Abstract
Macrobrachium rosenbergii is the most important cultured freshwater prawn in the world and it is now farmed on a large scale in many countries. Generally, freshwater prawn is considered to be tolerant to diseases but a disease of viral origin is responsible for severe mortalities in larval, post-larval and juvenile stages of prawn. This viral infection namely white tail disease (WTD) was reported in the island of Guadeloupe in 1995 and later in Martinique (FrenchWest Indies) in Taiwan, the People’s Republic of China, India, Thailand, Australia and Malaysia. Two viruses, Macrobrachium rosenbergii nodavirus (MrNV) and extra small virus-like particle (XSV) have been identified as causative agents of WTD. MrNV is a small icosahedral non-enveloped particle, 26–27 nm in diameter, identified in the cytoplasm of connective cells. XSV is also an icosahedral virus and 15 nm in diameter. Clinical signs observed in the infected animals include lethargy, opaqueness of the abdominal muscle, degeneration of the telson and uropods, and up to 100 % within 4 days. The available diagnostic methods to detect WTD include RT-PCR, dot-blot hybridization, in situ hybridization and ELISA. In experimental infection, these viruses caused 100 % mortality in post-larvae but failed to cause mortality in adult prawns. The reported hosts for these viruses include marine shrimp, Artemia and aquatic insects. Experiments were carried out to determine the possibility of vertical transmission of MrNV and XSV in M. rosenbergii. The results indicate that WTD may be transferred from infected brooders to their offspring during spawning. Replication of MrNV and XSV was investigated in apparently healthy C6/36 Aedes albopictus and SSN-1 cell lines. The results revealed that C6/36 and SSN-1cells were susceptible to these viruses. No work has been carried out on control and prevention of WTD and dsRNA against protein B2 produced RNAi that was able to functionally prevent and reduce mortality in WTD-infected redclaw crayfish.
Introduction
Macrobrachium rosenbergii, commonly known as ‘scampi’, is an economically important cultured freshwater prawn throughout the world. It is the most favoured species for farming and farmed on a large scale in many countries. The scampi is native to Southeast Asian countries and being cultured in Thailand, Vietnam, Kampuchea, Malaysia, Myanmar, Bangladesh, India, Sri Lanka, and the Philippines. It is also produced in Israel, Japan, Taiwan, and some African, Latin American, and Caribbean countries [10]. In the western hemisphere, examination of the possibility of intensive freshwater prawn culture began in the 1960s, but a considerable proportion of the global farmed freshwater prawn output still originates from the Indian sub-continent, Southeast Asia, and tropical areas of Latin America. Considering its high export potential, the giant freshwater prawn enjoys immense potential for culture in India. About 4 million hectares of impounded freshwater bodies in India offer great potential for freshwater prawn culture. In India its culture was introduced to compensate the heavy economic losses due to the epidemic white spot syndrome (WSS) in penaeid shrimp farming, hypothesizing the resistance of the giant freshwater prawn to WSS [19]. Freshwater prawn farming in India has increased dramatically in the last decade and total production has reached 30,450 MT in 2003 in India. The prawns are exported to eastern European countries and the USA. Since the world market for scampi is expanding at attractive prices, there is great potential for scampi production and export.
Three species of Macrobrachium are very important for culture purpose and being cultured in different countries. M. rosenbergii is cultured in many countries, the oriental river prawn M. nipponens in China [9, 35], and the monsoon river prawn M. malcolmsonii in India [8, 9]. Disease has been considered as one of the important constraints to limit the production of freshwater prawn worldwide. Generally, M. rosenbergii is considered to be moderately disease-resistant in comparison to penaeid shrimp. Very few viral pathogens such as Macrobrachium hepatopancreatic parvo-like virus (MHPV), Macrobrachium muscle virus (MMV), infectious hypodermal and hematopoietic necrosis virus (IHHNV), white spot syndrome virus (WSSV), Macrobrachium rosenbergii nodavirus (MrNV), and extra small virus like particle (XSV) have been reported in freshwater prawn. Vijayan et al. [33] have reported the incident of mortality in post-larvae of M. rosenbergii due to possible viral pathogen resembling to Macrobrachium muscle virus (MMV). Among these viruses, MrNV and XSV are important and responsible for huge economic losses, particularly in the hatchery and nursery phases. Hence this review will focus on white tail disease caused by MrNV and XSV.
Geographical Distribution
White tail disease (WTD) caused by MrNV and XSV is responsible for large-scale mortalities in hatchery and nursery phases of prawn culture system. The disease was first reported in the French West Indies [1], later in China [13], India [20], Chinese Taipei [34], Thailand [37], Australia [11] and Malaysia [17].
Clinical Signs and Histopathology
The clinical signs of WTD-infected post-larvae of prawn include lethargy and opaqueness of the abdominal muscle starting at second or third abdominal segment and gradually extend from the center to the anterior and the posterior sections of the muscle (Fig. 1). The whitish muscle appearance gave the name to the disease. Degeneration of the telson and uropods is observed in severe cases. Larvae, post-larvae and juveniles of M. rosenbergii are highly susceptible to WTD, which often causes high mortalities in these life stages. Mortality may reach a maximum in about 5 or 6 days after the appearance of the first gross signs. Very few post-larvae with WTD survive beyond 15 days in an outbreak, and post-larvae that survive may grow to market size like any other normal post-larvae. Adults are resistant to WTD, but act as carriers [13, 20].
Fig. 1.
Post-larvae of Macrobrachium rosenbergii with whitish muscle (arrow) and normal post-larvae with transparent body
The most affected tissue in infected PL is striated muscle of the cephalothorax, abdomen and tail. Histological features include the presence of acute Zenker’s necrosis of striated muscles, characterised by severe hyaline degeneration, necrosis and muscular lysis. Moderate oedema and abnormal open spaces among the affected muscle cells are also observed. There are large oval or irregular basophilic cytoplasmic inclusion bodies with a diameter of 1–4 μm in infected muscles of the abdomen, cephalothorax, and intra tubular connective tissue of the hepatopancreas. No viral inclusions were observed in epithelial cells of the hepatopancreatic tubules or in midgut mucosal epithelial cells. Pathognomonic oval or irregular basophilic cytoplasmic inclusion bodies are demonstrated in the target tissues by histology [1, 6]. The presence of MrNV in infected cells can be demonstrated in histological sections using a DIG-labelled DNA in situ hybridisation probe specific for MrNV [24].
Causative Organisms
Two viruses namely Macrobrachium rosenbergii nodavirus (MrNV) and extra small virus-like particle (XSV) are responsible for WTD [2]. MrNV is a small, icosahedral, non-enveloped particle, 26–27 nm in diameter (Fig. 2a). It contains two single-stranded RNAs: RNA1 (2.9 kb) and RNA2 (1.26 kb). Its capsid contains a single polypeptide of 43 kDa (Fig. 3). With these characteristics, it is closely related to the Nodaviridae family. XSV is also a non-enveloped icosahedral virus, 15 nm in diameter (Fig. 2b), with a linear ssRNA genome of 0.9 kb encoding two overlapping structural proteins of 16 and 17 kDa (Fig. 3). Because of its small size and absence of gene-encoding enzymes required for replication, it has been suggested that XSV may be a satellite virus and MrNV a helper virus.
Fig. 2.
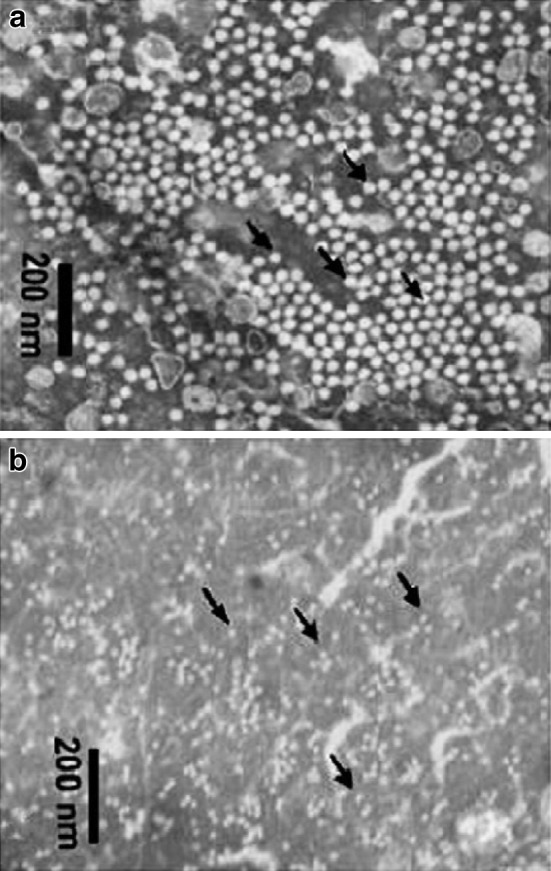
Negatively stained purified MrNV (a) and XSV (b)
Fig. 3.
SDS-PAGE analysis of MrNV and XSV particles purified by sucrose and CsCl density gradients. Lane M Marker; Lanes 1 Purified MrNV (43 kDa); Lanes 2 Purified XSV (17 and 16 kDa)
Nucleotide sequencing of the MrNV genome indicated that RNA-1 was composed of 3,202 nucleotides (Acc. No. AY222839) and that RNA-2 contained 1,175 nucleotides (Acc. No. AY222840). Both RNAs appeared to be in sense orientation, allowing thus their direct translation. Partial amino-acid sequencing of the N-terminal end of CP-43 established that this structural protein was encoded by RNA-2 and its estimated size corresponds to that determined by SDS-PAGE analysis of CP-43. No other coding sequences were found in RNA-2. RNA-1 contained the coding sequences of two non-structural proteins, A protein, i.e. an RNA-dependent RNA polymerase (RdRp) consisting of ca 1,000 amino-acids (ca 100 kDa) and B protein with an estimated size of 13 kDa. B protein was encoded by the 3′ region of RNA-1 (2,725–3,126 nucleotides). It turned out therefore that the genome contains genes required for its replication and most probably also for the regulation of its development within host-cells as it codes for an RdRp which is absent in the host-cell, the capsid protein and most probably the regulator protein B. Except for the RdRp needed for the genome replication, all the necessary enzymes are provided by the host-cells. Nothing is yet known on the mechanism of the virus development. In other noda viruses so far studied, the regulation of RNA replication involves the sub genomic RNA-3 and the protein B [7]. The nucleotide sequence analysis of 850-bp segment of Indian isolate of MrNV showed 98 % nucleotide and 99 % amino acid sequence identity with the reported sequence of an MrNV isolate from the West Indies [22].
Nucleotide sequencing of the XSV genome showed that the viral RNA is composed of 796 nucleotides and in sense orientation. A short poly (A) tail was found as well as a polyadenylation signal AAUAAA, localized 195 nucleotides downstream of the second stop codon and six nucleotides upstream of the poly(A) [25]. XSV genome contained the coding sequence of the capsid protein CP-17 or CP-16 and no other coding sequences were found. Amino acid sequencing of CP-17 and CP-16 clearly indicated that both polypeptides were encoded by the same reading frame and that CP-16 was a truncated form of CP-17, lacking the first 11N-terminal amino acids and it is worthwhile mentioning that the N-terminal end of both polypeptides is methionine. CP-16 was not a degraded form of CP-17 as both polypeptides were found in equimolar ratio and analysis was carried out by directly dissolving the virus particles in Laemmli buffer [2]. Besides, the ATG codon of CP-16 is localized in a favorable Kozak context as the ATG codon of CP-17 (7 out of 11 nucleotides matching with the Kozak consensus sequence). The organization of MrNV and XSV genomes and the encoded proteins are shown in Fig. 4.
Fig. 4.
Organization and encoded structural and non-structural proteins of MrNV and XSV genomes
Genes of MrNV such as RNA-dependent RNA polymerase (RdRp), B2 and capsid genes were amplified, cloned and sequenced. Expression of recombinant RdRP (44.5 kDa) B2 (32.2 kDa) and capsid (58.4 kDa) proteins was confirmed by Western blot analysis using anti-His mouse monoclonal antibodies [23].
Pathogenicity
These two viruses caused 100 % mortality in post-larvae 7–10 days post infection by immersion. In the virus-infected group, post-larvae started showing whitish muscles 5 days p.i. and reached the highest proportion at 7 days p. i. When infected by the intramuscular route, the viruses failed to cause mortality in adult prawns. RT-PCR analysis confirmed the infection in experimentally infected post-larvae [13, 21] and presence of viruses in gill tissue, head muscle, stomach, intestine, heart, hemolymph, pleopods, ovaries, and tail muscles of experimentally injected adult prawns [21]. MrNV and XSV were purified and post-larvae were challenged with different combinations of these two viruses by immersion [38]. Clinical signs of WTD were observed in post-larvae challenged with combinations containing a relatively high proportion of MrNV and low proportion of XSV. In contrast, there was little sign of WTD in post-larvae challenged with a higher proportion of XSV than MrNV, indicating that MrNV plays a key role in WTD of M. rosenbergii.
Transmission of Disease
Experiments were carried out to determine the possibility of vertical transmission of MrNV and XSV in M. rosenbergii [28]. Prawn brooders inoculated with MrNV and XSV by oral or immersion challenge survived without any clinical signs of WTD. Brooders spawned 5–7 days p. i. and eggs hatched. The survival rate of larvae gradually decreased, and 100 % mortality was observed at the post-larvae stage. Whitish muscle, the typical sign of WTD, was seen in advanced larvae stages. Ovarian tissue and fertilized eggs were positive for MrNV/XSV when tested by RT-PCR whereas larvae were positive by nested RT-PCR [28].
Host Susceptibility
The susceptibility of three species of marine shrimp (Penaeus indicus, P. japonicus, and P. monodon) to MrNV and XSV was tested by oral route and intramuscular injection [27]. The viruses failed to produce mortality in the shrimp but RT-PCR analysis revealed the presence of MrNV and XSV in the gills, abdominal muscle, stomach, intestine, and hemolymph of shrimp injected with the viruses. Re-inoculation using inoculum of MrNV and XSV prepared from the marine shrimp caused 100 % mortality in freshwater prawn post-larvae and moribund post-larvae showed positive for these viruses by RT-PCR. Our study indicates the possibility that marine shrimp act as a reservoir for MrNV and XSV and that these viruses maintain virulence in the shrimp tissue system. Nodavirus tentatively named PvNV (Penaeus vannamei nodavirus) was reported causing muscle necrosis in P. vannamei found in Belize in 2004 [31]. Numerous similarities were noted between PvNV and MrNV, particularly 69 % of similarity was found between the amino-acid sequence of the MrNV CP 43, the coat protein encoded by RNA-2 and the deduced amino-acid sequence of a PvNV cloned genome fragment. Based on alignment of known amino acid sequences of the coat protein of alpha- and beta nodavirus, prawn Nodavirus (MrNV) and shrimp Nodavirus (PvNV), three distinct groups emerge from the phylogenetic tree obtained in the noda viridae family: alpha nodavirus (terrestrial environment of invertebrates—insects), betanodavirus (aquatic environment of vertebrates—fish) and prawn and shrimp nodavirus (aquatic environment of invertebrates—crustacean).
Some farmers have considered culturing shrimp (P. monodon) with M. rosenbergii, or crop rotation between these two species, as an alternative for sustenance and economic viability. However, such a possibility invites the transmitting of pathologically significant organisms from native to non-native hosts, as observed in our study. Recently, a natural WTD infection was observed in hatchery-reared P. indicus in a hatchery near Chennai where prawn and shrimp seed are produced simultaneously. Clinical signs included lethargy and opaqueness of the abdominal muscle. Opaqueness appeared at the centre of the abdominal muscle and gradually extended anteriorly and posteriorly with 100 % mortality within 2–3 days after appearance of the whitish muscle. RT-PCR assay confirmed the presence of MrNV and XSV [15].
In horizontal transmission experiments, five developmental stages of Artemia were exposed to MrNV and XSV by immersion and oral routes to determine whether Artemia acts as a reservoir or carrier of the viruses [26]. There was no significant difference in percent mortality between Artemia control groups and virus-challenged groups although Artemia of all stages tested positive for both viruses by nested RT-PCR, regardless of the challenge route. Mortality was 100 % in M. rosenbergii post larvae fed Artemia nauplii exposed to MrNV and XSV by either immersion or oral challenge routes while no mortality was observed in post-larvae fed virus-free Artemia. RT-PCR analysis of the M. rosenbergii post larvae confirmed the presence of MrNV and XSV in the challenge group and its absence in the control group. Thus, virus exposed Artemia are capable of transmitting the disease to M. rosenbergii post-larvae.
Among the 5 species of aquatic insects examined, the giant water bug Belostoma sp., dragonfly nymphs Aesohna sp., diving beetles Cybister sp. and back swimmers Notonecta sp. gave positive RT-PCR results for MrNV and XSV. By contrast, results for the water scorpion Nepa sp. were negative [30].
Susceptibility of Cell Lines to MrNV/XSV Infection
MrNV/XSV could be easily propagated in the C6/36 mosquito Aedes albopictus cell line [29] and this cell line can be cultured easily in Leibovitz L-15 medium containing 100 International Units ml–1 penicillin, 100 μg ml–1 streptomycin and 2.5 μg ml−1 fungizone supplemented with 10 % fetal bovine serum at 28 °C [29]. Other cell lines, namely the fish SSN-1 cell line, partially support the multiplication of these viruses [5].
Diagnostic Methods
The protocol for the RT-PCR for detection of MrNV/XSV developed by Sri Widada et al. [24] and Sahul Hameed et al. [20, 21] is recommended for all situations. MrNV and XSV can be detected by RT-PCR separately using a specific set of primers or these two viruses can be detected simultaneously using a single-tube one-step multiplex RT-PCR [32, 36]. Nested RT-PCR (nRT-PCR) is also available and recommended for screening brood stock and seed [26].
Quantitative RT-PCR (RT-qPCR) assay can be performed to quantify the MrNV/XSV in the infected samples using the SYBR green dye based on the method described by Zhang et al. [38].
Haridas et al. [3] and Pillai et al. [12] have applied loop-mediated isothermal amplification (LAMP) for rapid diagnosis of MrNV and XSV in the freshwater prawn. A set of four primers, two outer and two inner, have been designed separately for detection of MrNV and XSV. In addition, a pair of loop primers specific to MrNV and XSV has been used to accelerate LAMP reaction.
Antibody-based diagnostic methods for MrNV include the ELISA described by Romestand and Bonami [15] or the triple-antibody sandwich (TAS) ELISA based on a monoclonal antibody [14].
Control and Prevention
No work has been carried out on control and prevention of WTD. However, proper preventive measures, such as screening of brood stock and PL, and good management practices may help to prevent WTD in culture systems. As the life cycle of M. rosenbergii is completed under controlled conditions, specific pathogen free (SPF) brood stock and PL can be produced by screening using sensitive diagnostic methods such as reverse-transcription polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA) [16, 24, 36]. Hayakijkosol and Owens [4] reported a sequence specific dsRNA against protein B2 produced RNAi that was able to functionally prevent and reduce mortality in WTD-infected redclaw crayfish. RNAi is an effective tool to protect against MrNV infection. Significant up-regulation of immune-related genes was observed in prawn treated with recombinant RdRp protein and this indicates the possibility of inducing the resistance against WTD in prawn using viral proteins [18].
References
- 1.Arcier JM, Herman F, Lightner DV, Redman R, Mari J, Bonami JR. A viral disease associated with mortalities in hatchery-reared post larvae of the giant freshwater prawn Macrobrachium rosenbergii. Dis Aquat Org. 1999;38:177–181. doi: 10.3354/dao038177. [DOI] [Google Scholar]
- 2.Bonami JR, Shi Z, Qian D, Sri Widada J. White tail disease of the giant freshwater prawn, Macrobrachium rosenbergii: separation of the associated virions and characterization of MrNV as a new type of nodavirus. J Fish Dis. 2005;28:23–31. doi: 10.1111/j.1365-2761.2004.00595.x. [DOI] [PubMed] [Google Scholar]
- 3.Haridas Divya V, Pillai D, Manojkumar B, Mohanakumaran C, Nair PM. Sherief Optimisation of reverse transcriptase loop-mediated isothermal amplification assay for rapid detection of Macrobrachium rosenbergii noda virus and extra smallvirus in Macrobrachium rosenbergii. J Virol Methods. 2010;167:61–67. doi: 10.1016/j.jviromet.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayakijkosol O, Owens L. B2 or not B2: RNA interference reduces Macrobrachium rosenbergii nodavirus replication in redclaw crayfish (Cherax quadricarinatus) Aquaculture. 2012;326–329:40–45. doi: 10.1016/j.aquaculture.2011.11.023. [DOI] [Google Scholar]
- 5.Hernandez-Herrera RI, Chappe-Bonnichon V, Roch P, Sri Widada J, Bonami JR. Partial susceptibility of the SSN-1 fish cell line to a crustacean virus: a defective replication study. J Fish Dis. 2007;30:673–679. doi: 10.1111/j.1365-2761.2007.00852.x. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh CY, Chuang PC, Chen LC, Tu C, Chien MS, Huang KC, Kao HF, Tung MC, Tsai SS. Infectious hypodermal and haematopoietic necrosis virus (IHHNV) infections in giant freshwater prawn, Macrobrachium rosenbergii. Aquaculture. 2006;258:73–79. doi: 10.1016/j.aquaculture.2006.04.007. [DOI] [Google Scholar]
- 7.Iwamoto T, Mise K, Takeda A, Okinaka Y, Mori KI, Arimoto M, Okuno T, Nakai T. Characterization of striped jack nervous necrosis virus sub genomic RNA3 and biological activities of its encoded protein B2. J Gen Virol. 2005;86:2807–2816. doi: 10.1099/vir.0.80902-0. [DOI] [PubMed] [Google Scholar]
- 8.Kanaujia DR, Mohanty AN, Tripathi SD. Growth and production of Indian river prawn Macrobrachium malcolmsonii (H. Milne Edwards) under pond conditions. Aquaculture. 1997;154:79–85. doi: 10.1016/S0044-8486(97)00013-6. [DOI] [Google Scholar]
- 9.Kutty MN, Herman F, Le Menn H. Culture of other prawn species. In: New MB, Valenti WC, editors. Freshwater prawn culture: the farming of Macrobrachiumrosenbergii. Oxford: Blackwell Sci; 2000. pp. 393–410. [Google Scholar]
- 10.New MB. Freshwater prawn farming: a review. Aquaculture. 1990;88:99–143. doi: 10.1016/0044-8486(90)90288-X. [DOI] [Google Scholar]
- 11.Owens L, La Fauce K, Juntunen K, Hayakijkosol O, Zeng C. Macrobrachium rosenbergii nodavirus disease (white tail disease) in Australia. Dis Aquat Org. 2009;85:175–180. doi: 10.3354/dao02086. [DOI] [PubMed] [Google Scholar]
- 12.Pillai D, Bonami JR, Sri Widada J. Rapid detection of Macrobrachium rosenbergii nodavirus (MrNV) and extra small virus (XSV), the pathogenic agents of white tail disease of Macrobrachium rosenbergii (De Man), by loop-mediated isothermal amplification. J Fish Dis. 2006;29:275–283. doi: 10.1111/j.1365-2761.2006.00718.x. [DOI] [PubMed] [Google Scholar]
- 13.Qian D, Shi Z, Zhang S, Cao Z, Liu W, Li L. Extra small virus-like particles (XSV) and nodavirus associated with whitish muscle disease in the giant freshwater prawn. Macrobrachium rosenbergii. J Fish Dis. 2003;26:521–527. doi: 10.1046/j.1365-2761.2003.00486.x. [DOI] [PubMed] [Google Scholar]
- 14.Qian D, Liu W, Jianxiang W, Yu L. Preparation of monoclonal antibody against Macrobrachium rosenbergii nodavirus and application of TAS-ELISA for virus diagnosis in post-larvae hatcheries in east China during 2000–2004. Aquaculture. 2006;261:1144–1150. doi: 10.1016/j.aquaculture.2006.05.022. [DOI] [Google Scholar]
- 15.Ravi M, Nazeer Basha A, Sarathi M, Rosa Idalia HH, Sri Widada J, Bonami JR. Studies on the occurrence of white tail disease (WTD) caused by MrNV and XSV in hatchery-reared post-larvae of Penaeus indicus and P. monodon. Aquaculture. 2009;292:117–120
- 16.Romestand B, Bonami JR. A sandwich enzyme linked immunosorbent assay (S-ELISA) for detection of MrNV in the giant freshwater prawn, Macrobrachium rosenbergii (de Man) J Fish Dis. 2003;26:71–75. doi: 10.1046/j.1365-2761.2003.00432.x. [DOI] [PubMed] [Google Scholar]
- 17.Saedi Tayebeh A, Moeini H, Wen Tan S, Yusoff K, Hassan Daud M, Kua Chu B, Soon Tan G, Bhassu S. Detection and phylogenetic profiling of nodavirus associated with white tail disease in Malaysian Macrobrachium rosenbergii (de Man) Mol Biol Rep. 2012;39:5785–5790. doi: 10.1007/s11033-011-1389-7. [DOI] [PubMed] [Google Scholar]
- 18.Sahoo PK, Shekhar MS, Abhilipsa D, Manickam D, Pillai RB, Sahul Hameed AS. Immunomodulatory effect of recombinant RNA-dependent RNA polymerase protein of Macrobrachium rosenbergii nodavirus in giant freshwater prawn M. rosenbergii. Aquacult Res 2011;1–11.
- 19.Sahul Hameed AS, Xavier Charles M, Anilkumar M. Tolerance of Macrobrachium rosenbergii to white spot syndrome virus. Aquaculture. 2000;183:207–213.
- 20.Sahul Hameed AS, Yoganandhan K, Sri Widada J, Bonami JR. Studies on the occurrence of Macrobrachium rosenbergii nodavirus and extra small virus-like particles associated with white tail disease of M. rosenbergii in India by RT-PCR detection. Aquaculture. 2004;238:127–133. doi: 10.1016/j.aquaculture.2004.06.009. [DOI] [Google Scholar]
- 21.Sahul Hameed AS, Yoganandhan K, Sri Widada J, Bonami JR. Experimental transmission and tissue tropism of Macrobrachium rosenbergii nodavirus (MrNV) and its associated extra small virus (XSV) Dis Aquat Org. 2004;62:191–196. doi: 10.3354/dao062191. [DOI] [PubMed] [Google Scholar]
- 22.Shekhar MS, Azad IS, Ravichandran P. Comparison of dot blot and PCR diagnostic techniques for detection of white spot syndrome virus in different tissues of Penaeus monodon. Aquaclture. 2006;261:1122–1127. doi: 10.1016/j.aquaculture.2006.05.017. [DOI] [Google Scholar]
- 23.Shekhar MS, Sahoo PK, Manickam D, Abhilipsa D. Cloning, expression and sequence analysis of Macrobrachium rosenbergii nodavirus genes: Indian isolate. Aquacult Res. 2011;42:1778–1788. doi: 10.1111/j.1365-2109.2010.02775.x. [DOI] [Google Scholar]
- 24.Sri Widada J, Durand S, Cambournac I, Qian D, Shi Z, Dejonghe. Genome-based detection methods of Macrobrachium rosenbergii nodavirus, a pathogen of the giant freshwater prawn, Macrobrachium rosenbergii dot blot, in situ hybridization and RT-PCR. J Fish Dis. 2003;26:583–90. [DOI] [PubMed]
- 25.Sri Widada J, Bonami JR. Characteristics of the monocistronic genome of extra small virus, a virus-like particle associated with Macrobrachium rosenbergii nodavirus: possible candidate for a new species of satellite virus. J Gen Virol. 2004;85:643–646. doi: 10.1099/vir.0.79777-0. [DOI] [PubMed] [Google Scholar]
- 26.Sudhakaran R, Yoganandhan K, Ishaq Ahmed VP, Sahul Hameed AS. Artemia as a possible vector for Macrobrachium rosenbergii nodavirus (MrNV) and extra small virus transmission (XSV) to Macrobrachiumrosenbergii post-larvae. Dis Aquat Org. 2006;70:161–166. doi: 10.3354/dao070161. [DOI] [PubMed] [Google Scholar]
- 27.Sudhakaran R, Syed Musthaq S, Haribabu P, Mukherjee SC, Gopal C, Sahul Hameed AS. Experimental transmission of Macrobrachiumrosenbergii noda virus (MrNV) and extra small virus (XSV) in three species of marine shrimp (Penaeus indicus, Penaeus japonicus and Penaeus monodon) Aquaculture. 2006;257:136–141. doi: 10.1016/j.aquaculture.2006.02.053. [DOI] [Google Scholar]
- 28.Sudhakaran R, Ishaq Ahmed VP, Haribabu P, Mukherjee SC, Sri Widada J, Bonami JR, et al. Experimental vertical transmission of Macrobrachium rosenbergii nodavirus (MrNV) and extra small virus (XSV) from brooders to progeny in Macrobrachium rosenbergii and Artemia. J Fish Dis. 2007;30:27–35. doi: 10.1111/j.1365-2761.2007.00774.x. [DOI] [PubMed] [Google Scholar]
- 29.Sudhakaran R, Parameswaran V, Sahul Hameed AS. In vitro replication of Macrobrachium rosenbergii noda virus (MrNV) and extra small virus (XSV) in C6/36 cell line. J Virol Methods. 2007;146:112–118. doi: 10.1016/j.jviromet.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Sudhakaran R, Haribabu P, Rajesh Kumar S, Sarathi M, Ishaq Ahmed VP, Venkatesan C, Sahul Hameed AS. Natural aquatic insect carriers of Macrobrachium rosenbergii noda virus (MrNV) and extra small virus (XSV) Dis Aquat Org. 2008;79:141–145. doi: 10.3354/dao01886. [DOI] [PubMed] [Google Scholar]
- 31.Tang KFJ, Pantoja CR, Redman RM, Lightner DV. Development if in situ hybridization and RT-PCR assay for the detection of a nodavirus (PvNV) that causes muscle necrosis in Penaeus vannamei. Dis Aquat Org. 2007;75:183–190. doi: 10.3354/dao075183. [DOI] [PubMed] [Google Scholar]
- 32.Tripathy S, Sahoo PK, Kumari J, Mishra BK, Sarangi N, Ayyappan S. Multiplex RT-PCR detection and sequence comparison of viruses MrNV and XSV associated with white tail disease in Macrobrachium rosenbergii. Aquaculture. 2006;258:134–139. doi: 10.1016/j.aquaculture.2006.04.016. [DOI] [Google Scholar]
- 33.Vijayan KK, Stalin Raj V, Alavandi SV, Thillai Sekhar V, Santiago TC. Incidence of white muscle disease, a viral like disease associated with mortalities in hatchery-reared post larvae of the giant freshwater prawn Macrobrachium rosenbergii (De Man) from the South-east coast of India. Aquacult Res. 2005;36:311–316. doi: 10.1111/j.1365-2109.2005.01246.x. [DOI] [Google Scholar]
- 34.Wang CS, Chang JS. RT-PCR amplification and sequence analysis of Macrobrachium rosenbergii nodavirus and extra small virus (XSV) associated with white tail disease of M. rosenbergii (de Man) cultured in Taiwan. GenBank Direct Submission (2006). [DOI] [PubMed]
- 35.Wang G, Qianhong S. Culture of freshwater prawns in China. Aquaculture Asia. 1999;4:14–7.
- 36.Yoganandhan K, Sri Widada J, Bonami JR, Sahul Hameed AS. Simultaneous detection of Macrobrachium rosenbergii nodavirus and extra small virus by a single tube, one-step multiplex RT-PCR assay. J Fish Dis. 2005;28:65–69. doi: 10.1111/j.1365-2761.2004.00606.x. [DOI] [PubMed] [Google Scholar]
- 37.Yoganandhan K, Leartvibhan M, Sriwongpuk S, Limsuwan C. White tail disease of the giant freshwater prawn Macrobrachium rosenbergii in Thailand. Dis Aquat Org. 2006;69:255–258. doi: 10.3354/dao069255. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, Wang J, Yuan J, Li L, Zhang J, Bonami JR. Quantitative relationship of two viruses (MrNV and XSV) in white-tail disease of Macrobrachium rosenbergii. Dis Aquat Org. 2006;71:11–17. doi: 10.3354/dao071011. [DOI] [PubMed] [Google Scholar]





