Abstract
Withania somnifera (L.) Dunal, is an important medicinal plant being the source of extremely important compounds like withanolides and withaferin. Influence of different plant growth regulators (PGRs) were evaluated for induction of callus, callus mediated regeneration and production of secondary metabolites in them. Explants for callusing were collected from plants grown in vitro and maximum callusing (98 %) was obtained on MS medium supplemented with a combination of 2,4-dichlorophenoxy acetic acid (2,4-D) (0.5 mg l-1) and kinetin (KN) (0.2 mg l−1). Among different types of calli, best shoot regeneration was observed on green, compact calli produced on MS medium with a combination of 6-benzylamino purine (BAP) and indole butyric acid (IBA). MS medium supplemented with BAP (2 mg l−1) showed highest frequency (98 %) of shoot bud regeneration. The micro-shoots were efficiently rooted on MS media supplemented with 0.5 mg l−1 IBA. Rooted plants were transferred to soil-vermi-compost (1:3; w/w) medium in greenhouse for acclimatization. Presence of withanolide A and withaferin A in calli was validated through high performance thin layer chromatography (HPTLC). It was interesting to observe that the PGRs showed significant influence on the secondary metabolites production in callus and 2,4-D having the least effect. Histological studies revealed the origin of shoot tip in the callus during regeneration.
Keywords: Callusing, High performance thin layer chromatography, Histology, Plant growth regulator, Shoot bud regeneration, Withaferin A, Withanolide A
Introduction
Withania somnifera (L.) Dunal commonly known as Ashwagandha or Winter Cherry is mentioned as an important, ancient, ayurvedic drug of India. This plant is reputed to have adaptogenic, tonic, analgesic, antipyretic and abortifacient properties. Its roots are prescribed for hiccup, female disorders, cough, rheumatism and dropsy (Kiritikar and Basu 1975). The root of this plant is not only a major source of several alkaloids viz. tropine, pseudotropine & somniferine but also important steroids like withaferin A and withanolides (Pati et al. 2008).
Ashwagandha belonging to family Solanaceae (Gamble 1918), contains withanolides, which are anti-bacterial, anti-inflammatory and enhance body’s defense against infection and tumor (Jaffer et al. 1988; Devi et al. 1992). Roots are also used as sedative for senile debility and for the prevention and for the inhibition of Alzheimer’s disease (Kiritikar and Basu 1975).
This important medicinal plant has now become rare since it is not available in wild condition; the observation has been corroborated by earlier reports (Sivanesan 2007). Also, it is worth mentioning that withanolide A, a commercially important chemical, constitute a very small part of the plants in vivo. However, there are reports regarding scope of bio-generation of this an important chemical in vitro (Sangwan et al. 2007).
Protocols for in vitro plant production via direct and indirect morphogenesis have many potential applications to any species particularly that of tremendous economic use and medicinal importance such as Withania somnifera. There are reports on in vitro culture of Withania somnifera using different explants (Sen and Sharma 1991; Kulkarni et al. 2000; Manickam et al. 2000; Sivanesan and Murugesan 2005; Sabir et al. 2007; Sivanesan 2007) as well as generation of withanolides in vitro (Roja and Heble 1991; Furmanowa et al. 2001; Ray and Jha 2001; Sangwan et al. 2005; Sangwan et al. 2007). There is also a report on the production of withanolide A in cell-suspension cultures of W. somnifera (Nagella and Murthy 2010). Das et al. (2010) reported production of withaferin A and withanolide A in stage-III (fully differentiated calli) but no production in stage-I (undifferentiated calli). However, few reports have so far been made to observe influence of PGR on the production of secondary metabolite in vitro.
The present study, targeted to optimize the production of callus from different explants, regeneration of whole plants from such calli, origin of the micro-shoot formed within callus and the relation between the PGR used and the synthesis of withnolides in different types of calli.
Materials and methods
Plant material
Seeds of Withania somnifera of ‘Jawahar’ variety were obtained from the medicinal plant garden of Ramakrishna Mission Ashrama, Narendrapur, Kolkata, India.
Surface sterilization and seed germination
Seeds were washed in running tap water for 2 min followed by washing in Teepol (4 %; v/v) solution for 5 min After that they were disinfected with 0.1 % (v/v) HgCl2 for 6 min followed by three rinses with sterile distilled water (Sen and Sharma 1991). The seeds were then inoculated on Murashige and Skoog (1962) medium supplemented with GA3 (0.5 mg l−1).
Preparation of media
MS medium was used universally for callusing, regeneration and rooting of micro-shoot. Medium supplemented with 2,4-D, IBA, KN & BAP, used alone or in combination, ranging from 0.2 mg l−1 - 2 mg l−1, were tried for callusing. For regeneration purpose, BAP & IBA, either alone or in combination, were used (Table 2 for detail). In all these cases, pH was adjusted to 5.8 by using 2N hydrochloric acid and 2(N) sodium hydroxide. The media were solidified with 0.8 % (v/v) agar and dispensed in tissue culture bottle, approx 40 ml in each bottle. Finally, those media containing bottles were sterilized by autoclaving at 103,421 N/m2 (15 lb) pressure, 121 °C temperature and for 15 min.
Table 2.
Effect of plant growth regulators on shoot regeneration from leaf explant derived callus tissue of W. somnifera
| Growth regulators (mg/l) | % of shoot response | Mean number of shoots | Response initiation (in days) | |
|---|---|---|---|---|
| BAP | IBA | |||
| – | – | 15.0 ± 5.77d | 3.4 ± 0.4dc | 25 |
| 0.5 | – | 6.66 ± 1.66d | 2.4 ± 0.5e | 25 |
| 1.0 | – | 46.67 ± 4.4c | 6.0 ± 0.44c | 20 |
| 2.0 | – | 98.33 ± 1.66a | 7.6 ± 0.4ab | 7 |
| – | 0.5 | 63.34 ± 7.26b | 6.8 ± 0.37bc | 20 |
| – | 1.0 | 51.67 ± 6.0bc | 5.8 ± 0.2c | 18 |
| – | 2.0 | 41.66 ± 3.33c | 4.0 ± 0.32d | 20 |
| 0.5 | 0.2 | 95.0 ± 2.88a | 8.6 ± 0.24a | 10 |
| 0.5 | 0.5 | 88.34 ± 4.4a | 6.8 ± 0.38bc | 15 |
| 0.5 | 1.0 | 18.33 ± 3.3d | 2.6 ± 0.4e | 25 |
Values represent mean ± standard error of 10 replicates per treatment in three repeated experiments. Means sharing by the same letter are not significantly different (P = 0.05) using Duncan’s multiple range test
Cultural conditions
Cultures were incubated at 25 ± 2 °C under white fluororescent light (3,000 lux) (Philips, Kolkata, India) for 16 h photo period and 55–60 % relative humidity. The explants were sub-cultured at an interval of 4–5 weeks.
Induction of callus and plant regeneration
From 4–6 weeks old seedlings shoot tip, node and leaf segments of 0.6–1.2 cm were isolated and aseptically placed in lug jar bottles containing MS medium supplemented with of 2,4-D, IBA, KN and BAP ranging from 0.2 – 2 mg l−1 (Table 1) either alone or in combination, for callusing. After 3–4 weeks growing calli obtained from different media were transferred for shoot induction on MS medium supplemented with various concentrations (ranging from 0.2 to 2 mg l−1) and combinations of BAP and IBA (Table 2). Responses were evaluated for callus induction in terms of number of explants producing callus, type of callus produced and also time required for initiation. In case of shoot induction responses were evaluated in terms of frequency of calli showing regeneration, number of shoots regenerated per unit of callus (20 × 20 mm) and time required for initiation of response.
Table 1.
Effect of plant growth regulators on callus formation from leaf explant of W. somnifera
| Growth regulators (mg/l) | % Callus response | Response initiation (in days) | |||
|---|---|---|---|---|---|
| 2,4-D | IBA | KN | BAP | ||
| 0 | 0 | 0 | 0 | 0 ± 0i | – |
| 0.5 | – | – | – | 85.0 ± 2.88ab | 7 |
| 0.5 | – | 0.2 | – | 98.33 ± 1.66a | 4 |
| 0.5 | – | 0.5 | – | 73.33 ± 4.46bc | 9 |
| 1.0 | – | 0.5 | – | 61.66 ± 1.67cd | 10 |
| 1.0 | – | 1.0 | – | 56.66 ± 6.0d | 10 |
| 1.0 | – | 2.0 | – | 28.33 ± 8.33efg | 18 |
| 1.0 | – | – | 0.5 | 13.33 ± 4.40ghi | 25 |
| 1.0 | – | – | 1.0 | 31.67 ± 7.21ef | 20 |
| 1.0 | – | – | 2.0 | 3.33 ± 1.66hi | 25 |
| – | 1.0 | – | 0.5 | 61.66 ± 6.0cd | 15 |
| – | 1.0 | – | 1.0 | 50.0 ± 7.63d | 17 |
| – | 1.0 | – | 2.0 | 65.0 ± 7.63cd | 20 |
| – | 1.0 | 0.5 | – | 35.0 ± 5.0e | 25 |
| – | 1.0 | 1.0 | – | 21.66 ± 6.0efg | 22 |
| – | 1.0 | 2.0 | – | 16.66 ± 1.67fgh | 25 |
| – | – | – | 2.0 | 6.66 ± 2.35hi | 50 |
Values represent mean ± standard error of 20 replicates per treatment in three repeated experiments. Means sharing the same letter are not significantly different (P = 0.05) using Duncan’s multiple range test
Root formation
Micro-shoots regenerated from calli (2–3 cm) were transferred to the rooting medium. Half strength of MS medium without PGR, MS basal medium and MS medium containing IBA (0.5–2 mg l−1) were used as rooting medium. However, only the significant results were recorded. The number of roots per shoot, root lengths as well as time of initiation was recorded.
Acclimatization
After proper development of root, plants were removed from medium and gently washed under tap water and then transferred to the plastic pots (net-pots) containing sterile soil-vermi-compost (1:3; w/w) and kept in green house for 3 weeks. After that plants were transferred to the earthen pots containing garden soil.
Histological analysis
Solid callus tissue whether bearing shoot bud or not were fixed in FAA solution (Formalin: Acetic acid: Absolute ethanol) in a ratio 5: 5: 90 (v/v/v) for 48 h before further processing. The fixation process was followed by gradual dehydration and finally embedded in paraffin according to procedure of Johansen (1940) with slight modification. Serial sections (4–10 μm) were cut using LEICA RM 2,235 microtome instrument and then stained with Haematoxylin and Eosin. Sections were examined under a light microscope (BX41, Olympus India Pvt. Ltd. New Delhi), at the power of 4X.
Analysis of pharmaceutically important constituents
According to different types of callus tissue (i.e. friable, solid non-regenerating and solid regenerating) each 0.2 g were air dried, finely powdered and subjected to methanol (5–10 ml) extraction, finally the methanol extracts were distilled and vacuum dried (Pati et al. 2008) . The samples, dissolved in 2 ml of AR grade methanol, were used for analysis of active principles particularly withaferin A and withanolide A by HPTLC. Presence of different active principle along with withanolide A and withaferin A was determined in reference to standard Atlas provided with the Camag HPTLC instrument. A number of secondary metabolites were identified; however, only withaferin A and withanolide A were taken into consideration during the study of secondary metabolites.
A Camag HPTLC system equipped with an automatic TLC sampler ATS 4, TLC scanner 3 and integrated software Win CATS version 1.2.3 was used for the analysis. HPTLC was performed on a pre-coated silica gel HPTLC 60F254 (10 × 10 cm) plate with layer thickness of 0.2 mm. Samples were applied to the plate as 6 mm wide bands with an automatic TLC sampler (ATS 4) under a flow of N2 gas.
The linear ascending development was carried out in a CAMAG twin trough chamber (20 × 10 cm) which was pre saturated with 25 ml mobile phase Toluene: Ethyl Acetate: Formic Acid in a ratio 5:5:1(v/v/v) for 30 min at room temperature (25 ± 2 °C) and 60 % ± 5 RH. The length of the chromatogram was 9 cm. Subsequent to the development; TLC plates were dried by using a drier. Evaluation of the plate was performed in the absorption reflection mode at 230 nm, using a slit width of 5 × 0.45 mm, data resolution 100 μm/step and scanning speed 20 mm/s with a computerized CAMAG TLC Scanner-3, Win CATS software version 1.2.3. The source of radiation utilized was a deuterium lamp emitting a continuous UV spectrum 190 to 400 nm.
Statistical analysis
Each experiment contained 20 replicates and each experiment was repeated three times. The data were analyzed using a one-way analysis of variance (ANOVA) test and means were compared by Duncan’s multiple range test (DMRT) at 5 % level of significance (P < 0.05) using SPSS software version 17.0.
Results and discussions
Callus initiation and regeneration
Out of three types of explants viz. leaf, shoot tip and node, obtained from 4–6 weeks old in vitro seed germinated plants, leaves proved to be the best explant followed by shoot tip and nodal explants as evaluated in terms of callus formation; the former two types of explants often associated with shoot multiplication. So for further experiments, leaves were used for induction of callus. 2,4-D and IBA either alone, or in combination with KN and BAP were used as shown in (Table 1) and 2,4-D alone was found to be adequate for induction of callus. However optimum results were obtained when a combination of 2,4-D (0.5 mg l−1) & KN (0.2 mg l−1) was used and maximum number of explants showed callusing in minimum number of days (Table 1). This corroborates with earlier reports in Withania somnifera (Nagella and Murthy 2010; Rani and Grover 1999; Roja and Heble 1991). The callus developed on media containing various combinations of 2,4-D and KN were soft, friable and greenish white in color (Fig. 1a). Here, increase in the concentration of PGR varied inversely with frequency of explants showing callus as well as time taken for callusing (Table 1). The combination of IBA and BAP was found to be less suitable, both for induction of callus as well as frequency of explants responded. The other combinations of PGR like 2,4-D & BAP and IBA & KN were also tried without much success. Though they could induce callus, their frequency was insignificant and mostly the calli turned brown immediately after induction. However, it was clear that the PGR were essential both for induction of callus and their maintenance since no calli were observed on MS basal medium alone (Table 1).
Fig. 1.
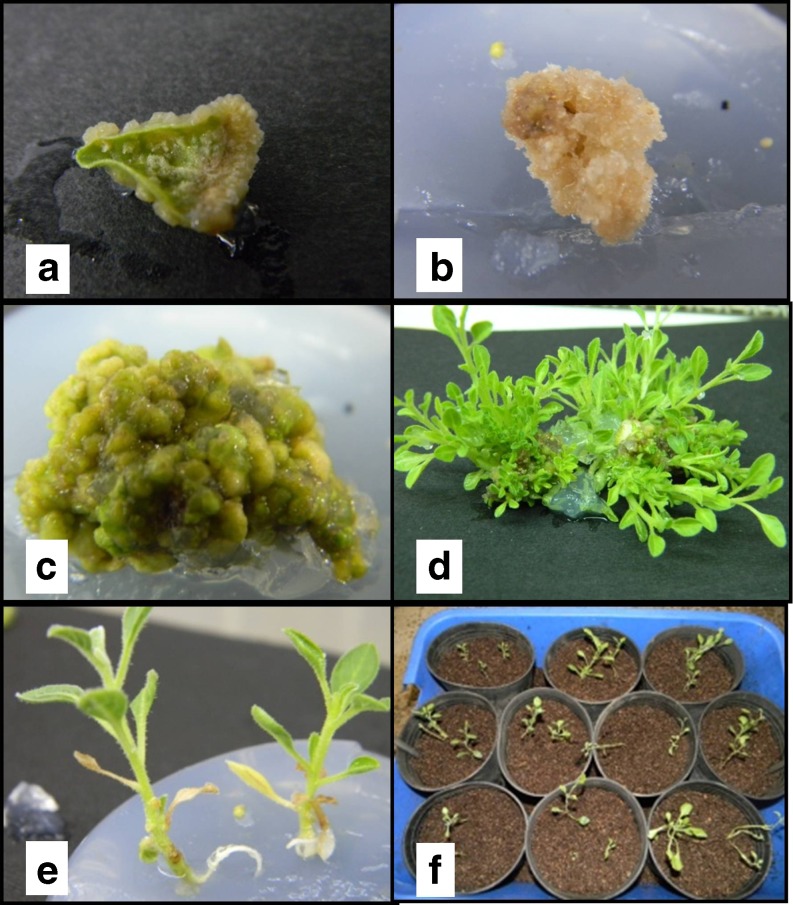
a Leaf explant derived callus tissue of W. somnifera in 2,4-D and KN containing media b Full grown callus tissue in turning brown in appearance c Full grown solid callus tissue in IBA and BAP containing media d Multiple shoot induction in from solid, partial brown callus tissue eIn vitro rooting in regenerated shoot f Rooted plantlets transplanted in plastic pot in green house for hardening
In contrast to earlier reports of induction of calli with BAP (Dewir et al. 2010) – it was observed that BAP alone was not adequate for induction of callus (Table 1). A combination of BAP (1–2 mg l−1) with IBA (0.5–1 mg l−1) could induce calli in 61 % – 65 % of explants though a longer period of time was required for such induction (Table 1). The callus thus produced was soft but compact and light green in color (Fig. 1c). BAP alone was not at all suitable for induction of callus but it was observed that BAP at a concentration of 2 mg l−1 was the best for maintenance of the calli after induction (Rani and Grover 1999; Kulkarni et al. 2000; Rani et al. 2003; Sivanesan and Murugesan 2008; Ray and Jha 2001; Blakesley and Constantine 1992). The fact that BAP is important for regeneration and multiplication was also supported by earlier established reports (Manickam et al. 2000; Sivanesan and Murugesan 2008). However, in contrast to earlier report (Kulkarni et al. 2000) de novo shoot formation from leaf or any other explant in presence of BAP, was not observed. It was interesting to note when shoot-tip and nodes were used as explant BAP alone induced shoot multiplication but mostly associated with callus formation at the base.
Regeneration was never observed in friable calli and it was observed that regardless of the nature of explant or PGR used, both white-friable calli and green compact calli gradually turned brown and necrotic in appearance in course of 4–5 cycles. However, in spite of their appearance they retained their capacity to grow & regenerate new shoot as confirmed through serial microtome sectioning. Though regeneration was observed in 2,4-D containing medium in one instance only, regeneration was best observed in MS medium supplemented with BAP alone or in combination with IBA. This finding is supported by earlier reports for other tissue cultured plants like Camellia sinensis (Agarwal et al. 1992), Neem (Salvi et al. 2001), Ocimum sanctum (Singh and Sehgal 1999), Dendrocalamus asper (Banerjee et al. 2011), Piper nigrum (Abbasi et al. 2010).
In contrast to lack of callus formation in MS basal medium, regeneration could be induced in such medium though the frequency of regeneration was few and delayed (Table 2). Regeneration was best observed in MS medium supplemented with BAP 2 mg l−1 where 98 % of the calli showed regeneration in about 7 days (Table 2) of inoculation and it may continue up to 2 years. As mentioned earlier the soft, compact, greenish calli, derived from BAP containing medium gradually turned brown and necrotic in appearance (Fig. 1b). This coloration may be due enzymatic activities rather than accumulation of phytotoxic chemicals since their capacity for regeneration never ceased - a phenomenon never reported earlier. However, the specific reason behind their change in appearance without altering their physiological characters needs further research. Regeneration was also observed in MS medium with BAP (0.5 mg l−1) and IBA (0.2 mg l−1) though the frequency of regeneration was slightly less (Fig. 1d). It was interesting to observe that this medium supported the growth of micro-shoot which was not observed when BAP was used alone for regeneration of shoot along with MS medium. Also, this last combination of PGR supported proliferation of shoot as well as their multiplication when nodes or shoot apex were used as explants.
Rooting, hardening and field trial
In all the cases, frequency of root initiation was 100 % provided IBA was used for root induction (Fig. 1e). Induction of root varied not only with the PGR but also with the strength of the medium and in contrast to earlier reports it was observed that full strength MS Medium performed better (6–8 rootlets/plant) than ½ strength of MS Medium (4–5 rootlets/plant) when the PGR remained constant (IBA 2 mg l−1). Rooting could be induced in MS basal medium with IBA 2 mg l−1 within 7 days of inoculation but it was almost always associated with formation of callus at the bottom. Also the roots are longer in this case than in any other combination (11.5 cm as an average). However, length of root was never considered as an important parameter for evaluation of the rooting media. The micro-shoots, when placed on MS medium supplemented with IBA (0.5 mg l−1), showed rooting after 3 weeks or even later. But no callus formation was associated with rhizogenesis in this case. Thus, MS basal medium supplemented with IBA 0.5 mg l−1 was found to be the most suitable rooting media since the earlier media (MS supplemented with 2 mg l−1 IBA) caused callusing which hampers acclimatization. This finding contradict with a recent report on the same material (Fatima and Anis 2012) but somewhat agrees with earlier report (Ghimire et al. 2010). More than 95 % of the plants could be successfully acclimatized (Fig. 1f), in contrast to the earlier reports of 83 % (Rani and Grover 1999), 84 % (Manickam et al. 2000), 87 % (Sivanesan and Murugesan 2008). Similar results were reported by Dewir et al. (2010) and Kulkarni et al. (2000). These plants were found to be extremely susceptible to excess humidity, during & immediately after hardening which may be the plausible reason for increased mortality. Thus, controlling the level of moisture during & immediately after hardening was found to be extremely important.
Histological analysis
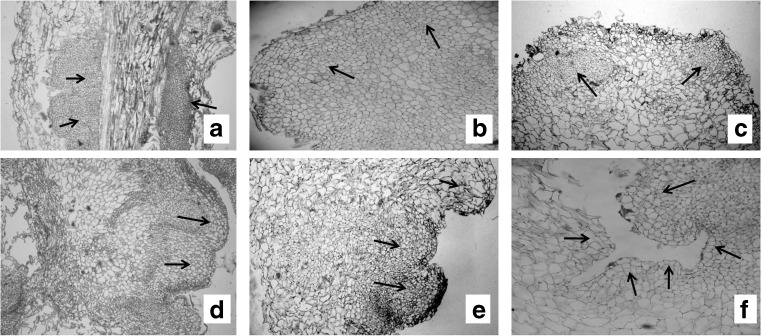
Since three types of explants were used for initiation of callus, a histological study was carried out to confirm the origin of the shoot tip. In this case it was observed that the undifferentiated mass of cells, called primary callus was derived from the parenchymatous cells of the explant (Fig. 2a). A detailed study showed that small group of cells were surrounded by larger cells and these two types of cells together gave rise to shoot apical meristem. It appears that certain small group of callus cells undergo divisions in such a way that produce a larger derivative and together remains in the meristem called initials as proposed by Easu (1977). In our sections, we have also found similar kind of meristematic division which shows in (Fig. 2b to d). Histological studies made through serial sections, through differentiating calli further shows the shoot bud initiation along with leaf primordia (Fig. 2e). The figure (Fig. 2f) shows multiple shoot bud forming region. The detailed histological analysis shows that the shoots regenerated from the leaf derived callus of W. somnifera have no organized cellular connection with the original explant tissue, indicating an adventitious origin.
Fig. 2.
Histological sections of developmental stages involved during callusing and plant regeneration in Withania somniferaa Histological section of primary callus developed from the node (arrows). b-d Meristemoid development within the callus mass (arrows). e Development of Shoot bud with leaf primordia (arrows). f Development of multiple shoot primordia flanked by leaf primordia (arrow)
Analysis of active principles
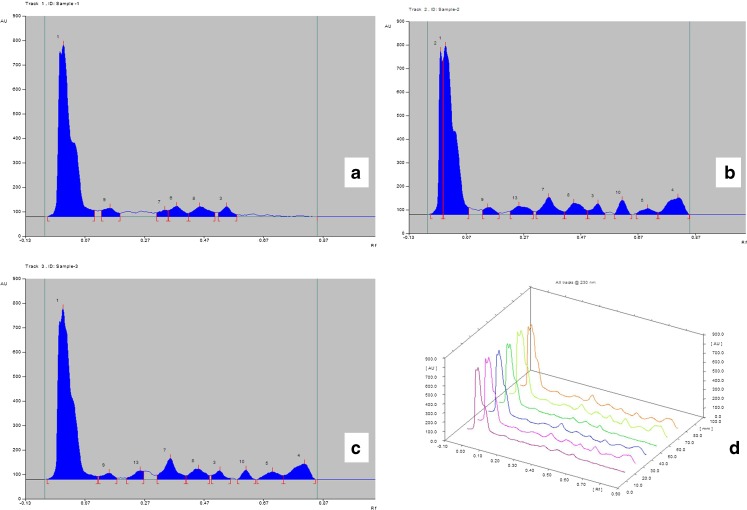
The results of HPTLC analysis of different types of callus tissues derived from varieties of media containing various combination of PGR are presented in (Fig. 4). Out of different types of secondary metabolites only withanolide A and withaferin A were taken into consideration. It was interesting to observe that friable callus derived from media containing 2,4-D and KN did not contain withanolide A and withaferin A (Fig. 4a) as compared with the standard chromatogram from CAMAG atlas (Fig. 3). However solid callus of both non-regenerating (Fig. 4b) and regenerating (Fig. 4c) types derived from media containing IBA and BAP have both of the compounds mentioned above. A comparative study of these three types of calli (Fig. 4d) shows that there is hardly any difference between the regenerating & non regenerating types with respect to their chemical content and it may be assumed that the process of regeneration do not inhibit synthesis of secondary metabolites. This observation though contradicts a recent report (Das et al. 2010) but may be due to the influence of specific plant growth regulator used in the media. It is worth mentioning, that reported absence of withanolides in the callus cultures as reported by Roja and Heble (1991) may be due to particular PGR used.
Fig. 4.
Graphical representation of active principle present in callus tissue derived from different PGR containing media as analyzed by CAMAG TLC Scanner-3, Win CATS software version 1.2.3. a Friable callus derived from 2,4-D and KN containing media b Solid non-regenerating callus tissue derived from BAP and IBA containing media c Solid regenerating callus tissue derived from BAP and IBA containing media d Line graph of previous samples, from left in 1st and 4th lane-friable callus tissue loaded 15 μl and 20 μl respectively, in 2nd and 5th lane- Solid non-regenerating callus tissue loaded 15 μl and 20 μl respectively, in 3rd and 6th lane- Solid regenerating callus tissue loaded 15 μl and 20 μl respectively
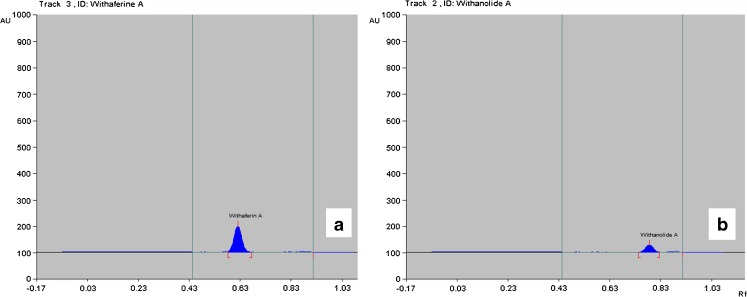
Fig. 3.
Chromatogram of withanolide A (a) and withaferin A (b) analytical standard from CAMAG atlas. According to CAMAG atlas Rf value− 0.60 represents withaferin A and Rf value− 0.80 represents withanolide A
In the native plant, withanolide A constitute a very minor proportion of withanolide complement (Nagella and Murthy 2010) which may vary due to several reasons including seasonal variation (Das et al. 2009), plant to plant variation in chemical content (Ciddi 2006) and variation in agro-climatic condition (Ciddi 2006). It appears to be relevant to adopt in vitro system which may serve as an alternative source of withanolides and thus may be exploited for efficient biogeneration of such substances throughout the year, which are pharmacologically promising but are severely limited in production. Production of withanolide D, withanolide A, withaferin A, withanone have been reported in organogenetic cultures including hairy roots (Banerjee et al. 1994; Furmanowa et al. 2001; Murthy et al. 2008; Ray and Jha 1999, 2001; Ray et al. 1996; Roja and Heble 1991; Sangwan et al. 2007; Vitali et al. 1996). Recently Nagella and Murthy (2010) reported production of withanolide A in the suspension cultures of Withania somnifera. However no study has so far been observed reporting the presence of withanolide A and withaferin A in solid callus and the influence of PGR in their synthesis. However, selection of high yielding cell line from the calli needs further investigation.
Conclusion
Most of the studies made on regeneration of this plant are mediated through direct regeneration of different types of explants (Sen and Sharma 1991; Ghimire et al. 2010; Fatima and Anis 2012 etc.). Callus mediated regeneration has special significance since it provides the scope of exploiting the somaclonal variation. IBA and BAP appear to be most suitable PGR for establishment of regenerative callus, regeneration and shoot multiplication of regenerants. The browning of the callus observed in this case may be a result of the stress condition induced naturally by the in vitro system. The synthesis of secondary metabolites like withanolide-A and withaferin-A in the in vitro system appears to be influenced by the nature of plant growth regulator, a phenomenon not reported earlier and absence of these two chemicals were clearly related to the presence of PGR like 2,4-D and KN.
Acknowledgements
The authors are indebted to Dr. Chhanda Mondal, Ramakrishna Mission Quality Testing Laboretory, Narendrapur, Kolkata for chemical analysis of the samples.
Abbreviations
- PGR
Plant Growth Regulator
- MS
Murashige and Skoog (1962) medium
- GA3
Gibberelic Acid A3
- 2,4-D
2,4-dichlorophenoxy Acetic Acid
- KN
kinetin
- BAP
6-benzylamino Purine
- IBA
indole-3-butyric Acid
- HPTLC
High Performance Thin Layer Chromatography
References
- Abbasi BH, Ahmad N, Fazal H, Mahmood T. Conventional and modern propagation techniques in Piper nigrum. J Med Plants Res. 2010;4(1):007–012. [Google Scholar]
- Agarwal B, Singh U, Banerjee M. In vitro clonal propagation of tea (Camellia sinensis (L.) Kuntze) Plant Cell Tissue Organ Cult. 1992;30:1–5. doi: 10.1007/BF00039995. [DOI] [Google Scholar]
- Banerjee S, Naqvi AA, Mandal S, Ahuja PS. Transformation of Withania somnifera (L.) Dunal by Agrobacterium rhizogenes: infectivity and phytochemical studies. Phytother Res. 1994;8:452–455. doi: 10.1002/ptr.2650080803. [DOI] [Google Scholar]
- Banerjee M, Gantait S, Pramanik BR. A two step method for accelerated mass propagation of Dendrocalamus asper and their evaluation in field. Physiol Mol Biol Plants. 2011;17(4):387–393. doi: 10.1007/s12298-011-0088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakesley D, Constantine D (1992) Uptake and Metabolism of 6- Benzyladenine in shoot cultures of a range of species. Plant Cell Tissue Organ Cult 28:1183–1186
- Ciddi V (2006) Withaferin A from cell cultures of Withania somnifera. Indian J Pharm Sci 68(4):490–492
- Das A, Datta AK, Ghosh S, Bhattacharyya A. Growth and cultivation of two varieties of Withania somnifera. J Trop Med Pl. 2009;10:225–230. [Google Scholar]
- Das A, Ghosh S, Bhattacharyya A, Datta AK (2010) In vitro biogeneration of alkaloids and withanolides in Withania somnifera (L.) Dunal (Solanaceae) var. ‘Poshita’ and ‘Jawahar 22’. Int J Plant Dev Biol 4(1):42–46
- Devi PU, Sharada AC, Solomon FE, Kamath MS. In vivo growth inhibitory effect of Withania somnifera (Ashwagandha) on a transplantable mouse tumor, Sarcoma 180. Indian J Exp Biol. 1992;30:169–172. [PubMed] [Google Scholar]
- Dewir YH, Chakrabarty D, Lee SH, Hahn EJ, Paek KY. Indirect regeneration of Withania somnifera and comparative analysis of withanolides in In vitro and greenhouse grown plants. Biol Planta. 2010;54(2):357–360. doi: 10.1007/s10535-010-0063-6. [DOI] [Google Scholar]
- Easu K. Anatomy of seed plants. New York: John Wiley and Sons; 1977. pp. 271–285. [Google Scholar]
- Fatima N, Anis M. Role of growth regulators on in vitro regeneration and histological analysis in Indian ginseng (Withania somnifera L.) Dunal. Physiol Mol Biol Plants. 2012;18(1):59–67. doi: 10.1007/s12298-011-0099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furmanowa M, Gajdzis-Kuls D, Ruszkowska J, Czarnocki Z, Obidoska G, Sadowska A, Rani R, Upadhyay SN. In vitro propagation of Withania somnifera and isolation of withanolides with immunosuppressive activity. Planta Med. 2001;67:146–149. doi: 10.1055/s-2001-11494. [DOI] [PubMed] [Google Scholar]
- Gamble JS. Flora of presidency of Madras 2. Calcutta: Botanical survey of India; 1918. [Google Scholar]
- Ghimire BK, Seong ES, Kim EH, Lamsal K, Yu CY, Chung M. Direct shoot organogenesis from petiole and leaf discs of Withania somnifera (L.) Dunal. African. J Biotechnol. 2010;9(44):7453–7461. [Google Scholar]
- Jaffer HJ, Jawad ALM, Saber HS, Al-Naib A. Evaluation of antimicrobial activity of Withania somnifera extracts. Fitoterapia. 1988;59:497–500. [Google Scholar]
- Johansen DA. Plant Microtechnique. NewYork: McGraw-Hill; 1940. pp. 126–154. [Google Scholar]
- Kiritikar KR, Basu BD (1975) Indian Medicinal Plants 3, 1 st Edn. Dehra Dun
- Kulkarni AA, Thengane SR, Krishnamurthy KV. Direct shoot regeneration from node, internode, hypocotyl and embryo explants of Withania somnifera. Plant Cell Tissue Organ Cult. 2000;62:203–209. doi: 10.1023/A:1006413523677. [DOI] [Google Scholar]
- Manickam VS, Mathavan RE, Antonisamy R. Regeneration of Indian ginseng plantlets from stem callus. Plant Cell Tissue Organ Cult. 2000;62:181–185. doi: 10.1023/A:1006499522799. [DOI] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Murthy HN, Dijkstra C, Anthony P, White DA, Davey MR, Power JB, Hahn EJ, Paek KY. Establishment of Withania somnifera hairy root cultures for the production of withanolide A. J Int Plant Biol. 2008;50:975–981. doi: 10.1111/j.1744-7909.2008.00680.x. [DOI] [PubMed] [Google Scholar]
- Nagella P, Murthy HN. Establishment of cell suspension cultures of Withania somnifera for the production of withanolide A. Bioresource Technol. 2010;101:6735–6739. doi: 10.1016/j.biortech.2010.03.078. [DOI] [PubMed] [Google Scholar]
- Pati PK, Sharma M, Salar RK, Sharma A, Gupta AP, Singh B. Studies on leaf spot disease of Withania somnifera and its impact on secondary metabolites. Indian J Microbiol. 2008;48:432–437. doi: 10.1007/s12088-008-0053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani G, Grover IS. In vitro callus induction and regeneration studies in Withania somnifera. Plant Cell Tissue Organ Cult. 1999;57:23–27. doi: 10.1023/A:1006329532561. [DOI] [Google Scholar]
- Rani G, Virk GS, Nagpal A. Callus induction and plantlet regeneration in Withania somnifera (L.) Dunal. In Vitro Cell Dev Biol Plant. 2003;39:468–474. [Google Scholar]
- Ray S, Jha S. Withanolide synthesis in cultures of Withania somnifera transformed with Agrobacterium tumefaciens. Plant Sci. 1999;146:1–7. doi: 10.1016/S0168-9452(99)00077-1. [DOI] [PubMed] [Google Scholar]
- Ray S, Jha S. Production of withaferin A in shoot cultures of Withania somnifera Dunal. Planta Med. 2001;67:432–437. doi: 10.1055/s-2001-15811. [DOI] [PubMed] [Google Scholar]
- Ray S, Ghosh B, Sen S, Jha S. Withanolide production by root cultures of Withania somnifera transformed with Agrobacterium rhizogens. Planta Med. 1996;62:571–573. doi: 10.1055/s-2006-957977. [DOI] [PubMed] [Google Scholar]
- Roja G, Heble MR. Tissue cultures of Withania somnifera: morphogenesis and withanolide synthesis. Phytother Res. 1991;5:185–187. doi: 10.1002/ptr.2650050411. [DOI] [Google Scholar]
- Sabir F, Sangwan NS, Chaurasiya ND, Misra LN, Tuli R, Sangwan RS (2007) Rapid micropropagation of Withania somnifera L. accessions from axillary meristems. J Herbs Spices Med Plants 13(4):123–133
- Salvi ND, Singh H, Tivarekar S, Eapen S. Plant regeneration from different explants of neem. Plant Cell Tissue Organ Cult. 2001;65:159–162. doi: 10.1023/A:1010672809141. [DOI] [Google Scholar]
- Sangwan RS, Chaurasiya ND, Misra LN, Lal P, Uniyal GC, Sharma R, Sangwan NS, Suri KA, Qazi GN, Tuli R (2005) Process for isolation of withaferin A from plant materials and products therefrom. US Patent (AppFT) 20050226950
- Sangwan RS, Chaurasiya NS, Lal P, Misra L, Uniyal GC, Tuli R, Sangwan NS. Withanolide A biogeneration in in vitro shoot cultures of ashwagandha (Withania somnifera Dunal), a main medicinal plant in Ayurveda. Chem Pharm Bull. 2007;55:1371–1375. doi: 10.1248/cpb.55.1371. [DOI] [PubMed] [Google Scholar]
- Sen J, Sharma AK. Micropropagation of Withania somnifera from germinating seeds and shoot tips. Plant Cell Tissue Organ Cult. 1991;26:71–73. doi: 10.1007/BF00036108. [DOI] [Google Scholar]
- Singh NK, Sehgal CB. Micropropagation of Holy Basil (Ocimum sanctum Linn.) from young inflorescences of mature plants. Plant Growth Regul. 1999;29:161–166. doi: 10.1023/A:1006201631824. [DOI] [Google Scholar]
- Sivanesan I. Direct regeneration from apical bud explants of Withania somnifera Dunal. Indian J Biotechnol. 2007;16:125–127. [Google Scholar]
- Sivanesan I, Murugesan K. In vitro adventitious shoot formation from leaf explants of Withania somnifera Dunal. Plant Cell Biotechnol Mol Biol. 2005;6:163–166. [Google Scholar]
- Sivanesan I, Murugesan K. An efficient regeneration from Nodal Explants of Withania somnifera Dunal. Asian J Plant Sci. 2008;7(6):551–556. doi: 10.3923/ajps.2008.551.556. [DOI] [Google Scholar]
- Vitali G, Conte L, Nicoletti M. Withanolide composition and in vitro culture of Italian Withania somnifera. Planta Med. 1996;62:287–288. doi: 10.1055/s-2006-957884. [DOI] [PubMed] [Google Scholar]