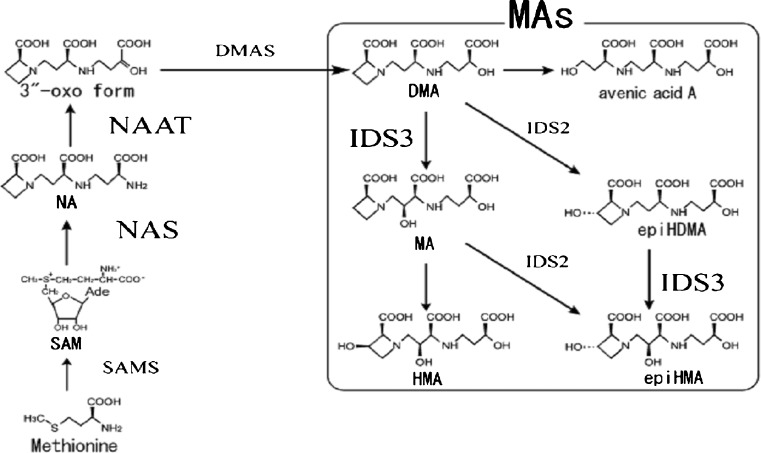
In response to metal deficiency graminaceous plants cells release phytosiderophores (PS) which belong to muginic acid (MA) family (Fig. 2) and are derived from the precursor nicotinamine. These molecules bind to metals and chelate them and specific plasma membrane transporter proteins import these metal-PS complexes into the plants (Lee et al. 2012).
Fig. 2.
The biosynthetic pathway of mugineic acid family phytosiderophores (MAs) in graminaceous plants. SAM S-Adenosyl-methionine, NA nicotianamine, DMA 2′-deoxymugineic acid, MA mugineic acid, HMA 3-hydroxymugineic acid, epiHDMA 3-epihydroxy-2′-deoxymugineic acid, epiHMA 3-epihydroxymugineic acid, SAMS S-adenosyl-methionine synthetase, NAS nicotianamine synthase, NAAT nicotianamine aminotransferase, DMAS 2′-deoxymugineic acid synthase, IDS2 dioxygenase catalyzing the hydroxylation of three positions of DMA and MA, IDS3 dioxygenase catalyzing the hydroxylation of 2′-positions of DMA and epiHDMA. (Source: Masuda et al. 2008)
For uptake Zn needs to be solubilized in the rhizosphere which occurs via plant mediated acidification and secretion of low molecular weight chelators (Sinclair and Kramer 2012). Zn crosses the root cell plasma membrane predominantly as free ion, which is then chelated by low molecular weight ligands in the cytosol to prevent cytosolic precipitation and non-specific binding to biomolecules (Freisinger 2008). Once in the root symplast, Zn can be immobilized in root through transport into vacuoles, or it can undergo symplastic transport, which is thought to occur through plasmodesmata, towards and into the vascular cylinder (Deinlein et al. 2012). The export from cells is required for the loading of Zn into the apoplastic xylem for the translocation of Zn from root to shoot. Once inside xylem Zn flow towards shoot is mass flow driven (Hanikenne et al. 2008). Inside the shoot Zn is taken up from xylem across the plasma membrane of the adjacent cells, although, little is known about how Zn is distributed between cells (Sinclair and Kramer 2012). Further the remobilization of Zn, in particular in photosynthetic source tissues and the senescing leaves, for the translocation into the sink tissues i.e. meristems, developing leaves, influorescenses and developing seeds via long distance mass flow-driven transport in the phloem is common (Erenoglu et al. 2010). In contrast to the xylem, phloem transport pathway consists of strongly modified living cells that are symplastically connected among each other and with the adjacent companion cells (Van Bel et al. 2011). Therefore because of high concentration of off-target metal binding compounds and the higher pH inside the phloem, the chelation of Zn is particularly important for long distance transport inside the phloem (Sinclair and Kramer 2012).
Zn transporters play a central role in plant acquisition of zinc from soil and its distribution. Many different Zn transporters have been identified distributed throughout the plant system. The major players include the OsZIP 5 which is present in root cells and help in Zn uptake (Lee et al. 2007). Besides this it has also been shown that maintenance of Zn homeostasis in whole plant relies on a variety of transporters, including the members of zinc-regulated transporter (ZRT) and iron-regulated transporter (IRT)- like protein (ZIP) which are involved in the cellular uptake of Zn from soil to root cells at soil root interface (Colangelo and Guerinot 2006); natural resistance associated macrophage protein (Nramp) families which regulate the proton driven transport of Zn and other transition metal ions (Thomine et al. 2000); to the same class of proton/metal antiporters also belong another family of transporters called the cation diffusion facilitator (CDF) family proteins (Sinclair and Kramer 2012). Yet another family of proton/metal antiporters is the yellow-stripe like-1 (YSL1) family of proteins which resemble the YS1 transporter of maize (Curie et al. 2001). Finally Arabidopsis HMA 1 to 4 proteins (Heavy Metal ATPases of the P1B-type ATPases) found a role in Zn uptake and transport from roots to shoots, which is yet to be explored (Sinclair and Kramer 2012). A more indepth insight on Zn transporters in presented in Table 2.
Table 2.
Different types of Zn transporters, their function(s) and the plants in which the function was established
| Name of Transporter(s) | Function(s) | Plant from | Reference |
|---|---|---|---|
| AtZIF1, AtZIF2, AtZIF3 | Zinc transport and distribution between vacuole and cytoplasm | Arabidopsis thaliana | Haydon and Cobbett 2007 |
| PCR2 | Export of Zn at high concentration and transport of Zn from root to shoot | A. thaliana | Song et al. 2010 |
| ZIP1, ZIP2, ZIP3 | Zn uptake from the soil | A. thaliana | Grotz et al. 1998 |
| OsZIP1 | Zn uptake from soil and redistribution of Zn in leaves | Oryza sativa | Ramesh et al. 2003 |
| OsZIP3 | Zn transport in vascular cells and Zn redistribution in epidermal cells | O. sativa | Ramesh et al. 2003 |
| OsZIP4 | Zn uptake in roots from soil | O. sativa | Ishimaru et al. 2005 |
| AtMTP1 | Vacuolar-cytosolic transport of Zn and maintaining Zn concentration | A. thaliana | Kobae et al. 2004 |
| HMA2 | Zn efflux from cells at high Zn concentration | A. thaliana | Eren and Arguello 2004 |
| OsHMA9 | Efflux of Zn from cells for remobilization | O. sativa | Lee et al. 2007 |
| OsZIP7a and OsZIP8 | Zn upake from soil in root cells | O. sativa | Yang et al. 2009 |
| ZNT1 | Zn uptake from soil in root cells | Thlaspi caerulescens | Pence et al. 1998 |
| OsHMA2 | Root to shoot translocation in rice | O. sativa | Takahashi et al. 2012 |
Partitioning of zinc
Around 75 % of total grain Zn was reported to be present in aleurone layer of brown rice (Jiang et al. 2008), the results were confirmed by XRF imaging which revealed that Zn is most abundant in aleurone layer and embryo (Takahashi et al. 2009). Most of this aleurone layer is removed during polishing resulting in grains with reduced Zn content. Hence, it becomes evident that Zn should be targeted to endosperm of the grain so as to be available to the consuming population (Waters and Sankaran 2011).
Movement of minerals to the developing seeds follow several paths, one such path involves the flag leaf. Flag leaves are the major source of phloem-delivered photoassimilates for rice developing seeds, and are believed to be one of the sources of remobilized metals for the seeds (Narayanan et al. 2007; Sperotto et al. 2009). In another cereal (wheat), Zn and Fe remobilization from flag leaves to seeds was clearly demonstrated (Uauy et al. 2006; Waters et al. 2009). It is possible that other mechanisms of mineral allocation to the rice grain, such as remobilization from other leaves, phloem delivery of minerals acquired from the xylem through a transfer process or even direct xylem loading, may contribute with higher Fe and Zn amounts than flag leaf remobilization during grain filling in rice (Sperotto et al. 2010).
Conclusion
Though regarded as important, Zn availability suffers a major set back in the populations mainly dependent on cereal grains for their major food requirements as in most cereals large amounts of Zn are present in outer layers which get removed during milling. Also, this unavailability of the Zn through the cereals has been due to the unavailability of the Zn to the cereals in soil because of certain physical characters like pH and other unavoidable conditions prevailing in most cereal cultivating areas of the world making them deficient in Zn. One other important factor that contributes to the unavailability of Zn in edible parts is the inability of plant to move the absorbed Zn from roots to shoot to the grains. To rectify this problem such cereal varieties should be made via physiological breeding or biotechnology programme or by a combined effort of both, that accumulate higher amounts of Zn in bioavailable form and can help provide this important nutrient to poor those who cannot afford costly chemical feed supplements.
Acknowledgement
The financial assistance received from CSIR, New Delhi, India is duly acknowledged.
References
- Alloway BJ. Zinc in soils and crop nutrition. Brussels, Belgium: Online book published by the International Zinc Association; 2008. [Google Scholar]
- Alloway BJ. Soil factors associated with zinc deficiency in crops and humans. Environ Geochem Heal. 2009;31:537–548. doi: 10.1007/s10653-009-9255-4. [DOI] [PubMed] [Google Scholar]
- Andreini C, et al. Zinc through the three domains of life. J Proteome Res. 2006;5:3173–3178. doi: 10.1021/pr0603699. [DOI] [PubMed] [Google Scholar]
- Fifth report on the world nutrition situation. Geneva: United Nations, Administrative Committee on Coordination/Sub-Committee on Nutrition; 2004. [Google Scholar]
- Barak P, Helmke PA. The chemistry of zinc. In: Robson AD, editor. Zinc in soils and plants. Dordrecht: Kluwer Academic Publishers; 1993. pp. 90–106. [Google Scholar]
- Black RE. Zinc deficiency, infectious disease and mortality in the developing world. J Nutr. 2003;133:1485–1489. doi: 10.1093/jn/133.5.1485S. [DOI] [PubMed] [Google Scholar]
- Borlaug NE. Ending world hunger. The promise of biotechnology and the threat of antiscience zealotry. Plant Physiol. 2000;124:487–490. doi: 10.1104/pp.124.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouis HE, Welch RM. Biofortification—A sustainable agricultural strategy for reducing micronutrient malnutrition in the global south. Crop Sci. 2010;50:20–32. doi: 10.2135/cropsci2009.09.0531. [DOI] [Google Scholar]
- Broadley MR, et al. Zinc in plants. New Phytol. 2007;173:677–702. doi: 10.1111/j.1469-8137.2007.01996.x. [DOI] [PubMed] [Google Scholar]
- Cakmak I. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000;146:185–205. doi: 10.1046/j.1469-8137.2000.00630.x. [DOI] [PubMed] [Google Scholar]
- Cakmak I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil. 2008;302:1–17. doi: 10.1007/s11104-007-9466-3. [DOI] [Google Scholar]
- Cakmak I. Biofortification of cereal grains with zinc by applying zinc fertilizers. Biozoom. 2009;1:2–7. [Google Scholar]
- Chandel G, Banerjee S, See S, Meena R, Sharma DJ, Verulkar SB. Effects of different nitrogen fertilizer levels and native soil properties on rice grain Fe, Zn and Protein Contents. Rice Sci. 2010;17:213–227. doi: 10.1016/S1672-6308(09)60020-2. [DOI] [Google Scholar]
- Clemens S, Persoh D. Multi-tasking phytochelatin synthases. Plant Sci. 2009;177:266–271. doi: 10.1016/j.plantsci.2009.06.008. [DOI] [Google Scholar]
- Colangelo EP, Guerinot ML. Put the metal to the petal: Metal uptake and transport throughout plants. Curr Opin Plant Biol. 2006;9:322–330. doi: 10.1016/j.pbi.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Curie C, Panaviene Z, Loulergue C, Dellaporta SL, Briat JF, Walker EL. Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature. 2001;409:346–349. doi: 10.1038/35053080. [DOI] [PubMed] [Google Scholar]
- Deb DL (1992) Development of soil and plant analytical methods for micronutrients and sulphur in Srilanka. GCPF/SRI/047/NET field document No. 11.
- Deinlein U, Weber M, Schmidt H, Rensch S, Trampczynska A, Hansen TH, Husted S, Schjoerring JK, Talke IN, Kramer U, Clemens S. Elevated nicotianamine levels in Arabidopsis halleri roots play a key role in Zn hyperaccumulation. Plant Cell. 2012;24:708–723. doi: 10.1105/tpc.111.095000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide DJ. Homeostatic and adaptive responses to zinc deficiency in Saccharomyces cerevisiae. J Biol Chem. 2009;284:18565–18569. doi: 10.1074/jbc.R900014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren E, Arguello JM. Arabidopsis HMA2, a divalent heavy metal-transporting PIB-Type ATPase, is involved in cytoplasmic Zn2+ homeostasis. Plant Physiol. 2004;136:3712–3723. doi: 10.1104/pp.104.046292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erenoglu EB, Kutman UB, Ceylan Y, Yildiz B, Cakmak I. Improved nitrogen nutrition enhances root uptake, root to shoot translocation and remobilization of zinc (65Zn) in wheat. New Phytol. 2010;189:438–448. doi: 10.1111/j.1469-8137.2010.03488.x. [DOI] [PubMed] [Google Scholar]
- Frank R, Ishida K, Suda P. Metals in agricultural soils of Ontario. Can J Soil Sci. 1976;56:191–196. [Google Scholar]
- Freisinger E. Plant MTs – long neglected members of the metallothionein superfamily. Dalton Trans. 2008;47:6663. doi: 10.1039/b809789e. [DOI] [PubMed] [Google Scholar]
- Frossard E, Bucher M, Machler F, Mozafar A, Hurrell R. Potential for increasing the content and bioavailability of Fe, Zn and Ca in plants for human nutrition. J Sci Food Agric. 2000;80:861–879. doi: 10.1002/(SICI)1097-0010(20000515)80:7<861::AID-JSFA601>3.0.CO;2-P. [DOI] [Google Scholar]
- Gomez-Galera S, Rojas E, Sudhakar D, Zhu C, Pelacho AM, Capell T, Christou P. Critical evaluation of strategies for mineral fortification of staple food crops. Transgenic Res. 2010;19:165–180. doi: 10.1007/s11248-009-9311-y. [DOI] [PubMed] [Google Scholar]
- Goto F, Yoshihara T, Shigemoto N, Toki S, Takaiwa F. Iron fortification of rice seed by the soybean ferritin gene. Nat Biotechnol. 1999;17:282–286. doi: 10.1038/7029. [DOI] [PubMed] [Google Scholar]
- Graham RD. Micronutrient deficiencies in crops and their global significance. In: Alloway BJ, editor. Micronutrient deficiencies in global crop production, vol. 105. Dordrecht: Springer; 2008. pp. 41–61. [Google Scholar]
- Graham RD, Humphries JM, Kitchen JL. Nutritionally enhanced cereals: A sustainable foundation for a balanced diet. Asia Pac J Clin Nutr. 2000;9:S91–S96. doi: 10.1046/j.1440-6047.2000.00185.x. [DOI] [PubMed] [Google Scholar]
- Graham RD, Senadhira D, Beebe S, Iglesias C, Montasterio I. Breeding for micronutrient density in edible portions of staple food crops: conventional approaches. Field Crop Res. 1999;60:57–80. doi: 10.1016/S0378-4290(98)00133-6. [DOI] [Google Scholar]
- Graham RD, Welch RM, Bouis HE. Addressing micronutrient malnutrition through enhancing the nutritional quality of staple foods: Principles, perspectives and knowledge gaps. Adv Agron. 2001;70:77–142. doi: 10.1016/S0065-2113(01)70004-1. [DOI] [Google Scholar]
- Grotz N, Fox T, Connolly E, Park W, Guerinot ML, Eide D. Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc. Natl. Acad. Sci. USA. 1998;95:7220–7224. doi: 10.1073/pnas.95.12.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot ML. It’s elementary: Enhancing Fe3+ reduction improves rice yields. PNAS. 2007;104:7311–7312. doi: 10.1073/pnas.0701954104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanikenne M, Talke IN, Haydon MJ, Lanz C, Nolte A, Motte P, Kroymann J, Weigel D, Kramer U. Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nauture. 2008;453:391–395. doi: 10.1038/nature06877. [DOI] [PubMed] [Google Scholar]
- Hansch R, Mendel RR. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl) Curr. Opin. Plant Biol. 2009;12:259–266. doi: 10.1016/j.pbi.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Hao H, Wei Y, Yang X, Feng Y, Wu C. Effects of different nitrogen fertilizer levels on Fe, Mn, Cu and Zn concentrations in shoot and grain quality in rice (Oryza sativa) Rice Sci. 2007;14:289–294. doi: 10.1016/S1672-6308(08)60007-4. [DOI] [Google Scholar]
- HarvestPlus. 2005. Breeding crops for better nutrition. Washington, DC: International Food Policy Research Institute. http://www.harvestplus.org/.
- Haydon MJ, Cobbett CS. A novel major facilitator superfamily protein at the tonoplast influences zinc tolerance and accumulation in Arabidopsis. Plant Physiol. 2007;143:1705–1719. doi: 10.1104/pp.106.092015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschfinkel M, Silverman WF, Sekler I. The zinc sensing receptor, a link between zinc and cell signaling. Mol Med. 2007;13:331–336. doi: 10.2119/2006-00038.Hershfinkel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton S. The economics of food fortification. J Nutr. 2006;136:1068–1071. doi: 10.1093/jn/136.4.1068. [DOI] [PubMed] [Google Scholar]
- Hotz C, Brown KH. Assessment of the risk of zinc deficiency in populations and options for its control. Food and Nutrition Bulletin. 2004;25:S91–S204. [PubMed] [Google Scholar]
- Ishimaru Y, Suzuki M, Kobayashi T, Takahashi M, Nakanishi H, Mori S, Nishizawa NK. OsZIP4, a novel zinc-regulated zinc transporter in rice. J Exp Bot. 2005;56:3207–3214. doi: 10.1093/jxb/eri317. [DOI] [PubMed] [Google Scholar]
- International Zinc Nutrition Consultative Group (2007) Technical Brief No. 4. Food Fortification. http://www.izincg.org/publications/briefs
- Jiang W, Struik PC, Keulen HV, Zhao M, Jin LN, Stomph TJ. Does increased zinc uptake enhance grain zinc mass concentration in rice? Ann Appl Biol. 2008;153:135–147. doi: 10.1111/j.1744-7348.2008.00243.x. [DOI] [Google Scholar]
- Katyal JC, Sharma BD. DTPA extractable and total Zn, Cu, Mn and Fe in Indian soils. Geoderma. 1991;49:165–179. doi: 10.1016/0016-7061(91)90099-F. [DOI] [Google Scholar]
- Kawachi M, Kobae Y, Mori H, Tomioka R, Lee Y, Maeshima M. A mutant strain Arabidopsis thaliana that lacks vacuolar membrane zinc transporter MTP1 revealed the latent tolerance to excessive zinc. Plant Cell Physiol. 2009;50:1156–1170. doi: 10.1093/pcp/pcp067. [DOI] [PubMed] [Google Scholar]
- Khan MU, Quasim M, Jamil M. Response of rice to zinc fertilizer in calcareous soils of D. I. Khan. Asian J Plant Sci. 2002;1:1–2. doi: 10.3923/ajps.2002.1.2. [DOI] [Google Scholar]
- Kobae Y, Uemura T, Sato MH, Ohnishi M, Mimura T, Nakagawa T, Maeshima M. Zinc transporter of Arabidopsis thaliana AtMTP1 is localized to vacuoler membranes and implicated in zinc homeostasis. Plant Cell Physiol. 2004;45:1749–1758. doi: 10.1093/pcp/pci015. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim SA, Lee J, Guerinot ML, An G. Zinc deficiency-inducible osZIP8 encodes a plasma membrane-localized zinc transporter in rice. Mol cells. 2010;29:551–558. doi: 10.1007/s10059-010-0069-0. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim YY, Lee Y, An G. Rice P1B-Type heavy-metal ATPase, OsHMA9, is a metal efflux protein. Plant Physiol. 2007;145:831–842. doi: 10.1104/pp.107.102236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Ryoo N, Jeon JS, Guerinot ML, An G (2012) Activation of rice yellow stripe1-like 16 (OsYSL16) enhances iron efficiency. Mol cells. doi:10.1007/s10059-012-2156-9 [DOI] [PMC free article] [PubMed]
- Lewinsohn E, Schalechet F, Wilkinson J, Matsui K, Tadmor Y, Nam K, Amar O, Lastochkin E, Larkov O, Ravid U. Enhanced levels of the aroma and flavor compound S-linalool by metabolic engineering of the terpenoid pathway in tomato fruits. Plant Physiol. 2001;127:1256–1265. doi: 10.1104/pp.010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CW, Chang HB, Huang HJ. Zinc induces mitogen-activated protein kinase activation mediated by reactive oxygen species in rice roots. Plant Physiol Biochem. 2005;43:963–968. doi: 10.1016/j.plaphy.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Lucca P, Hurrell R, Potrykus I. Genetic engineering approaches to improve the bioavailability and the level of iron in rice grains. Theor Appl Genet. 2001;102:392–397. doi: 10.1007/s001220051659. [DOI] [Google Scholar]
- Lucca P, Hurrell R, Potrykus I. Fighting iron deficiency anemia with iron-rich rice. J Am Coll Nutr. 2002;21:184–190. doi: 10.1080/07315724.2002.10719264. [DOI] [PubMed] [Google Scholar]
- Machado CT, De T, Furlani AMC. Kinetics of phosphorous uptake and root morphology of local and improved varieties of maize. Sci Agric. 2004;61:1–12. doi: 10.1590/S0103-90162004000100001. [DOI] [Google Scholar]
- Mandal B, Mandal LN, and Ali MH (1993) Chemistry of zinc availability in submerged soils in relation to zinc nutrition of rice crop. In: Proceedings of the workshop on nicronutrients, Bhubaneswar, India, 22–23 January 1992, pp 240–253.
- Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol. 2006;20:3–18. doi: 10.1016/j.jtemb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Masuda H, Suzuki M, Morikava KC, Kobayashi T, Nakanishi H, Takahashi M, Saigusa M, Mori S, Nishizawa NK. Increase in iron and zinc concentrations in rice grains via the introduction of barley genes involved in phytosiderophore synthesis. Rice. 2008;1:100–108. doi: 10.1007/s12284-008-9007-6. [DOI] [Google Scholar]
- Mayer JE, Pfeiffer WH, Beyer P. Biofortified crops to alleviatemicronutrient malnutrition. Curr. Opin. Plant Biol. 2008;11:166–170. doi: 10.1016/j.pbi.2008.01.007. [DOI] [PubMed] [Google Scholar]
- McDonald GK, Genc Y, Graham RD. A simple method to evaluate genetic variation in grain zinc concentration by correcting for differences in grain yield. Plant Soil. 2008;306:49–55. doi: 10.1007/s11104-008-9555-y. [DOI] [Google Scholar]
- Naik SK, Das DK. Relative performance of chelated zinc and zinc sulphate for lowland rice (Oryza sativa L.) Nutrition Cycle in Agroecosystem. 2008;81:219–227. doi: 10.1007/s10705-007-9158-7. [DOI] [Google Scholar]
- Nandi S, Suzuki YA, Huang JM, Yalda D, Pham P, Wu LY, Bartley G, Huang N, Lonnerdal B. Expression of human lactoferrin in transgenic rice grains for the application in infant formula. Plant Sci. 2002;163:713–722. doi: 10.1016/S0168-9452(02)00165-6. [DOI] [Google Scholar]
- Narayanan NN, Vasconcelos MW, Grusak MA. Expression profiling of Oryza sativa metal homeostasis genes in different rice cultivars using a cDNA macroarray. Plant Physiol Biochem. 2007;45:277–286. doi: 10.1016/j.plaphy.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Nene YL. Symptoms, cause and control of Khaira disease of Paddy. Bulletin Indian Phytopathology Society. 1966;3:97–191. [Google Scholar]
- Neue HU, Lantin RS. Micronutrient toxicities and deficiencies in rice. In: Yeo AR, Flowers TJ, editors. Soil mineral stresses: Approaches to Crop Improvement. Berlin: Springer; 1994. pp. 175–200. [Google Scholar]
- Palmer CM, Guerinot ML. Facing the challenges of Cu, Fe and Zn homeostasis in plants. Nat Chem Biol. 2009;5:333–340. doi: 10.1038/nchembio.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren MG, Clemens S, Williams LE, Kramer U, Borg S, Schjorring JK, Sanders D. Zinc biofortification of cereals: problems and solutions. Trends Plant Sci. 2008;13:464–473. doi: 10.1016/j.tplants.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Pence N, Larsen P, Ebbs S, Garvin D, Eide D, Kochian L. Cloning and characterization of a heavy metal transporter (ZNT1) from the Zn/Cd hyperaccumulator Thlaspi caerulescens. Plant Physiol. 1998;118:356–363. [Google Scholar]
- Pfeiffer WH, McClafferty B. HarvestPlus: breeding crops for better nutrition. Crop Sci. 2007;47:88–105. doi: 10.2135/cropsci2007.09.0020IPBS. [DOI] [Google Scholar]
- Phattarakul N, Rarkasem B, Li LJ, Wu LH, Zou CQ, Ram H, Sohu VS, Kang BS, Surek H, Kalayci M, Yazici A, Zhang FS, Cakmak I (2012) Biofortificaiton of rice grain with zinc through zinc fertilization in different countries. Plant Soil. doi:10.1007/s11104-012-1211-x
- Prasad AS. Zinc in human health: Effect of zinc on immune cells. Mol Med. 2008;14:353–357. doi: 10.2119/2008-00033.Prasad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh SA, Choimes S, Schachtman DP. Over-expression of an Arabidopsis zinc transporter in Hordeum vulgare increases short-term zinc uptake after zinc deprivation and seed zinc content. Plant Mol Biol. 2004;54:373–385. doi: 10.1023/B:PLAN.0000036370.70912.34. [DOI] [PubMed] [Google Scholar]
- Ramesh SA, Shin R, Edie DJ, Schachtman DP. Differential metal selectivity and gene expression of two zinc transporters from rice. Plant Physiol. 2003;133:126–134. doi: 10.1104/pp.103.026815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair SA, Kramer U (2012) The zinc homeostasis network of land plants. Biochim Biophys Acta. doi:10.1016/j.bbamcr.2012.05.016 [DOI] [PubMed]
- Singh AK, Khan SK, Nongkynrih P. Transformation of zinc in wetland rice soils in relation to nutrition of rice crop. J Indian Soc Soil Sci. 1999;47:248–253. [Google Scholar]
- Singh B, Natesan SKA, Singh BK, Usha K. Improving zinc efficiency of cereals under zinc deficiency. Curr Sci. 2005;88:36–44. [Google Scholar]
- Slaton NA, Wilson CE, Norman RJ, Boothe DL. Evaluation of zinc seed treatments for rice. Agron J. 2001;93:152–157. doi: 10.2134/agronj2001.931152x. [DOI] [Google Scholar]
- Song WY, Choi KS, Kim DY, Geisler M, Park J, Vincenzetti V, Schellenberg M, Kim SH, Lim YP, Noh EW, Lee Y, Martinoiaa E. Arabidopsis PCR2 is a zinc exporter involved in both zinc extrusion and long-distance zinc transport. Plant Cell. 2010;22:2237–2252. doi: 10.1105/tpc.109.070185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperotto RA, Boff T, Duarte GL, Santos LS, Grusak MA, Fett JP. Identification of putative target genes to manipulate Fe and Zn concentrations in rice grains. J Plant Physiol. 2010;167:1500–1506. doi: 10.1016/j.jplph.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Sperotto RA, Ricachenevsky KF, Duarte GL, Boff T, Lopes KL, Sperb ER. Identification of up-regulated genes in flag leaves during rice grain filling and characterization of OsNAC5, a new ABA-dependent transcription factor. Planta. 2009;230:985–1002. doi: 10.1007/s00425-009-1000-9. [DOI] [PubMed] [Google Scholar]
- Sudhalakshmi C, Krishnasamy R, Rajarajan R. Influence of zinc deficiency on shoot/root dry weight ratio of rice genotypes. Res J Agric Biol Sci. 2007;3:295–298. [Google Scholar]
- Swaine DJ (1955). Trace element content of soils. Commonwealth Bureau of Soil Science, Tech. Commun. 48, U.K.
- Takahashi M, Nozoye T, Kitajima N, Fukuda N, Hokura A, Terada Y. In vivo analysis of metal distribution and expression of metal transporters in rice seed during germination process by microarray and X-ray fluorescence Imaging of Fe, Zn, Mn, and Cu. Plant Soil. 2009;325:39–51. doi: 10.1007/s11104-009-0045-7. [DOI] [Google Scholar]
- Takahashi R, Ishimaru Y, Shimo H, Ogo Y, Senoura T, Nishizawa NK, Nakanishi H (2012) The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant Cell Environ. doi:10.1111/j.1365-3040.2012.02527.x [DOI] [PubMed]
- Tapeiro H, Tew KD. Trace elements in human physiology and pathology: Zinc and metallothioneins. Biomed Pharmacother. 2003;57:399–411. doi: 10.1016/S0753-3322(03)00081-7. [DOI] [PubMed] [Google Scholar]
- Thomine S, Wang R, Ward JM, Crawford NM, Schroeder JI. Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc. Natl. Acad. Sci. USA. 2000;97:4991–4996. doi: 10.1073/pnas.97.9.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uauy C, Distelfeld A, Fahima T, Blechl A, Dubcovsky J. A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science. 2006;314:1298–1301. doi: 10.1126/science.1133649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood BA, Smitasiri S. Micronutrient malnutrition: policies and programs for control and their implications. Annu Rev Nutr. 1999;19:303–324. doi: 10.1146/annurev.nutr.19.1.303. [DOI] [PubMed] [Google Scholar]
- Valle BL, Falchuk KH. The biochemical basis of zinc physiology. Plant Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- Van Bel AJE, Furch ACU, Hafke JB, Knoblauch M, Patrick JW. (Questions)n on phloem biology. 2. Mass flow, molecular hopping, distribution patterns and molecular signalling. Plant Sci. 2011;181:325–330. doi: 10.1016/j.plantsci.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Vasconcelos M, Datta K, Oliva N, Khalekuzzaman M, Torrizo L, Krishnan S, Oliveira M, Goto F, Datta SK. Enhanced iron and zinc accumulation in transgenic rice with the ferritin gene. Plant Sci. 2003;164:371–378. doi: 10.1016/S0168-9452(02)00421-1. [DOI] [Google Scholar]
- Vinogradov AP. The geochemistry of rare and dispersed chemical elements in soils. New York: Consultants Bureau; 1959. [Google Scholar]
- Virk P, Barry G. Biofortified rice—towards combating human micronutrient deficiencies. DAPO Box 7777, Metro Manila, Philippines: International Rice Research Institute; 2009. [Google Scholar]
- Waters BM, Sankaran RP. Moving micronutrients from the soil to the seeds: Genes and physiological processes from a biofortification perspective. Plant Sci. 2011;180:562–574. doi: 10.1016/j.plantsci.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Waters BM, Uauy C, Dubcovsky J, Grusak MA. Wheat (Triticum aestivum) NAM proteins regulate the translocation of iron, zinc, and nitrogen compounds from vegetative tissues to grain. J Exp Bot. 2009;60:4263–4274. doi: 10.1093/jxb/erp257. [DOI] [PubMed] [Google Scholar]
- Welch RM. Micronutrients, agriculture and nutrition; linkages for improved health and well being. In: Singh K, Mori S, Welch RM, editors. Perspectives on the micronutrient nutrition of crops. Jodhpur, India: Scientific Publishers; 2001. pp. 247–289. [Google Scholar]
- Welch RM, Graham RD. Breeding for micronutrients in staple food crops from a human nutrition perspective. J Exp Bot. 2004;55:353–364. doi: 10.1093/jxb/erh064. [DOI] [PubMed] [Google Scholar]
- White CL (1993) In Zn in soils and plants (A. D. Robson ed.), Kulwer Academic Pub., Dordrecht, The Netherlands.
- White PJ, Broadley MR. Biofortifying crops with essential mineral elements. Trends Plant Sci. 2005;10:586–593. doi: 10.1016/j.tplants.2005.10.001. [DOI] [PubMed] [Google Scholar]
- White PJ, Broadley MR. Biofortification of crops with seven mineral elements often lacking in human diets: iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009;182:49–84. doi: 10.1111/j.1469-8137.2008.02738.x. [DOI] [PubMed] [Google Scholar]
- Wissuwa M, Ismail AM, Graham RD. Rice grain zinc concentrations as affected by genotype, native soil-zinc availability, and zinc fertilization. Plant Soil. 2008;306:34–48. doi: 10.1007/s11104-007-9368-4. [DOI] [Google Scholar]
- World Health Organization (2002) World Health Rep. 2002 (http://www.who.int/whr/2002/)
- World Health Organization (2007) UNICEF India, Children Issues. Global Database on Child Growth and Malnutrition in United Nations Administrative Committee on Coordination/Sub-Committee on Nutrition, Low Birth Weight, Nutrition Policy, Paper 18. http://www.who.int/nutgrowthdb/en/
- Yamaguchi N, Ishikawa S, Abel T, Baba K, Arao T, Terada Y (2012) Role of the node in controlling traffic of cadmium, zinc, and manganese in rice. J Exp Bot. doi:10.1093/jxb/err455 [DOI] [PMC free article] [PubMed]
- Yang X, Huang J, Jiang Y, Zhang HS. Cloning and functional identification of two members of the ZIP (Zrt, Irt-like protein) gene family in rice (Oryza sativa L.) Mol Biol Rep. 2009;36:281–287. doi: 10.1007/s11033-007-9177-0. [DOI] [PubMed] [Google Scholar]
- Ye X, Babili SA, Klöti A, Zhang J, Lucca P, Beyer P, Potroykus I. Engineering the provitamin A (β-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science. 2000;287:303–305. doi: 10.1126/science.287.5451.303. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Ahn JS, Forne DA. Occurrence, diagnosis and correction of zinc deficiency of low land rice. Soil Sci Plant Nutr. 1973;19:89–93. [Google Scholar]
- Yoshida S, Tanaka A. Zinc deficiency of the rice plant in calcareous soils. Soil Sci. Plant Nutr. 1969;15:75–80. doi: 10.1080/00380768.1969.10432783. [DOI] [Google Scholar]
- Zeng Y, Zhang H, Wang L, Pu X, Du J, Yang S, Liu J. Genotypic variation in element concentrations in brown rice from Yunnan landraces in China. Environ Geochem Heal. 2010;32:165–177. doi: 10.1007/s10653-009-9272-3. [DOI] [PubMed] [Google Scholar]
- Zhao FJ, McGrath SP. Biofortification and phytoremidiation. Curr. Opin. Plant Biol. 2009;12:373–380. doi: 10.1016/j.pbi.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Zimmermann MB, Hurrell RF. Improving iron, zinc and vitamin A nutrition through plant biotechnology. Curr Opin Biotechnol. 2002;13:142–145. doi: 10.1016/S0958-1669(02)00304-X. [DOI] [PubMed] [Google Scholar]