Abstract
Plant growth and development are greatly affected due to changes in environmental conditions and become a serious challenge to scientific people. Therefore, present study was conducted to determine the role of secondary metabolites on the growth and development of maize under abiotic stress conditions. Cinnamic acid (CA) is one of the basic phenylpropanoid with antioxidant activity, produced by plants in response to stressful conditions. Response of maize seeds to the presoaking treatment with 0.5 mM CA was studied under different concentrations of NaCl stress. Exogenous CA increased growth characteristics in saline and non-saline conditions, while effects of CA were more significant under saline conditions in comparison to non-saline conditions in maize plants. CA also reduced oxidative damage through the induction of ROS scavenging enzymes such as supperoxide dismutase (SOD) (EC 1.15.1.1), peroxidase (POD) (EC 1.11.1.7), while the activity of enzyme catalase (CAT) (EC 1.11.1.6) was decreased. The content of malondialdehyde (MDA) was reduced significantly in maize leaf under CA treatment. Changes in protein banding patterns in the maize leaves showed a wide variation in response to NaCl-stress, while in the presence of CA salt-induced expression of polypeptides was reduced significantly. Present study clearly reports the alleviative effects of CA in response to salinity stress on growth, metabolic activity and changes in protein profile of 21 days old maize plants.
Keywords: Cinnamic acid, Maize (Zea mays L), Lipid peroxidation, Phenylpropanoids, Antioxidant enzymes, Salt-induced proteins
Introduction
Environmental stresses such as light, temperature, water, drought and salinity lead to increased production of free radicals and other oxidative species in plants. From agricultural perspective, these oxidative stresses are among the most significant factors responsible for substantial losses in crop productivity (Koca et al. 2007). The primary effects of abiotic stress is ion imbalance and hyper-osmotic stresses responsible for the induction of molecular network, which in turn activates stress responsive mechanism to reestablish homeostasis through repair mechanism of damaged proteins and membranes (Ahmad et al. 2010). During stress, electrons that have a high-energy state are transferred to molecular oxygen (O2) to form reactive oxygen species (ROS) (Mittler 2002). ROS, such as singlet oxygen (O−), superoxide ions (O−2) and peroxides are toxic molecules. ROS is capable of inducing damage to almost all cellular macromolecules including DNA (Tuteja and Sopory 2008). Salinity is one of the major stresses responsible for changes in metabolic activity of plants. Plants have evolved several adaptive mechanisms to handle with the salinity in their environments, but the understanding of these mechanisms still remains incomplete. Thus development of methods for inducing stress tolerance in plants is vital and is a major focus of research over many decades. In recent years, it has been shown that endogenous phenolic content increases dramatically not only due to pathogen infection but also due to environmental stressors (e.g. ozone, low and high temperature and salinity). These compounds are involved in the induction of gene expression, signal transduction pathways and metabolic homoeostasis in plants under adverse environmental conditions to modulate the stress responses for survival strategies (Singh et al. 2011). Stress induced phenylpropanoids are derived from CA (Dixon and Paiva 1995; Guo et al. 2011) and is a basic nucleus for the synthesis of numerous phenylpropanoids induced under adverse environmental conditions (Wang et al. 2007; Zhang et al. 2010). CA and its derivatives are appeared as an antioxidant and antibacterial activities in plants in response to stressful conditions (Korkina 2007). However, it’s physiological and molecular action to develop salt tolerance mechanisms in plants is not known. Keeping this in view, CA was used as a plant growth regulator under saline conditions. Primary objectives were to study the effects of CA on growth, ROS-scavenging enzymes, lipid peroxidation and on pattern of proteins profile in maize plants grown under different NaCl.
Materials and methods
Plant materials and growth conditions
Seeds of Zea mays L. (Var. Jaunpuri) were procured from Genetics and Plant Breeding Department, Institute of Agricultural Sciences, BHU, India. Seeds were surface sterilized with 0.01 % HgCl2 followed by cetramide solution and thoroughly rinsed with glass-distilled water. Homogenous lots of surface sterilized seeds were presoaked for six hrs in 0.5 mM trans-CA and distilled water respectively. Pre-soaked seeds were placed in acid washed petridishes lined with Whatman no. 1 filter paper in an incubator at 27 ± 1 °C for germination. Thereafter, germinated seeds were transferred to pots filled with perceived homogenous garden soil (sandy loam) under natural light condition (in the range of 27–35 °C air temperature, 450–500 μmol m−2 s−1 light intensity and 75 % relative humidity). Each pot was supplied with half strength Hoagland's nutrient solution (Hoagland and Arnon 1950) along with different salinity levels (0, 50, 100, 150, 200 mM NaCl).
Plant measurement and analysis
Plants were harvested after 3 weeks of germination and dried in a thermo-ventilated oven at 90 °C until constant weight was obtained. Growth parameters such as fresh weight, dry weight, root length, shoot length, leaf area, and relative water content (RWC) were analyzed according to the standard methods.
Extraction and assay of antioxidant enzymes
Two g of frozen leaf tissues of three weeks old corn were homogenized in mortar with 8 ml extraction buffer containing 50 mM Tris–HCl buffer (pH 7.2), 5 mM EDTA, 0.3 % PVP and 5 mM mercaptoethanol under ice-cold condition. The homogenate was centrifuged at 12,000 g for 20 min and the supernatant was used for the assay of superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) enzymes. SOD activity was assayed according to Das et al. (2000). The reaction mixture containing 200 μl of enzyme supernatant, three ml of K phosphate buffer (pH 7.4), 0.2 ml of 20 mM L-methionine, 0.1 ml of 1 % (v/v) triton x-100, 0.2 ml of 100 μM EDTA and 0.2 ml of 10 mM hydroxylamine HCl was incubated at 37 °C for 5 min. 0.2 ml of 100 μM riboflavin was added to the tubes under fluorescent lamp for 10 min. Then, 2 ml of freshly prepared Greiss reagent was added to the reaction mixture and readings were recorded at 543 nm, blank and controls were run in the same way without riboflavin and enzyme respectively. SOD activity was expressed in unit mg−1 protein. POD activity was assayed following the method of Rao et al. (1997). Three ml of the assay mixture was containing 100 mM of potassium phosphate buffer (pH 6.8), 16 mM guaicol, 10 % H2O2 and 200 μl of enzyme extract. Peroxidase activity was determined by increase in absorbance at 470 nm (tetraguaicol formation) for 5 min. The enzyme activity was expressed in terms of nmol guaicol oxidized min−1 mg−1 protein. CAT activity was assayed following the method of Aebi (1983). Three ml of the assay mixture was containing 100 mM of potassium phosphate buffer (pH 6.5), 10 mM H2O2 and 200 μl enzyme extract. Catalase activity was determined by decrease in absorbance at 240 nm for 5 min. The enzyme activity was expressed in terms of μmol H2O2 decomposed min−1 mg−1 protein.
Assay of lipid peroxidation
Lipid peroxidation in terms of malondialdehyde (MDA) content was determined following the method of Heath and packer (1968). For the measurement of MDA, 0.5 g leaf tissues were homogenized in 8 ml of 5 % TCA (trichloroacetic acid). The homogenate was centrifuged at 12,000 g for 15 min. 2 ml aliquot of the supernatant was mixed with 2 ml of 0.5 % thiobarbituric acid in 20 % TCA and the mixture was heated at 95 °C for 30 min and then quickly cooled in an ice bath. After centrifugation at 5,000 g for 10 min, absorbance of the supernatant was recorded at 532 nm. The value for non-specific absorption at 600 nm was subtracted. The MDA equivalent was calculated as follows; MDA (nmol/ml FW) = {(A532-A600)/ 155,000} x 106, where FW = fresh weight and A = absorbance.
Protein extraction and one-dimensional SDS-PAGE
Approximately 2 g of frozen leaf tissues were used to prepare protein extract from treated and control plants. Excised leaf tissues were extracted with sample buffer under reducing condition (2 % v/v 2-ME). After grinding in a homogenizer the suspension was centrifuged at 4,000 g for 20 min at 4 °C. The supernatant was collected and protein content was estimated as per Lowry et al. (1951). One-dimensional SDS-PAGE separation was performed using 12 % polyacrylamide gels (3.6 % stacking and 12 % separating gel) at a constant voltage (80 V). 20 μl of leaf extract was loaded on the gel and run in a Laemmli buffer system according to Laemmli (1970). Molecular weight standards in a range from 20 to 210 kDa were obtained from Sigma. Proteins were stained with a 0.3 % (by weight) solution of Coomassie Brilliant Blue R-250 in 45 % methanol: 10 % acetic acid: 45 % water (v: v: v). The resulting pattern was scanned at 300 dpi (canon-520).
Statistical analysis
Results were presented as means values ± standard error designed with five replications. Data were analyzed by one way analysis of variance (ANOVA). Means were compared by the least significant difference test of the 0.05 % level of significance.
Results
Effects of CA on growth dynamics
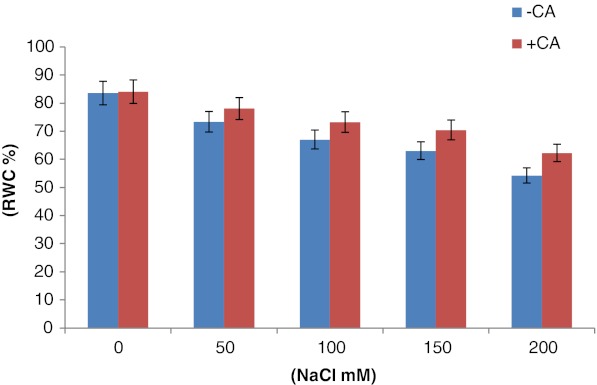
Growth pattern of maize plants were adversely affected with exposure to increasing concentrations of NaCl stress. A gradual decrease in fresh and dry weight as well as root/shoot length was observed with the increasing salt concentrations (Table 1). Reduction in growth characteristics of maize plants was severe at 150 mM of NaCl in comparison to aqueous control in absence of CA. Whereas, in presence of CA, fresh weight & dry matter of plants was restored. Growth promotory action of CA was more pronounced in saline condition in comparison to non-saline one. CA enhanced the growth of 21 days old maize plants significantly grown under 150 mM of NaCl concentration and alleviates the destructive effects of salt stress. Interestingly, the salt induced reduction in dry matter of maize plants was encountered by the treatment of exogenous CA (Table 1). Root length of 21 days old maize plants decreased up to 67 % at 200 mM of NaCl in comparison to aqueous control, whereas, exogenous CA application alleviated the damaging effect by 7 % of aqueous control at 200 mM NaCl and 47 % at 150 mM of NaCl (Table 1). Similar trend was observed in the case of shoot length; however reduction in shoot length was high in comparison to that of root length. Leaf area (photosynthetic surface) decreased gradually with increasing salt concentrations and the reduction was severe at 200 mM NaCl treatment as compared to the aqueous control (Table 1). This salt induced reduction in leaf area was encountered up to 56.9 % in the presence of exogenous CA. Present study stated that CA affects as a growth regulator and its impact was more significant under saline conditions (Table 1). In the absence of CA the relative water content (RWC) decreased gradually with the increasing concentrations of NaCl in the corn leaves (Fig. 1), whereas in the presence of CA level of RWC maize plants increased significantly in comparison to the aqueous control.
Table 1.
Effects of CA on growth characteristics in 3 weeks old maize plants grown under increasing concentrations of NaCl stress
| NaCl mM | Fresh weight | Dry weight | Root length | Shoot length | Leaf area | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (g plant−1) | (g plant−1) | (cm) | (cm) | (cm2) | ||||||
| CA − | CA + | CA − | CA + | CA − | CA + | CA − | CA + | CA − | CA + | |
| 0 | 5.70 | 7.6 | 0.78 | 1.00 | 15 | 16 | 10 | 10 | 72 | 109 |
| ± 0.013 | ± 0.033 | ± 0.004 | ± 0.003 | ± 0.210 | ± 0.039 | ± 0.037 | ± 0.020 | ± 0.003 | ± 0.005 | |
| 50 | 5.69 | 5.8 | 0.75 | 0.76 | 14 | 15 | 8.5 | 10 | 69.1 | 88.7 |
| ± 0.008 | ± 0.039 | ± 0.002 | ± 0.004 | ± 0.110 | ± 0.105 | ± 0.015 | ± 0.020 | ± 0.004 | ± 0.007 | |
| 100 | 4.70 | 4.8 | 0.46 | 0.62 | 9.0 | 10 | 8.0 | 9.0 | 62.3 | 73.0 |
| ± 0.103 | ± 0.051 | ± 0.003 | ± 0.003 | ± 0.015 | ± 0.032 | ± 0.034 | ± 0.035 | ± 0.007 | ± 0.005 | |
| 150 | 2.51 | 2.9 | 0.23 | 0.65 | 8.0 | 9.8 | 6.5 | 7.0 | 33.0 | 46.8 |
| ± 0.009 | ± 0.070 | ± 0.005 | ± 0.003 | ± 0.011 | ± 0.010 | ± 0.015 | ± 0.051 | ± 0.007 | ± 0.004 | |
| 200 | 2.30 | 2.4 | 0.22 | 0.67 | 5.0 | 6.0 | 4.0 | 6.0 | 31.0 | 37.2 |
| ± 0.010 | ± 0.070 | ± 0.003 | ± 0.004 | ± 0.008 | ± 0.007 | ± 0.005 | ± 0.007 | ± 0.005 | ± 0.019 | |
| LSD | 0.032 | 0.052 | 0.041 | 0.062 | 0.055 | 0.048 | 0.031 | 0.035 | 0.015 | 0.021 |
Data presented are mean ± SE (n = 5). LSD values were determined at p < 0.05
Fig. 1.
Effect of CA on relative water content (RWC) in 3 weeks old maize plants grown under increasing concentrations of NaCl 0.5 mM CA was given as presoaking seed treatment
Effects of CA on ROS scavenging enzymes
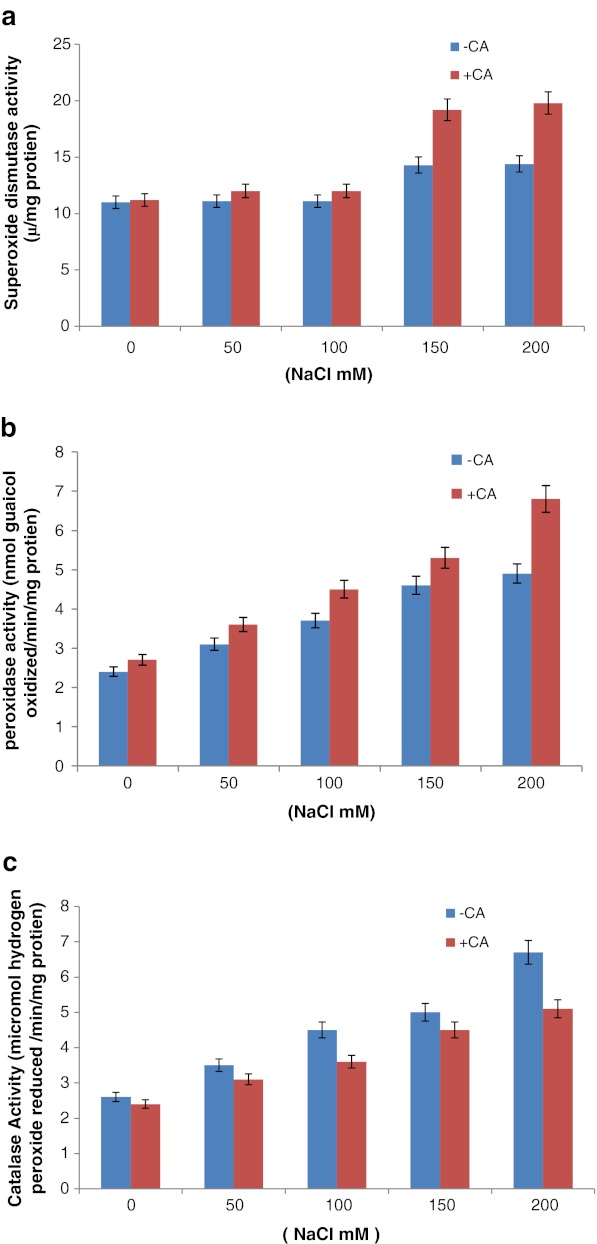
To determine the adaptive role of CA on oxidative damage in maize plants grown under different concentrations of NaCl-stress, levels of superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) activity were analyzed in the presence of 0.5 mM CA. The activity of SOD was significantly enhanced in response to NaCl-stress with CA in comparison to without CA treatment (Fig. 2a) and enhancement in the enzyme activity was more than two fold at 150 mM of NaCl stress as compared to aqueous control. The activity of the enzyme peroxidase was also enhanced significantly in response to NaCl-stress with CA in comparison to without CA in three weeks old maize plants (Fig. 2b). Induction in the action rate of enzyme was observed in both presence and absence of CA, however in the presence of exogenous CA (0.5 mM); enzyme activity was higher in comparison to NaCl stress without CA. Catalase activity increased significantly with increasing salinity levels in three weeks old maize seedlings. This enhancement was very gradual with the increasing concentrations (50, 100, 150, 200 mM) of NaCl whereas, in the presence of 0.5 mM CA, activity of enzyme catalase was reduced significantly in the maize plants (Fig. 2c). These contrasting observations showed specific interaction of CA and salinity levels in maize plants.
Fig. 2.
(a) Effect of CA on superoxide dismutase activity in 3 week old maize plants grown under increasing concentrations of NaCl stress. (b) Effect of CA on peroxidase activity in 3 weeks old maize plants grown under increasing concentration of NaCl stress. (c) Effect of CA on catalase activity in 3 weeks old maize plants grown under increasing concentration of NaCl stress
Effects of CA on MDA content
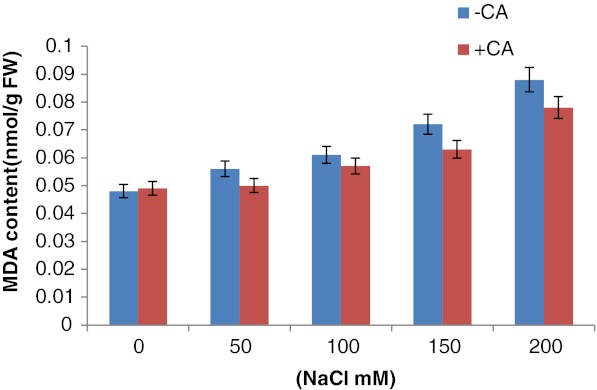
The oxidative effect of salt on the degree of lipid peroxidation was measured as accumulation of MDA (malondialdehyde) content in leaf tissue of three weeks old maize plants. Accumulation of MDA was very high (two fold) in NaCl stressed plants than that of control, whereas in the presence of 0.5 mM exogenous CA, content of MDA (a product of lipid peroxidation) was reduced significantly (Fig. 3). The increase in MDA content was 75 % and 100 % in comparison to aqueous control at 150 mM and 200 mM of NaCl concentrations respectively. CA pretreatment counteracted with the effect of salinity exerted on the degree of lipid peroxidation in maize plants.
Fig. 3.
Effect of CA on malondialdehyde content in 3 weeks old maize plants grown under increasing concentrations of NaCl. 0.5 mM CA was given as presoaking seed treatment
Effects of CA on proteins profile
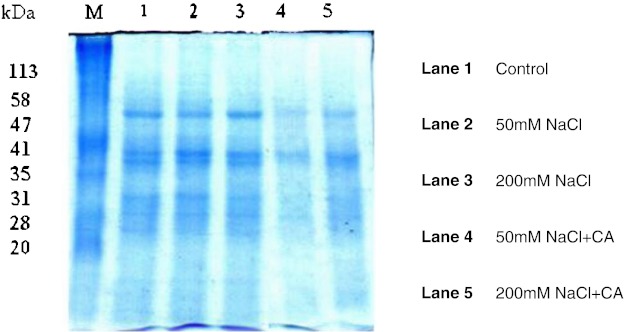
Total protein was extracted from the fourth leaf of three weeks old maize plants and then analyzed by SDS-PAGE (Fig. 4). Electrogram visualized from SDS-PAGE expressed several protein bands with molecular weight of 48, 42, 41, 40, 31, 29, 28 kDa and some unidentified polypeptides for leaves of control plants (lane 1). Salinity stress reasoned changes in the pattern and levels of protein profile in maize plants. Changes were observed with molecular weight of 26 kDa for 50 mM NaCl (Lane 2) and disappearance of some important protein bands with the molecular weight of 42, 28 kDa were observed at 200 mM of NaCl-stress. Application of exogenous CA induced the biosynthesis of some new polypeptides with the molecular weight of 48, 33 kDa, for leaf tissues of maize plants (lane 5). These alterations in protein levels in the presence of CA showed effect of phenylpropanoids in terms of salt acclimation.
Fig. 4.
One-dimensional SDS-PAGE of protein extracted from leaf tissues of Z. mays L. grown under different concentrations (50 and 200 mM) of NaCl stress in presence and absence of CA
Discussion
Salinity is a major environmental restraint to crop productivity throughout the arid and semi arid regions of the world. Salinity-stress inflicts various physiological, biochemical and molecular changes in plants, which leads to biosynthesis and accumulation of phenylpropanoids in plants (Dixon and Paiva 1995). Genotypes with high concentration of organic osmolytes (proline, glycinebetaine and amino acids) and high antioxidant activity (SOD, POD, CAT) had high salt tolerance, so it is also concluded that salt tolerance potential of plants is highly associated with concentration of osmolytes and antioxidant enzymes (Shahid et al. 2012). Over production of ROS and the presences of oxidative damage due to salt stress have been reported (Khallal et al. 2009). Trans-CA is a fundamental phenylpropanoid involved in the restoration of damage caused by various abiotic stresses (Wang et al. 2007; Singh et al. 2008). Lower concentrations of CA improved the growth of cucumber seedlings grown under saline conditions while higher concentrations were found inhibitory (Wang et al. 2007). In the present study, adverse effect of salinity stress on the plant growth is encountered significantly by the exogenous application of CA. CA increases the water content and other growth characteristics of maize seedlings grown under saline conditions, which in turn, could be related to the salt adaptation mechanisms in plants (Singh et al. 2011). Environmental stresses often damage the plant organic membrane permeability and integrity through the high degree of lipid peroxidation (release of MDA, a product of membrane lipid peroxidation). In our study, the MDA content was higher in salinity stressed maize seedlings than that of control, exogenous application of CA decreased the MDA content in maize seedlings under salt stress, and developed a moderate effect over NaCl-stress. Present findings supported by the report of Li et al. (2011) in which, CA was observed as a restoration activity of plasma membrane through the reduction of lipid peroxidation in cucumber leaves. Salt stress stimulated reactive oxygen species or peroxide free radicals, damaged membrane protective system in plants. CA appeared as potential scavenger of free radicals and other oxidative species under abiotic stress conditions (Li et al. 2011). The present findings indicated that CA increases activity of SOD and POD in three weeks old maize plants grown under different concentrations of NaCl. However, activity of enzyme catalase is reduced in leaf tissue. This might be due to adaptability capacity of CA under abiotic stress conditions. Earlier reports on CA induced up regulation of NADPH oxidase confirm the ROS regulatory property of exogenous trans-CA in cucumber under aerobic metabolism (Ding et al. 2007; Li et al. 2011). CA might relatively increased the leaf area, leaf relative water content (RWC) and develop a repairing ability to plasma membrane permeability through reduction in MDA production under salt stress conditions. Analysis of the plant proteome is an important alteration to analyze the functional genome, because gene expression is altered under adaptation to environmental stresses (Hopkins 1995). Present results showed that the appearance of number of protein bands in maize leaves treated with the increasing concentrations of NaCl in presence as well as absence of CA. Effects of exogenous CA on maize plants were more significant under saline conditions in comparison to non-saline conditions in terms of protein biosynthesis. Salinity and CA altered the protein profile and was responsible for induction of several osmoresponsive genes, which may be involved in adaptation to salt stress. CA induced accumulation of proteins such as 48 and 42 kDa, have a possible role in salt adaptation and osmotic adjustment as reported by Mikolajczyk et al. (2000) with salicylic acid, an analog of CA. Salicylic acid and its analog can alleviate the effect of salt stress, and improve the ability of plants to adopt salt stress. Present results showed that intensity of protein bands with molecular weight of 26 kDa, increased significantly in response to salt stress with or without CA. It is of keen interest whether the interaction and accumulation of specific polypeptides observed in plants have possible involvement in acquiring resistance directly or by some other mechanisms (Singh 2006). Although, all above indication that CA regulates some cellular and molecular aspects of plant metabolism are under research but its role in defense mechanism of plants is well documented.
A possible survival strategy of plants under saline condition is induction of secondary metabolism that could alleviate the deleterious effect of salt stress. Results of present study proved that trans-CA relatively increased the RWC and maintained the osmotic balance of maize leaves under salt stress, increased in membrane integrity through reduction in the degree of lipid peroxidation. CA was found with the up regulation ability of ROS scavenging enzymes as well as induction of new proteome biosynthesis in maize plants under saline condition. These findings may be translated into efforts aimed to develop salt tolerant genotypes and maximize the use of CA under saline environment.
Acknowledgement
The authors are thankful to University Grant Commission, New Delhi, India for financial support under Major Research Project (MRP No. F 38-255/2009, SR).
Abbreviations
- CA
Cinnamic acid
- MDA
Malondialdehyde
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
- POD
Peroxidase
- CAT
Catalase
- PVP
Polyvinylpyrrolidone
- SDS-PAGE
Sodium dodecyl sulfate-Polyacrylamide gel electrophoresis
Contributor Information
Pramod Kumar Singh, Phone: +91-9415388189, FAX: +91-5422282799, Email: baispkupc@sify.com.
Ramendra Singh, Email: ramendraparihar88@gmail.com.
References
- Aebi HE (1983) Methods of enzymatic analysis. VCH, Weinheim, Germany-Deerfield FL 3:273–286
- Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol. 2010;30(3):161–175. doi: 10.3109/07388550903524243. [DOI] [PubMed] [Google Scholar]
- Baque MA, Lee E-J, Paek K-Y. Medium salt strength induced changes in growth, physiology and secondary metabolite content in adventitious roots of Morinda citrifolia, the role of antioxidant enzymes and phenylalanine ammonia lyase. Plant Cell Rep. 2010;29:685–694. doi: 10.1007/s00299-010-0854-4. [DOI] [PubMed] [Google Scholar]
- Das K, Samanta L, Chainy GBN. A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. Ind J Biochem Biophys. 2000;37:201–204. [Google Scholar]
- Ding J, Sun Y, Xiao CL, Shi K, Zhou YH, Yu JQ. Physiological basis of different allelopathic reactions of cucumber and fig leaf gourd plants to cinnamic acid. J Exp Bot. 2007;58:765–3773. doi: 10.1093/jxb/erm227. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Paiva NL. Stress-induced phenylpropanoid metabolism. Plant Cell. 1995;7:1085–1097. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Wong WS, Xu WZ, Sun FF, Qing DJ, Li N. Cis-cinnamic acid-enhanced 1 gene plays a role in regulation of Arabidopsis bolting. Plant Mol Biol. 2011;75:481–495. doi: 10.1007/s11103-011-9746-4. [DOI] [PubMed] [Google Scholar]
- Heath R, Packer L. Photo oxidation in isolated chloroplast 1.Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125(b):189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. Calif Agric Exp Stn Cir. 1950;347:32. [Google Scholar]
- Hopkins W. The physiology of plants under stress. In: Introduction to plant physiology. New York: John Wiley and Sons Inc; 1995. pp. 422–423. [Google Scholar]
- Khallal E, Hathout SM, Raheim TA, Ahsour AE, Kerrit AA. Brassinolide and salicylic acid induced antioxidant enzymes, hormonal balance and protein profile of maize plants grown under salt stress. Res J Agr Biol Sci. 2009;5(4):391–402. [Google Scholar]
- Koca H, Bor M, Ozdemir F, Turkan I. The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Env Exp Bot. 2007;60:344–351. doi: 10.1016/j.envexpbot.2006.12.005. [DOI] [Google Scholar]
- Korkina LG. Phenylpropanoids as naturally occurring antioxidants: from plant defense to human health. Cell Mol Biol. 2007;53:15–25. [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li Q, Yu B, Gao Y, Dai A, Bai J. Cinnamic acid pretreatment mitigates chilling stress of cucumber leaves through altering antioxidant enzyme activity. J Plant Physiol. 2011;168:927–934. doi: 10.1016/j.jplph.2010.11.025. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rose rough NJ, Farr A, Randall RJ. Protein measurement with the folin phenol reagent. J BiolChem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mikolajczyk M, Awotunde OS, Muszynska G, Klessig DF, Dobrowolska G. Osmotic stress induces rapid activation of a salicylic acid-induced protein kinase and a homolog of protein kinase ASK 1 in tobacco cells. Plant Cell. 2000;12:165–178. [PMC free article] [PubMed] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Rao MV, Paliyath G, Ormrod P, Murr DP, Watkins CB. Influence of SA on H2O2 production, oxidative stress and H2O2-metabolizing enzymes. Plant Physiol. 1997;115:137–149. doi: 10.1104/pp.115.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid MA, Balal RM, Pervez MA, Abbas T, Ashfaq M, Ghazanfar U, Afzal M, Rashid A, Garcia-Sanchez F, Mattson NS. Differential response of pea (Pisum sativum L.) genotypes to salt stress in relation to the growth, physiological attributes antioxidant activity and organic solutes. Australian J. Crop Sci. 2012;6(5):828–838. [Google Scholar]
- Singh PK. Changes in Nitrate assimilation and protein content in isolated cucumber cotyledons in presence of cinnamic acid. Physiol Mol Biol Plants. 2006;12(31):253–257. [Google Scholar]
- Singh PK, Bose B, Sharma MK, Singh A. Physiological and molecular action of salicylate in plants. New Delhi: Development in Physiology, Biochemistry and Molecular Biology of Plants, New India Publishing Agency; 2008. pp. 135–155. [Google Scholar]
- Singh PK, Singh HB, Chaturvedi VK. Cross talk signaling: an emerging defense strategy in plants. Curr Sci. 2011;100(3):288–289. [Google Scholar]
- Tuteja N, Sopory SK. Plant signaling in stress, G-protein coupled receptors, heterotrimeric G-proteins and signal coupling via phospholipases. Plant Signal Behav. 2008;3:79–86. doi: 10.4161/psb.3.2.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang H, Wu F, Liu B. Effects of cinnamic acid on the physiological characteristics of cucumber seedlings under salt stress. Front Agric China. 2007;1(1):58–61. doi: 10.1007/s11703-007-0010-2. [DOI] [Google Scholar]
- Zhang E-P, Zhang S-H, Li W-BZL-L, Li T-L. Effects of exogenic benzoic acid and cinnamic acid on the root oxidative damage of tomato seedlings. J Hortic For. 2010;2(2):022–029. [Google Scholar]