Abstract
A micropropagation protocol was developed for multiplication of seedless lemon (Citrus limon L. cv. Kaghzi Kalan) using nodal explants. The maximum shoot regeneration was observed on low level of BAP (0.1 mg l−1) or kinetin (0.5 mg l−1). BA was recorded to be better than kinetin in terms of number of days taken to bud break. The maximum number of shoots per explant was observed on 0.1 mg l−1 BA and 0.5 mg l−1 kinetin. Shoot proliferation decreased with increasing concentration of BA alone, but in case of a combination of BA and NAA (0.1 mg l−1 each), it increased with increasing concentration of BA up to 10.0 mg l−1. None of the treatments including BA or kinetin alone or BA in combination with NAA produced significantly more shoots for commercial exploitation. In the case of a combination of BA + kinetin + IBA, the maximum (5.5 shoots per explants) proliferation was observed on MS medium containing 1.0 mg l−1 BA + 0.5 mg l−1 kinetin + 0.5 mg l−1 IBA or 0.25 mg l−1 BA + 1.0 mg l−1 kinetin + 1.0 mg l−1 IBA. Regenerated shoots showed root induction on MS basal medium or on MS medium containing 1.0 mg l−1 IBA. It is concluded that a five-fold increase (1.0 mg l−1 BA + 0.5 mg l−1 kinetin + 0.5 mg l−1 IBA) in axillary shoot proliferation, while seven-fold increase (0.25 mg/l mg l−1 BA + 1.0 mg l−1 kinetin + 1.0 mg l−1 IBA) during the second cycle of multiplication could be obtained using the two plant growth regulator combinations. PCR amplification with 14 different random primers confirmed no somaclonal variant up to two cycles of shoot multiplication.
Keywords: Micropropagation, Regeneration, In vitro, Genetic fidelity, RAPD
Introduction
Citrus is one of the most widespread fruit crops consumed throughout the globe with high economic and medicinal values. Citrus is an important genus of evergreen aromatic small trees, mostly spines, and having fruits called hespiridium. Among the citrus fruits of commercial value, sweet orange (Citrus sinensis L. Osbeck), mandarin (C. reticulate Blanco) and sour orange (C. auranticum L.) contribute approximately 80 % of the world citrus fruit production (FAOSTAT 2010). Other commercially important fruits are lime (C. aurantifolia Swingle), lemon (C. limon Burm f.), and pummelo (C. grandis L. Osbeck), but they are grown on relatively smaller scales. Eastern Asia, mainly the south-east Asia is considered to be the centre of origin of Citrus, in this region, still many citrus types are still found in their wild state. In India, citrus is grown in many states, but the states of Andhra Pradesh and Maharashtra have the largest production share.
Citrus can be propagated by both sexual and asexual methods; generally, rootstocks are propagated through seeds, while most of the commercial varieties are propagated through various asexual methods (Chaudhary et al. 1994). Conventional vegetative propagation of citrus plants is time-consuming and mainly dependent on season and availability of plant material; which restricts the faster adoption and replacement of new varieties. Seeds of most citrus species are polyembryonic and thus nucellar seedlings are used both for raising uniform rootstock as well as for direct planting. It also helps to raise healthy plants as citrus viruses are not transmitted through seeds, however, the same technique cannot be exploited for seedless genotypes. The availability of natural poly-embryony in different Citrus species provides an efficient means for mass multiplication without sacrificing the adult tree (Rangaswamy 1961; Sabharwal 1963; Rangan and Murashige 1968; Tisserat and Murashige 1977; Gavish et al. 1991, 1992). In addition, a few commercially important seed-less clones of Citrus are also available, the only option for their mass multiplication is through vegetative tissues. Alternatively, axillary bud proliferation is widely practiced for in vitro propagation of citrus because it ensures maximum genetic uniformity of the resulting plants
In case of a seedless lemon variety ‘Kaghzi Kalan,’ air-layering of plants never yield the required quantity of planting materials. Therefore, to build-up the stock of this very precious planting material, tissue culture techniques, especially micropropagation assume significance and remains the only viable alternative. There are different strategies for micropropagation of citrus, viz., somatic embryogenesis, adventitious shoot bud production and axillary enhancement which are routinely used. Among these, axillary enhancement using nodal segment as explant is considered best as it does not involve a callus phase, thus minimize the risk of somaclonal variation and it offers economically optimum multiplication rate. However, efforts have not yet been made to develop a method for axillary enhancement in Kaghzi Kalan. Efforts have also been made to put regenerated primary branches to multiple shooting. Although this method offers least risk of somaclonal variation, nevertheless, variatians may arise during successive culture cycles mainly due to prolonged exposure on plant growth regulators. In view of the above, the present investigation was carried out to develop an efficient regeneration system for multiplication of C. lemon cv. Kaghzi Kalan. The genetic fidelity of the micropropagation system needs to be ascertained before using it at commercial level. Thus, in present investigation, genetic fidelity of micropropagated plants was tested through RAPD analysis.
Materials and methods
Plant material and explant preparation
Young tender pencil-thick shoot pieces of seedless lemon (C. limon cv. Kaghzi Kalan) were collected from 2–3 years old plants growing in the orchard of Agricultural Research Station, Rajasthan Agriculture University, Bikaner during the month of November to March. In order to remove the micro-flora present on surface, shoot pieces were thoroughly washed under running tap water for 15–20 min, and then immersed in an aqueous solution of liquid detergent containing 2–3 drops of Tween 20 (HiMedia, India), 250 mg l−1 cefotaxime and 1 % Bavistin (Hindustan Insecticide limited,India) for 15 min followed by three to four rinses in sterile distilled water. The nodal segments of 2.5–3.0 cm containing at least one node from these shoot pieces were used as explant. Finally, inside a laminar air-flow cabinet, the nodal explants were surface sterilized with 0.1 % (w/v) mercuric chloride (HgCl2) for 4–5 min followed by 5–6 rinses with sterile double distilled water.
Culture media and growth conditions
MS (Murashige and Skoog 1962) medium containing 3 % sucrose and 0.8 % agar was used throughout the experiments; different growth regulators were supplemented as per the requirement of the experiment. The pH of the medium was adjusted to 5.8 prior to autoclaving for 15 min at 121 °C temperature and 15 p.s.i. For shoot proliferation, surface sterilized explants were inoculated onto MS medium supplemented with different concentration of growth regulators (Table 1). Cultures were incubated in a growth room at 25 ± 2 °C with 16/8 h (light/dark) photoperiod at a photon flux of 50–70 mmol m−2 s−1 from cool white fluorescent tubes (Philips, India). Subculture was done at an interval of 20–25 days. Shoots initiated on each treatment in each culture were cultured for further shoot proliferation and root induction. Data was recorded on (i) frequency of responding cultures, (ii) days to shoot induction, (iii) number of shoots per explant cultures, and (iv) shoot length (cm), after 60 days after culture.
Table 1.
Details of the medium, which were tested in the present study. Murashige and Skoog medium (1962) was used throughout the study
| Medium | BA | Kin | NAA | IAA |
|---|---|---|---|---|
| B-1 | 0.1 | – | – | – |
| B-2 | 1.0 | – | – | – |
| B-3 | 5.0 | – | – | – |
| B-4 | 10.0 | – | – | – |
| B-5 | 15.0 | – | – | – |
| B-6 | 20.0 | – | – | – |
| B-7 | 25.0 | – | – | – |
| B-8 | 30.0 | – | – | – |
| K-1 | – | 0.5 | – | – |
| K-2 | – | 1.0 | – | – |
| K-3 | – | 2.0 | – | – |
| BN-1 | 0.1 | – | 0.1 | – |
| BN-2 | 1.0 | – | 0.1 | – |
| BN-3 | 5.0 | – | 0.1 | – |
| BN-4 | 10.0 | – | 0.1 | – |
| BI-1 | 5.0 | – | – | 1.0 |
| BI-2 | 10.0 | – | – | 1.0 |
| BI-3 | 15.0 | – | – | 1.0 |
| BT-1 | 0.1 | – | – | – |
| BT-2 | 0.1 | – | – | – |
| BKI-1 | 0.25 | 0.5 | – | 0.5 |
| BKI-2 | 0.5 | 0.5 | – | 0.5 |
| BKI-3 | 1.0 | 0.5 | – | 0.5 |
| BKI-4 | 2.0 | 0.5 | – | 0.5 |
| BKI-5 | 0.25 | 1.0 | – | 1.0 |
| BKI-6 | 0.5 | 1.0 | – | 1.0 |
| BKI-7 | 1.0 | 1.0 | – | 1.0 |
| BKI-8 | 2.0 | 1.0 | – | 1.0 |
BA 6-benzyl adenine; Kin kinetin; NAA Naphthalene acetic acid; IAA Indole-3 acetic acid. Values are in mg l−1
Root induction
For root induction, three different media, each having MS medium supplemented with 1.0 mg/l of either of IBA, IAA or NAA was tested. Data was recorded on (i) frequency of cultures showing root induction, (ii) days to root induction, (iii) numbers of roots per explant, and (iv) root length (cm), 60 days after culture.
Experimental scheme
The experiments were laid out according to a randomized complete block design (RCBD) with three replications, each replication had 15 cultures/plantlets per treatment. Analysis of variance (ANOVA) appropriate for the design was carried out to detect the significance of differences among the treatment means (Gomez and Gomez 1984). The treatment means were compared using Duncan’s Multiple Range Test (DMRT, Duncan 1955). Both ANOVA and DMRT analyses were carried out using the software SPAR ver. 2.0 (Statistical Package for Agricultural Research, SPAR 2008).
Hardening of plantlets and transfer to soil
For hardening, healthy plantlets obtained from shoots rooted on agar medium were removed from culture tubes, washed under running tap water to remove agar sticking to the roots and transplanted into plastic cups filled with sterilized soilrite (KelPerlite, Bangalore, India) and covered with polyethylene bags. The pots were then kept in a culture room at 25 ± 2 °C, and 16-h light and 8 h dark period. The pots were irrigated every alternate day with distilled water. After 7 days, the polyethylene bags were gradually removed over a period of 15 days. The plantlets were kept in the culture room for 10 days, and then transferred to room temperature for another 10 days before they were transferred to a glasshouse. After 2 to 3 months in the greenhouse the plants were transplanted under the field conditions. The genetic integrity of the in vitro regenerated citrus plants was checked using random amplified polymorphic DNA (RAPD) analysis.
RAPD analysis
Genetic fidelity between the mother plant and randomly selected in vitro regenerated plants established in soil was assessed by polymerase chain reaction (PCR)-based RAPD analysis. Genomic DNA was isolated from juvenile leaves of the mother plant and in vitro propagated plants by CTAB method (Doyle and Doyle 1990). The concentration of DNA was determined by a UV–vis spectrophotometer (Perkin Elmer 2380) and quality of genomic DNA was checked following electrophoresis on 0.8 % agarose gel. RAPD assay was performed using the 12 random decanucleotide primers ((Operon Biotechnologies, California; Table 1). PCR was performed in a volume of 25 μl reaction mixture containing 1 μl template DNA (80 ng), 2.5 μl 10 × PCR buffer, 1 μl of dNTPs (25 mM), 1.5 μl MgCl2 (1.5 mM), 1 μl randomprimer (10 pM), 0.5 μl Taq polymerase (three units) and 17.5 μl, sterile distilled water. DNA amplification was carried out in a DNA thermal cycler (Biorad, USA). The reaction was set in a thermal cycler with following thermal profile. Initial denaturation at 94 °C for 5 min, primer annealing at 33 °C for 1 min, and primer extension 72 °C for 1 min, than 38 cycles of 94 °C 1 min, 37 °C 1 min, 72 °C 2 min. Amplification with each primer was repeated twice to confirm reproducibility of the results. The PCR amplification products were resolved on 1.2 % agarose gel through electrophoresis. Lambda DNA Hind III/EcoR І double digest was used as molecular size marker. After electrophoresis, amplification products were visualised in a gel documentation system (Alfa Imager, Alfa Innotech Corp., USA).
Results
Effect of BA and Kin on shoot proliferation
The morphogenic response of nodal segments of C. limon inoculated on MS medium supplemented with various concentrations of BA (0.1–30 mg l−1) and kin (0.5–2 mg l−1) are presented in Table 2. A significant effect was observed due to different concentrations of BA and kin. The highest (62.66 %) frequency of explants showing bud break was observed in the medium containing 0.1 mg l−1 BA followed by the medium with 0.5 mg l−1 Kin (50.26 %; Fig. 1a). Frequency of response from nodal explants decreased with a progressive increase in the level of cytokinins and the lowest frequency (10.46 %) was observed on 30 mg l−1 BA (Table 1). Statistically, the induction frequencies were highly significant at various levels of cytokinins used. Nodal explants inoculated on lower level (0.1 mg l−1 BAP) of cytokinins took lesser time to bud break (9.5 days) compared to higher levels. Similarly, a lower level of BA (0.1 mg l−1) also effected higher shoot length (2.0 cm) followed by 0.5 mg l−1 kinetin (1.9 cm). A decline in the shoot length was noticed as the levels of cytokinins increased in the medium (0.9 cm on media containing 30 mg l−1 BA). Number of shoots per explant varied with the varying concentration of BA and Kin (Table 2). The maximum (1.6) number of shoots per explants was recorded in the medium supplemented with 0.1 mg l−1 BA, followed by medium with 0.5 mg l−1 Kin (1.2); at higher levels, a decline was recorded with respect to the number of shoots.
Table 2.
Effect of BA and Kin on shoot proliferation from nodal explants of C. limon cv. Kaghzi Kalan. Data was recorded 8 weeks after inoculation
| Treatment* (mg l−1) | Frequency of explants showing bud break (%) | Days to shoot induction | Shoot length (cm) | Number of shoots per explant |
|---|---|---|---|---|
| B-1 (0.1) | 62.66 | 9.5 ± 0.61 | 2.0 ± 0.29 | 1.6 ± 0.18 |
| B-2 (1.0) | 55.50 | 10.4 ± 0.67 | 1.9 ± 0.31 | 1.2 ± 0.16 |
| B-3 (5.0) | 42.59 | 11.5 ± 0.57 | 1.6 ± 0.28 | 1.2 ± 0.19 |
| B-4 (10.0) | 29.62 | 13.2 ± 0.72 | 1.4 ± 0.29 | 1.0 ± 0.18 |
| B-5 (15.0) | 20.36 | 13.6 ± 0.63 | 1.3 ± 0.32 | 1.0 ± 0.17 |
| B-6 (20.0) | 17.49 | 15.3 ± 0.62 | 1.2 ± 0.25 | 0.9 ± 0.17 |
| B-7 (25.0) | 13.32 | 16.7 ± 0.71 | 1.0 ± 0.33 | 0.8 ± 0.15 |
| B-8 (30.0) | 10.46 | 18.5 ± 0.78 | 0.9 ± 0.22 | 0.8 ± 0.14 |
| K-1 (0.5) | 50.26 | 12.2 ± 0.62 | 1.9 ± 0.25 | 1.2 ± 0.14 |
| K-2 (1.0) | 42.00 | 13.2 ± 0.67 | 1.5 ± 0.18 | 1.1 ± 0.18 |
| K-3 (2.0) | 30.00 | 14.1 ± 0.66 | 1.3 ± 0.27 | 1.0 ± 0.13 |
| Mean | 34.02 | |||
| Error | ±5.35 |
Treatments are as per the medium shown in Table 1.
The value in parenthesis represents the concentration in mg l−1
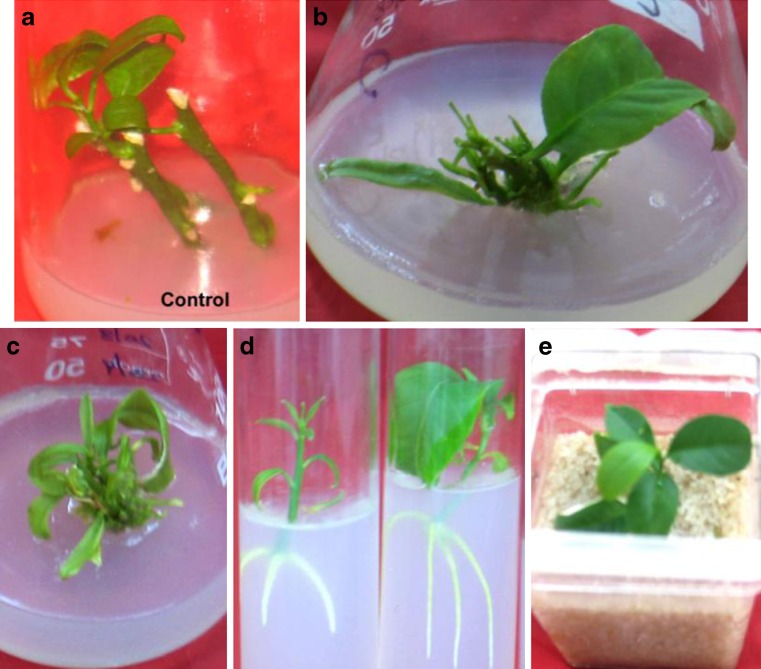
Fig. 1.
Shoot initiation from nodal segment explants of C. limon cv. Kaghazi Kalan cultured on growth regulator-free MS medium (a); Multiple shoot formation from nodal explants cultured on MS medium supplemented with 1.0 mg/l BA + 0.5 mg/l kin + 0.5 mg/l IBA (b); Multiple shoot formation from nodal explants cultured on MS medium supplemented with 0.25 mg/l BA + 1.0 mg/l kin + 1.0 mg/l IBA (c); Root induction from in vitro-raised shoots on growth regulator-free MS medium (d); A plantlet kept on soil: sand mixture for hardening (e)
Effect of combination of BA and NAA on shoot proliferation
A combination of BA and NAA had a significant effect on frequency of bud break, days to bud break, number of regenerated shoots and shoot length (Table 3). Frequency of shoot induction was directly proportional to concentration of BA in the medium. The highest (67.68 %) shoot induction frequency was recorded with the medium supplemented with 10.0 mg l−1 BA and 0.1 mg l−1 NAA, while the minimum (52 %) frequency was recorded with 0.1 mg l−1 BA and 0.1 mg l−1 NAA. Days to shoot induction was the earliest (8.28) days on media containing 10.0 mg l−1 BA and 0.1 mg l−1 NAA, while a delayed shoot induction was observed with increasing levels of BA.
Table 3.
Effect of a combination of BA and NAA on shoot proliferation from nodal explants of C. limon cv. Kaghzi Kalan. Data recorded after 8 weeks of culture
| Treatment (mg l−1) | Frequency of explants showing bud break (%) | Days to shoot induction | Shoot length (cm) | Number shoots per explant |
|---|---|---|---|---|
| BN-1 (10.0 + 0.1) | 67.86 | 8.28 ± 0.91 | 3.0 ± o.25 | 1.3 ± 0.65 |
| BN-2 (5.0 + 0.1) | 62.50 | 9.20 ± 0.86 | 2.3 ± 0.14 | 1.3 ± 0.46 |
| BN-3 (1.0 + 0.1) | 53.57 | 10.45 ± 0.95 | 1.9 ± 0.20 | 1.2 ± 0.25 |
| BN-4 (0.1 + 0.1) | 52.00 | 12.15 ± 0.75 | 1.9 ± 0.25 | 1.2 ± 0.19 |
| Mean | 58.98 | |||
| Error | ±3.75 |
Treatments are as per the medium shown in Table 1
The values in parenthesis represent the concentration in mg l−1
Similarly, the highest shoot length (3.0 cm) was recorded at 10 mg l−1 BA and 0.1 mg l−1 NAA, while a declined (1.9 cm) in shoot length was recorded at a lower level (0.1 mg l−1 BA and 0.1 mg l−1 NAA). Increasing concentration of BA and in combination with NAA had a promotory effect on number of shoots per explants. The number of shoots per explants decrease with an increase in concentration of BA alone but in case of BA and NAA combination, it was observed that number of shoots was the maximum (1.3) on MS media containing 10.0 mg l−1 BA, while the lowest (1.2) shoot number was observed in the medium with 0.1 mg l−1 BA and 0.1 mg l−1 NAA.
Effect of combination of BA, kinetin and IBA on axillary shoot enhancement
Combinations of BA, kinetin and IBA had a significant effect on frequency of bud break, days to bud break, number of regenerated shoots and shoot length (Table 4). The number of shoots increased with the increasing levels of BA in combination with 0.5 mg l−1 Kin and 0.5 mg l−1 IBA. The maximum (5.5) number of shoots per explants was obtained on a medium containing 1.0 mg l−1 BA + 0.5 mg l−1 kin + 0.5 mg l−1 IBA. A further increase in BA with the same levels of Kin and NAA, number of shoots reduced to 2.5. Different concentrations of Kin in combination with BA and IBA were also found to significantly promote the number of shoots per plant. At 1.0 mg l−1 of Kin and 1.0 mg/l of IBA, the lowest concentration of BA (0.25 mg l−1) produced 5.5 shoots per explants, which was a similar response as observed for 1.0 mg l−1 BA + 0.5 mg l−1 Kin + 0.5 mg l−1 IBA. A further increase in BA at a same level of Kin and IBA (1.0 mg l−1 each) drastically reduced the number of shoots per explant. The frequency of responding explants was the highest (70 %) on the medium containing 1.0 mg l−1 BA + 0.5 mg l−1 Kin + 0.5 mg l−1 IBA (Fig. 1b) or 0.25 mg l−1 BA + 1.0 mg l−1 Kin + 1.0 mg l−1 IBA (Fig. 1c). The higher levels of Kin and IBA (1.0 mg l−1) drastically reduced (<40 %) the number of responding explants, whereas, most of the other treatments in the study induced more than 60 %. These results are in agreement with the results of Al-Khayri and Al-Bahrany (2001), wherein, the best (8 shoots per explants) results for multiple shoot formation were obtained with 1.0 mg l−1 BA + 0.5 mg l−1 kin from nodal explants of mature trees.
Table 4.
Effect of a combination of BA, kinetin and IBA on shoot proliferation from nodal explants of C. limon cv. Kaghzi Kalan. Data recorded after 8 weeks of culture
| Treatment (mg l−1) | Frequency of explants showing bud break (%) | Days to Shoot induction | Shoot length (cm.) | Number of shoots per explant |
|---|---|---|---|---|
| BKI-1 (0.25 + 0.5 + 0.5) | 61.66 | 12.5 ± 0.65 | 1.75 ± 0.50 | 1.5 ± 0.25 |
| BKI-2 (0.5 + 0.5 + 0.5) | 58.33 | 11.5 ± 0.40 | 1.65 ± 0.40 | 2.5 ± 0.15 |
| BKI-3 (1.0 + 0.5 + 0.5) | 73.33 | 10.5 ± 0.24 | 2.25 ± 0.25 | 5.5 ± 0.20 |
| BKI-4 (2.0 + 0.5 + 0.5) | 66.66 | 13.5 ± 0.43 | 1.75 ± 0.15 | 2.5 ± 0.25 |
| BKI-5 (0.25 + 1.0 + 1.0) | 70.00 | 11.0 ± 0.50 | 2.25 ± 0.50 | 5.5 ± 0.30 |
| BKI-6 (0.5 + 1.0 + 1.0) | 65.00 | 12.5 ± 0.25 | 1.50 ± 0.15 | 3.5 ± 0.50 |
| BKI-7 (1.0 + 1.0 + 1.0) | 38.33 | 13.0 ± 0.30 | 1.25 ± 0.25 | 1.5 ± 0.60 |
| BKI-8 (2.0 + 1.0 + 1.0) | 35.00 | 13.5 ± 0.40 | 1.25 ± 0.40 | 1.5 ± 0.25 |
| Mean | 58.53 | |||
| Error | ±5.05 |
Treatments are as per the medium shown in Table 1.
The values in parenthesis represent the concentration in mg l−1
Similarly, the two concentrations found better for number of shoots per explant were also found better to support shoot growth and at the same time shoots were induced quickly. At 1.0 mg l−1 BA + 0.5 mg l−1 Kin + 0.5 mg l−1 IBA, shoot induction was observed in 10.5 days, while at 0.25 mg l−1 BA + 1.0 mg l−1 Kin + 1.0 mg l−1 IBA, shoot induction was observed in 11 days. The highest (2.25 cm) shoot length was observed in MS media containing 1.0 mg l−1 BA + 0.5 mg l−1 Kin + 0.5 mg l−1 IBA and 0.25 mg l−1 BAP + 1.0 mg l−1 Kin + 1.0 mg l−1 IBA. Whereas, the maximum shoot length was obtained on medium having 0.5 mg l−1 BA + 1 mg l−1 kin + 0.5 mg l−1 NAA. Similar results were also reported in case of C. aurantifolia, wherein, multiple shoots were produced from nodal explants on MS medium supplemented with 2 mg l−1 BA, 1 mg l−1 kin + 1 mg l−1 NAA (Al-Bahrany 2002).
The addition of IBA, in most cases appeared to have negative effect on shoot elongation. Kin appeared to have little effect on shoot length, particularly with 0.25 to 1.0 mg l−1 BA, but in combination with 2.0 mg l−1 BA, kinetin generally inhibited elongation (1.25 cm). The best combinations for lime shoot elongation consisted of 0.25 mg l−1 BA + 1.0 mg l−1 kin + 1.0 mg l−1 IBA or 1.0 mg l−1 BA + 0.5 mg l−1 kin + 0.5 mg l−1 IBA. These results are in contrast to the reports of Ali and Bushra (2006), wherein, direct shoot regeneration was reported from nodal explants of C. jambhiri on MS medium containing only 3.0 mg l−1 BA.
In Citrus cultivars, the multiple shoot induction has been reported to be directly proportional to the increasing levels of BA and NAA in medium (Usman et al. 2005). In the case of C. depresssa, Cancino et al. (2001) reported that Pluronic F-68 (0.5 % w/v) enhanced shoot regeneration in micropropagated explants cultured on MS medium with BA and significantly enhanced bud induction and the number of buds regenerated per cotyledon explants. Though, BA and Kin alone and in combination with NAA influenced shoot proliferation, shoot growth along with number of shoots induced and days taken to induction, the influence was not good enough for commercial exploitation. A previous report by Al-Khayri and Al-Bahrany (2001) in the case of C. aurantifolia Christm. Swing suggests use of low level of IBA instead of NAA in combination with BAP and KN. Therefore; a few combinations of these have been tried in the present investigation. Since, various levels of BA and Kin alone (Table 2) failed to provide appreciable axillary enhancement. However, when a few combinations of these two were tried with IBA it resulted in multiple shooting. Various levels of the three hormones have been tried in the present study and found a good response on shoot proliferation.
Effect of combination of BAP, kinetin and IBA on second cycle of shoot multiplication
In case of the combination of two cytokinins (BA and Kin) and auxin (IBA), number of shoots induced to multiple shooting was found the highest (75 %) on MS media containing 0.25 BA + 1.0 Kin + 1.0 IBA mg l−1 followed by 70 % on media containing 1.0 BA + 0.5 Kin + 0.5 IBA mg l−1 (Table 5). Multiple shoot induction percentage was found lowest (40 %) on media containing (0.5 BA +1.0 Kin + 1.0 IBA). However, remaining four combinations did not induce additional shoots (Table 5). The earliest (23 days) multiple shoot induction was observed on MS media containing 0.25 BA + 1.0 Kin + 1.0 IBA mg l−1 followed by 24 days on media containing 1.0 BA + 0.5 Kin + 0.5 IBA mg l−1. But overall, the differences were non-significant. The maximum shoot length (3.5 cm) was observed on MS media containing 1.0 BA + 0.5 Kin + 0.5 IBA mg l−1 while the minimum (1.5 cm) was observed on test of the media tested (Table 5). The number of shoots was maximum (7) on media containing 0.25 BA + 1.0 Kin + 1.0 IBA followed by (6) on media containing 1.0 BA + 0.5 Kin + 0.5 IBA while the minimum (2.5) was observed on media containing 0.5 BA + 1.0 Kin + 1.0 IBA. In order to overcome the limitations of shoots that could be produced on primary explant, second cycle of shoot proliferation is followed taking the shoot born in vitro. However, this method has not been well-studied in citrus particularly in cultivar Kaghzi Kalan.
Table 5.
Effect of a combination of BA, kinetin and IBA on proliferation from nodal explants of C. limon cv. Kaghzi Kalan during the second cycle of in vitro multiplication. Data recorded after 8 weeks of culture
| Treatment (mg l−1) | Frequency of cultures showing multiple shoots (%) | Days to shoot induction | Shoot length (cm) | Number of shoots per explant |
|---|---|---|---|---|
| BKI-1 (0.25+0.5+0.5) | – | – | 1.5 ± 0.19 | – |
| BKI-2 (0.5+0.5+0.5) | – | – | 1.5 ± 0.18 | – |
| BKI-3 (1.0+0.5+0.5) | 70.00 | 23.0 ± 1.12 | 3.5 ± 0.19 | 7.0 ± 0.51 |
| BKI-4 (2.0+0.5+0.5) | 60.00 | 25.0 ± 1.04 | 2.0 ± 0.21 | 2.5 ± 0.48 |
| BKI-5 (0.25+1.0+1.0) | 75.00 | 24.0 ± 1.50 | 3.0 ± 0.17 | 6.0 ± 0.62 |
| BKI-6 (0.5+1.0+1.0) | 40.00 | 25.5 ± 0.97 | 2.0 ± 0.15 | 2.5 ± 0.37 |
| BKI-7 (1.0+1.0+1.0) | – | – | 1.5 ± 0.17 | – |
| BKI-8 (2.0+1.0+1.0) | – | – | 1.5 ± 0.18 | – |
| Mean | 61.25 | |||
| Error | ±12.11 |
Treatments are as per the medium shown in Table 1
The values in parenthesis represent the concentration in mg l−1
Root induction
Roots could be induced from the in vitro-raised shoots on MS medium containing 1.0 mg l−1 IBA or IAA. But different concentrations of NAA failed to induce rooting in any of the shoots. None of the treatments induced significantly more root induction than control (MS basal salts). MS basal medium without any induced roots in 60 % of shoots cultured (Table 6, Fig. 1e). IBA could only marginally increase the rooting shoots (66.7 %), whereas effect of IAA was lesser (46.66 %). Overall, it was concluded that MS basal medium without any growth regulator was recorded to the best rooting medium as far as number of roots per explant and root length is concern (Fig. 1d). However, IBA resulted in earlier (13.5 days) root induction compared to that of only MS basal medium (17 days). Plantlets produced in vitro were transferred to magenta boxes, especially designed pre-acclimation chambers (PACS) containing autoclaved soilrite for hardening (Fig. 1e).
Table 6.
Effect of IBA, IAA, or NAA (1 mg l−1) on root induction from the in vitro regenerated shoots of C. limon cv. Kaghzi Kalan
| Growth regulator (mg l−1) | Frequency of shoots showing root induction (%) | Days to root induction | Root length | Number of root/explant (cm) |
|---|---|---|---|---|
| IBA (1.0) | 66.66 | 13.5 ± 1.24 | 4.5 ± 0.41 | 2.5 ± 0.21 |
| IAA (1.0) | 46.66 | 16.0 ± 2.10 | 2.5 ± 0.31 | 3.5 ± 0.19 |
| NAA (1.0) | – | – | – | – |
| Control | 60.00 | 17.0 ± 2.43 | 4.5 ± 0.51 | 4.5 ± 0.24 |
| Mean | 43.33 | |||
| Error | ±15.02 |
Treatments are as per the medium shown in Table 1
The values in parenthesis represent the concentration in mg l−1
In case of citrus spp., in vitro produced shoots have been induced to root both in vitro as well as ex vitro, the latter is preferred, as it provides a better establishment of plants during hardening that it cuts down per se the cost of production, but needs high techniques that are costlier to operate. In grape vine, auxins like IBA, IAA and NAA were mostly used to induce roots in in vitro shoots either through continuous exposure or through pulse treatment (Barreto and Nookaraju 2007). In the present study, both IAA and IBA (1.0 mg l−1) were found to induce a high frequency (66.66 %) of rooting on IBA followed by IAA (46.66 %) and no rooting was observed on NAA supplemented media. However, in the control even 60 % micropropagules induced rooting with more root number and vigour compared to hormonal treatments, though induction was delayed, compared IBA the earliest one and comparable in frequency.
However, contrary to the present findings, Al-Bahrany (2002) reported the positive effect of phytohormones on in vitro rooting of lime Citrus aurantifolia (Christm.) Swing. The highest rooting percentage was obtained on a medium containing either 1.0 mg l−1 NAA alone or 0.5 mg l−1 NAA + 2.0 mg l−1 IBA, whereas the highest numbers of roots were produced on a treatment containing both 2.0 mg l−1 NAA and 2.0 mg l−1 IBA. Roots elongated most on treatments containing 0.5 mg l−1 of either NAA (2.7 μM) or IBA (2.4 μM). Shoot growth associated with the rooting phase was the highest in response to 2.0 mg l−1 IBA (9.6 μM) or 0.5 mg l−1 NAA (2.7 μM). Ali and Bushra (2006) reported 0.5 mg l−1 NAA in MS medium was the best for root induction.
Rooting of regenerated shoots was best on MS medium supplemented with 2.7 μM NAA. In vitro and ex vitro rooted plantlets survived (on an average 83.3 %) after being transferred to the soil mixture consisting of soil, sand and organic material (1:1:1) and kept in the glasshouse (Normah et al. 1997). The perusals of present results regarding rooting behaviour of in vitro produced shoots of C.limon indicate that IBA as an auxin and control, as plain MS are method of choice for root induction.
Molecular analysis of regenerated plantlets
In order to find out the genetic uniformity of the micropropagated plants, three plants from each of the treatment and 3 from control were analyzed for RAPD. The present study is concerned with 8 different regenerated somaclones of Citrus limon by micropropagation method. Of the 12 primers screened, OPG-01 and OPG-02 did not generate any amplification product. Thus the remaining 10 decamer primers (Table 7) were selected for final RAPD-PCR analysis. The details of amplification pattern generated by each primer have been presented in Table 7. The number of amplification products was primer dependent and ranged from 2 to 7. The primer, OPG-17 amplified the maximum number of fragments (7) followed by 5 bands with OPG-08 (Table 7). Figure 2a shows the agarose gel image of amplification patters generated with primer OPG-07 and Fig. 2b with OPG-08. Of the total 42 amplification products, none was found to be of novel type and all the bands were found to be monomorphic and resembled with the parental type. From these results, it could be concluded that the regeneration system maintained high genetic fidelity and can be used for micropropagation at commercial level at least with two cycles of multiple shooting. RAPD analysis, in case of Citrus species has previously been reported to confirm the genetic fidelity of the micropropagated plants (Khawale and Singh 2005). It has also been used in a number of species for confirmation of genetic uniformity of micropropagated plants (e.g., Populus deltoids (Rani et al. 1995); Prunus persica (Hashmi et al. 1997); Cavendish banana (Bairu et al. 2006); Momordica dioica (Rai et al. 2012). DNA-based marker techniques have been employed to detect somaclonal variations. Such studies have usually involved searches for RFLPs and have lead to reports of mutations in regenerants (Nelke et al. 1993). RAPD may also be useful to assess the level of background genetic changes resulting from the tissue culture processes (Munthali et al. 1996) and to verify the “true-to-type” genotype of micropropagated plants.
Table 7.
Details of amolification products obtained after PCR amplification of genomic DNA from micropropagated plants of C. limon cv. Kaghzi Kalan
| Primer | Sequence (5′-3′) | Total Number of amplified products | Presence of novel bands |
|---|---|---|---|
| OPG-1 | CTACGGAGGA | – | – |
| OPG-2 | GGCACTGAGG | – | – |
| OPG-7 | GAACCTGCGG | 2 | – |
| OPG-8 | TCACGTCCAC | 6 | – |
| OPG-10 | AGGGCCGTCT | 5 | – |
| OPG-12 | CAGCTCACGA | 4 | – |
| OPG-13 | CTCTCCGCCA | 5 | – |
| OPG-14 | GGATGAGACC | 3 | – |
| OPG-15 | ACTGGGACTC | 3 | – |
| OPG-16 | AGCGTCCTCC | 5 | – |
| OPG-17 | ACGACCGACA | 7 | – |
| OPG-18 | GGCTCATGTG | 2 | – |
| Total | 42 | – |
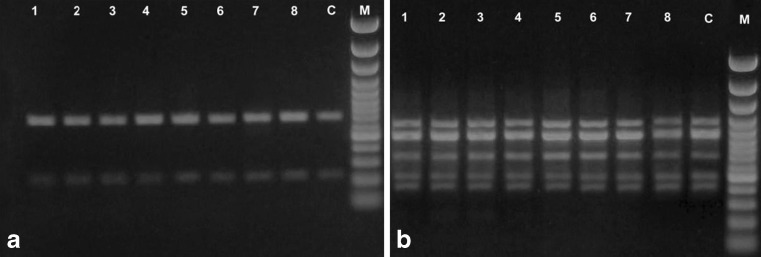
Fig. 2.
Agarose gel image showing the DNA amplification profile generated by primer OPG07 (a) and OPG08 (b) from eight seedless individuals plants (lanes 1–8); M, 100 bp ladder; and C is normal plant
Micropropagation is a powerful tool for large-scale multiplication of horticultural crops. It is a rapid technique, where mass multiplication of ornamental, timber and fruit trees can be achieved in a relatively short time with high fidelity index. Micropropagation is a boon in cases where no other vegetative propagation method is available such as in citrus, and in cases where an acceptable rate of multiplication cannot be achieved by conventional tools. It is also an ideal system for production of disease-free plants. Rapid plant multiplication can be achieved through axillary enhancement, organogenesis or somatic embryogenesis, the latter two being often accompanied by the induction of somaclonal variation that are less preferred. In vitro micropropagation has been successfully used for many horticultural fruit trees (Das et al. 1996). Multiple shoot production from axillary buds obtained from mature trees is now recognized as a better alternative of micropropagation in fruit trees where fidelity of the propagule is of prime importance (Tavares et al. 1996; Quraishi and Mitra 1998). However, it is from seedling explants whose genetic composition would differ from the mother plant and micropropagation studies are lacking. Such studies are either had not been conducted or have not been reported under patent conditions. Previous studies reveal that direct regeneration from explant obtained from mature field grown trees are lacking altogether. This situation warrants an immediate need to develop our own protocol for in vitro micropropagation and morphogenesis in Citrus limon.
There are various factors, which affect tissue culture of woody fruits and ornamental trees such as levels of cytokinins and auxins, the growth status of the mother plant, time of explants harvest and quality of micro flora present on the explants. In the present study, a lower level (0.1 mg l−1) of BA and (0.5 mg l−1) Kin induced the highest frequency of shoot regeneration in the nodal explants compared to their respective higher concentrations. Frequency of shoot regeneration from nodal explants decreased with a progressive increase in the level of cytokinins. BA was observed to be superior to kinetin in taking less time for induction of shoot regeneration and for number of shoots per explant.
Although BA has been reported to be more conducive for shoot proliferation from nodal explants, supplementation of kinetin and BA in medium has been found to enhance axillary branching of nodal segments (Fougat et al. 1997; Pereira et al. 1995 and Dewan et al. 1992). In the present study, it was observed that increase in the level of cytokinins from 5.0 mg l−1 to 30.0 mg l−1 BA produced a negative effect on all parameters. Effect of BA in combination with NAA on regeneration capacity of shoots per explant was found to be dependent on concentration of BAP. The number shoots of responsive explants decreased with an increase in concentration of BAP alone but in the presence of BAP and NAA, higher concentration of BAP (10 mg l−1) induced more nodal explants. The promoter effect of NAA in combination with BA was evident on the time taken for shoot induction and on average shoot length as compared to that observed in presence of cytokinins alone. Usman et al. (2005) found multiple shoot induction directly proportionate to the increase in the levels of BA and NAA in the modified MS medium. Whereas, Drateza et al. (1997) observed complementary effect of cytokinins and auxins and the best production of shoots was achieved on medium containing 1.0 mg l−1 BA and 0.1 mg l−1 NAA. In a similar study, combination of BA (1.0 mg l−1) and NAA (0.1 mg l−1) was reported to enhance axillary bud proliferation from nodal segments of Sapium sebiferum (Siril and Dhar 1997).
References
- Al-Bahrany AM. Effects of phytohormone on in vitro shoot multiplication and rooting of lime Citrus aurantifolia (Christm.) Swing. Sci Hortic. 2002;95:285–295. doi: 10.1016/S0304-4238(01)00349-1. [DOI] [Google Scholar]
- Al-Khayri JM, Al-Bahrany AM. Reported in vitro micropropagation of Citrus aurantifoliya (Lime) Curr Sci. 2001;81(9):1242–1246. [Google Scholar]
- Ali S, Bushra M. Micropropagation of rough lemon (Citrus jambhiri Lush.): Effect of explant type and hormone concentration. Acta Bot Croat. 2006;65(2):137–146. [Google Scholar]
- Bairu MW, Fennell CW, VanStaden J. The effect of plant growth regulators on somaclonal variation in Cavendish banana (Musa AAA cv. ‘Zelig’) Sci Hortic. 2006;108:347–351. doi: 10.1016/j.scienta.2006.01.039. [DOI] [Google Scholar]
- Barreto MS, Nookaraju A. Effect of auxin types on in vitro and ex vitro rooting and acclimatization of grapevine as influenced by substrates. Indian J Hort. 2007;64(1):5–11. [Google Scholar]
- Cancino GO, Gill MIS, Antony P, Davey MR, Power JB, Lowe KC. Pluronic F-68 enhanced shoot regeneration in a potentially novel citrus rootstock. Artif Cells Blood Substit Immobil Biotech. 2001;23(4):317–324. doi: 10.1081/BIO-100104233. [DOI] [PubMed] [Google Scholar]
- Chaudhary NA, Siddique MI, Agha SA. Characteristics of some local and exotic citrus rootstocks. Pb Fr J. 1994;47(1–4):22–28. [Google Scholar]
- Das S, Timir BJ, Jha S. In vitro propagation of cashew nut (Anacardium occidentale) Plant Cell Rep. 1996;15:615–619. doi: 10.1007/BF00232464. [DOI] [PubMed] [Google Scholar]
- Dewan A, Nanda K, Gupta SC. Plant Cell Rep. 1992;12:18–21. doi: 10.1007/BF00232415. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. Isolation of plant genomic from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- Drazeta L. Pomegranate (Punica granatum L.) propagation by in vitro method of tissue culture. Rev Res Fac Agri. 1997;42(1):49–59. [Google Scholar]
- Duncan DB. Multiple range and multiple F tests. Biometrics. 1955;11:1–42. doi: 10.2307/3001478. [DOI] [Google Scholar]
- FAOSTAT (2010) FAOSTAT database. Available at www.faostat.fao.org
- Fougat RS, Pandya SB, Ahmad T, Godhani PR. Micropropagation of pomegranate. J Appl Hort. 1997;3(1–2):23–29. [Google Scholar]
- Gavish H, Vardi A, Fluhr R. Extracellular proteins and early embryo development in Citrus nucellar cell cultures. Physiol Plant. 1991;82:606–616. doi: 10.1111/j.1399-3054.1991.tb02954.x. [DOI] [Google Scholar]
- Gavish H, Vardi A, Fluhr R. Suppression of somatic embryogenesis in cell cultures by extracellular proteins. Planta. 1992;186:511–517. doi: 10.1007/BF00198030. [DOI] [PubMed] [Google Scholar]
- Gomez KA, Gomez AA. Statistical procedures for agricultural research. USA: John Willey and Sons Inc.; 1984. [Google Scholar]
- Hashmi G, Huettel R, Meyer R, Krusberg L. RAPD analysis of somaclonal variants derived from embryo callus cultures of peach. Plant Cell Rep. 1997;16:24. doi: 10.1007/BF01275503. [DOI] [PubMed] [Google Scholar]
- Khawale RN, Singh SK. In vitro adventive embryony in citrus. Curr Sci. 2005;88(8):1309–1311. [Google Scholar]
- Munthali MT, Newburry HJ, Ford-Llyod BV. The detection of somaclonal variants of beets using RAPD. Plant Cell Rep. 1996;16:474–478. doi: 10.1007/BF00232977. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Nelke M, Nowak I, Wright JM, McLean NL. DNA-based marker techniques to detect somaclonal variations. Plant Cell Rep. 1993;13:72–78. doi: 10.1007/BF00235293. [DOI] [PubMed] [Google Scholar]
- Normah MN, Hamidah S, Ghani FD. Micropropagation of Citrus halimii stone. Plant Cell Tiss Organ Cult. 1997;50(3):225–227. doi: 10.1023/A:1005933417685. [DOI] [Google Scholar]
- Pereira AMS, Moro JR, Cerdeira RMM, Franca SC. Plant Cell Tiss Org Cult. 1995;42:295–297. doi: 10.1007/BF00030003. [DOI] [Google Scholar]
- Quraishi A, Mitra SK. Micropropagation of nodal explants from adult trees of Cleistanthus collinus. Plant Cell Rep. 1998;17:430–433. doi: 10.1007/s002990050420. [DOI] [PubMed] [Google Scholar]
- Rai GK, Singh M, Rai NP, Bhardwaj DR, Kumar S (2012) In vitro propagation of spine gourd (Momordica dioica Roxb.) and assessment of genetic fidelity of micropropagated plants using RAPD analysis. Physiol Mol Biol Plants. Online first (doi:10.1007/s12298-012-0109-7) [DOI] [PMC free article] [PubMed]
- Rangan TS, Murashige T. In vitro initiation of nucellar embryo in embryonic Citrus. HortSci. 1968;3:226–227. [Google Scholar]
- Rangaswamy NS. Experimental study on female reproductive structure of Citrus microcarpa Bunge. Phytomorphology. 1961;11:109–127. [Google Scholar]
- Rani V, Parida A, Raina SN. RAPD markers for genetic analysis in micropropagated plants of Populus deltoieds Marsh. Plant Cell Rep. 1995;14:459–462. doi: 10.1007/BF00234055. [DOI] [PubMed] [Google Scholar]
- Sabharwal PS (1963) In vitro culture of ovules, nucelli and embryos of Citrus reticuleta Blanco var Nagpuri. In: Maheshwari P, Rangaswamy NS (Eds.) Plant tissue and organ culture, Delhi, pp. 265–274
- Siril EA, Dhar U. Micropropagation of mature Chinese tallow tree (Sapium sebifeum Roxb.) Plant Cell Rep. 1997;16:637–640. doi: 10.1007/BF01275506. [DOI] [PubMed] [Google Scholar]
- Statistical package for agricultural research data analysis version 2.0. New Delhi: Indian Agricultural Statistics Research Institute; 2008. [Google Scholar]
- Tavares AC, Pimentra MC, Gonclaves Micropropagation of Melissa officinalis L. through proliferation of axillary shoots. Plant Cell Rep. 1996;15:441–444. doi: 10.1007/BF00232072. [DOI] [PubMed] [Google Scholar]
- Tisserat B, Murashige T. Probable identity of substances in citrus that repress asexual embryogenesis in vitro. Plant Tissue Cult. 1977;13:785–789. doi: 10.1007/BF02627858. [DOI] [PubMed] [Google Scholar]
- Usman M, Sana M, Fatima B. In vitro multiple shoot induction from nodal explant of Citrus cultivars. J Cent Euro Agri. 2005;6(4):435–442. [Google Scholar]