Abstract
Hepatopancreatic parvovirus (HPV) is one of the major shrimp parvovirus which is known to cause slow growth in penaeid shrimps. HPV has been found in wild and cultured penaeid shrimps throughout the world and there is high genetic variation among the different geographic isolates/host species. Given its high prevalence, wide distribution and ability to cause considerable economic loss in shrimp aquaculture industry, HPV deserves more attention than it has received. Till date, a total of four complete genome sequences of HPV have been reported in addition to a large number of partial sequences. HPV infection is seldom observed alone in epizootics and has occurred in multiple infections with other more pathogenic viruses and in most cases, heavy infections result in no visible inflammatory response. A great deal of information has accumulated in recent years on the clinical signs, geographical distribution, transmission and genetic diversity of HPV infection in shrimp aquaculture. However, the mechanism by which HPV enters the shrimp tissues and pathogenesis of virus is still unknown. To date, no effective prophylactic measures are available to reduce the infection in shrimps. To control and prevent HPV infection, considerable research efforts are on. This review provides information on current knowledge on HPV infection in penaeid shrimp aquaculture.
Keywords: HPV, PmoDNV, PCR, Penaeid shrimps, Diagnosis
Introduction
Aquaculture is one of the fastest growing food producing sectors in the world. World aquaculture has grown at an average annual rate of 8.3 % from 1970 to 2008 [18]. The reported global production of food fish from aquaculture, including finfish, crustaceans, molluscs and other aquatic animals reached 52.5 million tonnes in 2008. Shrimp farming has rapidly expanded in Asia and generated substantial income for farmers in many developing countries. In India, the shrimp aquaculture industry started only during the mid-eighties, flourished well and proved lucrative initially until the sector was affected by diseases. The increased occurrence of devastating viral diseases in shrimp culture systems threatens the sustainability of both the aquaculture industry and the commercial shrimp fishery. In 1993, the shrimp aquaculture industry was hit by viral diseases and during that period, infections reached epizootic proportions in culture ponds all along the coast causing mass mortalities and extensive damage to the system. The main virus associated with these cases was the White spot syndrome virus (WSSV) [58]. However, the presence of other viruses like monodon baculovirus (MBV), hepatopancreatic parvovirus (HPV) [50], Laem-Singh virus (LSNV) [70] and more recently the infectious hypodermal and hematopoietic necrosis virus (IHHNV) [71, 72] together with WSSV caused the problems to be more serious. To date, there are almost 20 viruses known to infect penaeid shrimps in the world and many of them are able to cause mass mortalities in culture [27]. Thus the impact of viral diseases in terms of economic loss is very high and is approximately US $3–4 billion per year, with all major species of shrimp being affected [12, 16, 39, 42, 44, 91]. Of all viral diseases, parvoviral diseases are emerging as a major threat to penaeid shrimp culture due to their ability to cause slow growth in shrimp. Among the crustacean parvoviruses, only those of shrimp have been studied in detail. The two major parvoviruses which are known to cause slow growth in shrimp include HPV [67, 82] and IHHNV [4, 40, 77]. Even though the two densoviruses of shrimp, HPV and IHHNV are having similar structure, nearly identical virion size and shape, HPV infects hepatopancreatic epithelial cells of shrimps whereas IHHNV infects all organs of ectodermal and mesodermal origin except the midgut [45]. Spawner-isolated mortality virus from Penaeus monodon (SMVmon) is another major shrimp parvovirus which causes mid-crop mortality syndrome [26, 60, 61]. The present review provides current advances on HPV infection in penaeid shrimps.
Hepatopancreatic Parvovirus (HPV)
HPV or Penaeus monodon densovirus (PmoDNV) of penaeid shrimp is an emerging shrimp virus reported to cause considerable economic loss in shrimp aquaculture [25] and infects several penaeid shrimp species. It was first reported in Penaeus (Fenneropenaeus) chinensis from Korea, Penaeus merguiensis from Singapore, Penaeus semisulcatus from Kuwait and P. monodon from the Philippines [13, 41]. This virus is reported to cause mortalities in early larval and postlarval stages of shrimp [45, 80] and stunted growth in juveniles [24, 48].
HPV infection is associated with reduced growth rates of juvenile shrimp without showing any gross signs of disease [24]. Usually HPV infection appears as co-infections with other hepatopancreatic pathogens. The HPV-associated cumulative mortality was reported to be 50–100 % after 4–8 weeks in juvenile P. merguiensis [41]. Chantanachookin et al. [10] reported triple infection of P. monodon with yellow head virus (YHV), HPV and MBV from ponds. Due to nonspecific clinical signs, the importance of PmoDNV as a disease causing agent is under estimated and there is no quantitative information regarding the impact of this disease in Indian shrimp aquaculture. Till date, there is no HPV susceptible shrimp cell lines developed to study the pathology, modes of transmission and virulence mechanism of the virus. Recently an insect model, Acheta domesticus has been used to describe the viral etiology of Penaeus merguiensis densovirus (PmergDNV) of shrimp and this study points to the use of animal models as an alternative bioassay to cell lines [37].
Geographical Distribution
HPV was first discovered in Asia and all initial reports of HPV infected shrimp or prawns were from Asia or the Indo-Pacific region and hence thought to be of Indo-Pacific origin but later spread to wild shrimp in the Americas via importation of live Asian shrimp for aquaculture [47]. HPV is widely distributed in wild, cultured and hatchery reared shrimps throughout the world including Australia, Asia, Africa, China, Korea, Taiwan, the Philippines, Indonesia, Malaysia, Singapore, Kenya, Israel, Kuwait, North and South America and India [47, 50] (Fig. 1). HPV was introduced to areas throughout North and South America, the Pacific coast of western Mexico and along the coast of coastal El Salvador with the movement of infected Penaeus (Litopenaeus) vannamei [47]. Through the importation of infected Penaeuspenicilliatus from Kenya and P. (Fenneropenaeus) chinensis from Korea, this virus was introduced into Israel and Hawaii respectively. HPV was first reported in Australia from samples of Penaeus esculentus from Moreton Bay and the Gulf of Carpentaria [66]. Later, it was reported in P. merguiensis,P. monodon and Penaeus japonicus [47, 74, 80] from Australia. In 1992, HPV infection in the black tiger shrimp P. monodon was first reported from Thailand [20–22]. In India, the presence of HPV in hatchery-reared, early postlarvae (PL) and the mass mortalities in shrimp larvae due to HPV infection along with MBV and WSSV has been reported [50]. The prevalence of HPV infection in samples of P. monodon PL have been reported from India [87–89]. These findings suggest that HPV is distributed all over the world.
Fig. 1.
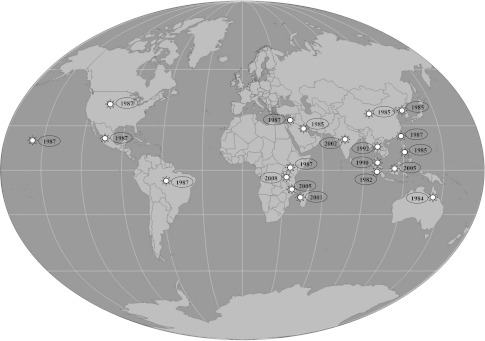
Global distribution of hepatopanreatic parvovirus (HPV). Asterisks indicates country and year of emergence
Host Range and Strains of HPV
A phylogenetic analysis based on genomic DNA of shrimp and insect parvoviruses suggests that IHHNV, SMVmon and HPVmon/HPVchin are a group of distantly related densoviruses having a host range outside the class Insecta that diverged from the Crustacea [73]. Its natural host range includes an ever increasing number of cultured and captured shrimp species [47, 66, 80] such as P. merguiensis, P. semisulcatus, Penaeus chinensis (orientalis), P. esculentus, P. monodon, Penaeus (Fenneropenaeus) indicus, P. penicillatus, P. japonicus, Penaeus (Litopenaeus) stylirostris and Penaeus vannamei from all around the world [41, 47, 74]. In India, HPV was detected in P. monodon, Penaeus indicus and P. semisulcatus by non-nested PCR and it was also detected from wild shrimps Parapenaeopsis stylifera, P. japonicus, Metapenaeus monoceros, M. affinis, M. elegans, M. dobsoni, M. ensis and Solenocera choprai by nested PCR [51]. A HPV-like agent has been reported in PL of Macrobrachium rosenbergii [2] and in Cray fish [36]. Recently PmeDNV was reported in the haemolymph of the mud crab, Scylla serata by PCR [62]. To date, ten strains of HPV have been described. The first strain (HPVchin) was reported in 1995 from P. chinensis in Korea [5] followed by HPV from P. monodon in Thailand (PmoDNV) in 1999 [82]. In 2005, HPVsemi was reported in wild stocks of P. semisulcatus from India [51] and the fourth penaeid shrimp strain was described from Australian P. merguiensis [34]. Recently, other strains of HPV have also been reported in P. monodon from India [76], Madagascar, New Caledonia and Tanzania [85] and two more strains have been reported in P. chinensis from South Korea [29] and China (GU371276).
Transmission
The first report on successful horizontal transmission of HPV by oral challenge in PL of P. monodon has been reported in 2003 [8]. HPV is believed to be transmitted both vertically and horizontally [44]. Recent report on the horizontal transmission of HPV from the infected Artemia to healthy post-larvae of P. monodon by feeding experiment suggest that this vector could also horizontally transmit HPV into rearing systems [79]. The horizontal transmission of HPV by cannibalism may also be a serious problem in transmission of this virus in shrimp farms. The report on HPV infection from parental broodstock to F1 progeny of P. chinensis suggested that this virus can also be transmitted vertically [47]. HPV infections in hatchery reared PL in India [50, 89] also suggested the vertical transmission of this viral infection. La Fauce and Owens [36] reported the susceptibility of crayfish, Cherax quadricarinatus to the Australian isolate of hepatopancreatic parvovirus (PmeDNV) infection. The juvenile crayfishes challenged with PmeDNV showed signs of disease within 1 week and mortalities were recorded 1 day after commencement of the clinical signs.
HPV infection starts by attaching to the microvilli followed by its entry into the host cell by pinocytosis [53]. The HPV maturation occurs in the nucleoplasm and later accumulated in the main inclusion bodies [65]. The accumulation of mature parvovirus particles during the last stage of the replication cycle, cause disruption of the nuclear membrane and release of virions into the cytoplasm [30]. On the liberation of whole intranuclear inclusion into the hepatopancreatic tubule lumens, HPV spreads to new host cells [65].
Clinical Signs of Disease
High levels of HPV infections have been reported during the early larval and postlarval stages [23, 45, 80]. This virus has been attributed to 100 % mortalities during outbreaks and result in stunted growth during the juvenile stages [20, 24, 48]. Some of the nonspecific clinical signs of HPV infected animals are poor growth rate, atrophy of the hepatopancreas, anorexia, decreased preening activity, increased surface and gill fouling by epicommensal organisms and sporadic opacity of tail musculature [11, 41, 43, 82]. Other than smaller size, HPV infected shrimps are often normal in coloration or length of antennae and has even been reported in apparently healthy hatchery reared larvae and in wild shrimp from India [51, 89]. HPV has been reported to cause serious crop losses in farms as the HPV infected small shrimp don’t fetch any market value. Increased mortality has been associated with stress and overcrowding conditions. However mortalities due to HPV infections are difficult to document as it is seldom observed alone in epizootics and usually occurs in association with other pathogens.
Taxonomic Affiliation and Morphology
HPV is considered as a member of the family Parvoviridae based on virion characteristics [5]. However, because of their distinctively different genome structure, unusual capsid proteins and size, HPV is considered as a new member of Densovirinae, subfamily which are able to infect vertebrates and invertebrates. HPV is a non-enveloped icosahedral virus with an average diameter of 22–24 nm and contains a single stranded linear DNA having an approximate size of 6 kb. HPV genome has hairpin structures on both the end of the linear DNA like any other parvoviruses. The buoyant density value of HPV by isopycnic centrifugation in the CsCl density gradient was estimated to be 1.412–1.425 g/ml [5, 84].
Genome Organization
Till date, four complete genome sequences of HPV are available, and these have been reported from Thailand (Penaeus monodon densovirus (PmoDNV) [84], Australia (Penaeus merguiensis densovirus (PmeDNV) [34], India (Penaeus monodon densovirus (PmoDNV) [76] and South Korea (F. chinensis hepatopancreatic densovirus-FcDNV) [29]. The South Korean isolate had the largest genome (6,336 bp; JN082231) followed by Thai (6,321 bp), Indian (6,310 bp) and Australian (6,299 bp) strains. Though a nucleotide sequence of a HPV strain from F. chinensis in China (6,085 bp) is available in GenBank (GU371276), it lacks the 3′- and 5′-terminal sequences compared to the other complete genome sequences of HPV [29]. Partial sequences are available from India (HPVsemi) [51], Korea (GenBank accession no. AY008257), Tanzania (GenBank accession no. EU588991), New Caledonia (GenBank accession no. EU346369) and Madagascar [85]. The viral genome of the HPV genome has high A+T content (58.2 %) [76] which is similar to the shrimp parvovirus IHHNV (56.96 %) [77] and the mosquito brevidensoviruses Aedes aegypti densovirus (AaeDNV), 62.5 % [1] and Aedes albopictus densovirus (AalDNV), 61.8 % [6]. Based on the phylogenetic tree constructed from the amino acid sequence of the right ORF, HPV isolates has been tentatively divided into 3 genotypes: Type I (Korea, Madagascar, Tanzania), Type II (Thailand, Indonesia, India) and Type III (Australia, New Caledonia) and the genetic variation was primarily associated with the geographic distribution of the host [29, 85].
A complete genome sequence analysis of HPV strains indicated that three ORFs have their own potential promoters with TATA boxes, transcription start signals (Inr-box), and downstream promoter elements. Upstream from the ORF1, ORF2 and ORF3, there were three functional promoters namely p2, p22 and p48. The putative promoter (p22) found upstream to the ORF2 controls the transcription of the ORF2 and the third set of putative transcription regulatory sequences (p48) controls transcription of the right ORF3 [84]. The complete genome of HPV virus was organized as monosense and the organization of genes from 5′ end of the complementary strand was in the order of ORF1 (NS2 gene), ORF2 (NS1 gene) and then ORF3 (VP gene) (Fig. 2). This organization was different from other parvoviruses in which the genes are organized as left ORF (NS1), mid ORF (NS2) and right ORF (VP) [1, 6, 77]. It was reported that in the Indian HPV isolate, there was a slight overlap (16 bp) between the non structural protein 2 (first ORF) and the non structural protein 1 (second ORF) but in different reading frames (reading frame 1 and 2) (Fig. 2). By analogy to this, in PstDNV a shrimp densovirus, the mid ORF (NS2) is located entirely within the left ORF (NS1) in a different reading frame [77]. HPV genome is the biggest among other parvoviruses (>6 kb) so far reported since the coding regions of HPV are non-overlapping. Both the 5′ and 3′ terminal sequences of the genome contained palindromic sequences capable of forming hairpin-like structures by base pairing which is necessary for replication and encapsidation of viral genome. Even though the nucleotide sequence at 5′ end could be folded into a hairpin-like structure it could not generate a typical Y-, T-, or J-form similar to other parvoviruses [84]. Due to the presence of asymmetrical generation of flip and flop at the 5′ and 3′ ends of HPV genome this viral DNA may replicate by using the modified rolling hairpin model as in case of autonomous parvovirus MVM [3].
Fig. 2.

Organization of open readings frames (ORFs) in the genome of Indian hepatopancreatic parvovirus (HPV) strain. The genome is shown in plus strand. The three open reading frames (ORFs; ORF1, ORF2 and ORF3) are indicated by boxes
Analysis of the complete genome sequences of different strains of HPV isolate suggests that HPV isolated from different shrimp species and/or different geographical region is genetically different. The nucleotide sequence similarity between different strains of HPV reported so far was determined by using BLAST searches for the sequence at the GenBank NCBI database (Table 1). The 10 % sequence variation between the Chinese and Korean strains explains that the diversity of HPV strains is affected by geographical distribution of the host species. Generally the genetic variation among different strains of parvovirus is only up to 4 % [17]. However, the HPV sequences isolated from different host and geographical region have unexpectedly high variation (Table 1). The sequence variation among HPV isolates from populations of P. chinensis from South Korea and Korea showed a very low nucleic acid variation (1 %) and these findings again shows that the genetic diversity of HPV strains is affected by geographical distribution of the host. It could be due to the genetic adaptation of the virus to the different environmental factors in different geographical areas.
Table 1.
Pairwise distance (%) among eight hepatopancreatic parvovirus (HPV) isolates
| HPV isolates | India (FJ410797) | Thailand (DQ002873) | Australia (DQ458781) | Tanzania (EU247528) | Madagascar (EU588991) | Korea (AY008257) | South Korea (JN082231) | China (GU371276) |
|---|---|---|---|---|---|---|---|---|
| India (FJ410797) | 100 | |||||||
| Thailand (DQ002873) | 86.37 | 100 | ||||||
| Australia (DQ458781) | 77.98 | 74.74 | 100 | |||||
| Tanzania (EU247528) | 80.63 | 80.05 | 81.33 | 100 | ||||
| Madagascar (EU588991) | 80.30 | 80.42 | 82.41 | 87.68 | 100 | |||
| Korea (AY008257) | 81.69 | 80.08 | 83.15 | 85.24 | 84.58 | 100 | ||
| South Korea (JN082231) | 80.82 | 76.22 | 82.15 | 85.18 | 84.56 | 98.76 | 100 | |
| China (GU371276) | 78.01 | 77.40 | 77.27 | 81.74 | 82.89 | 89.54 | 86.39 | 100 |
The similarity was estimated based on the complete genome sequences of Indian, Thai, Australian, South Korean HPV isolates and partial sequence from Korean, Tanzanian, Chinese and Madagascan HPV isolates
By analyzing the open reading frames of all four complete genome of HPV the ORF1 of Thai, Indian, Australian and South Korean isolate is 1287, 1281, 1026 and 1278 bp respectively. The function of NS2 protein in the viral genome is still unknown. It has been previously reported that NS2 proteins from minute virus of mice (MVM), a parvovirus, play a critical role in viral capsid assembly and in the generation of viral single-stranded DNA [14, 54, 55]. NS2 proteins have also been involved in the production of other viral replicative forms [9, 54, 55]. Furthermore, NS2 proteins seems to enhance NS1 associated parvovirus-induced cell killing in some, but not all cell lines tested [7, 19, 38]. The nucleotide sequence of ORF2 (NS1) of all three complete genome of HPV from Thai, Indian, Australian and South Korean isolate is 1740, 1734, 1737 and 1737 bp respectively. NS1 is the major replicative protein of the autonomous parvovirus [15] which is highly conserved across the present known genomes of HPV. Two regions within the NS1 protein sequence contain highly conserved motifs namely replication initiator motifs and NTP-binding and helicase domains. In parvoviruses, the replication initiator motifs are involved in initiation and termination of rolling circle replication [77]. In all HPV strains, these two motifs in the NS1 polypeptide are highly conserved when compared to rest of the parvoviral sequences. The structural protein (VP) of HPV is encoded by the third open reading frame. The nucleotide sequence of ORF3 (VP) of all four complete genome of HPV from Thailand, India, Australia and South Korea is 2457, 2460, 2454 and 2463 bp respectively. Like other parvoviruses, a highly conserved glycine-rich sequence was found in structural protein of HPV where as phospholipase A2 (PLA2) signature sequence was found to be absent in this shrimp densovirus.
Diagnosis of HPV Infection
Histology
Since there are no distinctive gross signs of HPV, initially only conventional diagnostic methods like histopathology of affected cells was being used for the diagnosis of HPV [47]. HPV infection is characterized by the presence of single, large basophilic intranuclear inclusion bodies in hypertrophied nuclei of hepatopancreas tubules and adjacent midgut cells [47]. HPV inclusion bodies when fully formed cause nuclear hypertrophy and compression and displacement of the host cell nucleolus. The inclusions of HPV infection is confined to actively dividing cells (E-cells) at the distal ends of hepatopancreatic tubules. It can also be diagnosed by preparing smears of hepatopancreatic tissue on a microscope slide in a drop of 10 % formalin solution containing 2.8 % NaCl. HPV inclusions bodies can be easily visualized after drying and normal H&E staining (Fig. 3). Transmission electron micrograph of a thin section of hepatopancreatic tissue of P. monodon infected with HPV showed that the developing inclusion bodies appeared as ovoid to spherical in shape with a diameter from 5 to11 μm. These inclusion bodies were composed of electron-dense granular material and virions which caused the lateral displacement of the nucleoli and margination of the chromatin.
Fig. 3.
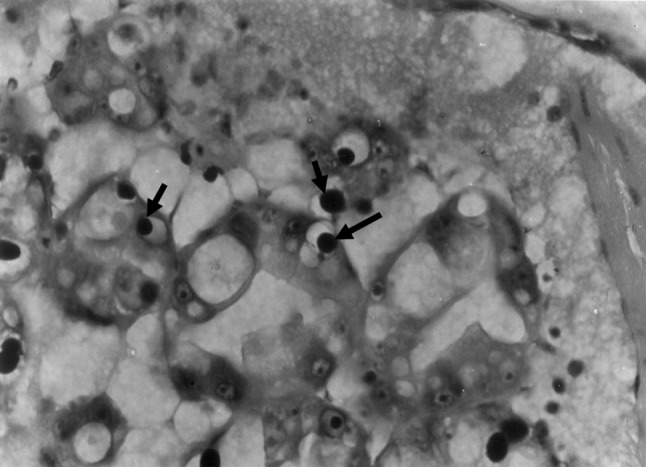
Histopathology of HPV infected P. monodon showing intranuclear inclusion bodies in hepathopancreatic tubular epithelial cells
Nucleic Acid Based Diagnostic Techniques
Due to the importance of this viral disease in shrimp aquaculture, many molecular diagnostic techniques were developed for easy and rapid detection of the virus that allow early diagnosis of the infection in shrimp larvae, parental broodstock and possible carriers of HPV in the shrimp aquaculture system. In comparison with conventional techniques, molecular diagnostic techniques are more rapid and non-destructive. Many DNA-based detection methods for HPV have been reported and some of these are commercially available.
Polymerase Chain Reaction (PCR)
PCR method has been widely used to detect HPV. Sequence insertions or duplications can generate size variations in the PCR product and the result may appear falsely negative. Hence, designing of the primer according to various strains of the virus is important to obtain greatest possible sensitivity and specificity. A PCR based detection method for HPVmon developed by Sukhumsirichart et al. [82] using the primer sets 121F and 276R yielded a product of 156 bp and this PCR assay could detect at least 1 fg purified HPV DNA. Pantoja and Lightner [64] designed another primer pairs (1120F/1120R) based on sequences of HPVchin that yielded a product of 592 bp. The commercial primer pair designed for HPVchin (7490F/7852R) from DiagXotics Inc. (Wilton, CT) yielded a 732 bp amplicon with HPVmon rather than the 350 bp amplicon with HPVchin [67]. It was due to the 30 % difference in DNA sequence of HPVchin and HPVmon. Thus, to improve the sensitivity for HPVmon detection, another primer pairs (H441F and H441R) were designed from the sequence of the 732 bp HPVmon. These primers yielded an amplicon of 441 bp and the sensitivity was as little as 1 fg of purified HPVmon DNA [68]. The primer pair HPV441F and HPV441R, specifically designed for HPVmon has been shown to be effective with HPV in P. monodon from India. By contrast, the primer pair designed for HPVchin (1120F and 1120R) was not able to detect HPV from India [89]. This could be due to the highest nucleotide similarity (88 %) of complete genome of HPV strain from India and Thailand when compared to HPVchin from Korea (i.e. 84 %) [76]. Later, two primers inner to 441 bp product (HPnF and HPnR) were designed to detect HPV infection in wild shrimp from India by nested PCR [90]. The 441 bp PCR fragment obtained from P. semisulcatus shows 86 % sequence similarity with the HPV from Thai P. monodon (HPVmon) [51]. In addition to this, a multiplex reverse transcription-polymerase chain reaction was developed for simultaneous detection of six major shrimp viruses including HPV, YHV, WSSV, TSV, IHHNV and MBV [31]. Though several PCR primers (Table 2) have been described for detection of HPV, a small nucleotide changes may give negative results with diagnostic methods like PCR. Hence, specific primer set should be used to detect different strains of virus from different geographical area. Strain variation may cause difficulties in performing specific diagnostic techniques like PCR [46, 67].
Table 2.
Primers used for detection of hepatopancreatic parvovirus (HPV) infection
| Primer code | Primer sequence (5′–3′) | Product size (bp) | Reference |
|---|---|---|---|
| H441F | GCATTACAAGAGCCAAGCAG | 441 | [68] |
| H441R | ACACTCAGCCTCTACCTTGT | ||
| HPnF | ATAGAACGCATAGAAAACGCT | 265 | [90] |
| HPnR | GGTGGCGCTGGAATGAATCGCTA | ||
| 121F | GCA CTT ATC ACT GTC TCT AC | 156 | [82] |
| 276R | GTG AAC TTT GTA AAT ACC TTG | ||
| 1120F | GGT GAT GTG GAG GAG AGA | 592 | [64] |
| 1120R | GTA ACT ATC GCC GCC AAC | ||
| 7490F | TGGAGGTGAGACAGCAGG | 732 | [67] |
| 7852R | CCA ACT GTC CTC GCT CTT | ||
| HPV140F | CTACTCCAATGGAAACTTCTGAGC | 140 | [35] |
| HPV140R | GTG GCG TTG GAA GGC ACT TC |
Real-Time PCR
Real-time PCR is an alternative method to conventional PCR which quantitates the number of viral copies in a tissue sample [33]. Real-time PCR monitors the fluorescence emitted during the reaction at the end of each PCR cycle as an indicator of amplicon production in real time as opposed to the endpoint detection. For the detection of Australian isolate HPV, a TaqMan based real-time PCR assay was developed to amplify a segment of the capsid protein gene [35]. The assay was very specific to Australian HPV and could not detect Thai and Korean strains which were having very low homology of the target sequence. Hence a generic TaqMan assay was developed for the detection of all strains of HPV by targeting relatively conserved regions within the genome [92]. This real-time PCR could detect HPV from nine geographic locations with a sensitivity of one copy of HPV DNA. Among the nine geographical locations, the real-time PCR primers designed for Korean HPV was most sensitive to the Korea, China-Yellow Sea and Taiwan HPV isolates. The high sensitivity was because of their geographic proximity. This real-time PCR could not detect the Indonesian and Mozambique HPV isolates, because of high nucleic acid sequence variation from the Korean HPV isolate.
Recently a duplex real-time PCR assay using TaqMan probes for the simultaneous detection of HPV and MBV was developed [86]. For the duplex PCR assay the primers/probe for HPV detection was designed based on a Thai isolate, because the Thai isolate showed highest number of nucleotide mismatches in the primers/probe targeted sequences of earlier real-time PCR primers reported from Australia and Korea [35, 92]. This real-time duplex assay had a detection limit of single copies for both HPV and MBV and used for the quantification of these viruses [86]. A highly specific and sensitive multiplex real-time PCR and high-resolution melting analysis was developed for simultaneous detection of PmoDNV, WSSV and YHV in penaeid shrimp [63]. To quantify HPV Type III infections in wild broodstocks and hatchery reared PL of P. chinensis from Korea, a highly sensitive type specific TaqMan real-time PCR was developed and the result showed that HPV Type III is widely distributed in Korea in addition to HPV Type I [28].
Loop-Mediated Isothermal Amplification (LAMP)
LAMP is a nucleic acid amplification technique by using a single temperature incubation of nucleic acid with 4 different primers to identify 6 distinct regions on the target gene. Hence, it avoids the need for expensive thermal cyclers used in conventional PCR and is highly specific. The LAMP is a quantitative technique in which the amplification can be followed in real-time either by measuring the turbidity or by the signals produced by fluorescent dyes that intercalate the DNA [57]. In addition to being inexpensive, isothermal amplification this technique is further simplified by the use of chromatographic, lateral flow dipstick (LFD) [32, 56]. For the specific detection of HPV, a LAMP based diagnostic technique was developed in combination with LFD [56] and this method was 10 times more sensitive than the one-step PCR [83] and the sensitivity was equivalent to nested PCR [51].
PCR–Enzyme Linked Immunosorbent Assay (PCR–ELISA)
The PCR–ELISA is an alternative method for the detection of nucleic acids which mimic enzyme linked immunosorbant assays. The technique mainly involves amplification of viral DNA by PCR and then hybridization of the PCR product with a specific probe and finally the detection of the hybridized product by ELISA technique [49, 93]. In this assay, the PCR products will be hybridized to an immobilized capture probe with sequences internal to the PCR product. Thus, it is an alternative and less expensive technique than real-time PCR. PCR–ELISA, a promising diagnostic tool has been developed for detection of HPV in P. monodon. A 156 bp HPV DNA fragment amplified and labeled with Digoxygenin-dUTP was detected using a biotin labeled specific internal sequence probe. This technique could detect up to three viral particles. Hence PCR–ELISA is more sensitive than conventional PCR and histological examination and can be used for field level applications where large numbers of samples can be analyzed simultaneously [83].
Probe Techniques
The development of non-radioactive labeling of nucleic acids methods has made gene probe technology readily available in shrimp diagnosis. This technology was first developed for the diagnosis of another parvovirus, IHHNV and now it is being used for other shrimp viruses [43, 52]. The two DIG-11-dUTP labeled probes developed for the detection of low grade infections of HPV infection which were specific to this virus [53]. Although these gene probes were very specific to HPV, it failed to explain the pathogenesis of the disease as individual HPV particles are very difficult to visualize. Hence, to overcome such problems, Pantoja and Lightner [65] used in situ hybridization combined with electron microscopy and could explain the aspects of the infection and development cycle of HPV. For this technique, a 592 bp HPV specific DNA probe labeled with DIG-11-dUTP was used to hybridize semi-thin and ultra thin sections of HPV infected tissues and examined by light and electron microscopy, respectively. A probe has also been developed from the 732 bp PCR amplicon of commercial PCR primers developed from HPVchin [68].
In Situ Hybridization
The DIG-11-dUTP labeled probes were used for in situ hybridization of HPV infected and uninfected shrimp to test the specificity. The probes produced a strong labeling of infected nuclei of HPV infected hepatopancreas [53]. At ultra structural level, a post-embedding in situ hybridization technique was developed to detect HPV infection of penaeid shrimp by using a probe labeled with DIG-11-dUTP and for the detection of hybridized probe in HPV infected juvenile P. monodon and P. chinensis PL using gold conjugated anti-DIG antibody was used [65]. The use of electron microscopic technique to detect in situ hybridized HPV nucleic acids could explain the replication cycle of HPV and the pathogenesis including the process of adsorption, penetration, and transport within the cytosol, penetration into the nucleus, replication and release [65]. The sensitivity of HPVmon detection with the commercial PCR primers (DiagXotics) and hybridization probes designed for HPVchin were significantly lower than that of HPVchin hence Phromjai et al. [68] designed PCR primers and hybridization probe using the sequence of the 732 bp HPVmon PCR amplicon obtained by the primer designed by Pantoja and Lightner [64]. Even though HPVchin (AY008257, 5,740 bp) and HPVmon (DQ002873, 6,321 bp) differ in DNA sequences by 20 %, both of them showed positive in situ hybridization reaction [67]. In addition, the Australian HPV strain from P. merquiensis and P. esculentis also reacted with gene probes from Asia [59] and showed positive in situ hybridization with a commercial DNA probe designed from HPVchin [47]. These results suggest that in situ hybridization technique cannot be used for strain identification of HPV and it may not be used as a highly sensitive technique to detect genomic sequence identity. Localization of virus in tissues is essential to detect them by in situ hybridization and it requires more technical skill and expensive equipments.
Dot Blot Hybridization
Dot blot is a type of hybridization technique to measure abundance of target sequences in a large number of samples. Phromjai et al. [68] used this hybridization technique to check the specificity of DIG-labeled probe for the specific detection of HPVmon. The sensitivity of dot blot hybridization using these probes were approximately 1 pg with field samples of shrimp feces and 0.1 pg with shrimp PL. A dot blot hybridization technique was also developed using 196 bp DNA probe labeled with DIG-11-dUTP to confirm the 441 bp PCR amplicon obtained from wild shrimp from India [51]. One drawback of dot blot detection was weak background signals probably due to non-specific probe binding. This would not lead to false positive results for heavy infections because of the inclusion of negative controls such as fecal or PL samples free of HPV together with sharply contrasting positive control samples. On the other hand, light infections would clearly go undetected and so the method would not be suitable for screening of carriers with low viral loads [68].
Monoclonal Antibody (MAb) Based Techniques
MAb based diagnosis, has proven to be an effective diagnostic method for the diagnosis of various viral diseases in shrimp like yellow head disease [78] and infectious hypodermal and hematopoietic necrosis [69]. Monoclonal antibodies specific to hepatopancreatic parvovirus was developed for HPV isolated from P. monodon [75]. These monoclonal antibodies were raised against a major 54 kDa protein of purified HPV and these antibodies could detect HPV infection in shrimp tissues by means of immunohistochemistry and by western blotting. Since the method for propagation of HPV in the laboratory has not been established, the availability of the antigen for monoclonal antibody production relied on the viral preparation from specimens collected from natural infection. To increase the sensitivity of the MAb based detection of HPV, various epitopes (PmoDNV-N and PmoDNV-C) of capsid protein were cloned and expressed and used for the production of monoclonal antibodies [81]. The detection limit of PmoDNV-N specific MAbs obtained was approximately 50 fmol μl−1 of antigen as determined by Dot blot and it was similar to the PmoDNV-MAb characterized by Rukpratanporn et al. [75] but the sensitivity of PmoDNV-C-specific MAbs was twofold less than the MAbs specific to PmoDNV-N. By using a combination of all three MAbs, the sensitivity of detection was improved by four fold for the PmoDNV-N protein and twofold for the partially purified PmoDNV [81]. Though the sensitivity of the immunological techniques is lower than PCR, they are more convenient, specific and less time-consuming and can be used for the detection of various shrimp pathogens with a large number of samples at much lower cost.
Concluding Remarks
HPV is a major causative agent of slow growth in shrimp that leads to considerable economic loss in shrimp aquaculture. HPV infection is associated with reduced growth rates of juvenile shrimp without showing any gross signs of disease. However, the absence of specific clinical signs of HPV infection and masking of HPV by other pathogens make the disease diagnosis of the disease is difficult by simple observation. Therefore, several robust detection methods have been developed for the specific and sensitive detection of HPV infection. However, because of its wide geographical distribution, wide host range and the presence of different genotypes, the continuous monitoring of this viral infection becomes difficult. Based on the information available to date, HPV is distributed worldwide and sequence analysis of their genome has shown variations among HPV isolates from different species and/or geographical areas. Due to the need to track and monitor genetic diversity, the complete genome sequence analysis of HPV strains from different areas throughout the world and from different host species is required. Variations in nucleotide sequence can cause closely related strains to have significantly different biological properties such as pathogenicity, tissue tropism or host range. Worldwide genetic monitoring of this virus in shrimp farms should be performed in order to verify the current prevalence, and a quarantine strategy to limit the impact of its spread. To date, no specific antiviral treatments or vaccinations against HPV are available and hence preventive measures are still the best way to reduce disease outbreaks. Thus, studies on prevention and control of HPV infection should be a priority. Further research on the proteins and their role in viral replication should form the basis for future studies in this area.
Acknowledgments
The authors gratefully acknowledge the financial support received from the Department of Biotechnology, Govt. of India and the facilities of the Bioinformatics centre of DBT.
References
- 1.Afanasiev BN, Galyov EE, Buchatsky LP, Kozlov YV. Nucleotide sequence and genomic organization of Aedes densonucleosis virus. Virology. 1991;185:323–336. doi: 10.1016/0042-6822(91)90780-F. [DOI] [PubMed] [Google Scholar]
- 2.Anderson IG, Law AT, Shariff M. A parvo-like virus in the giant freshwater prawn, Macrobrachium rosenbergii. J Invertebr Pathol. 1990;55:447–449. doi: 10.1016/0022-2011(90)90093-L. [DOI] [Google Scholar]
- 3.Astell CR, Chow MB, Ward DC. Sequence analysis of the termini of virion and replicative forms of minute virus of mice DNA suggests a modified rolling hairpin model for autonomous parvovirus DNA replication. J Virol. 1985;54:171–177. doi: 10.1128/jvi.54.1.171-177.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonami JR, Trumper B, Mori J, Brehelier M, Lightner DV. Purification and characterisation of the infectious hypodermal and hematopoietic necrosis virus of penaeid shrimps. J Gen Virol. 1990;71:2657–2664. doi: 10.1099/0022-1317-71-11-2657. [DOI] [PubMed] [Google Scholar]
- 5.Bonami JR, Mari J, Poulos BT, Lightner DV. Characterization of hepatopancreatic parvo-like virus, a second unusual parvovirus pathogenic for penaeid shrimps. J Gen Virol. 1995;76:813–817. doi: 10.1099/0022-1317-76-4-813. [DOI] [PubMed] [Google Scholar]
- 6.Boublik Y, Jousset FX, Bergoin M. Complete nucleotide sequence and genome organization of the Aedes albopictus parvovirus (AaPV) pathogenic for Aedes aegypti larvae. Virology. 1994;200:752–763. doi: 10.1006/viro.1994.1239. [DOI] [PubMed] [Google Scholar]
- 7.Brandenburger A, Legendre D, Avalosse B, Rommelaere J. NS-1 and NS-2 proteins may act synergistically in the cytopathogenicity of parvovirus MVMp. Virology. 1990;174:576–584. doi: 10.1016/0042-6822(90)90110-D. [DOI] [PubMed] [Google Scholar]
- 8.Catap ES, Lavilla-Pitogo CR, Maeno Y, Travina RD. Occurrence, histopathology and experimental transmission of hepatopancreatic parvovirus infection in Penaeus monodon postlarvae. Dis Aquat Org. 2003;57:11–17. doi: 10.3354/dao057011. [DOI] [PubMed] [Google Scholar]
- 9.Cater JE, Pintel DJ. The small non-structural protein NS2 of the autonomous parvovirus minute virus of mice is required for virus growth in murine cells. J Gen Virol. 1992;73:1839–1843. doi: 10.1099/0022-1317-73-7-1839. [DOI] [PubMed] [Google Scholar]
- 10.Chantanachookin C, Boonyaratanapalin S, Kasornchandra J, Direkbusarakom S, Ekpanithanpong U, Supamataya K, Siurairatana S, Flegel TW. Histology and ultrastructure reveal a new granulosis-like virus in Penaeus monodon affected by “yellow-head” disease. Dis Aquat Org. 1993;17:145–157. doi: 10.3354/dao017145. [DOI] [Google Scholar]
- 11.Chen D. An overview of the disease situation, diagnostic techniques, treatments and preventives used on shrimp farms in China. In: Fulks W, Main KL, editors. Diseases of cultured penaeid shrimp in Asia and the United States. Honolulu: The Oceanic Institute; 1992. pp. 47–55. [Google Scholar]
- 12.Chen SN, Chang PS, Kou GH. Observation on pathogenicity and epizootiology of Penaeus monodonBaculovirus (MBV) in cultured shrimp in Taiwan. Fish Pathol. 1989;24:189–195. doi: 10.3147/jsfp.24.189. [DOI] [Google Scholar]
- 13.Chong YC, Loh H. Hepatopancreas clamydial and parvoviral infections of farmed marine prawns in Singapore. Singap Vet J. 1985;9:51–56. [Google Scholar]
- 14.Cotmore SF, D’abramo AMJ, Carbonell LF, Bratton J, Tattersall P. The NS2 polypeptide of parvovirus MVM is required for capsid assembly in murine cells. Virology. 1997;231:267–280. doi: 10.1006/viro.1997.8545. [DOI] [PubMed] [Google Scholar]
- 15.Cotmore SF, Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/S0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- 16.Destoumieux-Garzon D, Saulnier D, Garnier J, Jouffrey C, Bulet P, Bachere E. Crustacean immunity: antifungal peptides are generated from the C terminus of shrimp hemocyanin in response to microbial challenge. J Biol Chem. 2001;276:47070–47077. doi: 10.1074/jbc.M103817200. [DOI] [PubMed] [Google Scholar]
- 17.Erdman DD, Durigon EL, Wang QY, Anderson LJ. Genetic diversity of human parvovirus B19: sequence analysis of the vp1/vp2 gene from multiple isolates. J Gen Virol. 1996;77:2767–2774. doi: 10.1099/0022-1317-77-11-2767. [DOI] [PubMed] [Google Scholar]
- 18.FAO. The State of world fisheries and aquaculture 2010. FAO Fisheries and Aquaculture Department, Food and Agriculture Organization of the United Nations, Rome; 2010.
- 19.Fauquet CP, Perros M, Brandenburger A, Spegelaere P, Rommelaere J. Programmed killing of human cells by means of an inducible clone of parvoviral genes encoding non-structural proteins. EMBO J. 1990;9:2989–2995. doi: 10.1002/j.1460-2075.1990.tb07491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flegel TW, Fegan DF, Kongsom S, Vuthikornudomkit S, Sriurairatana S, Boonyaratpalin S, Chantanachookhin C, Vickers JE, Macdonald OD. Occurrence, diagnosis and treatment of shrimp diseases in Thailand. In: Fulks W, Main KL, editors. Diseases of cultured penaeid shrimp in Asia and the United States. Honolulu: The Oceanic Institute; 1992. pp. 57–112. [Google Scholar]
- 21.Flegel TW, Sriurairatana S. Black tiger prawn diseases in Thailand. In: Akiyama DM, editor. Technical bulletin of the American Soybean Association. Technical Bulletin AQ39 1993/3. Singapore: American Soybean Association; 1993. p. 30. [Google Scholar]
- 22.Flegel TW, Sriurairatana S. Shrimp health management: an environmental approach. In: Shariff M, editor. Diseases in aquaculture: the current issues. Malaysian Fisheries Society publication no. 8, Faculty of Fisheries and Marine Science, University Pertanian Malaysia, Serdang; 1994. p. 1–48.
- 23.Flegel TW, Fegan DF, Sriurairatana S. Environmental control of infectious shrimp diseases in Thailand. In: Shariff M, Arthur JR, Subasinghe RP, editors. Diseases in asian aquaculture II. Fish Health Section, Asian Fish. Society, Manila; 1995. p. 65–79.
- 24.Flegel TW, Thamavit V, Passarawipas T, Alday-Sanz V. Statistical correlation between severity of Hepatopancreatic parvovirus (HPV) infection and stunting of farmed black tiger shrimp (Penaeus monodon) Aquaculture. 1999;174:197–206. doi: 10.1016/S0044-8486(98)00507-9. [DOI] [Google Scholar]
- 25.Flegel TW. Detection of major penaeid shrimp viruses in Asia, a historical perspective with emphasis on Thailand. Aquaculture. 2006;258:1–33. doi: 10.1016/j.aquaculture.2006.05.013. [DOI] [Google Scholar]
- 26.Fraser CA, Owens L. Spawner-isolated mortality virus from Australia Penaeus monodon. Dis Aquat Org. 1996;27:141–148. doi: 10.3354/dao027141. [DOI] [Google Scholar]
- 27.Hsu YL, Wang KH, Yang YH, Tung MC, Hu CH, Lo CF, Wang CH, Hsu T. Diagnosis of Penaeus monodon-type baculovirus by PCR and by ELISA of occlusion bodies. Dis Aquat Org. 2000;40:93–99. doi: 10.3354/dao040093. [DOI] [PubMed] [Google Scholar]
- 28.Jang IK, Suriakala K, Kim JS, Meng X, Choi TJ. TaqMan real-time PCR assay for quantifying type III hepatopancreatic parvovirus infections in wild broodstocks and hatchery-reared postlarvae of Fenneropenaeus chinensis in Korea. J Microbiol Biotechnol. 2011;21:1109–1115. doi: 10.4014/jmb.1107.07009. [DOI] [PubMed] [Google Scholar]
- 29.Jeeva S, Kang SW, Lee YS, Jang IK, Seo HC, Choi TJ. Complete nucleotide sequence analysis of a Korean strain of hepatopancreatic parvovirus (HPV) from Fenneropenaeus chinensis. Virus Genes. 2012;44:89–97. doi: 10.1007/s11262-011-0675-8. [DOI] [PubMed] [Google Scholar]
- 30.Kawase S, Garzon S, Su DM, Tijssen P. Insect parvovirus diseases. In: Tijssen P, editor. Handbook of parvoviruses. Boca Raton: CRC Press; 1990. pp. 213–228. [Google Scholar]
- 31.Khawsak P, Deesukon W, Chaivisuthangkura P, Sukhumsirichart W. Multiplex RT-PCR assay for simultaneous detection of six viruses of penaeid shrimp. Mol Cell Probes. 2008;22:177–183. doi: 10.1016/j.mcp.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Kiatpathomchai W, Jaroenram W, Arunrut N, Jitrapakdee S, Flegel TW. Shrimp Taura syndrome virus detection by reverse transcription loop-mediated isothermal amplification combined with a lateral flow dipstick. J Virol Methods. 2008;153:214–217. doi: 10.1016/j.jviromet.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 33.Kubista M, Andrade JM, Bengtsson M, Forootan, Jona KJ, Lind K, Sindelka R, Sjoback R, Sjogreen B, Strombom L, Stahlber G, Zoric A. Review on the real-time polymerase chain reaction. Mol Aspects Med. 2006;27:95–125. doi: 10.1016/j.mam.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 34.La Fauce KA, Elliman J, Owens L. Molecular characterization of hepatopancreatic parvovirus (PmergDNV) from Australian Penaeus merguiensis. Virology. 2007;362:397–403. doi: 10.1016/j.virol.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 35.La Fauce KA, Layton R, Owens L. TaqMan real-time PCR for detection of hepatopancreatic parvovirus from Australia. J Virol Methods. 2007;140:10–16. doi: 10.1016/j.jviromet.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 36.La Fauce KA, Owens L. Investigation into the pathogenicity of Penaeus merguiensis densovirus (PmergDNV) to juvenile Cherax quadricarinatus. Aquaculture. 2007;271:31–38. doi: 10.1016/j.aquaculture.2007.06.028. [DOI] [Google Scholar]
- 37.La Fauce KA, Owens L. The use of insects as a bioassay for Penaeus merguiensis densovirus (PmergDNV) J Invertebr Pathol. 2008;98:1–6. doi: 10.1016/j.jip.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Legrand C, Rommelaere J, Fauquet CP. MVM (p) NS-2 protein expression is required with NS-1 for maximal cytotoxicity in human transformed cells. Virology. 1993;195:149–155. doi: 10.1006/viro.1993.1355. [DOI] [PubMed] [Google Scholar]
- 39.Liao IC. Marine prawn culture industry of Taiwan. In: Fast AW, Lester LJ, editors. Marine shrimp culture: principles and practices. Amsterdam: Elsevier; 1992. pp. 289–320. [Google Scholar]
- 40.Lightner DV, Redman RM, Bell TA. Detection of IHHN virus in Penaeus stylirostris and Penaeus vannamei imported into Hawaii. J World Maricult Soc. 1983;14:212–225. doi: 10.1111/j.1749-7345.1983.tb00077.x. [DOI] [Google Scholar]
- 41.Lightner DV, Redman RM. A parvo-like virus disease of penaeid shrimp. J Invertebr Pathol. 1985;45:47–53. doi: 10.1016/0022-2011(85)90048-5. [DOI] [PubMed] [Google Scholar]
- 42.Lightner DV. Shrimp virus diseases: diagnosis, distribution and management. In: Wyban J, editor. Proceedings of the special session on shrimp farming. Baton Rouge: World Aquaculture Society; 1992. pp. 238–253. [Google Scholar]
- 43.Lightner DV, Poulos BT, Bruce L, Redman RM, Mari J, Bonami JR. New developments in penaeid virology: application of biotechnology in research and disease diagnosis for shrimp viruses of concern in the Americas. In: Fulks W, Main K, editors. Diseases of cultured penaeid shrimp in Asia and the United States. Honolulu: Oceanic Institute; 1992. pp. 233–253. [Google Scholar]
- 44.Lightner DV, Redman RM. Penaeid virus diseases of the prawn culture industry in the Americas. In: Fast AW, Lester LJ, editors. Marine prawn culture: principles and practices. Amsterdam: Elsevier; 1992. pp. 569–588. [Google Scholar]
- 45.Lightner DV, Redman RM, Moore DW, Park MA. Development and application of a simple and rapid diagnostic method to study hepatopancreatic parvovirus of penaeid shrimp. Aquaculture. 1993;116:15–23. doi: 10.1016/0044-8486(93)90218-N. [DOI] [Google Scholar]
- 46.Lightner DV, Redman RM, Poulos BT, Mari JL, Bonami JR, Shariff M. Distinction of HPV-Type viruses in Penaeus chinensis and Macrobrachium rosenbergii using a DNA probe. Asian Fish Sci. 1994;7:267–272. [Google Scholar]
- 47.Lightner DV. A handbook of shrimp pathology and diagnostic procedures for diseases of penaeid shrimp. Baton Rouge: World Aquaculture Society; 1996. p. 304. [Google Scholar]
- 48.Limsuwan CH. Shrimp culture in Thailand toward year 2000. In: Tonguthai K, Chinabut S, Somsiri T, Chanratchakul P, Kanchanakan S, editors. The AAHRI newsletter. Bangkok: Department of Fisheries, Kasertsart University; 1999. pp. 5–6. [Google Scholar]
- 49.Lungu O, Sun XW, Wright TC, Ferenczy A, Jr, Richard RM, Silverstein S. A polymerase chain reaction-enzyme-linked immunosorbent assay method for detecting human papillomavirus in cervical carcinomas and high-grade cervical cancer precursors. Obstet Gynecol. 1995;85:337–342. doi: 10.1016/0029-7844(94)00399-X. [DOI] [PubMed] [Google Scholar]
- 50.Manivannan S, Otta SK, Karunasagar I, Karunasagar I. Multiple viral infections in Penaeus monodon prawn post larvae in an Indian hatchery. Dis Aquat Org. 2002;48:233–236. doi: 10.3354/dao048233. [DOI] [PubMed] [Google Scholar]
- 51.Manjanaik B, Umesha KR, Karunasagar I, Karunasagar I. Detection of hepatopancreatic parvovirus (HPV) in wild prawn from India by nested polymerase chain reaction (PCR) Dis Aquat Org. 2005;63:255–259. doi: 10.3354/dao063255. [DOI] [PubMed] [Google Scholar]
- 52.Mari J, Bonami JR, Lightner DV. Partial cloning of the genome of infectious hypodermal and hematopoietic necrosis virus, an unusual parvovirus pathogenic for penaeid shrimps; diagnosis of the disease using a specific probe. J Gen Virol. 1993;74:2637–2643. doi: 10.1099/0022-1317-74-12-2637. [DOI] [PubMed] [Google Scholar]
- 53.Mari J, Lightner DV, Poulos BT, Bonami JR. Partial cloning of the genome of an unusual shrimp parvovirus (HPV): use of gene probes in disease diagnosis. Dis Aquat Org. 1995;22:129–134. doi: 10.3354/dao022129. [DOI] [Google Scholar]
- 54.Naeger LK, Cater J, Pintel DJ. The small nonstructural protein (NS2) of the parvovirus minute virus of mice is required for efficient DNA replication and infectious virus production in a cell-type-specific manner. J Virol. 1990;64:6166–6175. doi: 10.1128/jvi.64.12.6166-6175.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naeger LK, Salome N, Pintel DJ. NS2 is required for efficient translation of viral mRNA in minute virus of mice-infected murine cells. J Virol. 1993;67:1034–1043. doi: 10.1128/jvi.67.2.1034-1043.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nimitphaka T, Kiatpathomchaia W, Flegel TW. Shrimp hepatopancreatic parvovirus detection by combining loop-mediated isothermal amplification with a lateral flow dipstick. J Virol Methods. 2008;154:56–60. doi: 10.1016/j.jviromet.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 57.Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:e63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Otta SK, Shubha G, Joseph B, Chakraborty A, Karunasagar I, Karunasagar I. Polymerase chain reaction (PCR) detection of white spot syndrome virus (WSSV) in cultured and wild crustaceans in India. Dis Aquat Org. 1999;38:67–70. doi: 10.3354/dao038067. [DOI] [Google Scholar]
- 59.Owens L. Special topic review: the history of the emergence of viruses in Australian prawn aquaculture. World J Microbiol Biotechnol. 1997;13:427–431. doi: 10.1023/A:1018528317508. [DOI] [Google Scholar]
- 60.Owens L, Haqshenas G, McElnea C, Coelen R. Putative spawner-isolated mortality virus associated with mid-crop mortality syndrome in farmed Penaeus monodon from northern Australia. Dis Aquat Org. 1998;34:177–185. doi: 10.3354/dao034177. [DOI] [PubMed] [Google Scholar]
- 61.Owens L, McElnea C. Natural infection of the redclaw crayfish Cherax quadricarinatus with presumptive spawner-isolated mortality virus. Dis Aquat Org. 2000;40:219–223. doi: 10.3354/dao040219. [DOI] [PubMed] [Google Scholar]
- 62.Owens L, Liessmann L, La Fauce K, Nyguyen T, Zeng C. Intranuclear bacilliform virus and hepatopancreatic parvovirus (PmergDNV) in the mud crab Scylla serrata (Forskal) of Australia. Aquaculture. 2010;310:47–51. doi: 10.1016/j.aquaculture.2010.10.028. [DOI] [Google Scholar]
- 63.Panichareon B, Khawsak P, Deesukon W, Sukhumsirichart W. Multiplex real-time PCR and high-resolution melting analysis for detection of white spot syndrome virus, yellow-head virus, and Penaeus monodon densovirus in penaeid shrimp. J Virol Methods. 2011;178:16–21. doi: 10.1016/j.jviromet.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 64.Pantoja CR, Lightner DV. A non-destructive method based on the polymerase chain reaction for detection of hepatopancreatic parvovirus (HPV) of penaeid shrimp. Dis Aquat Org. 2000;39:177–182. doi: 10.3354/dao039177. [DOI] [PubMed] [Google Scholar]
- 65.Pantoja CR, Lightner DV. Detection of hepatopancreatic parvovirus (HPV) of penaeid shrimp by in situ hybridization at the electron microscope level. Dis Aquat Org. 2001;44:87–96. doi: 10.3354/dao044087. [DOI] [PubMed] [Google Scholar]
- 66.Paynter JL, Lightner DV, Lester RGJ. Prawn virus from juvenile Penaeus esculentus. In: Rothlisberg RC, Hill BY, Staples DY, editors. Proceedings of the second Australian national prawn seminar. Cleveland; 1985. p. 61–4.
- 67.Phromjai J, Sukhumsirichart W, Pantoja C, Lightner DV, Flegel TW. Different reactions obtained using the same DNA detection reagents for Thai and Korean hepatopancreatic parvovirus of penaeid shrimp. Dis Aquat Org. 2001;46:153–158. doi: 10.3354/dao046153. [DOI] [PubMed] [Google Scholar]
- 68.Phromjai J, Boosaeng V, Withyachumnarmkul B, Flegel TW. Detection of hepatopancreatic parvovirus (HPV) in Thai shrimp Penaeus monodon by in situ hybridization, dot blot hybridization and PCR amplification. Dis Aquat Org. 2002;51:227–232. doi: 10.3354/dao051227. [DOI] [PubMed] [Google Scholar]
- 69.Poulos BT, Lightner DV, Trumper B. Monoclonal antibodies to a penaeid shrimp parvovirus, infectious hypodermal and hematopoietic necrosis virus (IHHNV) J Aquat Anim Health. 1994;6:149–154. doi: 10.1577/1548-8667(1994)006<0149:MATAPS>2.3.CO;2. [DOI] [Google Scholar]
- 70.Prakasha BK, Ramakrishna RP, Karunasagar I, Karunasagar I. Detection of Laem-Singh virus (LSNV) in cultured Penaeus monodon from India. Dis Aquat Org. 2007;77:83–86. doi: 10.3354/dao01835. [DOI] [PubMed] [Google Scholar]
- 71.Rai P, Pradeep B, Karunasagar I, Karunasagar I. Detection of viruses in Penaeus monodon from India showing signs of slow growth syndrome. Aquaculture. 2009;289:231–235. doi: 10.1016/j.aquaculture.2008.12.035. [DOI] [Google Scholar]
- 72.Rai P, Pradeep B, Safeena MP, Karunasagar I, Karunasagar I. Simultaneous presence of infectious hypodermal and hematopoietic necrosis virus (IHHNV) and type A virus-related sequence in Penaeus monodon from India. Aquaculture. 2009;295:168–174. doi: 10.1016/j.aquaculture.2009.07.015. [DOI] [Google Scholar]
- 73.Roekring S, Nielsen L, Owens L, Pattanakitsakul S, Malasit P, Flegel TW. Comparison of penaeid shrimp and insect parvoviruses suggests that viral transfers may occur between two distantly related arthropod groups. Virus Res. 2002;87:79–87. doi: 10.1016/S0168-1702(02)00084-9. [DOI] [PubMed] [Google Scholar]
- 74.Roubal FR, Paynter JL, Lester RJG. Electron microscope observation of hepatopancreatic parvo-like virus (HPV) in the penaeid prawn, Penaeus merguiensis de Man, from Australia. J Fish Dis. 1989;12:199–201. doi: 10.1111/j.1365-2761.1989.tb00293.x. [DOI] [Google Scholar]
- 75.Rukpratanporn S, Sukhumsirichart W, Chaivisuthangkura P, Longyant S, Sithigorngul W, Menasveta P, Sithigorngul P. Generation of monoclonal antibodies specific to hepatopancreatic parvovirus (HPV) from Penaeus monodon. Dis Aquat Org. 2005;65:85–89. doi: 10.3354/dao065085. [DOI] [PubMed] [Google Scholar]
- 76.Safeena MP, Tyagi A, Rai P, Karunasagar I, Karunasagar I. Complete nucleic acid sequence of Penaeus monodon densovirus (PmDNV) from India. Virus Res. 2010;150:1–11. doi: 10.1016/j.virusres.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 77.Shike H, Dhar AK, Burns JC, Shimizu C, Jousset FX. Infectious hypodermal and hematopoietic necrosis virus of shrimp is related to mosquito brevidensoviruses. Virology. 2000;277:167–177. doi: 10.1006/viro.2000.0589. [DOI] [PubMed] [Google Scholar]
- 78.Sithigorngul P, Rukpratanporn S, Longyant S, Chaivisuthangkura P, Sithigorngul W, Menasveta P. Monoclonal antibodies specific to yellow-head virus (YHV) of Penaeus monodon. Dis Aquat Org. 2002;49:71–76. doi: 10.3354/dao049071. [DOI] [PubMed] [Google Scholar]
- 79.Sivakumar VK, Sarathi M, Venkatesan C, Sivaraj A, Sahul Hameed AS. Experimental exposure of artemia to hepatopancreatic parvo-like virus and subsequent transmission to post-larvae of Penaeus monodon. J Invert Pathol. 2009;102:191–195. doi: 10.1016/j.jip.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 80.Spann KM, Adlard RD, Hudson DA, Dyecroft SB, Jones TC, Voigt MOC. Hepatopancreatic parvo-like virus (HPV) of Penaeus japonicus cultured in Australia. Dis Aquat Org. 1997;31:239–241. doi: 10.3354/dao031239. [DOI] [Google Scholar]
- 81.Srisuk C, Chaivisuthangkura P, Sukhumsirichart W, Sridulyakul P, Longyant S, Rukpratanporn S, Sithigorngul P. Improved immunodetection of Penaeus monodon densovirus with monoclonal antibodies raised against recombinant capsid protein. Aquaculture. 2011;311:19–24. doi: 10.1016/j.aquaculture.2010.08.018. [DOI] [Google Scholar]
- 82.Sukhumsirichart W, Wongteerasupaya C, Boonsaeng V, Panyim S, Sriurairatana S, Withyachumnarnkul B, Flegel TW. Characterization and PCR detection of hepatopancreatic parvovirus (HPV) from Penaeus monodon in Thailand. Dis Aquat Org. 1999;38:1–10. doi: 10.3354/dao038001. [DOI] [PubMed] [Google Scholar]
- 83.Sukhumsirichart W, Kisytsyhomchai W, Wongteerasupaya C, Withyachumnarmkul B, Flegel TW, Boonsaeng V, Panyim S. Detection of hepatopancreatic parvovirus (HPV) Penaeus monodon using PCR–ELISA. Mol Cell Probes. 2002;16:409–413. doi: 10.1006/mcpr.2002.0435. [DOI] [PubMed] [Google Scholar]
- 84.Sukhumsirichart W, Pongsopee A, Boonsaeng V, Panyim S. Complete nucleotide sequence and genomic organization of hepatopancreatic parvovirus (HPV) of Penaeus monodon. Virology. 2006;46:266–277. doi: 10.1016/j.virol.2005.06.052. [DOI] [PubMed] [Google Scholar]
- 85.Tang KFJ, Pantoja CR, Lightner DV. Nucleotide sequence of Madagascar hepatopancreatic parvovirus (HPV) and comparison of genetic variation among geographic isolates. Dis Aquat Org. 2008;80:105–112. doi: 10.3354/dao01928. [DOI] [PubMed] [Google Scholar]
- 86.Tang KFJ, Lightner DV. Duplex real-time PCR for detection and quantification of monodon baculovirus (MBV) and hepatopancreatic parvovirus (HPV) in Penaeus monodon. Dis Aquat Org. 2011;93:191–198. doi: 10.3354/dao02293. [DOI] [PubMed] [Google Scholar]
- 87.Uma A, Koteeswaran A, Karunasagar I, Karunasagar I. Prevalence of hepatopancreatic parvovirus (HPV) in Penaeus monodon postlarvae from commercial shrimp hatcheries in Tamilnadu, southeast coast of India. Asian Fish Sci. 2006;9:113–116. [Google Scholar]
- 88.Uma A, Ramanathan N, Saravanabava K. Study by PCR on the prevalence of white spot syndrome virus, monodon baculovirus and hepatopancreatic parvovirus infection in Penaeus monodon post larvae in Tamil Nadu, Southeast coast of India. Asian Fish Sci. 2007;20:227–239. [Google Scholar]
- 89.Umesha KR, Uma A, Otta SK, Karunasagar I, Karunasagar I. Detection by polymerase chain reaction (PCR) of hepatopancreatic parvovirus (HPV) and other viruses in hatchery-reared postlarvae of Penaeus monodon. Dis Aquat Org. 2003;57:141–146. doi: 10.3354/dao057141. [DOI] [PubMed] [Google Scholar]
- 90.Umesha KR, Dass MKB, Manjanaik B, Venugopal MN, Karunasagar I, Karunasagar I. High prevalence of dual and triple viral infections in blacktiger shrimp ponds in India. Aquaculture. 2006;258:91–96. doi: 10.1016/j.aquaculture.2006.04.003. [DOI] [Google Scholar]
- 91.Vega-Villasante F, Puente ME. A review of viral diseases of cultured shrimp. Prev Vet Med. 1993;17:271–282. doi: 10.1016/0167-5877(93)90035-R. [DOI] [Google Scholar]
- 92.Yan DC, Tang KF, Lightner DV. A real-time PCR for the detection of hepatopancreatic parvovirus (HPV) of penaeid shrimp. J Fish Dis. 2010;33:507–511. doi: 10.1111/j.1365-2761.2010.01149.x. [DOI] [PubMed] [Google Scholar]
- 93.Zerbini M, Gallinella G, Manaresi E, Musiani M, Gentilomi G, Venturoli S. Standardization of a PCR-ELISA in serum samples: diagnosis of active parvovirus B19 infection. J Med Virol. 1999;59:239–244. doi: 10.1002/(SICI)1096-9071(199910)59:2<239::AID-JMV19>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]


