Abstract
Bamboos (family Poaceae) are the most beautiful and useful plants on the Earth, mainly found in the tropical and sub-tropical regions of the world. Bamboos are fast growing and early maturing, but lack of proper management of bamboo resources is leading to rapid reduction of the existing bamboosetum. Bamboo propagation through seeds is limited due to long flowering cycle of upto 120 years, seed sterility and short seed viability. Infrequent and unpredictable flowering events coupled with peculiar monocarpic behaviour i.e. flowering once before culm death, and extensive genome polyploidization are additional challenges for this woody group. Similarly, vegetative propagation by cuttings, offsets and rhizomes are also inadequate to cope up with the demand of planting stock due to large propagule size, limited availability, seasonal dependence, low multiplication rate and rooting percentage. Therefore, attempts have been made to propagate bamboos through in vitro techniques. In vitro flowering has also been achieved successfully in some bamboo species. Classification systems proposed to date need further support, as taxonomic delineation at lower levels is still lacking sufficient resolution. Tremendous advancement in molecular markers holds the promise to address the needs of bamboo taxonomy (systematics and identification) and diversity studies. Successful application of molecular marker techniques has been achieved in several bamboo species although, more studies are required to understand the population structure and genetic diversity of bamboos in a better way. In addition, some efforts have also been made to clone important genes from bamboos and also for genetic transformation using Agrobacterium and particle bombardment methods. An overview of the recent developments made in improvement of bamboos through in vitro propagation, molecular marker technologies, cloning, and transformation and transgenics has been presented. The future potential of improvement of bamboos using modern biotechnological tools has also been discussed.
Keywords: Bamboos, Genetic fidelity, Micropropagation, Molecular markers, Phylogenetic relationships, Transformation and transgenics
Introduction
Bamboos are the most unique, fascinating and versatile group of plants known to mankind which are commonly called “Green Gold” or “Poor Man’s Timber” and are unique with complex branching patterns, woody culms and gregarious, monocarpic flowering. It is one of the largest members of the grass subfamily Bambusoideae of family Poaceae (Gramineae), which includes ~1,575 species distributed mainly in tropical and subtropical countries of the world. Major species richness is found in Asia Pacific (China: 626, India: 102, Japan: 84, Myanmar: 75, Malaysia: 50 and few others) and South America (Brazil: 134, Venezuela: 68, Colombia: 56 and few others) while least (5) is found in Africa (Bystriakova et al. 2003, 2004). The herbaceous bamboos with ~110 species are mostly concentrated in the Neotropics of Brazil, Paraguay, Mexico, Argentina and West Indies (Judziewicz et al. 1999). Brazil is the most prominent place representing 89 % of the genera and 65 % of the species that are reported from the New World. The largest natural bamboo forests, known as ‘tabocais’ in Brazil and ‘pacales’ in Peru, cover approximately 600,000 ha across Brazil, Peru and Bolivia (Filgueiras and Goncalves 2004).
Conventionally, bamboos are propagated through seeds, offsets and culm cuttings. However, propagation through seeds is beset with several problems like long flowering cycle (upto 120 years), monocarpic nature of plant, poor seed set, short seed viability, highly heterogeneous seedling populations and consumption of seeds by birds, rodents and wild animals. Similarly, bulkiness and limited availability of propagules, difficulties in transportation over long distances, seasonal dependence, low survival rate and limited rooting of the propagules are the major constraints in bamboo propagation through vegetative methods. Shortage of bamboo planting material is expected to become a bottle neck in the reforestation process due to inefficacy of the conventional propagation techniques like seed propagation, clump division, rhizome and culm cuttings etc.
Pests and diseases also play an important role in the success or failure of the establishment of nursery and plantation of bamboo stands. Tar spot caused by Phyllachora shiriana complex and leaf rust caused by Phakopsora louditiae are the most common diseases of B. blumeana, Bambusa sp. and D. latiflorus. However, mite (Schizotetranycus floresi) was the most prevalent pest observed (Dayan 1988). Similarly, it is susceptive to the Bamboo mosaic virus (BaMV), a potexvirus which infects 13 species of bamboo and is considered as an important limiting factor in the production of B. edulis in Taiwan. The virus reduces the quality and yield of bamboo by over 50 %. No chemicals effectively control or eliminate BaMV from infected plants (Hsu et al. 2000). Development of disease-resistant bamboo may be a solution to this problem. However, it is difficult to obtain virus-resistant bamboos using traditional breeding methods (Chang and Ho 1997; John and Nadgauda 1999).
The conventional methods of taxonomical classification are based on the morphological and flowering features of any plant species. However, in bamboos, taxonomic delineation has been done predominantly on the basis of various morphological features due to erratic and long flowering cycles, which severely restricts the study of reproductive features. Hence, the identification keys are mostly dependent on various vegetative features that need further refinement and re-investigation. In particular, the taxonomic demarcation of woody bamboos at lower ranks, such as genera and species, is not well resolved and requires additional efforts.
Peculiar flowering habits have made it almost impossible to undertake breeding programs for superior traits in woody bamboos. In addition, the characteristic death of bamboo clumps after flowering makes the study of bamboo flowering quite difficult. Tissue culture has been used for rescue of hybrid seeds produced by conventional breeding methods. Alexander and Rao (1968) were the first to report aseptic germination of seeds of hybrid bamboo (Bambusa x Saccharum) on a sucrose enriched medium, heralding the start of tissue culture of bamboos. However, no breakthrough has been achieved in bamboo breeding using conventional methods.
In spite of enormous volume of research work undertaken in bamboos, no compilation of data is available which can provide a ready reference to the various aspects of biotechnological improvements being undertaken in bamboo, therefore this review has been compiled to encompass most of the available literature on bamboo biotechnology.
Progress made using modern biotechnological tools
A number of successful reports documenting propagation of bamboos through in vitro techniques have been published during the last three decades. An attempt has been made to summarize the available information regarding micropropagation of bamboos through tissue culture. Many groups have also attempted to induce flowering in vitro in bamboos to study the floral details. In addition, an overview of the information available on use of various molecular markers in diversity analysis, phylogenetic and taxonomic studies; attempts being made to develop transgenics in bamboos using various transformation techniques and cloning of genes has been presented. Figure 1 summarizes the uses, limitations and the possible areas wherein biotechnological interventions can be made for improvement of bamboos.
Fig. 1.
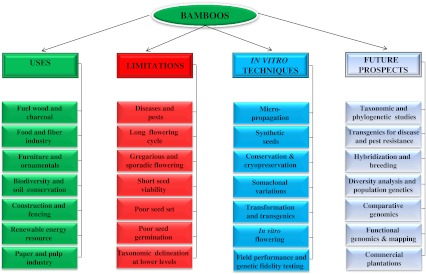
Uses, limitations, applications of in vitro techniques and future prospects of biotechnological interventions in bamboos
Micropropagation
Micropropagation is a valuable technique for rapid multiplication of difficult-to-propagate plants, both for commercial production and germplasm conservation. Micropropagation using tissue culture techniques offers substantial advantage over largely insufficient and inefficient classical techniques used for mass scale propagation of bamboos. Two distinct patterns of in vitro micropropagation used for bamboos are organogenesis and somatic embryogenesis (Tables 1, 2, 3).
Table 1.
Micropropagation of bamboos through enhanced axillary branching using juvenile and mature explants
| Species | Explant | Medium + PGRs | References | |
|---|---|---|---|---|
| Induction | Rooting | |||
| Juvenile explants | ||||
| 54 Bamboo species | Node | MS + BAP | MS + NAA | Prutpongse and Gavintertvatana 1992 |
| Bambusa balcooa, B. bambos | Node | MS + BAP + NAA | MS + IBA/NAA | Rathore et al. 2009 |
| B. bambos | Node | MS + BAP | MS + NAA | Arya and Sharma 1998 |
| Embryonic axis of caryopsis | MS + BAP | MS + BAP + GA3 + NAA | Kapoor and Rao 2006 | |
| B. nutans | Node | MS + BAP | MS + IBA | Yashoda et al. 1997 |
| B. oldhamii | Shoot apices | MS + TDZ | MS + NAA | Lin et al. 2007a |
| Node | MS + BAP + AdS | MS + IBA + NAA | Thiruvengadam et al. 2011 | |
| B. tulda | Shoot apices | MS + BAP + Kn | ½ MS + IAA + Cou | Saxena 1990 |
| B. ventricosa | Node | MS + BAP + NAA + AC | MS + BAP + NAA + AC | Dekkers and Rao 1989 |
| Shoot apices | MS + BAP | MS + BAP + NAA | Huang and Huang 1995 | |
| Dendrocalamus asper | Seed | MS + BAP | MS + IBA + NAA | Arya and Arya 1996; Arya et al. 1999, 2002a |
| D. brandisii | Seed | MS + BAP + CW | MS + IBA | Nadgauda et al. 1990 |
| D. giganteus | Node | MS + BAP + Kn | ½ MS + IBA + Cou | Ramanayake and Yakandawala 1997 |
| Node | MS + BAP + NAA | MS + IBA | Agnihotri et al. 2009 | |
| Node | MS + BAP | MS + IBA/IAA/NAA + Cou | Sood et al. 2002 | |
| D. membranaceus | Node | MS + BAP | MS + IBA | Yashoda et al. 1997 |
| D. strictus | Shoot apices | MS + BAP + CW | MS + IBA | Nadgir et al. 1984 |
| Node, Coleoptile | ½ MS + BAP | MS | Shirgurkar et al. 1996 | |
| Node | MS + GA3 + Kn | MS + GA3 + Kn | Maity and Ghosh 1997 | |
| Shoot apices | MS + BAP + triacontanol | MS + NAA + rice bran extract | Mishra et al. 2001 | |
| Shoot apices, node | ½ MS + TDZ | ½ MS + IBA | Singh et al. 2001 | |
| D. strictus, D. giganteus | Node | MS + BAP + AdS | ½ MS + IBA | Das and Rout 1991 |
| Oxytenanthera abyssinica | Node | MS + BAP + NAA | MS + IBA | Diab and Mohamed 2008 |
| Phyllostachys meyeri | Node | ½ MS | ½ MS | Ogita et al. 2008 |
| Thamnocalamus spathiflorus | Zygotic embryos | MS + BAP + IBA | MS + IBA | Bag et al. 2000 |
| Mature explants | ||||
| Bambusa balcooa | Node | MS + BAP | MS + BAP + NAA | Mudoi and Borthakur 2009 |
| Node | MS + BAP + Kn | ½ MS + IBA | Das and Pal 2005a | |
| B. balcooa, B. nutans, B. salarkhanii, B. vulgaris | Node | MS + BAP | ½ MS + NAA + IBA | Nurul Islam and Rahman 2005 |
| B. bambos | Node | MS + BAP | MS + NAA | Arya and Sharma 1998 |
| B. edulis | Inflorescence | MS + NAA + IBA + 2,4- D | – | Lin et al. 2005 |
| B. glaucescens | Node | MS + BA + AC | MS + BA + NAA + AC | Banik and Alam 1987 |
| Node | MS + BAP + Kn | MS + IBA | Shirin and Rana 2007 | |
| B. nutans | Node | MS + BAP | MS + IBA | Yashoda et al. 2007 |
| Node | MS + BAP + Kn | MS + IBA + IAA + NAA | Negi and Saxena 2011 | |
| B. oldhamii | Node | MS + BAP | MS + IBA + NAA | Thiruvengadam et al. 2011 |
| B. polymorpha | Node | MS + BAP | MS + NAA | Arya et al. 2005 |
| B. tulda | Node | MS + Glut + IAA + BAP | MS + Cou | Mishra et al. 2008 |
| B. ventricosa | Node | MS + BAP | MS + NAA | Arya et al. 2002b |
| B. vulgaris, B. arundinacea | Node | MS + BAP + Kn + CW | ½ MS + IBA | Nadgir et al. 1984 |
| B. vulgaris | Node | MS + BAP + AdS | MS + BAP + IBA | Das and Rout 1994 |
| Node | MS + BAP | MS + IBA | Ramanayake et al. 2006 | |
| B. wamin | Node | MS + BAP + Kn | ½ MS + IBA | Arshad et al. 2005 |
| Dendrocalamus asper | Node | MS + BAP | MS + IBA + NAA | Arya and Arya 1996; Arya et al. 1999, 2002a |
| Node | MS + BAP | MS + IBA | Banerjee et al. 2011 | |
| Node | MS + BAP + AdS | MS + IBA + NAA | Singh et al. 2011 | |
| D. giganteus | Node | MS + BAP + Kn + CW | ½ MS + IBA + Cou | Ramanayake and Yakandawala 1997 |
| Node | MS + BAP | – | Ramanayake et al. 2001 | |
| Node | MS + BAP | MS + IBA + NAA | Arya et al. 2006 | |
| D. hamiltonii | Node | MS + BAP + 2,4-D | ½ MS + IBA + NAA | Sood et al. 1994 |
| Node | MS + BAP + NAA | MS + IBA | Agnihotri and Nandi 2009 | |
| Node | MS + BAP + NAA | MS + IBA | Agnihotri et al. 2009 | |
| Node | MS + TDZ + AA | MS + IBA + CC | Singh et al. 2012a | |
| D. longispathus | Node | MS + BAP + Kn | ½ MS + IBA + Cou | Saxena and Bhojwani 1993 |
| D. strictus | Node | MS + IAA + AdS | MS + IBA + NAA + Phloroglucinol | Chaturvedi et al. 1993 |
| Node | MS + BAP + Kn | Ravikumar et al. 1998 | ||
| D. strictus | Node | MS + BAP + Kn + CW | ½ MS + IBA | Nadgir et al. 1984 |
| Guadua angustifolia | Node | MS + BAP | MS + BAP | Jimenez et al. 2006 |
| Pleioblastus pygmaeus | Node | MS + BAP | MS | Watanable et al. 2000 |
| Pseudoxytenanthera stocksii | Node | MS + BAP + NAA + AA + Cyst + Glut | ½ MS + BAP + IBA + AA + Cyst + Glut | Sanjaya et al. 2005 |
| Thamnocalamus spathiflorus | Node | MS + BAP + IBA | MS + IBA | Bag et al. 2000 |
| Thyrsostachys oliveri | Node | MS + BAP | ½ MS + NAA + IBA | Nurul Islam and Rahman 2005 |
AA ascorbic acid, AC activated charcoal, AdS adenine sulphate, BAP 6-benzylaminopurine, CC choline chloride, CW coconut water (milk), Cou coumarin, Cyst cysteine, 2, 4-D 2, 4 Dichlorophenoxy acetic acid, GA3 gibberellic acid, Glu glutamine, IAA indole-3-acetic acid, IBA indole-3-butyric acid, Kn kinetin, NAA α-napthaleneacetic acid, PGR plant growth regulator, PVP polyvinylpyrolidone, TDZ Thidiazuron
Table 2.
Callogenesis and indirect organogenesis in bamboos
| Species | Explants | Medium + PGRs | References | |
|---|---|---|---|---|
| Callus formation | Organogenesis | |||
| 54 Bamboo species | Shoot tips, leaf, inflorescence | MS + 2,4-D + CW | – | Prutpongse and Gavintertvatana 1992 |
| Bambusa glaucescens | Young leaves | MS + CH + CW + 2,4-D + PVP | – | Jullien and Van 1994 |
| B. multiplex | Shoot tip | MS + 2,4-D | – | Huang and Murashige 1983 |
| B. nutans | In vitro shoots | MS + 2,4-D + BAP + ABA | MS + 2,4-D + BAP | Kalia et al. 2004 |
| B. oldhamii, B. multiplex | Shoot apices | MS + BAP + NAA | MS + NAA | Huang et al. 1989 |
| B. vetricosa | Internode, sheath base | MS + 2,4-D | MS + 2,4-D | Dekkers and Rao 1989 |
| Dendrocalamus farinosus | Seed embryo, young shoots | MS + 2,4,5-T + Kn + IBA | MS + Kn + IAA | Hu et al. 2011 |
| D. giganteus | Shoots, spikelets, roots | MS + 2,4-D + NAA | MS + 2,4-D + NAA | Ramanayake and Wanniarachchi 2003 |
| D. hamiltonii | Node | MS + BAP + 2,4-D + GA3 | MS + IBA | Sood et al. 1994 |
| D. latiflorus | Internodes | MS + 2,4-D | MS + 2,4-D + BA | Zamora et al. 1989 |
| Inflorescence | MS + 2,4-D + Kn + CW + PVP | MS + TDZ | Lin et al. 2007b | |
| Phyllostachys aurea | Shoot apices | MS + BAP + NAA | MS + NAA | Huang et al. 1989 |
| P. nigra | Shoots | ½ MS + 2,4-D | ½ MS + 2,4-D | Ogita 2005 |
| Sasa pygmaea | Shoot apices | MS + BAP + NAA | MS + NAA | Huang et al. 1989 |
| Schizostachyum brachycladum, Thyrsostachys sinensis | Internode, sheath base | MS + 2,4-D | MS + 2,4-D | Dekkers and Rao 1989 |
ABA Abscisic acid, BAP 6-benzylaminopurine, CH casein hydrolysate, CW coconut water (milk), 2, 4-D 2, 4 Dichlorophenoxy acetic acid, GA3 Gibberellic acid, IAA indole-3-acetic acid, IBA indole-3-butyric acid, Kn kinetin, NAA α-Napthaleneacetic acid, PGR plant growth regulator, PVP polyvinylpyrolidone, TDZ thidiazuron, 2, 4, 5-T 2, 4, 5-Trichlorophenoxyacetic acid
Table 3.
Somatic embryogenesis in bamboos
| Species | Explants | Medium + PGRs | References | |
|---|---|---|---|---|
| Embryogenesis | Germination | |||
| Bambusa arundinacea | Embryonal axis | N6 + BAP + 2,4-D + PVP | N6 + BA + 2,4-D + PVP | Mehta et al. 1982 |
| B. balcooa | Pseudo-spikelet | MS + BAP | MS + BAP | Gillis et al. 2007 |
| B. beecheyana | Inflorescence | MS + 2,4-D + Kn | MS + 2,4-D + Kn | Yeh and Chang 1986b |
| Roots | MS + Kn + 2,4-D | MS + Kn + 2,4-D | Chang and Lan 1995 | |
| B. edulis | Node, internode | MS + Kn + 2,4-D + CW | MS + TDZ + NAA | Lin et al. 2004 |
| B. glaucescens | Leaves | MS + BAP + 2,4-D | – | Jullien and Van 1994 |
| B. oldhamii | Inflorescence | MS + 2,4-D + Kn | MS + 2,4-D + Kn | Yeh and Chang 1986a |
| Floral tissue | MS + BAP + NAA | MS + 2,4-D + Kn | Ho and Chang 1998 | |
| B. oldhamii, B. multiplex | Shoot apices | MS + BAP + NAA | MS + NAA | Huang et al. 1989 |
| B. ventricosa | Stem segment | MS + BAP + IBA | MS + BAP | Cheah and Chaille 2011 |
| B. vulgaris | Node, zygotic embryos | MS + 2,4-D + Kn + AdS | MS + 2,4-D + Kn + AdS | Rout and Das 1994 |
| Dendrocalamus asper | Seed | MS + 2,4-D | MS + 2,4-D | Kanyaratt 1991 |
| In vitro shoots | MS + BAP + 2,4-D + IAA | MS + BAP + IAA | Arya et al. 2008 | |
| Roots, leaves, node | MS + 2,4-D | MS + BAP | Ojha et al. 2009 | |
| D. giganteus, D. strictus | Node, zygotic embryos | MS + 2,4-D + Kn + AdS | MS + 2,4-D + Kn + AdS | Rout and Das 1994 |
| D. hamiltonii | Node | MS + BAP + 2,4-D | MS | Godbole et al. 2002 |
| Axillary bud | MS + BAP + 2,4-D | ½ MS + IBA | Bag et al. 2012 | |
| D. latiflorus | Meristems | MS + 2,4-D + BAP | MS + 2,4-D + BAP | Zamora et al. 1989 |
| D. longispathus | Internode | B5/MS + 2,4,5-T + 2,4-D | – | Saxena and Bhojwani 1993 |
| D. membranaceus | Node | MS + BAP + 2,4-D | – | Vongvijitra 1988 |
| D. strictus | Zygotic embryo | B5 + 2,4-D | ½ B5 + CW | Zamora and Gruezo 1990 |
| Seed | MS + 2,4-D + BAP + PVP | ½ MS + NAA + IBA | Saxena and Dhawan 1999 | |
| Seed | B5 + 2,4-D | B5 + IBA + NAA | Rao et al. 1985 | |
| Seed | MS + 2,4-D + CW | MS | Dekkers and Rao 1989 | |
| Seed | MS + 2,4-D + Kn | MS + 2,4-D + Kn | Kumar and Mathur 1992 | |
| Otatea acuminata | Seed | MS + 2,4-D + BAP | MS + 2,4-D + BAP | Woods et al. (1992) |
| Phyllostachys aurea | Shoot apices | MS + BAP + NAA | MS + NAA | Huang et al. 1989 |
| P. bambusoides | Node | MS + picloram | – | Komatsu et al. 2011 |
| Sasa pygmaea | Shoot apices | MS + BAP + NAA | MS + NAA | Huang et al. 1989 |
| Sinocalamus latiflora | Zygotic embryos | MS + 2,4-D + Kn + PVP | MS + 2,4-D + Kn + PVP | Yeh and Chang 1987 |
| Anthers | N6 + 2,4-D + BAP + AC | N6 + 2,4-D + BAP + AC | Tsay et al. 1990 | |
AC activated charcoal, AdS adenine sulphate, BAP 6-benzylaminopurine, CW coconut water (milk), 2, 4-D 2, 4 Dichlorophenoxy acetic acid, GA3 gibberellic acid, IAA indole-3-acetic acid, IBA indole-3-butyric acid, Kn kinetin, NAA α-Napthaleneacetic acid, PGR plant growth regulator, PVP polyvinylpyrolidone, TDZ thidiazuron, 2, 4, 5-T 2, 4, 5-Trichlorophenoxyacetic acid
Organogenesis
Clonal propagation via organogenesis is a two-staged process involving the proliferation (axillary meristems) or induction (adventitious meristems) of unipolar shoots on explants followed by shoot excision and induction of root meristems. It is generally agreed that plants regenerated from shoot tips or nodal buds are genetically stable and free from somaclonal variations associated with plants differentiated from callus. Therefore, a lot of studies are available wherein enhanced axillary branching has been utilized for micropropagation of bamboo species using juvenile and mature tissues (Table 1) and only few reports document indirect organogenesis (Table 2).
Factors controlling organogenesis
Explant
Success in micropropagation of bamboos was obtained using both juvenile and mature explants (Table 1). Sprouting of nodal buds into shoots is primarily determined by genotype, physiological state of the tissue, and time of the year when the explants are collected and cultured (Saxena and Dhawan 1994; Ramanayake et al. 1995; Ramanayake and Yakandawala 1997; Singh et al. 2011, 2012a). Saxena and Bhojwani (1993) reported that bud-break frequency in Dendrocalamus longispathus was strongly influenced by the juvenility of lateral shoots, position of axillary bud on the branch, and the season in which cultures were initiated. Explants collected during spring (February-April) gave better response in terms of decreased contamination, early shoot initiation and increased percent bud break with higher number of shoots in D. asper (Singh et al. 2011), while early summer (April–June) was best for explant collection and establishment of D. hamiltonii (Singh et al. 2012a).
Medium
The nutritional requirements for optimum growth of a tissue in vitro may vary with species. In bamboos, mostly MS medium has been used for both direct as well as indirect organogenesis. Singh et al. (2011, 2012a) compared four media viz. MS (Murashige and Skoog 1962), SH (Schenk and Hildebrandt 1972), B5 (Gamborg et al. 1968) and NN (Nitsch and Nitsch 1969) during axillary bud break and found better response in MS medium. Similarly, half strength rather than full strength MS was reported better for axillary shoot formation and organogenesis in some bamboo species (Shirgurkar et al. 1996; Singh et al. 2001; Ogita et al. 2008) (Tables 1, 2).
In general, agar or gellan gum solidified medium is used for tissue culture of plants, however, several workers have reported higher rates of shoot multiplication and improved growth in liquid medium in comparison to semi-solid medium (Saxena and Bhojwani 1993; Sood et al. 2002; Das and Pal 2005a; Arya et al. 2006; Shirin and Rana 2007; Ogita et al. 2008). The slower growth or poor shoot multiplication on semi-solid medium vis-a-vis liquid medium may be attributed to the fact that solubilized agar binds water, absorbs nutrients and PGRs resulting in reduced uptake of nutrients, PGRs and other essential constituents by cultured tissues, however, in some instances vitrification of shoots leading to reduced multiplication rates have also been reported in liquid medium.
Growth regulators
The frequency of bud break on PGR free basal medium is usually very low (Arya et al. 2006; Singh et al. 2011). The variable endogenous levels of growth regulators are known to be the cause of varied responses of species and genotypes to growth regulator supplemented media. Therefore, detailed information regarding the requirement of plant growth regulators (PGRs) is necessary before we can exploit plant tissue culture on commercial scale. The level and kind of PGRs included in the culture medium largely determine the success of tissue culture protocol. Incorporation of BAP into the medium improved the axillary bud proliferation (Nadgir et al. 1984; Dekkers and Rao 1989; Hirimburegama and Gamage 1995; Arya and Arya 1996; Arya et al. 2006), while Kinetin (Kn) alone was found to be less effective (Ramanayake and Yakandawala 1997; Arya et al. 2006; Singh et al. 2011). Synergistic effect of the two cytokinins BA and Kn was reported best for shoot multiplication in D. giganteus (Arya et al. 2006) and B. glaucescens (Shirin and Rana 2007). TDZ has been used during axillary shoot propliferation in B. oldhamii (Lin et al. 2007a), D. strictus (Singh et al. 2001) and D. hamiltonii (Singh et al. 2012a) while gibberellic acid (GA3) was used during in vitro propagation of D. strictus (Maity and Ghosh 1997).
In addition to cytokinins and auxins, other additives like adenine sulphate, activated charcoal and amino acids have also been included in the proliferation medium. The lethal browning or blackening of cultures due to phenolic compounds has been controlled using polyphenol adsorbents or antioxidants. Significant control of browning with enhanced shoot multiplication was achieved using ascorbic acid in D. hamiltonii while PVP and activated charcoal were ineffective in doing so (Singh et al. 2012a). On the contrary PVP improved shoot health in D. strictus cultures (Saxena and Dhawan 1999).
Medium pH
The hydrogen ion concentration of the media effect growth of the tissue by altering pH of cells. Higher ‘H’ ion concentration induced precipitation of phosphates, gelatinization of agar and destruction of vitamins and growth regulators. Though majority of plant tissues have optimum pH from 5.0 to 5.5 (Butenko et al. 1984) yet the pH range is variable for individual plant tissues. In an investigation, Arya et al. (2006) found that shoot growth was well in the pH range of 4.5 to 5.8 in D. giganteus, however, best shoot multiplication rate was obtained in the medium with pH of 4.5.
Carbon source
Sucrose is the most widely used carbon source in various plant tissue culture media, but its concentration varies from 2 to 6 %. The most commonly used sucrose concentration in bamboos is 3 %. Saxena (1990) found that 2 % sucrose was ideal for shoot multiplication in B. tulda. A high concentration (~6 %) of sucrose was used in medium for callus initiation and proliferation (Yeh and Chang 1986a, b, 1987; Tsay et al. 1990; Lin et al. 2004). Replacement of sucrose with less expensive table sugar had negligible effect on rate of shoot multiplication in D. asper and D. hamiltonii but reduced the cost of plant production considerably, however, the use of glucose showed deleterious effects on shoot multiplication (Singh et al. 2011, 2012a).
Propagule size
Single shoots usually do not survive under in vitro conditions. A propagule of three to five shoots has been reported best for multiplication of shoots in Bambusa tulda (Saxena 1990), Dendrocalamus longispathus (Saxena and Bhojwani 1993) and D. hamiltonii (Agnihotri and Nandi 2009; Agnihotri et al. 2009) in comparison to lesser or more shoots (Nadgir et al. 1984; Arya et al. 1999). Agnihotri and Nandi (2009) and Agnihotri et al. (2009) reported 20-fold shoot multiplication rate with propagule size of 3–5 shoots in D. hamiltonii. A propagule of 7–10 shoots was found optimum (supporting 5–6 fold multiplication rate) for large scale propagation of D. asper and D. hamiltonii (Singh et al. 2011, 2012a).
Culture duration
Sub-culturing of shoots is usually done at periodic interval of 3–4 weeks so as to maintain healthy cultures. Longer sub-culture durations usually lead to longer and pale shoots which gradually turn brown to black instead of enhancing the multiplication rate further (Mudoi and Borthakur 2009; Bisht et al. 2010; Singh et al. 2012a). The available nutrients in the culture medium become a limiting factor hampering the health of shoots.
Rooting of shoots
Induction of roots in excised shoots and subsequent survival of plantlets in the soil are the most crucial steps for success of any micropropagation protocol. Shirgurkar et al. (1996) and Watanable et al. (2000) reported rooting of Pleioblastus pygmaeus shoots in MS basal medium without growth regulators. The role of auxins in root development is well established and has been reviewed by Scott (1972) and Torrey (1976). Different auxins differ in their physiological activities depending upon the extent to which they move through tissues, remains bound inside the cells, or gets metabolized. Usually there is sufficient residual cytokinin in the shoots, thus little or no cytokinin is required for root induction. IBA alone or in combination with NAA are the most commonly used growth regulators for rooting in bamboos. Although, some reports are available where cytokinins (BAP or Kn) in combination with auxins were used for root induction in bamboos (Table 1). Addition of choline chloride along with IBA enhanced the rooting response in D. hamiltonii upto 89 % (Singh et al. 2012a) in comparison to the earlier report of Sood et al. (2002) where rooting response was not consistent and only 25–30 % of the shoots developed into plantlets. On the other hand, Saxena (1990) and Ramanayake and Yakandawala (1997) accomplished rooting of B. tulda and D. giganteus shoots respectively in the presence of coumarin. Usually a single step procedure is used for rooting of shoots in bamboos, however, a two step procedure has also been used for rooting of D. hamiltonii shoots (Agnihotri and Nandi 2009; Agnihotri et al. 2009) and a very high rooting rate (>90 %) was reported when the propagules were cultured on IBA supplemented medium for a week followed by transfer to IBA-free medium. Similarly, Singh et al. (2011) and Bag et al. (2012) also observed very high rooting efficiency (100 %) in D. asper and D. hamiltonii, respectively, while Singh et al. (2012a) reported 89 % rooting in D. hamiltonii. Shoots cultured on ½ MS medium gave better response among the four strengths tested (¼, ½, ¾ and full) in D. asper and D. hamiltonii (Singh et al. 2011, 2012a). This was attributed to reduction in total nitrogen required for rooting (Ajithkumar and Seeni 1998).
Acclimatization and field transfer
Lab to land transfer remains the major bottleneck in commercialization of tissue culture technique. This is mainly because of the shock which the in vitro raised plantlets experience when they are transferred from in vitro culture environment with low irradiance and high humidity to natural environment with high irradiance and low humidity. The in vitro raised plants usually have leaves with poor or no development of cuticular wax, impaired stomatal mechanism, low photosynthetic pigments, biochemicals e.g. carbohydrates, proteins, proline and phenols, poor photosynthetic activity, poor vascular development and connections, etc. Therefore, an efficient hardening and acclimatization technique is necessary to ensure better survival of in vitro raised plantlets in the field. Gradual reduction in the supply of nutrients and humidity during these procedures forces the plant to strengthen its own photosynthetic and defense mechanisms, and prepare them to grow under in vivo conditions. The healthy rooted plantlets are usually transferred to seedling trays or polybags containing different types of potting mix like soil, sand, soilrite, perlite, vermiculite, compost or farm yard manure either alone or in various ratios (Mishra et al. 2011; Singh et al. 2011) and maintained under high humidity. Initial application of reduced MS minerals to the plantlets has been found essential for their better acclimatization. After 2 to 3 weeks growth in mist chamber, the plants are transferred to net house for hardening for another 2 to 3 weeks. Addition of vermi-compost to the sand was found to improve the survival of plants probably due to increased porosity of sand and better aeration of roots (Singh et al. 2011). Verma and Arya (1998) studied the effect of arbuscular mycorrhizal fungal isolates and organic manure on growth and mycorrhization of micropropagated D. asper plantlets and spore production in their rhizosphere. Finally the acclimatized and hardened plants (1 to 2 ft height) are transferred to the field under natural conditions. Season of field transfer has also been found to influence the survival rate and growth of the plantlets (Mishra et al. 2011; Singh et al. 2011). Plantlets transferred in the months of July to August showed higher survival rate with sprouting of more new shoots than other months.
Macroproliferation, a method of plant multiplication by separating the rooted tillers has been used by many workers for enhancing the rate of multiplication of in vitro raised plants and for continuous supply of plantlets. Splitting of rooted tillers could double the production of D. asper plants (Singh et al. 2011), while three-fold increase was achieved in B. tulda (Mishra et al. 2011) and B. balcooa (Mudoi and Borthakur 2009).
Micropropagation has been widely used for rapid mass multiplication of bamboos, however, its application on commercial scale is restricted often due to high rate of plant loss when transferred to natural or ex vitro conditions. Only few reports are available regarding successful field transfer of micropropagated bamboos. Arya et al. (1999) reported 95 % field survival of D. asper and transferred 6,000 plants raised through seed tissue culture to the field. Sood et al. (2002) and Agnihotri et al. (2009) reported a survival percentage of 70 % in the field for the plants of D. hamiltonii. Mishra et al. (2011) reported 91 % survival of the plants of B. tulda in the green house. Negi and Saxena (2011) have successfully produced 2,500 plantlets with 95.83 % hardening rate up to nursery stage and transferred 12 plants with 100 % success in the field. Singh et al. (2011, 2012a) transferred 25,000 and 3,000 plants of D. asper and D. hamiltonii respectively to the Forest Department land in Yamunanagar, Haryana under the DBT’s Bamboo Mission. They reported a success rate of 92.34 % and 100 % for D. asper and D. hamiltonii in the green house, while 79.76 % and 85 % success was achieved in the field. Morphological growth variations were not observed among these plants over a period of 1–2 years. Few other reports have also documented good field performance of the tissue culture raised plantlets (Nadgir et al. 1984; Saxena 1990; Mudoi and Borthakur 2009; Agnihotri et al. 2009). Besides evaluating the morphological parameters, physiological parameters like photosynthesis, transpiration, water use efficiency, etc. have also been compared with mother plants in D. hamiltonii (Agnihotri and Nandi 2009; Agnihotri et al. 2009). The rate of photosynthesis increased from 3.55 CO2 l mol m−2 s−1 (hardened plants, ready for field transfer) to 5.44 l mol CO2 m−2 s−1 (6 months of field transfer); after a year of plantation, the rate of net photosynthesis was 14.0 l mol CO2 m−2 s−1, while after 1.5 years it was 12.76 CO2 l mol m−2 s−1. These values are comparable to those observed for the mother bush. Transpiration rate also increased simultaneously with the age of the plant. Water used efficiency also showed a similar pattern like net photosynthesis. A similar trend was also observed for Ci/Ca ratio for 18-month-old field transferred plants and the mother bush with values of 0.497 and 0.617, respectively. Similarly, Bag et al. (2012) compared 18 months old filed transferred in vitro propagated plants of the two age groups, and the corresponding mother plants (MPs) of D. hamiltonii in respect of gas and water vapour exchange rates, related parameters, morphological features and leaf anatomy. The rate of photosynthesis was significantly influenced by the age of the MPs and was found to be higher in the tissue culture (TC)-raised plants; plants (both TC-raised and MPs) of the younger age group performed better than the corresponding plants of the older age group. The same trend was found when the water-use efficiency was taken into consideration. Many groups have tested the genetic fidelity of the tissue culture raised plants using molecular markers also. The same will be discussed in the section on achievements made using molecular approaches.
Somatic embryogenesis
Micropropagation via somatic embryogenesis offers another easy and reliable method for mass propagation as both the root and the shoot primordia are produced in a single step. It can be used for large scale propagation of bamboo at minimum cost in a relatively shorter time and with lowest labor inputs. Encapsulation of somatic embryos in alginate beads to produce synthetic seeds holds great promise for establishment of bamboo plantations. Intensive research on tissue culture of bamboos related to somatic embryogenesis was initiated by Mehta et al. (1982) with the production of plantlets of Bambusa arundinacea. After that, somatic embryogenesis and plantlet regeneration has been reported in several bamboo species (Table 3). Recently, Bag et al. (2012) reported maximum embryogenesis (93.3 % and 90.0 %) with highest number of somatic embryos (38.7 and 37.3 per callus lump) and regenerated plantlets (11.9 and 11.3) per callus lump from 10- and 45-year-old D. hamiltonii bushes respectively, on MS medium supplemented with 5.0 μM BAP and 7.5 μM 2,4-D.
In general, embryogenic tissue is initiated on a medium containing low concentration of auxins, usually in the form of 2,4-D and NAA, and cytokinins (BA and TDZ). Mostly, MS medium has been used for embryogenesis in bamboos, however, in few studies other media such as B5 and N6 have also been used for somatic embryogenesis. The generation of morphologically developed somatic embryos does not guarantee satisfactory post-embryonic performance. Embryo development in bamboos is initiated by arresting cell proliferation through the removal of auxins and cytokinins and putting them on PGR free medium (Godbole et al. 2002). Although, Rout and Das (1994) reported development, maturation as well as germination of somatic embryos on (MS) basal medium supplemented with Kn, 2, 4-D and AdS. In general, maturation of somatic embryos is achieved on agar solidified media, however, as demonstrated by Hassan and Debergh (1987) somatic embryos can also be obtained in liquid medium. Mature somatic embryos germinate and convert to plantlets in a growth regulator free medium (Godbole et al. 2002). Although in some studies, cytokinin has been found to be an essential component in germination of bamboo somatic embryos. Kn was used to promote the germination of B. oldhamii, B. beecheyana and Sinocalamus latiflora somatic embryos (Yeh and Chang 1986a, b, 1987), while Lin et al. (2004) used TDZ for somatic embryo germination in B. edulis.
Flowering of bamboos in vitro
The most unique feature of bamboos is their monocarpic flowering behaviour (John and Nadgauda 1999). Most bamboos flower (and seed) gregariously at the end of long vegetative growth phases, ranging between 3 and 120 years or more (Janzen 1976; John and Nadgauda 1999) and usually die after flowering. This characteristic makes the study of bamboo flowering quiet difficult. It has been almost impossible to breed woody bamboos for superior traits due to these peculiar flowering habits. The first reports on flowering of bamboos in tissue culture (Nadgauda et al. 1990; Rao et al. 1990) created great excitement among the plant biologists. Since then tissue culture systems have been significantly used to reduce the juvenility stage of bamboo, and many studies have shown that bamboos flowered in vitro in just a few months (Table 4). It opened up the possibility of controlled flowering that can be used for breeding of bamboos. There is no synchrony in the timings of anthesis under in vitro conditions, whereas in nature the timing of anthesis is influenced by environmental conditions and usually takes place in the morning hours. Once in vitro methods are standardized for obtaining flowering comparable to that observed in nature, this technology can be used for attempting hybridization between bamboo species (Nadgauda et al. 1993).
Table 4.
In vitro flowering studies in bamboos
| Species | Medium + PGRs | References |
|---|---|---|
| Bambusa sp., Cephalostachyum pergacil, Dendeocalamus membranaceus | – | Prutpongse and Gavintertvatana 1992 |
| Bamboo sp. | B5 + BAP + 2,4-D | Rao and Rao 1990 |
| B. arundinacea | MS + BAP + NAA | Ansari et al. 1996 |
| MS + BAP + CW | Nadgauda et al. 1997 | |
| MS + BAP | Joshi and Nadgauda 1997 | |
| B. arundinacea, D. strictus, D. brandisii | MS + BAP + CW | Nadgauda et al. 1990 |
| B. arundinacea, D. strictus, D. brandisii | MS + BAP | John and Nadgauda 1999 |
| B. edulis | MS + TDZ + 2,4-D | Lin and Chang 1998 |
| MS + TDZ | Lin et al. 2003, 2004 | |
| MS + 2,4-D + IBA + NAA | Lin et al. 2005 | |
| B. oldhamii | MS + 2,4-D + Kn | Ho and Chang 1998 |
| B. vulgaris, D. strictus, D. giganteus | MS + IBA + AdS + GA3 | Rout and Das 1994 |
| D. asper | MS + BAP | Satsangi et al. 2001 |
| D. giganteus | MS + BAP | Ramanayake et al. 2001 |
| D. hamiltonii | MS + BAP | Chambers et al. 1991 |
| D. latiflorus | MS + 2,4-D | Lin et al. 2007b |
| D. strictus | ½ MS + TDZ | Singh et al. 2000 |
AdS adenine sulphate, BAP 6-benzylaminopurine, CW coconut water (milk), 2, 4-D 2, 4 Dichlorophenoxy acetic acid, GA3 gibberellic acid, IBA indole-3-butyric acid, Kn kinetin, NAA α-Napthaleneacetic acid, PGR plant growth regulator, TDZ Thidiazuron
In vitro flowering is an important phase in growth, development and physiological science. It is a difficult phenomenon sensitive to the environment. The conversion from vegetative to reproductive phase in vitro is thought to be regulated by external and internal factors, which include plant growth regulators, auxin-cytokinin equilibrium and genotypic variation, nutrients, pH of the medium and light conditions (Heylen and Vendrig 1988) which interact in complex and erratic ways (Van Tran Thanh 1973; Teixeira da Silva and Nhut 2003). More than 10 years after the first report on in vitro flowering in bamboo some significant results have been obtained, but practical and commercially exploitable results have not been reported yet.
Investigations have revealed that cytokinin is a key factor for in vitro flowering of bamboos (Nadgauda et al. 1990; Chambers et al. 1991; Rout and Das 1994; Lin and Chang 1998). Cytokinins are constituents of floral stimulus transported from phloem sap to the apical part stimulating in vitro flowering (Bernier et al. 1993). These are required to keep up the cell division cycle but might also be involved in promoting the transition from undifferentiated stem cells to differentiation (Werner et al. 2001). Lin et al. (2003) reported that multiple shoots grown from spikelet-derived somatic embryos of Bambusa edulis flowered on MS medium containing TDZ, while NAA was a negative regulator for cytokinin-dependent in vitro flowering. TDZ was also found effective in the induction of flowering in the cultures of D. latiflorus (Lin et al. 2004) and D. strictus (Singh et al. 2000). The cultures behave like natural plants during in vitro flowering as the rate of shoot proliferation gradually increases to over three-fold before flower induction. However, in vitro flowering was not the expression of a species specific mechanism believed to occur during gregarious flowering, as the mother clump did not flower (Ramanayake et al. 2001). In most of the studies, flowering was induced in medium supplemented with BA. Rout and Das (1994) used adenine hemisulphate, IBA and gibberellic acid for flower induction in B. vulgaris, D. giganteus and D. strictus. Many other cytokinins such as AdS, 2iP, Kn and zeatin tested were ineffective and presence of BA in the culture medium was absolutely essential for induction of flowering. Although, 2iP showed synergistic effect in combination with BA, zeatin showed antagonistic effect on induction of flowering (Joshi and Nadgauda 1997). However, Prutpongse and Gavintertvatana (1992) reported that in 8 species of bamboo, flowering was not affected by the culture conditions like light, medium, temperature, etc.
The long and unpredictable flowering cycles, and gregarious flowering is speculated to be genetically programmed. It is now known that genes control flowering in plants and that the expression of these genes is due to endogenous or exogenous signals. Some of these genes control the transition of the meristem from a vegetative to a reproductive state, while others control when to flower (Hempel et al. 1997). However, much needs to be done to understand the precise mechanisms controlling these unique flowering characteristics in bamboos.
Transformation and transgenics
Introduction of foreign genes into plant cells can be achieved by a variety of methods including particle bombardment, electroporation, silicon carbide, polyethylene glycol and Agrobacterium. Table 5 summarizes the reports pertaining to cloning and transgenic development in bamboos. Luciferase genes have been cloned from fireflies (Photinus pyralis) and successfully transferred into D. giganteus using Agrobacterium tumefaciens binary vector (Wiersma 2008). Optimization of the cell culture conditions of target plant cells/tissues is one of the most important factors for transformation studies. Transient expression of the GUS gene in the log phased suspension cells of Phyllostachys nigra indicated that cells having active growth efficiency were ideal targets for transformation using the particle bombardment method (Ogita et al. 2011). In order to construct transgenic bamboo cells with high efficiency, two problems needs to be resolved—establishment of an efficient suspension cell culture system and an improvement of the transformation procedure.
Table 5.
Cloning, transformation and transgenic studies in bamboos
| Tools used | Species | Achievements | References |
|---|---|---|---|
| Agrobacterium | Dendrocalamus giganteus | Luciferase genes cloned from fireflies (Photinus pyralis) were successfully transferred into D. giganteus using A. tumefaciens binary vector | Wiersma 2008 |
| Cloning | Bambusa oldhamii | Cloning and characterization of catechol-O-methyltransferase (COMT) gene | Li et al. 2007 |
| D. latiflorus | A cDNA named DlMADS8 was isolated from the young spikelets of D. latiflorus by rapid amplification of cDNA end (RACE) and transformed into Arabidopsis thaliana. Transgenic plants of DlMADS8 exhibited the phenotypes of curled leaves and early flowering. After bolting, three novel phenotypes related to inflorescence development were observed in different transgenic plants. No obvious homeotic conversions of floral organs were observed in all of the 35S::DlMADS8 transgenic Arabidopsis plants. These results indicated that DlMADS8 probably plays a role in floral meristem determinacy and is involved in controlling the flowering time of D. latiflorus. | Tian et al. 2006 | |
| Neosinocalamus affinis | Cloned 4-coumarate-coenzyme A ligase gene (EU327341) | Hu et al. 2009 | |
| Particle bombardment | Phyllostachys nigra | Generation of stable transgenic bamboo cells using constructs expressing hygromycin- phosphotransferase gene and enhanced fluorescent protein genes namely AcGFP1 and mCherry | Ogita et al. 2011 |
Achievements made using molecular approaches
Polymorphism and phylogenetic relationships
Vegetative and floral characteristics have been used for taxonomic studies and to assess diversity within and between populations in majority of plant species since ancient times. Morphological features are readily available visually and additionally do not require sophisticated equipments for data documentation. However, morphological determinations need to be taken by an expert taxonomist as they are subject to changes due to environmental factors and may vary at different developmental stages (Kalia et al. 2011a). Therefore, taxonomic studies mainly depend on the inflorescence and floral morphology however, flowering has been a major bottleneck in these studies and the basic knowledge of biology and genetics of bamboos is severely lacking (Janzen 1976).
The limitations of morphological markers were complimented with the markers developed at both protein and DNA level. Leaf isozymes were used by Heng et al. (1996) to detect polymorphism among five genera of bamboos. However, the number of isozyme loci that can be scored is limited. To date, only 40–50 reagent systems have been developed that permit the staining of a particular protein. A second drawback of biochemical markers is tissue variability. Therefore, several samplings of the segregating population are necessary to score all the available isozymes. Moreover, protein markers are also influenced by the environmental and developmental changes. Due to these reasons isozymes are not preferred markers for diversity analysis.
Under such circumstances, only molecular approaches are the useful techniques for characterizing the genetic diversity among different cultivars or species, for identifying genes of commercial interest and improvement through genetic transformation technology, and for taxonomic delineation of bamboos especially at lower levels of species and subspecies. Molecular markers are powerful enough to discriminate closely related varieties also. At the nuclear level, several markers viz. RFLP, RAPD, AFLP, SCARs, ISSR, SSRs, EST-SSRs and MITE-TD have been widely applied in genetic variation, systematic classification, and phylogenetic relationships among bamboos, with more or less success. Table 6 summarizes the applications of morphological, biochemical and molecular markers to study polymorphism and phylogenetic relationships in bamboos.
Table 6.
Application of morphological, biochemical and molecular markers to study polymorphism and phylogenetic relationships in bamboos
| Tools used | Species | Achievements | References |
|---|---|---|---|
| Morphological and biochemical markers | |||
| Morphological descriptors | Bamboo sp. | Evaluated phylogenetic relationships among 15 bamboo species using 32 key morphological descriptors | Das et al. 2007 |
| Isozymes | Bamboo sp. | Detected polymorphism in five bamboo species using leaf isozymes | Heng et al. 1996 |
| Molecular markers | |||
| RFLP | Phyllostachys sp. | Detected 380 polymorphic bands using 43 probe enzyme combinations in 12 species | Friar and Kochert 1991, 1994 |
| RAPD | Bambusa, Dendrocalamus, Sasa, Dinocloa, Cephalostachyum | Identified genetic relationship between 12 bamboo species belonging to 5 genera | Nayak et al. 2003 |
| Bambusa sp. | Investigated relationships between samples of Bambusa species from South Eastern China that have been placed in Bambusa or in several segregate genera, Dendrocalamopsis, Leleba, Lingnania, Neosinocalamus and Sinocalamus, by different authors | Sun et al. 2006 | |
| Bamboo sp. | Evaluated phylogenetic relationships among 15 bamboo species | Das et al. 2007 | |
| Dendrocalamus, Bambusa, Gigantochloa, Arundinaria sp. | Reported that genetic distances between genera Bambusa and Gigantochloa are smaller while Dendrocalamus and Arundinaria has greater and greatest distances from other species, respectively | Ramanayake et al. 2007 | |
| Phyllostachys sp. | Assessed phylogenetic relationships among 73 genotypes | Gielis et al. 1997 | |
| SCAR | B. balcooa, B. tulda | Generated species-specific SCAR fragments named ‘Balco836’ for B. balcooa and ‘Tuldo609’ for B. tulda | Das et al. 2005 |
| AFLP | Bambusa, Dendrocalamus, Gigantochloa, Thyrsostachys | Examined 15 species from 4 genera and found that 6 species of Bambusa separated into 2 clusters while 6 species of Gigantochloa formed a discrete cluster. Thyrsostachys was less similar to Bambusa while two Dendrocalamus species were very different and required further study | Loh et al. 2000 |
| Bambusa sp., Dendrocalamus sp. | Studied phylogenetic relationship and genetic variability among 12 edible bamboo species (Bambusa and Dendrocalamus genus) of North-Eastern India using six primer pair combinations. | Ghosh et al. 2011 | |
| Bamboo sp. | Analyzed phylogeny of world bamboos by AFLP of chloroplast DNA | Kobayashi 1997 | |
| Guadua angustifolia | Conducted AFLP analysis of Guadua germplasm in Colombia with emphasis on the coffee region. | Marulanda et al. 2002 | |
| Phyllostachys pubescens | Could clearly identify ten cultivars of P. pubescens that had high similarity and divided them into three groups based on genetic variation and similarity. | Lin et al. 2009 | |
| P. pubescens | Analysis of clonal structure and flowering traits of bamboo species | Isagi et al. 2004 | |
| Phyllostachys sp. | Phylogenetic studies in genus Phyllostachys | Hodkinson et al. 2000 | |
| Sasa senanensis | Studied clonal structure of a dense population of this dwarf bamboo in a 10-ha study plot at Sugadaira Montane Research Center, University of Tsukuba, Nagano, Japan | Suyama et al. 2000 | |
| SSR | Bamboo sp. | Evaluated the transferability of 98 SSR markers of rice and 20 EST-SSR markers of sugarcane for phylogenetic and genetic diversity analysis in 23 bamboo species. | Sharma et al. 2008 |
| Bamboo sp. | 120 rice SSR markers were assessed for their transferability to 21 different bamboo species. The transferability was 68.3 %. SSR markers located on rice chromosome 7 and 1 showed the highest and lowest transferability, respectively to the bamboo genome. | Chen et al. 2010 | |
| Bambusa arundinacea | Characterized 6 microsatellites, three polymorphic and three monomorphic, in B. arundinacea and tested cross species amplification in 18 other bamboo species. | Nayak and Rout 2005 | |
| Guadua sp. | Demonstrated the usefulness of rice and sugarcane microsatellite sequences to establish the relationships between genotypes, varieties and cultivars of Guadua | Marulanda et al. 2007 | |
| Phyllostachys sp. | Analyzed 1,532 P. pubescens SSR sequences available in public domain DNA databases, and found 3,241 SSR loci comprising repeats of two or more nucleotides in 920 genomic survey sequences (GSSs) and 68 cDNA sequences. SSR PBM014 transferred successfully to six other Phyllostachys species and showed rich polymorphism, therefore could serve as species-specific marker for Phyllostachys interspecies hybrid identification. | Tang et al. 2010 | |
| Phyllostachys pubescens | Studied 176 samples of Phyllostachys in Taiwan and found limited genetic variation. The region around Nantou County consisted of all of the nine identified clones while the remaining regions generally consisted of only one common clone which indicated that center of variation is in Nantou County. | Lai and Hsiao 1997 | |
| EST-SSR | Arundinaria, Bambusa, Brachystachyum, Hibanobambusa, Indocalamus, Phyllostachys, Pseudosasa, Sasa, Semiarundinaria, Shibataea, Sinobambusa | Used EST-SSR markers derived from major cereal crops to assess the genetic diversity and phylogenetic relationships of a temperate bamboo collection of USDA consisting of 92 accessions, 11 genera and 44 species | Barkley et al. 2005 |
| Bambusa edulis, B. oldhamii | Analyzed 3406 publically available ESTs from caespitose bamboo species (B. edulis and B. oldhamii) and found 245 non-redundant SSRs in 205 EST contigs that were used to develop 15 EST-SSR markers. The transferability of markers was 59.6 % among 14 additional caespitose bamboo species. The successfully transferred markers showed 51.4 % polymorphism. | Dong et al. 2011 | |
| B. oldhamii | Selected 10 EST-SSR markers from B. oldhamii public sequence data base and observed their transferability to 25 species of Bambusoideae. Transferability ranged from 30 to 100 %. | Sharma et al. 2009 | |
| Phyllostachys edulis | Development of EST-SSR markers | Zhi-jun et al. 2011 | |
| P. rubromarginata, P. flexuosa, P. glauca | Detection of contamination in a bamboo plot where P. rubromarginata stands were mixed with either P. flexuosa or P. glauca. | Yu et al. 2004 | |
| ISSR | Bamboo sp. | Evaluated genetic relationships among 22 taxa of bamboo using 12 ISSR and four EST-based random primers, resulting in amplification of 220 loci. | Mukherjee et al. 2010 |
| Phyllostachys pubescens | Reported that ten cultivars of P. pubescens having high similarity could be divided into three groups | Lin et al. 2009 | |
| P. violascens | Assessed genetic diversity within different cultivars of P. violascens using 15 ISSR primers and a total of 209 (136 polymorphic) bands were detected. Based on genetic diversity, all the cultivars of P. violascens could be divided into four groups, which are reflected by their morphologies. | Lin et al. 2011 | |
| MITEs | B. multiplex | Presence of Ac-like sequences was found | Huttley et al. 1995 |
| B. vulgaris, Sasa veitchii, Phyllostachys edulis | Isolated partial Ac-like transposon elements | Gielis 1998 | |
| B. vulgaris | Obtained sequence from B. vulgaris that revealed considerable homology to the HAT superfamily of transposons | Keukeleire et al. 2004 | |
| P. pubescens | Observed that 23.28 % of P. pubescens genome is enriched with repeat elements and majority of them (18.89 %) were LTR retrotransposons, mainly Gypsy/DIRS1 and Ty1/Copia type | Jie et al. 2007 | |
| Bamboo sp. | Isolated 79 full-length MLE (Mariner-like elements) transposase genes from 63 bamboo species representing 38 genera in six subtribes mainly found in China. The transposases were highly conserved, mostly uniform in length and contained intact DNA-binding motifs and DD39D catalytic domains with few notable frameshift, indel and nonsense mutations. | Zhou et al. 2011 | |
| Cp DNA | Asian bamboos | Examined restriction site mutations of cpDNA for 16 Asian bamboo genera | Watanable et al. 1994 |
| Bamboo sp. | Utilized rpl 16 intron data to study relationships between 23 species of Chusquea and 15 taxa from Bambusoideae | Kelchner and Clark 1997 | |
| Bamboo sp. | High-throughput sequencing of six bamboo chloroplast genomes | Zhang et al. 2011a | |
| Bamboo sp. | Studied polymorphism, similarities and relationships among 22 bamboo species using RAPD of chloroplast DNA (RACPD) | Zhang et al. 2011b | |
| ITS Sequences | Arundinaria sp. | Analyzed phylogenetic relationships of Arundinaria and related genera (Pleioblastus, Bashania, Pseudosasa, Oligostachyum, Clavinodum, etc.) using nrDNA ITS sequences and the cpDNAtrnL-F intergenic spacer (IGS) | Qiang et al. 2005 |
| Phyllostachys sp. | Made a comparison of nrDNA ITS sequences for phylogenetic studies in genus Phyllostachys to review the previous infra-generic classifications | Hodkinson et al. 2000 | |
| RT-PCR, cDNA library | B. oldhamii | Four cDNA clones, BoSus1, BoSus2, BoSus3 and BoSus4, were isolated by screening a cDNA library from etiolated bamboo shoots and suggested that, sucrose synthase (SuS) is encoded by at least four genes in bamboo, each with a specific role in providing substrates for the polysaccharide biosynthesis and/or energy production | Chiu et al. 2006 |
| SSH and Microarray analysis | B. edulis | Identified differentially expressed genes in an albino mutant. These genes were not related to photosynthesis | Lin et al. 2006 |
| RT-PCR and microarray analysis | Phyllostachys praecox | Identified several genes related to development of bamboo rhizome bud and cloned six genes, the expression patterns of these genes revealed significant differences in rhizome shoots, rhizome buds, bamboo shoots, leaves, and young florets | Wang et al. 2010 |
AFLP amplified fragment length polymorphism, Cp DNA chloroplast DNA, EST expressed sequence tag, ISSR inter simple sequence repeat, ITS internal transcribed spacer, MITEs miniature inverted-repeat transposable elements, RAPD randomly amplified polymorphic DNA, RFLP restriction fragment length polymorphism, RT-PCR real time polymerase chain reaction, SCAR sequence characterized amplified regions, SSH suppression subtractive hybridization, SSR simple sequence repeat (microsatellite)
Genetic fidelity testing
In vitro propagation has emerged as a powerful technique for large scale propagation of bamboos. However, culture conditions, explant source, ploidy level and in vitro culture age are known to induce somaclonal variation in vitro. These somaclonal variations may appear due to cell cycle disturbances caused by exogenously applied growth regulators, increased mutation rate per cell-generation over time, and accumulation of mutations over a period of time, alteration in DNA methylation patterns, DNA damage and mutation, and alteration of cell’s ability to repair damaged and mutated DNA (see Singh et al. 2012b). It is therefore extremely important to ascertain the clonal uniformity of the in vitro raised plants. Although, morphological, biochemical, physiological and anatomical parameters such as leaf shape, thickness, leaf mass, chlorophyll and relative water content, photosynthetic parameters and leaf anatomy etc. have been used for the purpose in D. asper and D. hamiltonii (Agnihotri et al. 2009; Singh et al. 2011; Bag et al. 2012), use of more reliable DNA based markers such as RAPD, ISSR, SSR, AFLP, etc., has also been done to test the genetic fidelity of in vitro raised bamboo plants. Amplification of monomorphic bands in tissue culture raised plants and mother bushes confirmed that the former were genetically uniform and true to type to the mother in these studies (Table 7). Use of more than one marker system has been suggested by various workers so as to target a wider region of the genome. Singh et al. (2012b) used a set of four markers namely RAPD, ISSR, SSR and AFLP to access the genetic fidelity of D. asper plants raised through axillary shoot proliferation. Effect of length of culture age on genetic stability was studied in B. balcooa (33 subculture cycles, Negi and Saxena 2010), B. nutans (27 passages, Negi and Saxena 2011) and D. asper (30 subculture passages, Singh et al. 2012b), however, it was not found to affect the genetic stability of the plants raised through enhanced axillary branching.
Table 7.
Genetic fidelity testing of in vitro raised bamboos
| Tools used | Species | Achievements | References |
|---|---|---|---|
| Morphological, biochemical, physiological and anatomical markers | |||
| Morphological descriptors | Dendrocalamus asper | Compared in vitro-raised plants with mother plants and found no variation. | Singh et al. 2011 |
| D. hamiltonii | Leaf thickness and specific leaf mass of the in vitro raised plants were found comparable to the mother plants. | Bag et al. 2012 | |
| Biochemical analysis | D. hamiltonii | Chlorophyll and relative water content of the in vitro raised plants were found comparable to the mother plants. | Bag et al. 2012 |
| Physiological studies | D. hamiltonii | The rate of net photosynthesis and water use efficiency were found comparable to those observed for the mother bush. | Agnihotri et al. 2009 |
| The rates of photosynthesis and water use efficiency of in vitro propagated and hardened plants were found to be comparable with the corresponding mother plants. | Bag et al. 2012 | ||
| Anatomical studies | D. hamiltonii | Leaf anatomy of the in vitro propagated and hardened plants was found to be similar with the corresponding mother plants. | Bag et al. 2012 |
| Molecular markers | |||
| RAPD | Bambusa balcooa, B. tulda | Confirmed clonal fidelity of in vitro raised plants and advocated the use of axillary meristem culture for true-to-type or clonal propagation. | Das and Pal 2005b |
| D. asper | Tested clonal fidelity of in vitro raised shoots uo to 30th passage, hardened plants growing in the ployhouse and randomly selected field transferred plants up to 2 years with mother plant and found no somaclonal variation. | Singh et al. 2012b | |
| D. hamiltonii | Reported genetic fidelity during various stages of growth and development of in vitro raised plants, up to 1.5 years after field plantation and found no somaclonal variation. | Agnihotri et al. 2009 | |
| Guadua angustifolia | Evaluated clonal fidelity of in vitro raised plants at various stages of subculture along with hardened plants and compared with mother plant. | Nadha et al. 2011 | |
| ISSR | B. balcooa | Clonal fidelity testing of in vitro raised plants up to 33rd passage and in vitro raised plants transferred to the field compared with parent plant and found true to type. | Negi and Saxena 2010 |
| B. nutans | In vitro raised shoots up to 27th cycle of shoot multiplication, hardened plants growing in the ployhouse, plants growing in the field and mother plant were found genetically similar. | Negi and Saxena 2011 | |
| D. asper | Tested clonal fidelity of in vitro raised shoots uo to 30th passage, hardened plants growing in the ployhouse and randomly selected field transferred plants up to 2 years with mother plant and found no somaclonal variation. | Singh et al. 2012b | |
| G. angustifolia | Evaluated clonal fidelity of in vitro raised plants at various stages of subculture along with hardened plants and compared with mother plant. | Nadha et al. 2011 | |
| SSR | D. asper | Tested clonal fidelity of in vitro raised shoots uo to 30th passage, hardened plants growing in the ployhouse and randomly selected field transferred plants up to 2 years with mother plant and found no somaclonal variation. | Singh et al. 2012b |
| AFLP | B. balcooa | Compared the tissue culture raised plants originating through axillary bud proliferation and somatic embryogenesis and reported that no epigenetic changes could be detected by methylation sensitive AFLP (MSAP) | Gillis et al. 2007 |
| B. nutans | Assessment of genetic fidelity of tissue culture raised plants at various stages from plant regeneration to field establishment. Reported 98.8 % genetic stability in regenerated plantlets. | Mehta et al. 2011 | |
| D. asper | Tested clonal fidelity of in vitro raised shoots uo to 30th passage, hardened plants growing in the ployhouse and randomly selected field transferred plants up to 2 years with mother plant and found no somaclonal variation. | Singh et al. 2012b | |
Future prospects of bamboo biotechnology
Bamboo is becoming an increasingly important economic asset in poverty eradication, and economic and environmental development. About 2.5 billion people in the world depend economically on bamboo and the international trade in bamboo amounts to $5–10 billion. With the growth in the demand for environment friendly green products, the world bamboo market is expected to double by 2015, from USD 10 billion to USD 20 billion (Xuhe 2003). Traditionally used as low-cost construction material in developing countries, bamboo is being processed into increasingly sophisticated products that serve consumers in developed countries and high end markets. Nowadays, with new technologies for processing, most products made from wood can be made from bamboos, resulting in the potential for a multi-billion dollar market. In such conditions, it is essential to adopt the techniques like tissue culture for mass multiplication of bamboo to fill the gap of demand and supply. This must be followed by genetic fidelity testing of tissue culture raised plants to ensure their true to type nature. In a similar endeavour, the Department of Biotechnology (DBT), Government of India established a Bamboo Mission, a network project on bamboo resources under which more than 800 ha of land was planted with tissue culture raised plants of eight bamboo species (DBT 2009–10) by more than 10 Institutions located in various parts of India.
Development of genetically engineered plants capable to counter biotic and abiotic stresses is imperative. However, an efficient regeneration protocol must be in place before genetic transformation studies can be initiated. Micropropagation, using mature as well as juvenile explants, through organogenesis and somatic embryogenesis has been attempted in many bamboo species. However, the problems of endogenous contamination, browning of explants or shoots during multiplication, instability of multiplication rate, somatic mutations and somaclones, albinism of plants, low rooting percentage and limited survival of plants during hardening and field transfer needs further attention. Vascular arbuscular mycorrhizal (VAM) fungal isolates may be incorporated in the hardening media to strengthen the growth and mycorrhization of micropropagated bamboo plantlets. This will improve the rhizosphere as well as the field survival rate of tissue culture raised plants.
Commercial scale application of micropropagation technology is still limited in bamboos. To date, somatic embryogenesis has been achieved in only 26 species (~1.5 % of the total species) belonging to six genera, i.e. Bambusa, Dendrocalamus, Phyllostachys, Sasa, Sinocalamus and Otatea. Therefore, additional efforts are required for standardizing the micropropagation techniques for majority of important bamboo species so that they can be used for improvement and clonal forestry programme using bamboos as well as for ex situ conservation and cryopreservation of rare species or populations. Research on haploid culture, callus culture against stress conditions, development of tolerant cell lines, etc. should also be started as a part of the long-term genetic improvement program. The most promising approach for improving the ornamental use of bamboo germplasm involves in vitro manipulations to exploit the somaclonal variations.
The in vitro regeneration techniques need to be improved further so that they can be used for genetic improvement of bamboo through transgenics. Successful introduction of foreign genes into plant cells is primarily governed by two factors, optimization of culture condition of target plant cells/tissues and transformation procedure. In bamboos, only few reports are available regarding transformation and transgenic development. Further inputs are required for establishment of an efficient suspension cell culture system and development of transformation procedure with high efficiency in bamboos.
Monocarpic flowering habit has made it almost impossible to breed for superior traits in woody bamboos. In vitro flowering has opened up the possibility of controlled flowering that can be used for bamboo breeding. Negligible progress has been made in improving the taxon through conventional breeding programs but some traits which need further improvement include yield, growth in all types of soils, wider climatic adaptation, thornlessness, thick walls, disease and pest resistance, and improved palatability, among others. Scientists are trying to develop hybrid bamboo that will be the solution for energy, paper pulp and bamboo charcoal production. Tissue culture technology can also be used for rescue of hybrid seeds produced by conventional breeding methods. Understanding of the precise mechanisms controlling flowering in vitro can lead to new avenues for hybridization between bamboo species. Conventional breeding programs are time, cost and labour intensive therefore marker assisted selection (MAS) must be used for selecting beneficial genetic traits as well as for assessing the genetic potential of specific genotypes prior to phenotypic evaluation. Molecular markers linked with QTL/major genes for traits of interest must be developed. In addition, availability of a broad genetic base is must for initiating breeding programmes in any given crop. The available genetic base can be broadened using modern tools of biotechnology including in vitro selection, mutagenesis and transgenics (Kalia et al. 2011b).
In addition, conservation of agronomically important cultivars through in vitro methods and cryopreservation must be done to defeat the biological and environmental calamities which may threat the germplasm maintained in situ in field genebanks and germplasm gardens. Cryopreserved material (stored as seeds, ovules, embryos, callus, etc.) can be used successfully for breeding in the future.
Taxonomic delineation of species and subspecies is still controversial and needs to be addressed more rigorously. Efforts have been made to classify the genus based on morphological, biochemical and molecular markers but more inputs are required to confirm the phylogeny and taxonomy of the genus. Major challenge associated with any molecular method is to determine the appropriate taxonomic level at which it is most informative and to correlate it with morphologically definable taxonomic groupings. Considerable progress has been made in this area, but much more needs to be done yet. In contrast to the vast majority of studies done to date on bamboo taxonomy and systematics, investigations on genetic diversity at the population level are in its infancy. Therefore, studies are required to better understand the level of population diversity and clonal structure in bamboos.
One of the potentially emerging areas for bamboo biology is the comparative genomic studies, wherein available genomic information of other well characterized cereal crops could be extrapolated to initiate functional genomics in bamboos. The genomic resources have accumulated rapidly for almost all major lineages of grasses except bamboos, which can seriously hamper our ability to take a full advantage of the wealth of grass genomic data for effective comparative studies and for better understanding of gene and genome evolution that underlies phenotypic and ecological divergence of plants. By January 2009, the number of ESTs deposited in the GenBank ranged from 436,535 to 2,018,337 for rice, wheat, maize, barley, sorghum, sugarcane, and switchgrass, but only 3,087 for bamboos. This creates a major missing link in the grass family for comparative genomics. Considering the high economic importance of bamboo in rural economies and industrial applications, genetic and genomic analyses of bamboo need to be significantly advanced (Peng et al. 2010).
Undoubtedly, the relationship between bamboos and man has travelled a long journey and both remain inseparable due to the outstanding capacity of bamboos to improve the human environment and economy. Judicious utilization and conservation of bamboo resources can bring more benefits to mankind throughout the world.
Acknowledgements
Research grant from Department of Biotechnology, Ministry of Science and Technology, Govt. of India, New Delhi, under Bamboo Mission project No. BT/PR/5261/AGR/16/459/2004, is gratefully acknowledged.
Contributor Information
Rohtas Singh, Phone: +91-1662-227088, FAX: +91-1662-271369, Email: rohtas_bot@rediffmail.com.
Rajwant K. Kalia, Email: rajwantkalia@yahoo.com
References
- Agnihotri RK, Nandi SK (2009) In vitro shoot cut: a high frequency multiplication and rooting method in bamboo Dendrocalamus hamiltonii. Biotechnology 8:259–263
- Agnihotri RK, Mishra J, Nandi SK. Improved in vitro shoot multiplication and rooting of Dendrocalamus hamiltonii Nees et Arn. ex Munro: production of genetically uniform plants and field evaluation. Acta Physiol Plant. 2009;31:961–967. doi: 10.1007/s11738-009-0311-6. [DOI] [Google Scholar]
- Ajithkumar D, Seeni S. Rapid clonal multiplication through in vitro axillary shoots proliferation of Aegle marmelos (L.) Corr., a medicinal tree. Plant Cell Rep. 1998;17:422–426. doi: 10.1007/s002990050418. [DOI] [PubMed] [Google Scholar]
- Alexander MP, Rao TC. In vitro culture of bamboo embryo. Curr Sci. 1968;37:415. [Google Scholar]
- Ansari SA, Kumar S, Palaniswamy K. Peroxidase activity in relation to in vitro rhizogenesis and precocious flowering in Bambusa arundinacea. Curr Sci. 1996;71:358–359. [Google Scholar]
- Arshad SM, Kumar A, Bhatnagar SK. Micropropagation of Bambusa wamin through shoot proliferation of mature nodal explants. J Biol Res. 2005;3:59–66. [Google Scholar]
- Arya ID, Arya S. In vitro culture and establishment of exotic bamboo Dendrocalamus asper. Indian J Exp Biol. 1996;35:1252–1255. [Google Scholar]
- Arya S, Sharma S. Micropropagation technology of Bambusa bambos through shoot proliferation. Indian Forester. 1998;124:725–731. [Google Scholar]
- Arya S, Sharma S, Kaur R, Arya ID. Micropropagation of Dendrocalamus asper by shoot proliferation using seeds. Plant Cell Rep. 1999;18:879–882. doi: 10.1007/s002990050678. [DOI] [Google Scholar]
- Arya S, Satsangi R, Arya ID. Rapid mass multiplication of edible bamboo Dendrocalamus asper. J Sustain For. 2002;4:103–109. [Google Scholar]
- Arya ID, Rana PK, Satsangi R, Muzaffar FS, Sharma S, Arya S. Rapid and mass multiplication of bamboos through tissue culture techniques. In: Nandi SK, Palni LMS, Kumar GB, editors. Role of Plant Tissue Culture in Biodiversity Conservation and Economic Development. Nainital: Gyanodaya Prakashan; 2002. pp. 29–39. [Google Scholar]
- Arya S, Rana PK, Arya S. Tissue culture studies on Bambusa polymorpha. In: Trivedi PC, editor. Advances in Biotechnology. India: Agrobios Jodhpur; 2005. pp. 229–240. [Google Scholar]
- Arya S, Rana PK, Sharma R, Arya ID. Tissue culture technology for rapid multiplication of Dendrocalamus giganteus Munro. Indian Forester. 2006;3:345–357. [Google Scholar]
- Arya S, Satsangi R, Arya ID. Large scale production of edible bamboo Dendrocalamus asper through somatic embryogenesis. J Am Bamboo Soc. 2008;21:13–23. [Google Scholar]
- Bag N, Chandra S, Palni LMS, Nandi SK. Micropropagation of dev-ringal [Thamnocalamus spathiflorus (Trin.) Munro]—a temperate bamboo, and comparison between in vitro propagated plants and seedlings. Plant Sci. 2000;156:125–135. doi: 10.1016/S0168-9452(00)00212-0. [DOI] [PubMed] [Google Scholar]
- Bag N, Palni LMS, Chandra S, Nandi SK. Somatic embryogenesis in ‘Maggar’ bamboo (Dendrocalamus hamiltonii) and field performance of regenerated plants. Curr Sci. 2012;102:1279–1287. [Google Scholar]
- Banerjee M, Saikat G, Pramanik BR. A two step method for accelerated mass propagation of Dendrocalamus asper and their field evaluation. Physiol Mol Biol Plant. 2011;17:387–393. doi: 10.1007/s12298-011-0088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banik RL, Alam MK. A note on the flowering of Bambusa balcooa Roxb. Bano Biggyan Patrika. 1987;16:25–29. [Google Scholar]
- Barkley NA, Newman ML, Wang ML, Hotchkiss MW, Pederson GA. Assessment of the genetic diversity and phylogenetic relationships of a temperate bamboo collection by using transferred EST-SSR markers. Genome. 2005;48:731–737. doi: 10.1139/g05-022. [DOI] [PubMed] [Google Scholar]
- Bernier G, Havelange A, Hussa C, Petitjean A, Lejeune P. Physiological signals that induce flowering. Plant Cell. 1993;5:1147–1155. doi: 10.1105/tpc.5.10.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht P, Pant M, Kant A. In vitro propagation of Gigantochloa atroviolaceae Widjaja through nodal explants. J Am Sci. 2010;6:1019–1026. [Google Scholar]
- Butenko RG, Lipsky AK, Chernyak ND, Arya HC. Changes in the culture medium pH by cell suspension cultures of Dioscorea deltoidea. Plant Sci Lett. 1984;35:207–212. doi: 10.1016/0304-4211(84)90230-X. [DOI] [Google Scholar]
- Bystriakova N, Kapos V, Lysenko I, Stapleton C. Distribution and conservation status of forest bamboo biodiversity in the Asia Pacific region. Biodivers Conserv. 2003;12:1833–1841. doi: 10.1023/A:1024139813651. [DOI] [Google Scholar]
- Bystriakova N, Kapos V, Lysenko I (2004) Bamboo biodiversity. Africa, Madagascar and the Americas. UNEP-WCMC/INBAR
- Chambers SM, Heuch JHR, Pirrie A. Micropropagation and in vitro flowering of the bamboo Dendrocalamus hamiltonii Munro. Plant Cell Tissue Organ Cult. 1991;27:45–48. doi: 10.1007/BF00048205. [DOI] [Google Scholar]
- Chang WC, Ho CW. Micropropagation of bamboo. In: Bajaj YPS, editor. Biotechnology in agriculture and forestry. Berlin Heidelberg New York: Springer; 1997. pp. 203–219. [Google Scholar]
- Chang WC, Lan TH. Somatic embryogenesis and plant regeneration from roots of bamboo (Bambusa beecheyana Munro Var beecheyana) J Plant Physiol. 1995;145:535–538. doi: 10.1016/S0176-1617(11)81784-0. [DOI] [Google Scholar]
- Chaturvedi HC, Sharma M, Sharma AK. In vitro regeneration of Dendrocalamus strictus Nees through nodal segments taken from field grown culms. Plant Sci. 1993;91:97–101. doi: 10.1016/0168-9452(93)90192-3. [DOI] [Google Scholar]
- Cheah KT, Chaille LC. Biotechnology. Honolulu: College of Tropical Agriculture and Human Resources (CTAHR); 2011. Somatic embryogenesis from mature Bambusa ventricosa; pp. 1–5. [Google Scholar]
- Chen SY, Lin YT, Lin CW, Chen WY, Yang CH, Ku HM. Transferability of rice SSR markers to bamboo. Euphytica. 2010;175:23–33. doi: 10.1007/s10681-010-0159-2. [DOI] [Google Scholar]
- Chiu WB, Lin CH, Chang CJ, Hsieh MH, Wang AY. Molecular characterization and expression of four cDNAs encoding sucrose synthase from green bamboo Bambusa oldhamii. New Phytol. 2006;170:53–63. doi: 10.1111/j.1469-8137.2005.01638.x. [DOI] [PubMed] [Google Scholar]
- Das M, Pal A. In vitro regeneration of Bambusa balcooa Roxb.: factors affecting changes of morphogenetic competence in the axillary buds. Plant Cell Tissue Organ Cult. 2005;81:109–112. doi: 10.1007/s11240-004-3017-x. [DOI] [Google Scholar]
- Das M, Pal A. Clonal propagation and production of genetically uniform regenerants from axillary meristem of adult bamboo. J Plant Biochem Biotechnol. 2005;14:185–188. [Google Scholar]
- Das P, Rout GR. Mass multiplication and flowering of bamboo in vitro. Orissa J Hort. 1991;19:118–121. [Google Scholar]
- Das P, Rout GR. Analysis of current methods and approaches on the micropropagation of bamboo. Proc Natl Acad Sci (India) LXIV B Biol Sci. 1994;64:235–246. [Google Scholar]
- Das M, Bhattacharya S, Pal A (2005) Generation and characterization of SCARs by cloning and sequencing of RAPD products: a strategy for species-specific marker development in Bamboo. Ann Bot 95:835–841 [DOI] [PMC free article] [PubMed]
- Das M, Bhattacharya S, Basak J, Pal A. Phylogenetic relationships among the bamboo species as revealed by morphological characters and polymorphism analyses. Biol Plant. 2007;51:667–672. doi: 10.1007/s10535-007-0140-7. [DOI] [Google Scholar]
- Dayan MP. Survey, identification and pathogenicity of pests and diseases of bamboos in the Philippines. Sylvatrop, The Philippine. Forest Res J. 1988;13:61–77. [Google Scholar]
- DBT (2009–10) DBT Annual Report 2009–10. Department of Biotechnology, Ministry of Science & Technology, Government of India, New Delhi, p 61. http://dbtindia.nic.in/annualreports/DBT-An-Re-2009-10.pdf
- Dekkers AJ, Rao AN (1989) Tissue culture of four bamboo genera. In: Rao AN, Yusoff AM (eds) Tissue Culture of Forest Species, FRIM/IDRC: 89–90
- Diab EEE, Mohamed SE. In vitro morphogenesis and plant regeneration of bamboos (Oxytenanthera abyssinica A. Rich. Munro) Int J Sustain Crop Prod. 2008;3:72–79. [Google Scholar]
- Dong WJ, Wu MD, Lin Y, Zhou MB, Tang DQ. Evaluation of 15 caespitose bamboo EST-SSR markers for cross-species/genera transferability and ability to identify interspecies hybrids. Plant Breed. 2011;130:596–600. doi: 10.1111/j.1439-0523.2011.01860.x. [DOI] [Google Scholar]
- Filgueiras TS, Goncalves APS. A checklist of the basal grasses and bamboos in Brazil. Bamboo Sci Cult. 2004;18:7–18. [Google Scholar]
- Friar E, Kochert G. Bamboo germplasm screening with nuclear restriction fragment length polymorphisms. Theor Appl Genet. 1991;82:697–703. doi: 10.1007/BF00227313. [DOI] [PubMed] [Google Scholar]
- Friar E, Kochert G. A study of genetic variation and evolution of Phyllostachys (bambusoideae: poaceae) using nuclear markers. Theor Appl Genet. 1994;89:265–270. doi: 10.1007/BF00225152. [DOI] [PubMed] [Google Scholar]
- Gamborg OL, Miller RA, Ojima L. Nutrient requirement of suspension culture of Soybean. Exp Cell Res. 1968;50:151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Devi SW, Mandi S, Talukdar NC. Amplified fragment length polymorphism based study of phylogenetic relationship and genetic variability among some edible bamboo species of North-East India. J Plant Mol Biol Biotechnol. 2011;2:8–15. [Google Scholar]
- Gielis J (1998) Upstream fundamental research in bamboo-possibilities and directions. Keynote lecture at Vth International Bamboo Congress, San Jose, Costa Rica. http://www.bamboonetwork.org/downloads/gielis03.pdf
- Gielis J, Everaert I, De Loose M. Genetic variability and relationships in Phyllostachys using random amplified polymorphic DNA. In: Chapman GP, editor. The bamboos. London: Academic; 1997. pp. 107–124. [Google Scholar]
- Gillis K, Gielis J, Peeters H, Dhooghe E, Oprins J. Somatic embryogenesis from mature Bambusa balcooa Roxb as basis for mass production of elite forestry bamboos. Plant Cell Tissue Organ Cult. 2007;91:115–123. doi: 10.1007/s11240-007-9236-1. [DOI] [Google Scholar]
- Godbole S, Sood A, Thakur R, Sharma M, Ahuja PS. Somatic embryogenesis and its conversion into plantlets in a multipurpose bamboo, Dendrocalamus hamiltonii Nees et Arn. ex Munro. Curr Sci. 2002;83:885–889. [Google Scholar]
- Hassan AAE, Debergh P. Embryogenesis and plantlet development in the bamboo Phyllostachys viridis (Young) McClure. Plant Cell Tissue Organ Cult. 1987;10:73–77. doi: 10.1007/BF00037499. [DOI] [Google Scholar]
- Hempel FD, Weigel D, Mandel MA, Ditta G, Zambryski PC, Feldman LJ, Yanofsky MF. Floral determination and expression of floral regulatory genes in Arabidopsis. Development. 1997;124:3845–3853. doi: 10.1242/dev.124.19.3845. [DOI] [PubMed] [Google Scholar]
- Heng HP, Yeho HH, Tan CKC, Rao AN. Leaf isozyme polymorphisms in bamboo species. J Singap Nat Acad Sci. 1996;22:10–14. [Google Scholar]
- Heylen C, Vendrig JC. The influence of different cytokinins and auxins on flower neoformation in thin cell layers of Nicotiana tabacum L. Plant Cell Physiol. 1988;29:665–671. [Google Scholar]
- Hirimburegama K, Gamage N. Propagation of Bambusa vulgaris (yellow bamboo) through nodal bud culture. J Hortic Sci. 1995;70:469–475. [Google Scholar]
- Ho CW, Chang WC. In vitro flowering of albino bamboo (Bambusa oldhamii Munro) regenerants derived from an eleven-year old embryogenic cell line. Acta Horticult. 1998;461:433–438. [Google Scholar]
- Hodkinson TR, Renvoize SA, Chonghaile GN, Stapleton CMA, Chase MW. A comparison of ITS nuclear rDNA sequence data and AFLP markers for phylogenetic studies in Phyllostachys (bambusoideae, poaceae) J Plant Res. 2000;113:259–269. doi: 10.1007/PL00013936. [DOI] [Google Scholar]
- Hsu YH, Annamalai P, Lin CS, Chen YY, Chang WC, Lin NS. A sensitive method for detecting bamboo mosaic virus (BaMV) and establishment of BaMV-free meristem tip cultures. Plant Pathol. 2000;49:101–107. doi: 10.1046/j.1365-3059.2000.00433.x. [DOI] [Google Scholar]
- Hu SL, Cao Y, Huang SX, Sun X, Lu XQ, Jiang Y. Cloning and bioinformation analysis of 4CL gene in Neosinocalamus affinis. J Northwest A F Univ (Nat Sci Ed) 2009;37:204–210. [Google Scholar]
- Hu S, Zhou J, Cao Y, Lu X, Duan N, Ren P, Chen K. In vitro callus induction and plant regeneration from mature seed embryo and young shoots in a giant sympodial bamboo, Dendrocalamus farinosus (Keng et Keng f.) Chia et H.L. Fungal Afr J Biotechnol. 2011;10:3210–3215. [Google Scholar]
- Huang LC, Huang BL. Loss of species distinguishing trait among regenerated Bambusa ventricosa McClure plants. Plant Cell Tissue Organ Cult. 1995;42:109–111. doi: 10.1007/BF00037688. [DOI] [Google Scholar]
- Huang L, Murashige T. Tissue culture of bamboo. Bot Bull Acad Sin. 1983;24:31–52. [Google Scholar]
- Huang LC, Huang BL, Chen WL. Tissue culture investigations of bamboo—IV. Organogenesis leading to adventitious shoots and plants in excised shoot apices. Environ Exp Bot. 1989;19:307–315. doi: 10.1016/0098-8472(89)90004-X. [DOI] [Google Scholar]
- Huttley GA, McRae AF, Clegg MT. Molecular evolution of the Ac/Ds transposable element family in pearl millet and other grasses. Genetics. 1995;139:1411–1419. doi: 10.1093/genetics/139.3.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isagi Y, Shimada K, Kushima H, Tanaka N, Ishikawa T, Onodera H, Watanabe S. Clonal structure and flowering traits of a bamboo [Phyllostachys pubescens (Mazel) Ohwi] stand grown from a simultaneous flowering as revealed by AFLP analysis. Mol Ecol. 2004;13:2017–2021. doi: 10.1111/j.1365-294X.2004.02197.x. [DOI] [PubMed] [Google Scholar]
- Janzen DH. Why bamboos wait so long to flower? Annu Rev Ecol Syst. 1976;7:347–391. doi: 10.1146/annurev.es.07.110176.002023. [DOI] [Google Scholar]
- Jie YG, Sheng W, Yan LQ, Ping CZ, Bao SL, Jun HZ, Liang J, Yin XZ, Xun NMA, Jiang LF. Genome size and sequence composition of moso bamboo: a comparative study. Sci China Ser C Life Sci. 2007;50:1–6. doi: 10.1007/s11427-007-0016-2. [DOI] [PubMed] [Google Scholar]
- Jimenez VM, Castillo J, Tavares E, Guevara E, Montiel M. In vitro propagation of the neotropical giant bamboo, Guadua angustifolia Kunth, through axillary shoot proliferation. Plant Cell Tissue Organ Cult. 2006;86:389–395. doi: 10.1007/s11240-006-9120-4. [DOI] [Google Scholar]
- John CK, Nadgauda RS. In vitro-induced flowering in bamboos. In Vitro Cell Dev Biol Plant. 1999;35:309–315. doi: 10.1007/s11627-999-0040-y. [DOI] [Google Scholar]
- Joshi M, Nadgauda RS. Cytokinins and in vitro induction of flowering in bamboo: Bambusa arundinacea (Retz.) Wild. Curr Sci. 1997;73:523–526. [Google Scholar]
- Judziewicz EJ, Clark LG, Londono X, Stern MJ. American Bamboos. Washington, DC: Smithsonian Institution Press; 1999. [Google Scholar]
- Jullien F, Van KTT. Micropropagation and embryoid formation from young leaves of Bambusa glaucescens golden goddess. Plant Sci. 1994;98:199–207. doi: 10.1016/0168-9452(94)90010-8. [DOI] [Google Scholar]
- Kalia S, Kalia RK, Sharma SK. In vitro regeneration of an indigenous bamboo (Bambusa nutans) from internode and leaf explant. J Bamboo Rattan. 2004;3:217–228. doi: 10.1163/1569159041765272. [DOI] [Google Scholar]
- Kalia RK, Singh R, Singh SR, Mishra GP, Rai MK, Dhawan AK. Biotechnological interventions in sea buckthorn (Hippophae L.)—current status and future prospects. Trees Struct Funct. 2011;25:559–575. doi: 10.1007/s00468-011-0543-0. [DOI] [Google Scholar]
- Kalia RK, Rai MK, Kalia S, Singh R, Dhawan AK. Microsatellite markers: an overview of the recent progress in plants. Euphytica. 2011;177:309–334. doi: 10.1007/s10681-010-0286-9. [DOI] [Google Scholar]
- Kanyaratt S (1991) In vitro culture of some economic bamboos. Dissertation in Thailand. M. Sc. Thesis, Kesetsart University, pp 266
- Kapoor P, Rao IU. In vitro rhizome induction and plantlet formation from multiple shoots in Bambusa bambos var. gigantea Bennet and Gaur by using growth regulators and sucrose. Plant Cell Tissue Organ Cult. 2006;85:211–217. doi: 10.1007/s11240-005-9074-y. [DOI] [Google Scholar]
- Kelchner SA, Clark LG. Molecular evolutions and phylogenetic utility of the chloroplast rpl 16 intron in Chusquea and Bambusiodeae (Poaceae) Mol Phylogenet Evol. 1997;8:385–397. doi: 10.1006/mpev.1997.0432. [DOI] [PubMed] [Google Scholar]
- Keukeleire PD, Schepper SD, Gielis J, Gerats T. A PCR-based assay to detect hAT-like transposon sequences in plants. Chromosom Res. 2004;12:117–223. doi: 10.1023/B:CHRO.0000013163.34505.96. [DOI] [PubMed] [Google Scholar]
- Kobayashi M. Phylogeny of world bamboos analyzed by restriction fragment length polymorphisms of chloroplast DNA. In: Chapman GP, editor. The Bamboos. UK: Linean Society Symposium Series. Linean Society of London; 1997. pp. 61–81. [Google Scholar]
- Komatsu YH, Batagin-Piotto KD, Brondani GE, Gonclaves AN, Almeida MD. In vitro morphogenic response of leaf sheath of Phyllostachys bambusoides. J For Res. 2011;22:209–215. doi: 10.1007/s11676-011-0152-1. [DOI] [Google Scholar]
- Kumar MS, Mathur J. Artificial seed production in the male bamboo Dendrocalamaus strictus L. Plant Sci. 1992;87:109–113. doi: 10.1016/0168-9452(92)90198-U. [DOI] [Google Scholar]
- Lai CC, Hsiao JY (1997) Genetic variation of Phyllostachys pubescens (Bambusoideae, Poaceae) in Taiwan based on DNA polymorphisms. Bot Bull Academia Sinica 38:145–152
- Li XP, Gao ZM, Peng ZH, Yue YD, Gao J, Cai CJ, Mou SH. Cloning and characterization of COMT gene from Bambusa oldhamii. For Res. 2007;20:722–725. [Google Scholar]
- Lin CS, Chang WC. Micropropagation of Bambusa edulis through nodal explants of field-grown culms and flowering of regenerated plantlets. Plant Cell Rep. 1998;17:617–620. doi: 10.1007/s002990050453. [DOI] [PubMed] [Google Scholar]
- Lin CS, Lin CC, Chang WC. In vitro flowering of Bambusa edulis and subsequent plantlet survival. Plant Cell Tissue Organ Cult. 2003;72:71–78. doi: 10.1023/A:1021281217589. [DOI] [Google Scholar]
- Lin CS, Lin CC, Chang WC. Effect of thidiazuron on vegetative tissue-derived somatic embryogenesis and flowering of bamboo Bambusa edulis. Plant Cell Tissue Organ Cult. 2004;76:75–82. doi: 10.1023/A:1025848016557. [DOI] [Google Scholar]
- Lin CS, Lin CC, Chang WC. Shoot regeneration, re-flowering and post flowering survival in bamboo inflorescence culture. Plant Cell Tissue Organ Cult. 2005;82:243–249. doi: 10.1007/s11240-005-0883-9. [DOI] [Google Scholar]
- Lin CS, Lai YH, Sun CW, Liu NT, Tsay HS, Chang WC, Jeremy JW. Identification of ESTs differentially expressed in green and albino mutant bamboo (Bambusa edulis) by suppressive subtractive hybridization (SSH) and microarray analysis. Plant Cell Tissue Organ Cult. 2006;86:169–175. doi: 10.1007/s11240-006-9105-3. [DOI] [Google Scholar]
- Lin CS, Kalpana K, Chang WC, Lin NS. Improving multiple shoot proliferation in bamboo mosaic virus-free Bambusa oldhamii Munro propagation by liquid culture. Hortic Sci. 2007;42:1243–1246. [Google Scholar]
- Lin CS, Liang CJ, Hsaio HW, Lin MJ, Chang WC. In vitro flowering of green and albino Dendrocalamus latiflorus. New Forest. 2007;34:177–186. doi: 10.1007/s11056-007-9045-8. [DOI] [Google Scholar]
- Lin XC, Ruan XS, Lou YF, Guo XQ, Fang W. Genetic similarity among cultivars of Phyllostachys pubescens. Plant Syst Evol. 2009;277:67–73. doi: 10.1007/s00606-008-0104-1. [DOI] [Google Scholar]
- Lin X, Lou Y, Zhang Y, Yuan X, He J, Fang W. Identification of genetic diversity among cultivars of Phyllostachys violascens using ISSR, SRAP and AFLP markers. Bot Rev. 2011;77:223–232. doi: 10.1007/s12229-011-9078-8. [DOI] [Google Scholar]
- Loh JP, Kiew R, Set O, Gan LH, Gan YY. A study of genetic variation and relationships within the Bamboo subtribe Bambusinae using amplified fragment length polymorphism. Ann Bot. 2000;85:607–612. doi: 10.1006/anbo.2000.1109. [DOI] [Google Scholar]
- Maity S, Ghosh A. Efficient plant regeneration from seeds and nodal segments of Dendrocalamus strictus using in vitro technique. Indian Forester. 1997;4:313–318. [Google Scholar]
- Marulanda ML, Marquez P, Londono X. AFLP analysis of Guadua angustifolia (Poaceae: Bambusoideae) in Columbia with emphasis on the coffee region. J Am Bamboo Soc. 2002;16:32–42. [Google Scholar]
- Marulanda ML, Lopez AM, Claroz JL. Analyzing the genetic diversity of Guadua spp. in Colombia using rice and sugarcane microsatellites. Crop Breed Appl Biotechnol. 2007;7:43–51. [Google Scholar]
- Mehta U, Rao IVR, Ram HYM (1982) Somatic embryogenesis in bamboo. Plant Tissue Culture. In: Fujiwara A (ed) Jpn. Assoc. Proc 5th Intl Cong Plant Tiss Cell Cult pp 109–110
- Mehta R, Sharma V, Sood A, Sharma M, Sharma RK. Induction of somatic embryogenesis and analysis of genetic fidelity of in vitro derived plantlets of Bambusa nutans Wall. using AFLP markers. Eur J Forest Res. 2011;130:729–736. doi: 10.1007/s10342-010-0462-4. [DOI] [Google Scholar]
- Mishra Y, Rana PK, Shirin F, Ansari SA. Augmenting in vitro shoot multiplication by vipul (triacontanol) and adventitious rhizogenesis by rice bran extract in Dendrocalamus strictus. Indian J Exp Biol. 2001;39:165–169. [PubMed] [Google Scholar]
- Mishra Y, Patel PK, Yadav S, Shirin F, Ansari SA. A micropropagation system for cloning of Bambusa tulda Roxb. Sci Hortic. 2008;115:315–318. doi: 10.1016/j.scienta.2007.10.002. [DOI] [Google Scholar]
- Mishra Y, Patel P, Ansari SA. Acclimatization and macroproliferation of micropropagated plants of Bambusa tulda Roxb. Asian J Exp Biol Sci. 2011;2:498–501. [Google Scholar]
- Mudoi KD, Borthakur M. In vitro micropropagation of Bambusa balcooa Roxb. through nodal explants from field-grown culms and scope for upscaling. Curr Sci. 2009;96:962–966. [Google Scholar]
- Mukherjee AK, Ratha S, Dhar S, Debata AK, Acharya PK, Mandal S, Panda PC, Mohapatra Genetic relationships among 22 taxa of bamboo revealed by ISSR and EST-based random primers. Biochem Genet. 2010;48:1015–1025. doi: 10.1007/s10528-010-9390-8. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assay with tobacco tissue culture. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Nadgauda RS, John CK, Masearenhas AF.Precocious flowering and seedling behaviour in tissue cultured bamboos Nature 1990344355–356. 10.1038/344335a02107403 [DOI] [Google Scholar]
- Nadgauda RS, John CK, Mascarenhas AF. Floral biology and breeding behavior in the bamboo Dendrocalamus strictus Nees. Tree Physiol. 1993;13:401–408. doi: 10.1093/treephys/13.4.401. [DOI] [PubMed] [Google Scholar]
- Nadgauda RS, John CK, Joshi MS, Masearenhas AF. A comparison of in vitro with in vivo flowering in bamboo; Bambusa arundinacea. Plant Cell Tissue Organ Cult. 1997;48:181–188. doi: 10.1023/A:1005800700024. [DOI] [Google Scholar]
- Nadgir AL, Phadke CH, Gupta PK, Parasharami VA, Nair S, Mascarenhas AF. Rapid multiplication of bamboo by tissue culture. Silvae Genet. 1984;33:219–223. [Google Scholar]
- Nadha HK, Kumar R, Sharma RK, Anand M, Sood A. Evaluation of clonal fidelity of in vitro raised plants of Guadua angustifolia Kunth using DNA-based markers. J Med Plants Res. 2011;5:5636–5641. [Google Scholar]
- Nayak S, Rout GR. Characterization of microsatellites in Bambusa arundinacea and cross species amplification in other bamboos. Z Naturforsch. 2005;60c:605–610. doi: 10.1515/znc-2005-7-816. [DOI] [PubMed] [Google Scholar]
- Nayak S, Rout GR, Das P. Evaluation of the genetic variability in bamboo using RAPD markers. Plant Soil Environ. 2003;49:24–28. [Google Scholar]
- Negi D, Saxena S (2010) Ascertaining clonal fidelity of tissue culture raised plants of Bambusa balcooa Roxb. using inter simple sequence repeat markers. New For 40:1–8
- Negi D, Saxena S. In vitro propagation of Bambusa nutans Wall. ex Munro through axillary shoot proliferation. Plant Biotechnol Rep. 2011;5:35–43. doi: 10.1007/s11816-010-0154-z. [DOI] [Google Scholar]
- Nitsch JP, Nitsch C. Haploid plants from pollen grains. Science. 1969;163:85–87. doi: 10.1126/science.163.3862.85. [DOI] [PubMed] [Google Scholar]
- Nurul Islam SAM, Rahman MM. Micro-cloning in commercially important six bamboo species for mass propagation and at large scale cultivation. Plant Tissue Cult Biotechnol. 2005;15:103–111. [Google Scholar]
- Ogita S. Callus and cell suspension culture of bamboo plant, Phyllostachys nigra. Plant Biotechnol. 2005;22:119–125. doi: 10.5511/plantbiotechnology.22.119. [DOI] [Google Scholar]
- Ogita S, Kashiwagi H, Kato Y. In vitro node culture of seedlings in bamboo plant, Phyllostachys meyeri Mcclure. Plant Biotechnol. 2008;25:381–385. doi: 10.5511/plantbiotechnology.25.381. [DOI] [Google Scholar]
- Ogita S, Kikuchi N, Nomura T, Kato Y. A practical protocol for particle bombardment-mediated transformation of Phyllostachys bamboo suspension cells. Plant Biotechnol. 2011;28:43–50. doi: 10.5511/plantbiotechnology.10.1101a. [DOI] [Google Scholar]
- Ojha A, Verma N, Kumar A. In vitro micropropagation of economically important edible bamboo (Dendrocalamus asper) through somatic embryos from root, leaves and nodal segments explants. Res Crops. 2009;10:430–436. [Google Scholar]
- Peng Z, Lu T, Li L, Liu X, Gao Z, Hu T, Yang X, Feng Q, Guan J, Weng Q, Fan D, Zhu C, Lu Y, Han B, Jiang Z. Genome-wide characterization of the biggest grass, bamboo, based on 10,608 putative full-length cDNA sequences. BMC Plant Biol. 2010;10:116. doi: 10.1186/1471-2229-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prutpongse, Gavintertvatana In vitro micropropagation of 54 species from 15 genera of bamboo. Hortic Sci. 1992;27:453–454. [Google Scholar]
- Qiang Z, Yu-long D, Chen X, Hui-yu Z, Min-ren H, Ming-xiu W. A preliminary analysis of phylogenetic relationships of Arundinaria and related genera based on nucleotide sequences of nrDNA (ITS region) and cpDNA (trnL-F intergenic spacer) J For Res. 2005;16:5–8. doi: 10.1007/BF02856844. [DOI] [Google Scholar]
- Ramanayake SMSD, Wanniarachchi WAVR. Organogenesis in callus derived from an adult giant bamboo (Dendrocalamus giganteus Wall. ex Munro) Sci Hortic. 2003;98:195–200. doi: 10.1016/S0304-4238(02)00204-2. [DOI] [Google Scholar]
- Ramanayake SMSD, Yakandawala K. Micropropagation of the giant bamboo (Dendrocalamus giganteus Munro) from nodal explants of field grown culms. Plant Sci. 1997;129:213–223. doi: 10.1016/S0168-9452(97)00185-4. [DOI] [Google Scholar]
- Ramanayake SMSD, Yakandawala K, Nilmini Deepika PKD, Ikbal MCM (1995) Studies on Micropropagation of Dendrocalamus gigateus and Bambusa vulgaris var. striata. In: Bamboo, People and The Environment, vol.1. Propagation and Management. INBAR Tech Rep 8: 75–85
- Ramanayake SMSD, Wanniarachchi WAVR, Tennakoon TMA. Axillary shoot proliferation and in vitro flowering in an adult giant bamboo, Dendrocalamus giganteus Wall. ex Munro. In Vitro Cell Dev Biol Plant. 2001;37:667–671. doi: 10.1007/s11627-001-0116-9. [DOI] [Google Scholar]
- Ramanayake SMSD, Meemaduma VN, Weerawardene TE. In vitro shoot proliferation and enhancement of rooting for the large-scale propagation of yellow bamboo (Bambusa vulgaris ‘Striata’) Sci Hortic. 2006;110:109–113. doi: 10.1016/j.scienta.2006.06.016. [DOI] [Google Scholar]
- Ramanayake SMSD, Meemaduma VN, Weerawardene TE. Genetic diversity and relationships between nine species of bamboo in Sri Lanka, using random amplified polymorphic DNA. Syst Evol. 2007;269:55–61. doi: 10.1007/s00606-007-0587-1. [DOI] [Google Scholar]
- Rao IVR, Rao IU. Tissue culture approaches to the mass propagation and genetic improvement of bamboos. In: Rao IVR, Gnanaharan R, Sastry CB, editors. Bamboo current research, Proc Intl Bamboo Workshop, 14–18 Nov 1988. Cochin: KFRI Peechi and IDRC Canada; 1990. pp. 151–158. [Google Scholar]
- Rao U, Rao IVR, Narang V. Somatic embryogenesis and regeneration of plants in the bamboo Dendrocalamus strictus. Plant Cell Rep. 1985;4:191–194. doi: 10.1007/BF00269286. [DOI] [PubMed] [Google Scholar]
- Rao IVR, Gnanaharan R, Sastry CB. Bamboo: Current Research. Singapore: Kerala Forest Res. Inst, Peechi and IDRC; 1990. [Google Scholar]
- Rathore TS, Kabade U, Jagadish MR, Somashekar PV, Viswanath S (2009) Micropropagation and evaluation of growth performance of the selected industrially important bamboo species in southern India. In: Proc 8th World Bamboo Cong Lucas S (ed) Bankok, Thailand p 41–55
- Ravikumar R, Ananthakrishnan G, Kathiravan K, Ganapathi A. In vitro shoot propagation of Dendrocalamus strictus Nees. Plant Cell Tissue Organ Cult. 1998;52:189–192. doi: 10.1023/A:1006092620731. [DOI] [Google Scholar]
- Rout GR, Das P. Somatic embryogenesis and in vitro flowering in 3 species of bamboo. Plant Cell Rep. 1994;13:683–686. doi: 10.1007/BF00231624. [DOI] [PubMed] [Google Scholar]
- Sanjaya, Rathore TS, Rai VR (2005) Micropropagation of Pseudoxytenanthera stocksii Munro. In Vitro Cell Dev Biol Plant 41:333–337
- Satsangi R, Kalia S, Arya ID, Arya S. Flowering in exotic bamboo Dendrocalamus asper in India. Indian Forester. 2001;127:1053–1057. [Google Scholar]
- Saxena S. In vitro propagation of the bamboo (Bambusa tulda Roxb.) through shoot proliferation. Plant Cell Rep. 1990;9:431–434. doi: 10.1007/BF00232266. [DOI] [PubMed] [Google Scholar]
- Saxena S, Bhojwani SS. In vitro clonal multiplication of 4-year-old plants of the bamboo, Dendrocalamus longispathus Kurz. In Vitro Cell Dev Biol Plant. 1993;29:135–142. [Google Scholar]
- Saxena S, Dhawan V. Micropropagation research in south Asia. Constraints to production of bamboo and rattan. INBAR Technol Rep. 1994;5:101–113. [Google Scholar]
- Saxena S, Dhawan V. Regeneration and large-scale propagation of bamboo (Dendrocalamus strictus Nees) through somatic embryogenesis. Plant Cell Rep. 1999;18:438–443. doi: 10.1007/s002990050600. [DOI] [Google Scholar]
- Schenk RU, Hildebrandt AC. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can J Bot. 1972;50:199–204. doi: 10.1139/b72-026. [DOI] [Google Scholar]
- Scott TK. Auxins and roots. Annu Rev Plant Physiol. 1972;23:235–258. doi: 10.1146/annurev.pp.23.060172.001315. [DOI] [Google Scholar]
- Sharma RK, Gupta P, Sharma V, Sood A, Mohapatra T, Ahuja PS. Evaluation of rice and sugarcane SSR markers for phylogenetic and genetic diversity analyses in bamboo. Genome. 2008;51:91–103. doi: 10.1139/G07-101. [DOI] [PubMed] [Google Scholar]
- Sharma V, Bhardwaj P, Kumar R, Sharma RK, Sood A, Ahuja PS. Identification and cross-species amplification of EST derived SSR markers in different bamboo species. Conserv Genet. 2009;10:721–724. doi: 10.1007/s10592-008-9630-1. [DOI] [Google Scholar]
- Shirgurkar MV, Thengane SR, Insiya S, Poonawala J, Nadgauda RS, Mascarenhas AF. A simple in vitro method of propagation and rhizome formation in Dendrocalamus strictus Nees. Curr Sci. 1996;70:940–944. [Google Scholar]
- Shirin F, Rana PK. In vitro plantlet regeneration from nodal explants of field-grown culms in Bambusa glaucescens Willd. Plant Biotechnol Rep. 2007;1:141–147. doi: 10.1007/s11816-007-0020-9. [DOI] [Google Scholar]
- Singh M, Jaiswal U, Jaiswal VS. Thidiazuron induced in vitro flowering in Dendrocalamus strictus Nees. Curr Sci. 2000;79:1529–1530. [Google Scholar]
- Singh M, Jaiswal U, Jaiswal VS. Thidiazuron-induced shoot multiplication and plant regeneration in bamboo (Dendrocalamus strictus Nees) J Plant Biochem Biotechnol. 2001;10:133–137. doi: 10.1007/BF03263122. [DOI] [Google Scholar]
- Singh SR, Dalal S, Singh R, Dhawan AK, Kalia RK. Micropropagation of Dendrocalamus asper (Schult. & Schult. F. Backer ex K Heyne): an exotic edible bamboo. J Plant Biochem Biotechnol. 2011;21:220–228. doi: 10.1007/s13562-011-0095-9. [DOI] [Google Scholar]
- Singh SR, Dalal S, Singh R, Dhawan AK, Kalia RK. Seasonal influences on in vitro bud break in Dendrocalamus hamiltonii Arn. ex Munro nodal explants and effect of culture microenvironment on large scale shoot multiplication and plantlet regeneration. Indian J Plant Physiol. 2012;17:9–21. [Google Scholar]
- Singh SR, Dalal S, Singh R, Dhawan AK, Kalia RK (2012b) Evaluation of genetic fidelity of in vitro raised plants of Dendrocalamus asper (Schult. & Schult. F.) Backer ex K. Heyne using DNA-based markers. Acta Physiol Plant doi:10.1007/s11738-012-1084-x
- Sood A, Palni LMS, Sharma M, Sharma OP (1994) Micropropagation of Dendrocalamus hamiltonii Munro using single node cutting taken from elite seedling plants. In: Proc Intl Bamboo Workshop Bamboo Asia Pacific, 27th to 30th Nov 1991, Chiangmai, Thailand, p 165–168
- Sood A, Ahuja PS, Sharma M, Sharma OP, Godbole S. In vitro protocols and field performance of elites of an important bamboo Dendrocalamus hamiltonii Nees et Arn. ex Munro. Plant Cell Tissue Organ Cult. 2002;71:55–63. doi: 10.1023/A:1016582732531. [DOI] [Google Scholar]
- Sun Y, Xia NH, Stapleton MA. Relationships between Bambusa species (Poaceae, Bambusoideae) revealed by random amplified polymorphic DNA. Biochem Syst Ecol. 2006;34:417–423. doi: 10.1016/j.bse.2005.10.015. [DOI] [Google Scholar]
- Suyama Y, Obayashi K, Hayashi I. Clonal structure in a dwarf bamboo (Sasa senanensis) population inferred from amplified fragment length polymorphism (AFLP) fingerprints. Mol Ecol. 2000;9:901–906. doi: 10.1046/j.1365-294x.2000.00943.x. [DOI] [PubMed] [Google Scholar]
- Tang DQ, Lu JJ, Fang W, Zhang S, Zhou MB. Development, characterization and utilization of gene bank microsatellite markers in Phyllostachys pubescens and related species. Mol Breed. 2010;25:299–311. doi: 10.1007/s11032-009-9333-4. [DOI] [Google Scholar]
- Teixeira da Silva JAT, Nhut DT. Thin cell layers and floral morphogenesis, floral genetics and in vitro flowering. In: Nhut DT, Le BV, Van TT, Thorpe T, editors. Thin Cell Layer Culture System: Regeneration and Transformation Applications. Dordrecht: Kluwer Academic Publishers; 2003. pp. 285–342. [Google Scholar]
- Thiruvengadam M, Rekha KT, Chung IM. Rapid in vitro micropropagation of Bambusa oldhamii Munro. Philipp Agric Sci. 2011;94:7–13. [Google Scholar]
- Tian B, Chen Y, Li D, Yan Y. Cloning and characterization of a bamboo Leafy Hull Sterile1 homologous gene. Mitochondrial DNA. 2006;17:143–151. doi: 10.1080/10425170600699877. [DOI] [PubMed] [Google Scholar]
- Torrey JG. Root hormones and plant growth. Annu Rev Plant Physiol. 1976;27:435–459. doi: 10.1146/annurev.pp.27.060176.002251. [DOI] [Google Scholar]
- Tsay HS, Yeh CC, Hsu JY. Embryogenesis and plant regeneration from another culture of bamboo (Sinocalamus latiflora (Munro) McClure) Plant Cell Rep. 1990;9:349–351. doi: 10.1007/BF00232396. [DOI] [PubMed] [Google Scholar]
- Van Tran Thanh M. In vitro control of de novo flower, bud, root and callus differentiation from excised epidermal tissues. Nature. 1973;446:44–45. doi: 10.1038/246044a0. [DOI] [Google Scholar]
- Verma RK, Arya ID. Effect of arbuscular mycorrhizal fungal isolates and organic manure on growth and mycorrhization of micropropagated Dendrocalamus asper plantlets and on spore production in their rhizosphere. Mycorrhiza. 1998;8:113–116. doi: 10.1007/s005720050221. [DOI] [Google Scholar]
- Vongvijitra R. Proc. Int. Bamboo Workshop. Canada: FRI, Kerela and IDRC; 1988. Bamboo - Current Research; p. 148. [Google Scholar]
- Wang K, Peng H, Lin E, Jin Q, Hua X, Yao S, Bian H, Han N, Pan J, Wang J, Deng M, Zhu M. Identification of genes related to the development of bamboo rhizome bud. J Exp Bot. 2010;61:551–561. doi: 10.1093/jxb/erp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanable M, Ito M, Kurita S. Chloroplast DNA phylogeny of Asian bamboos (Bambusoideae, Poaceae) and its systematic implication. J Plant Res. 1994;107:253–261. doi: 10.1007/BF02344252. [DOI] [Google Scholar]
- Watanable Y, Sawa Y, Nagaoka N, Kozai T (2000) A new micropropagation system for Pleioblastus pygmaeus Nakai. In: Proc Int Symp Royal Project Foundation. Chiang Mai, Thailand, 2–4 August, p 94–101
- Werner T, Motyka V, Strnad M, Schmulling T. Regulation of plant growth by cytokinin. Proc Natl Acad Sci. 2001;98:10487–10492. doi: 10.1073/pnas.171304098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersma R. Bioluminescent Bamboo. Newsl South Calif Chap Am Bamboo Soc. 2008;18:2–5. [Google Scholar]
- Woods SH, Philips GC, Woods JE, Collins GB. Somatic embryogenesis and plant regeneration from zygotic embryo explants in Mexican weeping bamboo, Otatea acuminata Aztecorum. Plant Cell Rep. 1992;11:257–261. doi: 10.1007/BF00235077. [DOI] [PubMed] [Google Scholar]
- Xuhe C. Promotion of bamboo for poverty alleviation and economic development. J Bamboo Rattan. 2003;2:345. doi: 10.1163/156915903322700386. [DOI] [Google Scholar]
- Yashoda R, Sumathi R, Mallinga P, Gurunurthi K. Genetic enhancement and mass production of quality propagaules of Bambusa nutans and Dendrocalamus membranaceus. Indian Forester. 1997;4:303–306. [Google Scholar]
- Yashoda R, Kamala S, Kumar ASP, Kumar PD, Kalaiarasi K. Effect of glucose on in vitro rooting of mature plants of Bambusa nutans. Sci Hortic. 2007;110:109–113. [Google Scholar]
- Yeh ML, Chang WC. Plant regeneration through somatic embryogenesis in callus culture of green bamboo (Bambusa oldhamii Munro) Theor Appl Genet. 1986;73:161–163. doi: 10.1007/BF00289269. [DOI] [PubMed] [Google Scholar]
- Yeh ML, Chang WC. Somatic embryogenesis and subsequent plant regeneration from inflorescence callus of Bambusa beecheyana Munro var. beecheyana. Plant Cell Rep. 1986;5:409–411. doi: 10.1007/BF00269628. [DOI] [PubMed] [Google Scholar]
- Yeh ML, Chang WC. Plant regeneration via somatic embryogenesis in mature embryo-derived callus culture of Sinocalamus latiflora (Munro) McClure. Plant Sci. 1987;51:93–96. doi: 10.1016/0168-9452(87)90224-X. [DOI] [Google Scholar]
- Yu JK, Rota ML, Kantety RV, Sorrells ME. EST derived SSR markers for comparative mapping in wheat and rice. Mol Genet Genomics. 2004;271:742–751. doi: 10.1007/s00438-004-1027-3. [DOI] [PubMed] [Google Scholar]
- Zamora AB, Gruezo SS. Embryo cell of bamboo (Dendrocalamus strictus Nees) Philipp Agric. 1990;73:199–206. [Google Scholar]
- Zamora AB, Gruezo SS, Damasco OP. Callus induction and plant regeneration from internode tissues of Dendrocalamus latiflorus cv Machiku. In: Rao AN, Yusoff AM, editors. Proc Seminar Tiss Cult Forest Sp. Singapore: FRI Malaysia and IDRC; 1989. pp. 76–82. [Google Scholar]
- Zhang YJ, Ma PF, Li DZ. High-throughput sequencing of six bamboo chloroplast genomes: Phylogenetic implications for temperate woody bamboos (Poaceae: Bambusoideae) PLoS One. 2011;6(5):e20596. doi: 10.1371/journal.pone.0020596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Yang YM, Liu XZ. Bamboo species relations revealed by Random Amplified Polymorphism of chloroplast DNA. Afr J Agric Res. 2011;6:1241–1245. [Google Scholar]
- Zhi-jun Z, Zhi-jun G, Li Y, Li Y, Shu-ping L. Analysis of SSRs information and development of SSR markers from Moso Bamboo (Phyllostachys edulis) ESTs. Acta Hortic Sin. 2011;38:989–996. [Google Scholar]
- Zhou MB, Liu XM, Tang DQ. Transposable elements in Phyllostachys pubescens (Poaceae) genome survey sequences and the full length cDNA sequences, and their association with simple sequence repeats. Genet Mol Res. 2011;10:3026–3037. doi: 10.4238/2011.December.6.3. [DOI] [PubMed] [Google Scholar]


