Abstract
The constant increase in the number of drug users and rapidly spread of Hepatitis C virus (HCV) and Human immunodeficiency virus (HIV) among drug users result in a serious public health problem in China. To investigate HCV prevalence among drug users in Zhenjiang city, Jiangsu, China, 207 drug users from Zhenjiang were enrolled in this study during 2009 and the prevalence of HCV, HIV and syphilis infection were detected. HCV prevalence among injection drug users (IDUs) was 81.6%, significantly higher than that (22.9%) among oral drug users (P < 0.001), suggesting a strong association of HCV infection with injection drug use (IDU). Most drug users were more than 25 years old (89.2%), single (60.5%, including single and divorced/widowed), and had a history of drug abuse over 6 years (92.9%). HCV prevalence among drug users with middle (72.6%) or high (83.8%) school diplomas was significantly higher than that among those with lower (46.9%) education level (P = 0.007). HCV prevalence among IDUs did not obviously change along with the increase in duration of drug use and in frequency of injection per day, suggesting less association of HCV infection with both variables. These results suggest that most Chinese addicts might start drug use after their middle/high school education. To reduce drug use and to prevent HIV and HCV transmission via IDU, large-scale drug prevention educations should be urgently conducted in all China’s middle and high schools.
Keywords: HCV, Injection drug use (IDU), Education level, Middle and high school, Jiangsu
Introduction
Hepatitis C virus (HCV) is a single-stranded RNA virus, which is a main cause of chronic hepatitis, cirrhosis and hepatocellular carcinoma [6]. HCV infection has become a very serious global public health problem [21, 22]. Approximately 200 million (3%) people are infected with HCV worldwide [21, 22]. In China, HCV prevalence has been estimated to be 3.2%, implying approximately 38 million Chinese people infected with HCV (http://www.cfhpc.org/detail.asp?id=283).
HCV is transmitted mainly through direct or indirect percutaneous exposure to contaminated blood or blood products from unscreened donors. Injection drug use (IDU), blood transfusion, and unsafe therapeutic injections are well-documented routes of HCV transmission [2, 21, 22, 28]. In addition, occupational exposure, mother-to-child transmission and sexual activities are also the routes of HCV transmission [1, 21, 23].
In developed countries, IDU appears to be the predominant mode of HCV transmission [1, 3, 7, 9, 10, 14, 21, 24]. For example, IDU accounted for 68 and 80% of current infections in USA and Australia, respectively. In some developing countries, illegal blood collection and blood transfusion had been identified as a primary source for HCV infection [15, 17, 19]. Besides blood transfusion, IDU is also the main form for HCV transmission in China and other southeastern Asian countries (e.g. Myanmar, Laos, and Thailand) neighboring the “Golden Triangle” [2, 22]. In China, the governments began to implement a strict management of blood products since 1992, and brought the new blood donation law into effect since 1998. As a consequence, HCV infection rate through blood transfusion and blood products had dropped dramatically down [19], and IDU became the most predominant mode of HCV transmission, which resulted in high HCV prevalence among injection drug users (IDUs) (15.6–98.7%) [4, 8, 11,26–29].
Recently, we characterized HCV subtypes among IDUs Zhenjiang city, Jiangsu, China and found that seven HCV subtypes were circulating in Zhenjiang, implying a crucial role of Zhenjiang in HCV Transmission in China [31]. In this study, we investigated the HCV prevalence among drug users in Zhenjiang and evaluated the potential risk factors associated with HCV infection. We found that IDU was significantly associated with HCV infection among drug users, whereas needle-sharing behavior, duration of drug use, and frequency of injection per day were not. Intriguingly, middle/high school education level appeared to significantly associate with HCV infection among drug users, providing new valuable information for future prevention of HCV transmission among IDUs in China.
Materials and Methods
Study Participants
Two hundreds and seven heroin drug users, including 129 participants from Zhenjiang Methadone Treatment Center (MTC) and 78 from Jiangsu Women’s Re-education through Labor Camp (WRTLC) were enrolled in this study during the March in 2009. Both institutions are located in Zhenjiang city, Jiangsu province. Under supervising of the Ethics Committee, all the participants agreed to participate in a questionnaire investigation on demographic characteristics and high risk behaviors for HCV infection after obtaining oral or written informed consents. The questionnaire included gender, age, marital status, education level, pattern of drug use, needle-sharing behavior among the last 6 months, frequency of injection per day, and sexual protection measure in the last 3 months. Among 207 drug users, 176 who agreed to provide their blood samples were subjected to serological assays of HCV, HIV and syphilis infections. They include 98 from Zhenjiang MTC and 78 from Jiangsu WRTLC.
Serological Assays
Whole blood samples (about 5 ml) were collected from these drug users using sterile ethylenediaminetetraacetic acid tubes, and transferred to the laboratory for detection of antibodies to HCV, HIV and syphilis. All assays were performed in the Bio-Safety Level 2 Lab at Zhenjiang Center for Disease Control and Prevention according to the manufacturer’s instructions. Infections of HCV and syphilis were tested by the corresponding enzyme immunoassay kits (Wantai Biotechnology Ltd., Beijing, China). HIV infection was detected by enzyme immunoassay kit from Lizhu Biotechnology Ltd., Zhuhai, China. Positive HIV results were confirmed by Western blot assay at HIV Confirmatory Center Laboratory at Jiangsu Center for Disease Control and Prevention.
Statistical Analyses
Statistical analysis was performed by the SPSS statistical package, version 16.0 (SPSS, Inc.). The significance level is chosen to be 0.05 and P values are two-sided. Statistical comparisons of the categorical variables were done by the chi-square (χ2) test or Fisher’s exact test. Multivariate analysis was done when factors identified as significant in univariate analysis.
Results
Prevalence of HCV Among Drug Users in Zhenjiang
Among 176 drug users, the prevalence of HCV infection was 69.9% (123/176, 95% confidence interval (CI): 63.1–76.7%) (Table 1). Among 123 HCV-infected individuals, although none was found to be co-infected with HIV, 9 (8.3%) were co-infected with syphilis. Among HCV-negative drug users, the HIV and syphilis infection rates were 2.0 and 11.4%, respectively (Table 1).
Table 1.
Prevalence of HCV/HIV and HCV/Syphilis co-infection in drug users in Zhenjiang, Jiangsu province, China
| HCV (number) | Co-infection with HIV (n = 171)a | Co-infection with syphilis (n = 153)a | ||
|---|---|---|---|---|
| Positive (infection rate %) | Negative | Positive (infection rate %) | Negative | |
| Positive (123) | 0 (0) | 120 | 9 (8.3) | 100 |
| Negative (53) | 1 (2.0) | 50 | 5 (11.4) | 39 |
| Total (176) | 1 (0.58) | 170 | 14 (9.2) | 139 |
aThe discrepancies in number of specimens were due to low volumes of some specimens thereby limiting the tests for HIV and/or syphilis
Demographic Information of Drug Users in Zhenjiang and Risk Factors for HCV Infection
Among 176 drug users, 110 are female, accounting for 62.5% (Table 2). Most drug users were single (98, 60.5%, including single and divorced/widowed), and had completed their middle/high school education (143, 81.7%). There was no significant difference in HCV prevalence between males (71.2%) and females (69.1%) (P = 0.766), and between different marital statuses (P = 0.530) (Table 2). Interestingly, however, a significantly higher HCV prevalence was observed among drug users who had middle (72.6%) and/or high (83.8%) school diplomas than that among those with lower education level (46.9%) (P ≦ 0.007).
Table 2.
Risk factors associated with HCV infection in Zhenjiang, Jiangsu Province, China
| Factors | Count (%) | HCV positive (%) | χ2 | P value |
|---|---|---|---|---|
| Gender | ||||
| Male | 66 (37.5) | 47 (71.2) | 0.880 | 0.766 |
| Female | 110 (62.5) | 76 (69.1) | ||
| Marital status | ||||
| Single | 75 (46.3) | 52 (69.3) | 1.269 | 0.530 |
| Married | 64 (39.5) | 42 (65.6) | ||
| Divorced/Widowed | 23 (14.2) | 18 (78.3) | ||
| Education levela | ||||
| ≤Primary school | 32 (18.3) | 15 (46.9) | 7.343 10.519 |
0.007 0.001 |
| Middle school | 106 (60.6) | 77 (72.6) | ||
| ≥High school | 37 (21.1) | 31 (83.8) | ||
| Pattern of drug usea | ||||
| Oral drug | 35 (19.9) | 8 (22.9) | 45.914 | <0.001 |
| Injecting | 141 (80.1) | 115 (81.6) | ||
| Sexual protection measures | ||||
| Every time | 10 (11.9) | 4 (40.0) | 4.968 | 0.083 |
| Occasionally | 12 (14.3) | 9 (75.0) | ||
| Nerve | 62 (73.8) | 46 (74.2) | ||
aα′ = 0.0125. The results represent the comparison between groups of ≦ Primary school and Middle school, and between groups of Primary school and ≧ High school
Previous studies demonstrated that IDU and sexual exposure are high-risk behaviors for HCV infection [11, 13, 24, 25, 28]. Among this cohort, most addicts were IDUs (141/176, 80.1%) and had engaged in sexual activity without protection (i.e. condom) (62/84, 73.8%). HCV prevalence among IDUs was 81.6%, significantly higher than that (22.9%) among oral drug users (P < 0.001) (Table 2), suggesting a strong association of HCV infection with IDU. In addition, HCV prevalence was slightly lower among those using sexual protection measures (59.1%) than that among those not using (74.2%) (P = 0.183) (Table 2).
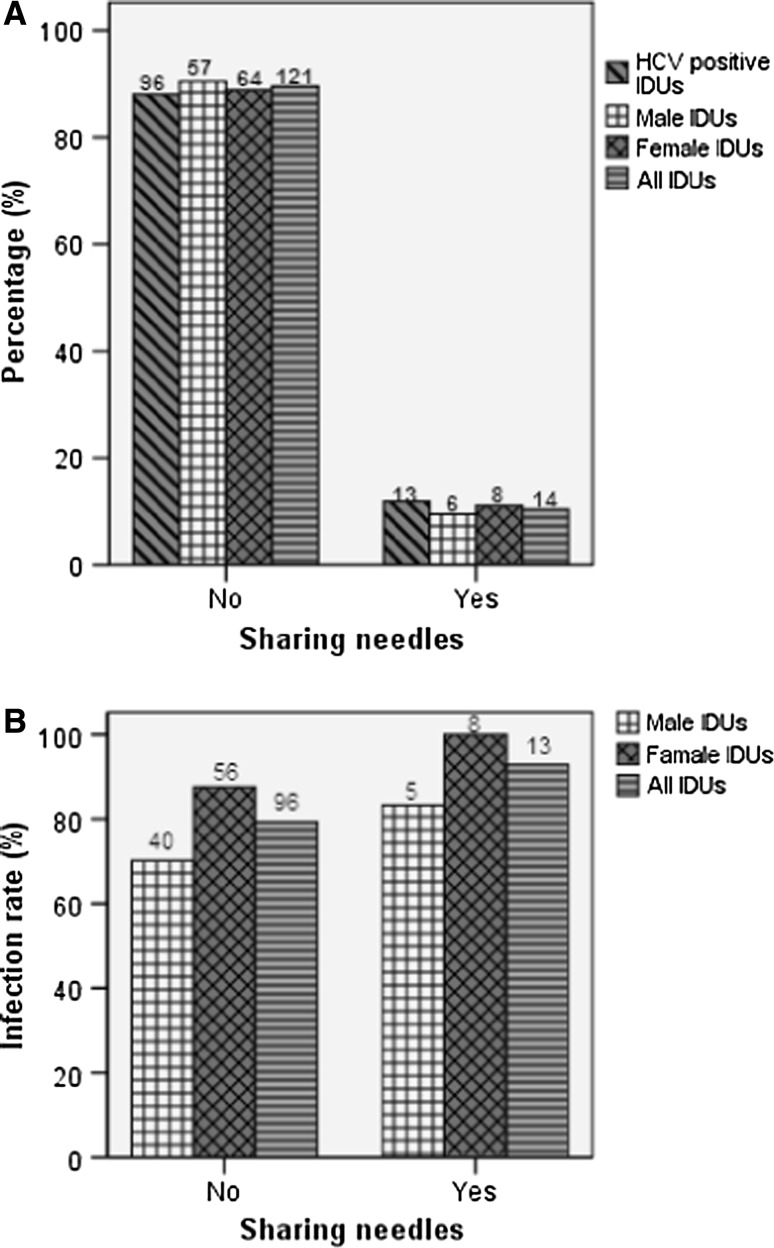
Further, we investigated whether needle-sharing was associated with high HCV prevalence among IDUs. Only 10.4% IDUs reported a history of sharing needles (Fig. 1a). Although HCV infection rate among those sharing needles (92.9%) was slightly higher than those not sharing needles (79.3%), there was no significant difference (P = 0.392) (Fig. 1b). Interestingly, HCV prevalence appeared to be slightly higher among female IDUs than male IDUs (Fig. 1b), which might be ascribed to sexual risk behaviors.
Fig. 1.
Comparison of HCV prevalence between needle-sharing IDUs and non-needle-sharing IDUs. a The percentage. b HCV Infection rate. The number above each bar is the number of individuals involved
Age Distribution of IDUs and HCV Infection
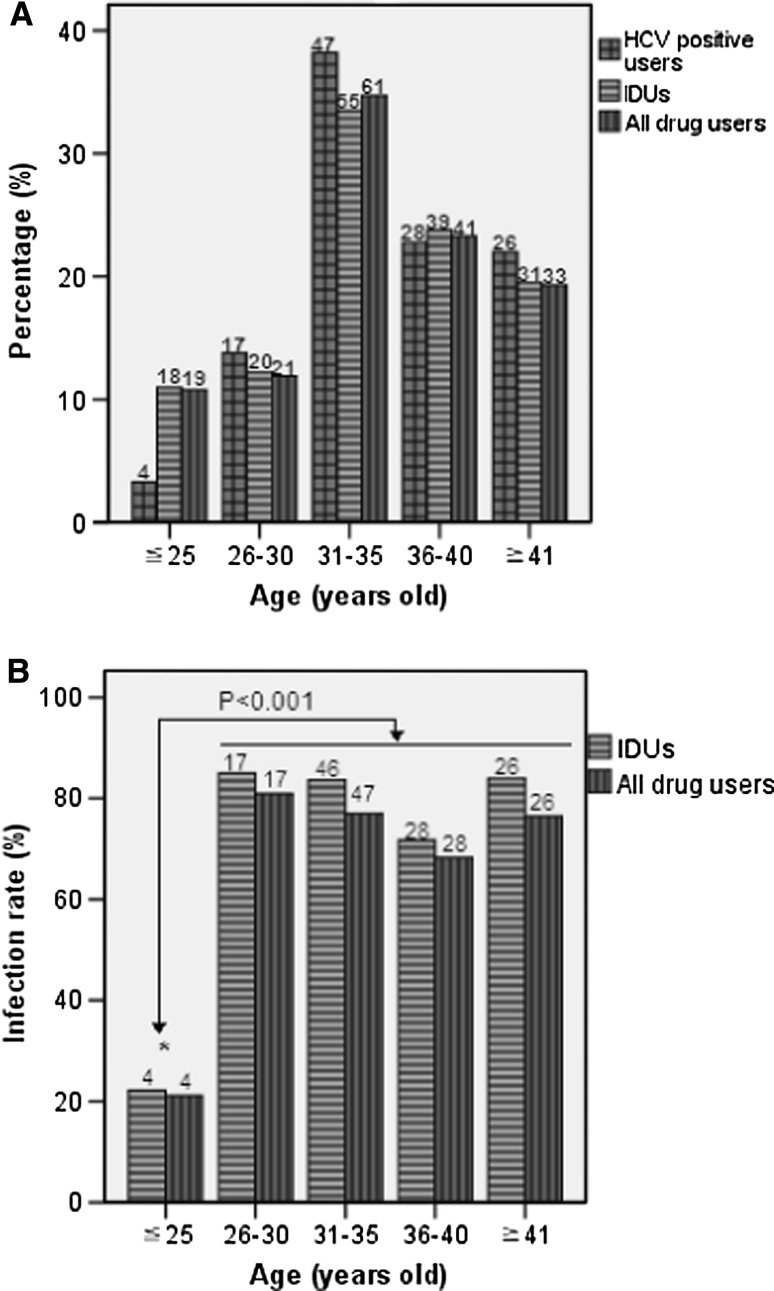
The age of drug users in this cohort ranged from 18 to 57 years old. The mean age was 35 (SD = 7.377, 95% CI: 33.90–36.10). All IDUs were divided into five age groups (Fig. 2). The age distributions of HCV-infected drug users, HCV-infected IDUs, and all drug users were well consistent with each other, and the greatest proportion (33.5–38.2%) appeared in the group of 31 to 35 years old in each cohort (Fig. 2a). HCV prevalence among both IDUs and drug users were significantly higher in the age groups over the age of 25 than the younger age group (below the age of 25) (P < 0.01) (Fig. 2b). However, HCV prevalence were similar among the four older age groups of >25 (Fig. 2b).
Fig. 2.
Comparison of HCV prevalence among IDUs and drug users between different age groups. a The age distribution. b HCV Infection rate. The number above each bar is the number of individuals involved
No Association of Duration of Drug use and Frequency of Injection per day With HCV Infection
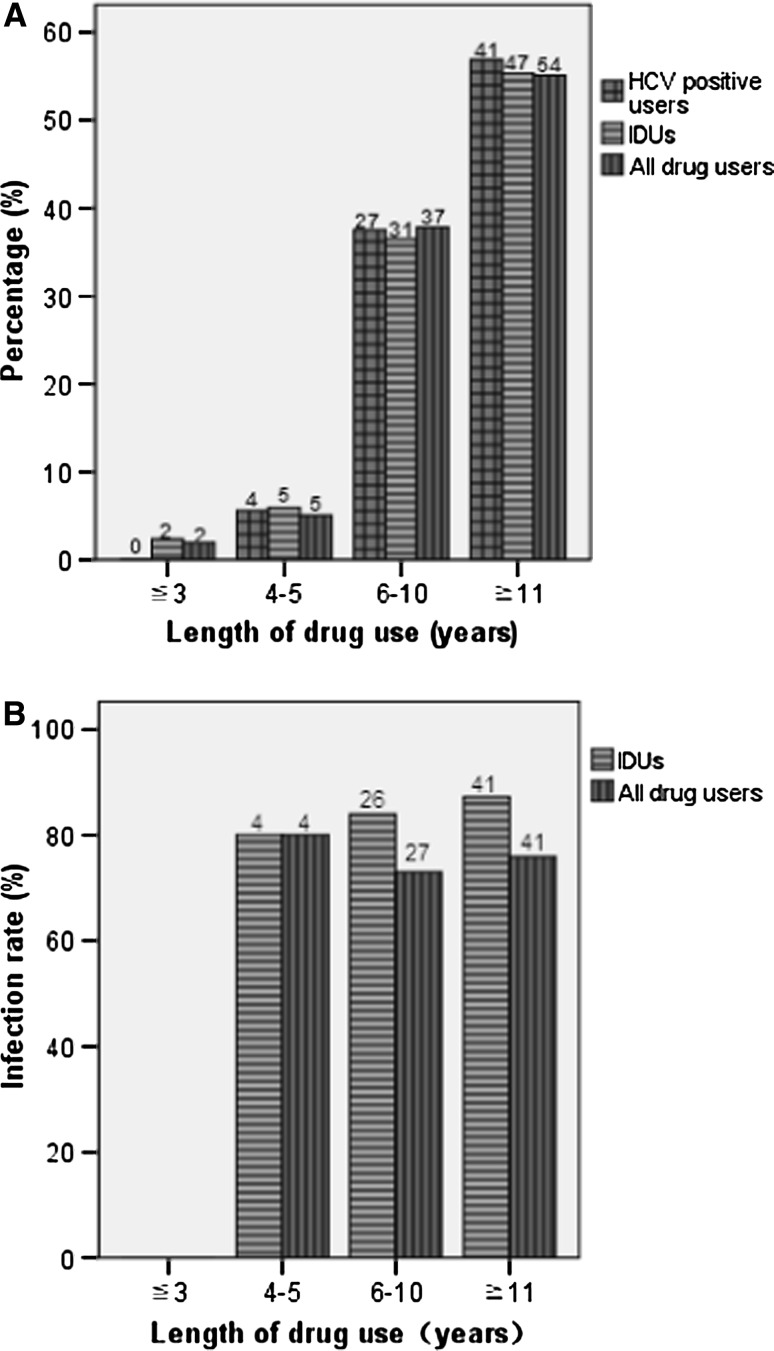
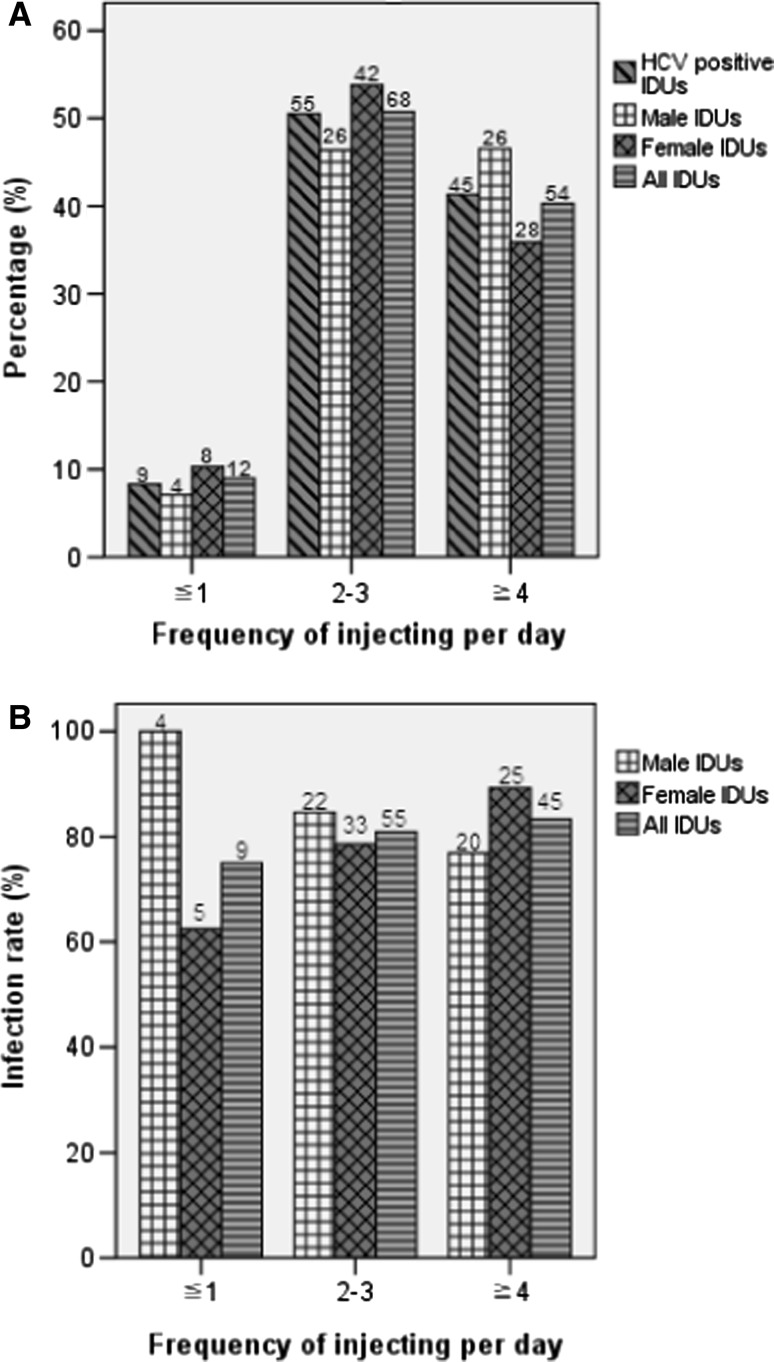
The period of drug abuse in this cohort ranged from 3 to 22 years. The mean period of drug abuse was 11 (SD = 3.708, 95% CI: 10.26–11.74) years. Most drug users (91.8–94.4%) had a history of drug abuse over 6 years (Fig. 3a). Except the group with the period of drug abuse ≦3 years and no HCV prevalence, three groups with the periods of drug abuse ≧4 years have similar high HCV prevalence among IDUs (80.0–87.2%) and among all drug users (73.0–80.0%) (Fig. 3b). Furthermore, more than half of IDUs (50.7%) had drug injection behavior over two times per day (Fig. 4a). HCV prevalence among IDUs did not obviously change along with an increase of frequency of injection per day (Fig. 4b). These results suggest that HCV infection among IDUs was less associated with duration of drug use and frequency of injection per day.
Fig. 3.
Comparison of HCV prevalence among IDUs and drug users with different lengths of drug use. a The distribution. b HCV Infection rate. The number above each bar is the number of individuals involved
Fig. 4.
Comparison of HCV prevalence among IDUs with different frequencies of injection per day. a The distribution. b HCV Infection rate. The number above each bar is the number of individuals involved
Multivariate logistic regression analysis shows that educational level and the pattern of drug use were independently associated with the HCV infection (P < 0.001), while the age distribution among these IDUs did not affect HCV infection (P = 0.279) (Table 3).
Table 3.
Multivariate logistic regression analysis for factors associates with HCV infection among drug users in Zhenjiang, Jiangsu province
| Factor | Multivariate adjusted OR (95% CI) | P value |
|---|---|---|
| Age | ||
| ≦25 | 1.201 (0.862–1.673) | 0.279 |
| 26–30 | ||
| 31–35 | ||
| 36–40 | ||
| ≧41 | ||
| Educational level | ||
| ≦Primary school | 3.306 (1.566–5.888) | 0.001 |
| Middle school | ||
| ≧High school | ||
| Pattern of drug use | ||
| Oral drug | 1.0 | <0.001 |
| Injecting | 14.055 (5.176–38.167) | |
Discussion
IDU plays an important epidemiologic role in the spread of HCV, as well as HIV [2, 21, 22, 28]. High HCV prevalence and co-infection rate with HIV were observed previously among IDUs in some areas of China [4, 12, 16, 18, 20, 28, 30]. Here, we found that HCV prevalence were 81.6 and 69.9% among IDUs and all drug users in Zhenjiang, Jiangsu (Table 1), respectively, slightly lower than 88.5 and 75.5% in other neighbor cities of Jiangsu, respectively [5]. Interestingly, HIV prevalence and HIV/HCV co-infection rate among Zhenjiang IDUs were 0.6 and 0%, respectively (Table 1), greatly lower than those (more than 20 and 90%, respectively) of some regions of Southern and Southwestern China [4, 12, 18, 28]. These results suggest that HCV transmission in Jiangsu (at least in Zhenjiang) might be geographically segregated from Southern and Southwestern China. In addition, we noted that the positive rates for syphilis were not only higher in HCV negative group than in HCV positive group, but also higher than for HIV in both HCV positive and negative groups (Table 1). These results might imply that there was less association in transmission routes between syphilis and HCV and/or HIV. The relatively higher rate of syphilis infection in HCV negative group than in HCV positive group might be due to that besides sexual contact, sores occurring on the lips and/or in the mouth also contribute to syphilis infection (http://www.cdc.gov/std/syphilis/STDFact-Syphilis.htm).
Compared with oral drug users (22.9%), IDUs had significantly higher HCV prevalence (81.6%) (P < 0.001) (Table 2), supporting a crucial role of IDU in HCV transmission [2, 21, 22, 28]. Interestingly, there was no obvious difference in HCV prevalence between needle-sharing IDUs (79.3%) and non-needle-sharing IDUs (92.9%) (P = 0.392) (Fig. 1b), consistent with a previous systematic review and meta-analysis [28]. This suggests that although sharing needles or syringes increases the risk of infection, it may be less important in HCV transmission than thought previously. Furthermore, this may also imply that besides needle-sharing there could be some potential unknown factors for HCV transmission among IDUs [28].
HCV prevalence among IDUs did not obviously increase along with the increases in duration of drug use (Fig. 3b) and frequency of injection per day (Fig. 4b), suggesting that HCV infection among IDUs was less associated with both risk factors. This implies that most IDUs (98.0%) might be infected by HCV within the first several (≦ 3) years of IDU [2, 21, 22, 28].
On the other hand, we noted that sexual protection measure appeared to result in a relatively lower HCV prevalence among drug users (Table 2), and female IDUs had a slightly higher HCV prevalence than male IDUs (Fig. 1b). These results, together with the fact that approximately 80% of female drug addicts were undertaking sex work [28], support that sexual transmission contributed to the total burden of HCV prevalence among drug users in China [21–23].
Consistent with previous investigations [27, 30], we found that most drug users (89.2%), HCV-infected drug users (96.7%), and IDUs (89.0%) in Zhenjiang were more than 25 years old, and the age peaks (33.5–38.2%) appeared in the groups of 31–35 years old (Fig. 2). The average duration of drug use among these cohorts were 11 year, and the average age of starting drug users was 25.8 years old, at which age most Chinese youngster should be soon after finishing their higher education. In addition, we found that most drug users (81.7%) had completed their middle or high school educations and they had significantly higher HCV prevalence than those with primary or lower education level (Tables 2, 3). These results, together with the finding that fewer addicts had received higher education (Table 2), strongly suggest that most Chinese addicts might start drug use soon after their middle/high school education, which might be ascribed to a severe lack of extensive school-based anti-drug education in most middle and/or high schools in China.
Currently, drug smuggling is strictly forbidden in China. The Chinese Government typically took some stringent measures to crackdown on drug smuggling and abuse [16, 28]. Drug users who are caught once, twice and more than twice will be sent to voluntary detoxification centers, compulsory rehabilitation centers, and re-education-through-labor centers, respectively. Furthermore, the Chinese Government also made great efforts to prevent HIV and HCV infection among IDUs. In spite of these measures and efforts, the number of drug users in China still increases constantly [4, 5], and these increased drug users will become new potential victims for HCV and HIV infection in China. The failure in control of the number of drug users may be ascribed, at least in part, to the severe lack of anti-drug education in most middle and/or high schools. Therefore, to reduce the drug use and to prevent the transmission of HIV and HCV among drug users, large-scale drug prevention education programs should be urgently conducted in all China’s middle and/or high schools.
Acknowledgments
We thank Yanhui Jia, Yan Huang, and Zhenzhen Wang for their technical assistance. This study was supported by the “Top-notch personnel” Project of Jiangsu University (to C. Zhang) and Jiangsu Health International Exchange Program (to G. He).
Contributor Information
Guangli He, Email: zjshgl@163.com.
Chiyu Zhang, Email: zhangcy1999@hotmail.com.
References
- 1.Alter MJ. Prevention of spread of hepatitis C. Hepatology. 2002;36:S93–S98. doi: 10.1002/hep.1840360712. [DOI] [PubMed] [Google Scholar]
- 2.Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13:2436–2441. doi: 10.3748/wjg.v13.i17.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balogun MA, Laurichesse H, Ramsay ME, Sellwood J, Westmoreland D, Paver WK, et al. Risk factors, clinical features and genotype distribution of diagnosed hepatitis C virus infections: a pilot for a sentinel laboratory-based surveillance. Commun Dis Public Health. 2003;6:34–39. [PubMed] [Google Scholar]
- 4.Bao YP, Liu ZM. Systematic review of HIV and HCV infection among drug users in China. Int J STD AIDS. 2009;20:399–405. doi: 10.1258/ijsa.2008.008362. [DOI] [PubMed] [Google Scholar]
- 5.Bao Y, Liu Z, Lu L. Review of HIV and HCV infection among drug users in China. Curr Opin in Psychiatr. 2010;23:187. doi: 10.1097/YCO.0b013e328338658b. [DOI] [PubMed] [Google Scholar]
- 6.Chen YD, Liu MY, Yu WL, Li JQ, Peng M, Dai Q, et al. Hepatitis C virus infections and genotypes in China. Hepatobiliary Pancreat Dis Int. 2002;1:194–201. [PubMed] [Google Scholar]
- 7.Dalgard O, Jeansson S, Skaug K, Raknerud N, Bell H. Hepatitis C in the general adult population of Oslo: prevalence and clinical spectrum. Scand J Gastroenterol. 2003;38:864–870. doi: 10.1080/00365520310004542. [DOI] [PubMed] [Google Scholar]
- 8.Deng LP, Gui XE, Wang X, Luo JL. A survey of HIV, HBV, HCV, HGV and TTV infections among drug abusers in Hubei Province (in Chinese) Hubei J Prev Med. 2003;14:2. [Google Scholar]
- 9.Dore GJ, Law M, MacDonald M, Kaldor JM. Epidemiology of hepatitis C virus infection in Australia. J Clin Virol. 2003;26:171–184. doi: 10.1016/S1386-6532(02)00116-6. [DOI] [PubMed] [Google Scholar]
- 10.Elghouzzi MH, Bouchardeau F, Pillonel J, Boiret E, Tirtaine C, Barlet V, et al. Hepatitis C virus: routes of infection and genotypes in a cohort of anti-HCV-positive French blood donors. Vox Sang. 2000;79:138–144. doi: 10.1046/j.1423-0410.2000.7930138.x. [DOI] [PubMed] [Google Scholar]
- 11.Garten RJ, Lai S, Zhang J, Liu W, Chen J, Vlahov D, et al. Rapid transmission of hepatitis C virus among young injecting heroin users in Southern China. Int J Epidemiol. 2004;33:182–188. doi: 10.1093/ije/dyh019. [DOI] [PubMed] [Google Scholar]
- 12.Garten RJ, Zhang J, Lai S, Liu W, Chen J, Yu XF. Coinfection with HIV and hepatitis C virus among injection drug users in southern China. Clin Infect Dis. 2005;41(Suppl 1):S18–S24. doi: 10.1086/429491. [DOI] [PubMed] [Google Scholar]
- 13.Hahn JA, Page-Shafer K, Lum PJ, Ochoa K, Moss AR. Hepatitis C virus infection and needle exchange use among young injection drug users in San Francisco. Hepatology. 2001;34:180–187. doi: 10.1053/jhep.2001.25759. [DOI] [PubMed] [Google Scholar]
- 14.Mele A, Tosti ME, Marzolini A, Moiraghi A, Ragni P, Gallo G, et al. Prevention of hepatitis C in Italy: lessons from surveillance of type-specific acute viral hepatitis. SEIEVA collaborating Group. J Viral Hepat. 2000;7:30–35. doi: 10.1046/j.1365-2893.2000.00179.x. [DOI] [PubMed] [Google Scholar]
- 15.Moore A, Herrera G, Nyamongo J, Lackritz E, Granade T, Nahlen B, et al. Estimated risk of HIV transmission by blood transfusion in Kenya. Lancet. 2001;358:657–660. doi: 10.1016/S0140-6736(01)05783-X. [DOI] [PubMed] [Google Scholar]
- 16.Qian HZ, Schumacher JE, Chen HT, Ruan YH. Injection drug use and HIV/AIDS in China: review of current situation, prevention and policy implications. Harm Reduct J. 2006;3:4. doi: 10.1186/1477-7517-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ray VL, Chaudhary RK, Choudhury N. Transfusion safety in developing countries and the Indian scenario. Dev Biol. 1999;102:195–203. [PubMed] [Google Scholar]
- 18.Ruan YH, Hong KX, Liu SZ, He YX, Zhou F, Qin GM, et al. Community-based survey of HCV and HIV coinfection in injection drug abusers in Sichuan Province of China. World J Gastroenterol. 2004;10:1589–1593. doi: 10.3748/wjg.v10.i11.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shan H, Wang JX, Ren FR, Zhang YZ, Zhao HY, Gao GJ, et al. Blood banking in China. Lancet. 2002;360:1770–1775. doi: 10.1016/S0140-6736(02)11669-2. [DOI] [PubMed] [Google Scholar]
- 20.Shang H, Zhong P, Liu J, Han X, Dai D, Zhang M, et al. High prevalence and genetic diversity of HCV among HIV-1 infected people from various high-risk groups in China. PLoS ONE. 2010;5:e10631. doi: 10.1371/journal.pone.0010631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 22.Sy T, Jamal MM. Epidemiology of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3:41–46. doi: 10.7150/ijms.3.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terrault NA. Sexual activity as a risk factor for hepatitis C. Hepatology. 2002;36:S99–S105. doi: 10.1002/hep.1840360713. [DOI] [PubMed] [Google Scholar]
- 24.Thorpe LE, Ouellet LJ, Levy JR, Williams IT, Monterroso ER. Hepatitis C virus infection: prevalence, risk factors, and prevention opportunities among young injection drug users in Chicago, 1997–1999. J Infect Dis. 2000;182:1588–1594. doi: 10.1086/317607. [DOI] [PubMed] [Google Scholar]
- 25.Thorpe LE, Ouellet LJ, Hershow R, Bailey SL, Williams IT, Williamson J, et al. Risk of hepatitis C virus infection among young adult injection drug users who share injection equipment. Am J Epidemiol. 2002;155:645–653. doi: 10.1093/aje/155.7.645. [DOI] [PubMed] [Google Scholar]
- 26.Wang NC, Qiao XC, Zhang LF, Liu YP, Wu LP. Seroepidemiology of HCV infection among different populations in Shanxi (in Chinese) Chin Public Health. 2001;17:1. [Google Scholar]
- 27.Xia X, Luo J, Dong J, You H, Qian Y, Qian X. Epidemiological survey on the HCV infection among drug users in some areas of Jiangsu (in Chinese) Mod Prev Med. 2007;34:3. [Google Scholar]
- 28.Xia X, Luo J, Bai J, Yu R. Epidemiology of hepatitis C virus infection among injection drug users in China: systematic review and meta-analysis. Public Health. 2008;122:990–1003. doi: 10.1016/j.puhe.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Yang J, Ding JJ, Li YY. Co-infection with HCV and HGV among 107 injection drug users (in Chinese) Chin J Prev Med. 2000;34:1. [Google Scholar]
- 30.Zhang C, Yang R, Xia X, Qin S, Dai J, Zhang Z, et al. High prevalence of HIV-1 and hepatitis C virus coinfection among injection drug users in the southeastern region of Yunnan, China. J Acquir Immune Defic Syndr. 2002;29:191–196. doi: 10.1097/00042560-200202010-00014. [DOI] [PubMed] [Google Scholar]
- 31.Zhang C, Wu N, Liu J, Ge Q, Huang Y, Ren Q, et al. HCV subtype characterization among injection drug users: implication for a crucial role of Zhenjiang in HCV transmission in China. PLoS ONE. 2011;6:e16817. doi: 10.1371/journal.pone.0016817. [DOI] [PMC free article] [PubMed] [Google Scholar]