Abstract
Among the emerging diseases in shrimp aquaculture, monodon slow growth syndrome (MSGS) is a major concern in South and Southeast Asia. Shrimp farming in Thailand was severely affected during 2000–2002 due to MSGS, which caused an economic loss, of about US$ 300 million. MSGS is characterized by abnormally slow growth with coefficients of size variation of >35 %, that has impacted P. monodon production in Thailand. A new shrimp virus, Laem-Singh virus (LSNV) was identified to be associated in MSGS affected shrimp. LSNV a RNA virus of about 25 nm diameter is phylogenetically related to the insect-borne viruses in the families Barnaviridae, Tymoviridae and Sobemoviridae an important histopathological observation is exclusively noticed in growth-retarded shrimp. The LSNV infections have been confirmed in various organs of infected shrimp such as lymphoid organ, gills and nervous tissues by various diagnostic techniques such as reverse transcription polymerase chain reaction (RT-PCR), in situ hybridization, quantitative real-time RT-PCR and reverse transcription loop-mediated isothermal amplification combined with a lateral flow dipstick (RT-LAMP-LFD) and these tools are available for the diagnosis of LSNV. Recently, an integrase containing element has been identified in absolute association with LSNV in stunted growth shrimp. The transmission of LSNV through horizontal and vertical routes has been experimentally demonstrated. The known natural host-range of LSNV includes P. monodon and other penaeid shrimp. The putative RdRp gene involved in replication of LSNV was targeted for dsRNA-mediated gene silencing and appeared to be effective in a dose-dependent manner. Since the discovery of LSNV in 2006 in Thailand, it has been added to the list of viruses to be excluded from domesticated specific pathogen-free stocks of P. monodon and it has been recommended that shrimp farmers avoid stocking post larvae positive for LSNV to prevent MSGS in their farms.
Keywords: LSNV, MSGS, Retinopathy, ICE, P. monodon
Introduction
Shrimp is the largest single seafood commodity accounting for 17 % by value, of internationally traded fishery products. Approximately 75 % of the production is from aquaculture, which is almost entirely dominated the black tiger shrimp (Penaeus monodon) and the Pacific white shrimp (Litopenaeus vannamei) [13, 57]. Diseases are major constraints in the sustainability of aquaculture worldwide. Intensification of aquaculture production systems continues to create new archetypes for disease expression. Among diseases with infectious causes, viruses are the most devastating agents. Approximately 20 viruses are currently known to infect penaeid shrimp worldwide [14, 23, 24, 27]. In stressful environments such as culture systems, some of these viruses can become more virulent and cause significant economic loss by causing mortality or retarded growth [55]. Besides the major viral diseases in shrimps, an emerging disease characterized by stunted growth and size variation in farmed black tiger shrimp called Monodon Slow Growth Syndrome (MSGS) is on the rise in recent past in South and Southeast Asian countries. The impact of MSGS in tiger shrimp has been substantial on national economies due to significant production losses and poor productivity.
Monodon Slow Growth Syndrome (MSGS)
The MSGS condition was first noticed in Thailand in 2001 when farmers found an unusual abnormally slow growth and a large size variation of their shrimp. In culture affected by MSGS, shrimp reached an average size of 5–10 g instead of the regular size of 24–40 g after 4 months of culture and very high (30–80 %) coefficient of variation (CV) in weight [58]. The term MSGS was coined by Thai shrimp farmers to refer this unusual retarded growth that has occurred in cultivated P. monodon [10, 16, 55] (http://www.biotec.or.th/rdereport/prjbioteceng.asp?Id=882). MSGS is considered as the most serious problem, probably ranks third next to WSSV and YHV in P. monodon [19, 42]. The cause of this slow growth is not determined but considered to be implication of an unknown infectious agent [10]. This contention was supported by laboratory trails suggesting involvement of a filterable infectious agent as injection of 0.45 μm filtered lymphoid extracts from MSGS shrimp caused similar slow growth symptoms in normal growing shrimp [58, 59]. It was reported based on a survey that there was no association of known shrimp viruses with MSGS [10]. MSGS in farmed P. monodon has reduced the farm profitability, caused serious economic loss, damaged export industries based on this species in Thailand [10, 55] and has been responsible for the major switch to L. vannamei farming. [10]. Subsequently, a similar condition was also reported in P. monodon from East Africa also [2]. Emergence of MSGS started following large-scale importations of Pacific white shrimp (L. vannamei). Hence it was thought that this syndrome would have arisen due to introduction of an exotic virus through such importation (http://www.biotec.or.th/rdereport/prjbioteceng.asp?Id=882).
Working Case Definition of MSGS
Thai researchers adopted a case definition to distinguish ponds exhibiting MSGS from slow growth caused by other problems, for surveillance and epidemiological purposes [19, 55] (http://www.biotec.or.th/rdereport/prjbioteceng.asp?Id=882) and suggested following working case definition: The suspected population should be RT-PCR positive for LSNV and must have a CV of more than 35 % by weight and absence of hepatopancreatic parvovirus (HPV) or of other severe hepatopancreatic infections by known agents while also complying with any three of the following gross signs: (i) unusually dark colour, (ii) average daily weight gain of less than 0.1 g day–1 g at 4 months, (iii) unusually bright yellow markings, (iv) “bamboo-shaped” abdominal segments, and (v) brittle antennae.
Nomenclature and Taxonomy
Laem-Singh virus (LSNV) was identified in 2006 while investigating the cause of MSGS in black tiger shrimp reared at Laem-Singh district, Chanthaburi Province, Thailand [55]. An initial study using random shotgun cloning technique yielded a partial deduced amino acid sequence homologous to viral RNA-dependent RNA polymerase (RdRp) sequences from tissues of MSGS shrimp. This fragment gave relatively low but significant homology to mushroom bacilliform virus (MuBV), type species and only member of genus Barnavirus of family Barnaviridae and to insect-plant viruses in the family Luteoviridae. However, phylogenetic analysis showed that the virus sequence did not cluster with the Luteoviridae or other known RNA virus sequences, and the MuBV and LSNV fell between clades of representatives from the families Togaviridae, Flaviviridae and the dsRNA-viruses, infectious bursal disease virus (IBDV) and bluetongue virus (BTV), with low bootstrap values. Thus, in accordance with frequent practice, it was named according to the area where it was first collected as LSNV [55]. In an attempt to identify the nature of viral genome, when the total nucleic acid extracted from the purified virions digested with RNase enzyme resulted in no RT-PCR product of LSNV. The presence of RdRp gene and the results from RNase digestion confirmed that LSNV is an RNA virus [54, 55]. The subsequent work on extending the fragment to a full-length RdRp gene using the rapid amplification of cDNA ends (RACE) followed by further phylogenetic analysis revealed possible relationships to insect-borne viruses in the families Barnaviridae, Tymoviridae and Sobemoviridae. Additional sequence information revealed the presence of a protease gene and other structural motifs suggestive of the family Sobemoviridae [49, 50, 54]. However, more genome information is required for proper classification of this virus (Table 1).
Table 1.
Details of LSNV—an emerging pathogen of shrimp
| Virus | Abbreviation | Genome | Taxonomic classificationa | Year emerged | Known geographic distribution | OIE listed diseaseb | Reference |
|---|---|---|---|---|---|---|---|
| Laem-Singh virus | LSNV | [+] RNA | Luteovirus-like (unclassified) | 2003 | South and Southeast Asia | No | [52] |
aICTV, 2009
bOIE, 2009
LSNV: Transmission Electron Microscopy (TEM)
Transmission electron microscopic description of the putative etiological agent of MSGS has been reported by several researchers [32, 36, 38, 53–55]. Detection of abundant, naked, icosahedral viral-like particles of approximately ~25–27 nm by TEM in the lymphoid organ (LO), gills, neural tissues including optic lobe, supra-esophageal ganglion or brain, thoracic ganglion, abdominal ganglion and ventral nerve cord of both small and normal shrimp from the MSGS pond has been reported by many investigators [1, 38, 49, 50]. Membrane-bound, vacuolated, intra-cytoplasmic inclusions that contained small granular bodies associated with naked, viral-like particles of 25–30 nm diameter located in LO tubule matrix cells at the outer tubule rim has been also observed [55]. It was reported that LSNV was present in the fasciculated zone and onion bodies of organ of Bellonci in the small shrimp, but not in the normal shrimp of both the MSGS and normal ponds. In this study the viral-like particles were observed in unidentified cells and in intercellular spaces in the fasciculated zone. In the neural tissues of the small shrimp, the virus particles were mostly in the connective tissue in intercellular spaces or in the cell cytoplasm. Few particles were seen in the cytoplasm of glial cells and neurons [36]. Icosahedral particles of about 25–33 nm diameter was reported by TEM in fractions between 45 and 60 % (w/w) sucrose gradients from ultra-purified gill tissue of affected shrimp by negative staining technique [32, 54]. Further, a second step purification yielded two bands at approximately 54 and 58 % (w/w) sucrose gradient but the particle sizes and substructure of virions were unclear though confirmed positive by RT-PCR [54]. Presumptive viral-like particles were observed from band obtained at 20–25 % (w/w) CsCl by second step ultracentrifugation. Further, a third round of gradient separation yielded a distinct band at 21 % CsCl (buoyant density 1.1843 g cm−3) which revealed the presence of non-enveloped, icosahedral viral-like particles of approximately 25 and 15 nm diameter using negative staining by TEM, similar to those of previously shown by TEM in tissue sections of stunted shrimp from MSGS ponds [32].
LSNV Genome
Initial work on LSNV showed significant deduced amino acid sequence similarity to RdRp of the RNA viruses and homology with viruses in the family Luteoviridae. The sequence alignment revealed 3 out of 8 conserved motifs of RdRp gene sequences of viruses in the family Luteoviridae. These were acidic SGT motif 2, a GDD (Gly-Asp-Asp) motif 3 and a modified FCG motif 5. The FCG consensus motif 5 was present as FMG in this sequence and as FCS in the others. As with the Luteoviridae, there was no basic motif 4 (conserved K between motifs 3 and 5) and motifs 6, 7 and 8 were not found (not ubiquitous in the Luteoviridae) and motif 1 fell outside the region of cloned fragment [55]. The GDD motif is found in many RdRp of plant, animal and bacterial viruses [8]. The GDD sequence in motif 3 is conserved in almost all polymerases and is postulated to be located at the enzyme active site [31]. Subsequent work and additional sequence information using RACE revealed the presence of a protease gene and other structural motifs suggestive of the family Sobemoviridae. A total sequence of 2,206 bp (LSNV2206) was assembled in a recent study. LSNV contains at least two overlapping open reading frames comprising a peptidase enzyme and RdRp. Although RdRp of LSNV showed high similarity to Luteoviridae and Sobemovirus, LSNV did not fit with both groups on phylogenetic analysis [54]. The large genome part (2,206 bp) of LSNV approximately constitutes 71 % of LSNV genome encodes a large polyprotein. Sequencing of LSNV2206 revealed part of consensus amino acid sequence (TXXGXSG) of the catalytic triad which is H(X-35)(D/E)(X61-62)TXXGXSG. The C-terminal of LSNV polyprotein predicted to encode RdRp. The putative LSNV-RdRp contains 1,149 bp encoding 382 deduced amino acids, with a predicted molecular mass of about 44 kDa. The conserved motifs reported in other viral RdRps including consensus amino acid sequence (GX3TX3NXnGDD) was found in LSNV-RdRp [6, 54]. The enzyme contains 8 conserved motifs of which I-VI were found to be conserved in LSNV and four of these motifs are in catalytic palm domain [54]. It was also reported that the fragments of the LSNV genome could be detected in the shrimp host genome by Southern blot analysis and RT-PCR, indicating that one or more inserts can occur in shrimp host genome at unknown locations. However, it is yet to be understood whether shrimp and other arthropods have mechanisms of viral integration into their host genomes that could lead to persistent infections without signs of disease [54]. It was reported that northern blot analysis indicated LSNV genome from infected shrimp samples was larger (3.4 kb) than those from the purified virus (2.8 kb). Sequence comparison with the Sobemo and Luteo viruses suggests that 3.4 kb is likely to be the total genome size for LSNV [49, 50, 54]. Further work is required to determine whether single genome fragment breaks during viral extraction or virus contain two RNA fragments [54].
Recently, a unique clone containing an integrase domain that appeared to arise from RNA of viral-like particles from ultra-purified tissue homogenates of MSGS shrimp was identified by shotgun cloning and sequencing. In this study, it was reported that a frame +1 translation of the 2,233 bp sequence yielded a single uninterrupted sequence of 744 deduced amino acids that had no known homology to any protein or translated protein sequence at GenBank except for an integrase protein of bacteriophage SH046 of Acinetobacter johnsonii near the 3′ end, with low identity (29 %). Further, it was observed that the deduced amino acids at positions 1,894-2,025 (44 amino acid residues) in the C-terminal portion of the sequence had homology to a conserved domain for phage integrases and DNA breaking enzymes in the DNA_BRE_C family. The sequence was called an integrase containing element (ICE) [32]. Northern blot of RNA from the 21 % (w/w) CsCl gradient band when subjected to northern blot analysis using ICE probe gave a positive hybridization signal at approximately 3.0 kb for ICE, while no signal was detected in the RNA extracted from specific pathogen-free (SPF) shrimp [32].
The consensus sequence of 2,233 bp of ICE identified together with LSNV exclusively in stunted shrimp revealed no significant identity to known nucleic acid sequences except for 100 % identity in 46 bases near the 3′ end of the sequence with thrombospondin of genus Penaeus (Marsupenaeus). Also, the ICE sequence shared no significant identity to the XSV genome sequence or any other known satellite virus sequences. Neither the XSV genome nor other known satellite genomes contain integrase genes [28]. Similarly, the ICE sequence showed no significant homology to the genomes of naked RNA viruses with integrase genes that can be found in the families Pseudoviridae [7] and Metaviridae [12] that are related to enveloped viruses in the family Retroviridae [26]. ICE contained an integrase domain with a conserved catalytic triad H-R-H and a conserved tyrosine (Y) in a binding region that may be involved in host genome insertion [22, 30]. The 44 amino acids of integrase domain found in LSNV affected shrimp showed homology only to integrases or recombinases of bacteria and bacteriophages and not viral-like members of the families Pseudoviridae and Metaviridae. The nature of ICE and its phylogenetic relationship to other viral-like entities known to occur in invertebrates still remains an enigma [32].
Genetic Lineage of LSNV
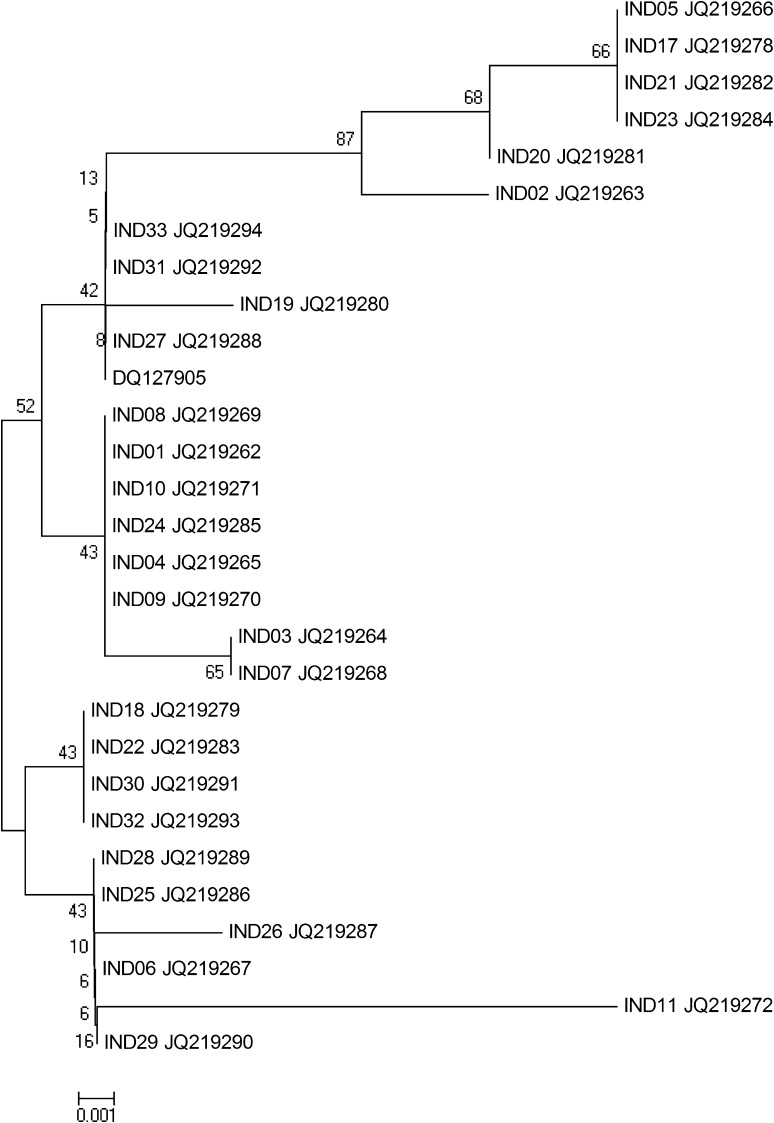
Many studies [47, 51, 52] (http://www.vienthuysan2.com/index.php?do=news&act=detail&id=32&lang=en) have found that the sequences obtained from different geographical locations had high homology (97–98 %) with the reference Thai isolates (Table 2). It was observed that sequences of eight viruses from Thailand, Malaysia and India, were identical to the Thai reference strain and there was no clustering according to geographic location or from the healthy or MSGS affected shrimp. Phylogenetic analysis based on nucleotide sequence of RdRp gene of ten Indian isolates and their comparison with published nucleotide sequences of LSNV suggested that LSNV exists as a single genetic lineage (Fig. 1) [33, 34] and occurs commonly in healthy P. monodon in parts of Asia [52]. The nucleotide sequence of RdRP gene fragment of LSNV was found to be highly conserved (99 % identity) across the four penaeid species [51]. Further phylogenetic studies reported LSNV peptidase had high sequence similarity to those of Sobemovirus whereas the LSNV-RdRp had high sequence similarity to RdRps of MuBV in the family Barnaviridae and viruses in the genus Polerovirus of the family Luteoviridae [54].
Table 2.
Sequencing of LSNV PCR products and genome fragments
| Species | Country | Milestone year | Genome | References | ||
|---|---|---|---|---|---|---|
| Disease observed | Virus observation | Sequence Accession | Product size (bp) | |||
| P. monodon | Thailand | 2001 | 2006 | DQ127905 | 615 | [55] |
| P. monodon | Vietnam | 2002 | 2009 | FJ811836 | 199 | [54] |
| P. monodon | Vietnam | 2002 | 2009 | FJ811835 | 199 | [54] |
| P. monodon | Malaysia | 2003 | 2009 | FJ811834 | 199 | [54] |
| P. monodon | India | 2006 | 2007 | EF593037 | 194 | [35] |
| P. monodon | Thailand | 2008 | 2008 | FJ356710 | 602 | |
| P. monodon | Thailand | 2008 | 2008 | FJ356709 | 604 | |
| P. monodon | India | 2007 | 2009 | FJ811832 | 199 | [54] |
| P. monodon | India | 2007 | 2009 | FJ811833 | 199 | [54] |
| P. monodon | India | 2007 | 2009 | FJ811829 | 199 | [54] |
| P. monodon | India | 2007 | 2009 | FJ811831 | 199 | [54] |
| P. monodon | India | 2007 | 2009 | FJ811830 | 199 | [54] |
| P. monodon | India | 2007 | 2009 | FJ811837 | 199 | [54] |
| P. monodon | India | 2007 | 2009 | EF593037 | 199 | [54] |
| P. monodon | India | 2007 | 2009 | FJ811838 | 199 | [54] |
| L. vannamei | India | 2010 | 2010 | HQ825248 | 205 | [51] |
| P. monodon | India | 2010 | 2010 | HQ728430 | 357 | [51] |
| P. monodon | Thailand | 2008 | 2011 | FJ498866 | 2214 | [32] |
| P. monodon and L. vannamei | India | 2008–2011 | 2008 | JQ219262–JQ219307 | 340–597 | [33] |
Fig. 1.
Phylogenetic tree of LSNV isolates based on the partial sequencing of RdRp coding region. The numbers at each node represent the percentage bootstrap scores (10,000 replicates) [33]
Geographical Distribution of MSGS and Prevalence of LSNV
LSNV was reported in association with MSGS in both healthy and infected P. monodon first from Thailand and later in Malaysia, Indonesia, India and Vietnam suggested a geographical distribution restricted to South and Southeast Asia [52]. Initial studies reported low incidence of LSNV from India [35, 40] probably the disease was newly reported from India. A later study reported 56.8 % prevalence of LSNV infection from 15 to 135 days culture period in Andhra Pradesh during 2007 and was no obvious clustering of positive samples according to the site of collection. Recent reports indicated much higher prevalence from the farms from both east and west coast of India with the history of extended culture period and growth retardation during past few years [10, 33]. Recently relatively high prevalence of LSNV was observed in brooders from different locations like Tamilnadu, Orissa and Maharashtra of India [33] including Andaman and Nicobar [33, 51]. LSNV was reported in brooders and post larvae for the first time in Sri Lanka in 2010 [39]. Case of growth retardation also found in P. monodon in East Africa in 2004. It was reported that the mean body weight of the shrimp at the sixth month in affected ponds was 19 ± 4 g, which was 30 % less than the expected size of normal shrimp grown within the same period. However, the phenomenon of MSGS occurring in this region and the growth retardation reported in farmed shrimp from East Africa is the same, remains unknown [2].
Economic Impact
Since 2000, economic losses due to MSGS became apparent. The most drastic consequence to the farmer is the uncertainty of final harvest yield and value. In a typical case, after 3-4 months of culture, the size variations ranges from 80 to 300 pieces kg−1 in a single pond [25] and at similar stocking density, the average growth rate in MSGS ponds is approximately half that of normally expected by shrimp farmers [36]. Shrimp farming in Thailand was reported to have suffered great economic damage as the production value of farmed P. monodon dramatically dropped to 40-68 % over a 2 year period. The accumulated damage caused by this phenomenon was estimated at 13 billion Baht (US$ 300 million) [10, 58]. In India, white spot disease, LSS and slow growth have been primarily responsible for economic losses to the shrimp farming sector. The production loss due to slow growth and white gut disease was estimated to be 5726 MT amounting to Rs.120 crores per year (about US$21.64 million annually) [5, 21].
Host-Range and Potential Vectors
The natural host-range of LSNV is presently unknown, but it occurs commonly in P. monodon [33, 35, 36, 40, 51, 52, 55]. LSNV was detected in P. monodon, Fenneropenaeus merguiensis, Metapenaeus dobsoni and L. vannamei, but not in F. indicus, Marsupenaeus japonicus and Scylla serrata. LSNV was most prevalent in P. monodon followed by M. dobsoni, F. merguiensis and L. vannamei in India [51]. L. vannamei may act as carrier as evidenced by experimental transmission study [1]. It was also detected in L. vannamei in Thailand affected by abdominal segment deformity disease [46]. An earlier study reported no evidence of LSNV infection in healthy juvenile or adult P. monodon, F. merguiensis or P. japonicus from northern and eastern Australia between 1998 and 2006. This is probably due to the fact that the numbers of Australian P. japonicus and P. mergiuensis tested were small and P. vannamei sampled from Thailand had origins as SPF stock imported from Hawaii [52].
Modes of Transmission
LSNV has been reported to be transmitted vertically as well as horizontally, as indicated by its widespread detection in wild and domesticated brood stock and post larval shrimp in Thailand and India [33, 41, 48, 51, 52]. High prevalence of LSNV was reported in female shrimp broodstock and various developmental stages of shrimp viz., nauplius, zoea, mysis, post larvae 5 (PL5) and PL15 [47]. It has been also reported that the slow growth syndrome in P. monodon could be induced by injection of LO extracts of MSGS affected shrimp into healthy P. monodon, that result in severe growth retardation and extreme size variation of 20–45 % in experimentally induced and 17–24 % in control groups [58, 59]. In another study, Pacific white shrimp, L. vannamei that were co-cultured with MSGS affected P. monodon were reported to grow normally. However, membrane filtered LO extracts from these co-cultured L. vannamei caused MSGS when injected into healthy P. monodon. These experimental transmission studies suggest L. vannamei might act as asymptomatic carrier [1]. Bioassay experiments by oral feeding of MSGS affected shrimp homogenates in healthy tiger shrimp showed 100 % LSNV-positives by 10 weeks along with signs of size variation, dark discoloration, bright yellow markings (Fig. 2) and 90 % mortalities by end of four and half months of experimental period in infected group [33]. Further, the cohabitation experiments of tiger shrimp showed 100 % positives by 5 weeks along with size variation and 65 % mortalities by end of five and half months of experimental period [33, 47]. In another study on experimental transmission in mud crab S. serrata a gradual increase in viral load 8 days post-injection was reported, which appeared to stabilize until 20 days post-infection. The ability of LSNV to cause infection when injected into a crustacean species other than shrimp suggests that other species might be potential natural carriers of LSNV infection [51].
Fig. 2.
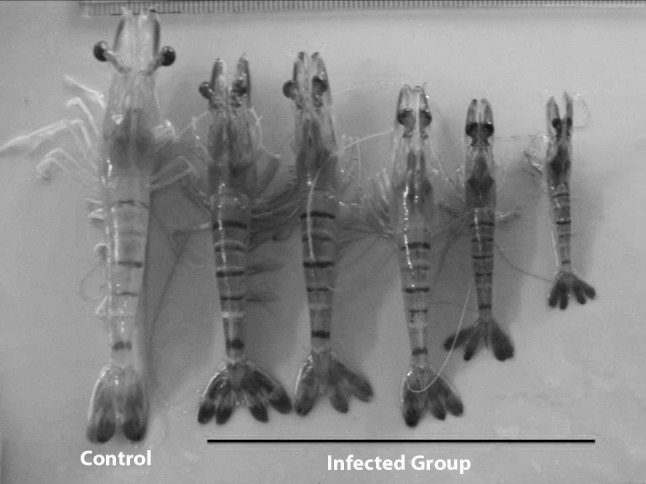
Shrimp exhibiting size variation with dark discoloration and stunted growth by experimental challenge study [34]
Predisposing Factors
It was concluded that LSNV is a necessary but insufficient cause of MSGS. The other component cause (s) that leads to LSNV-associated stunting of shrimp in MSGS ponds is still unknown but may involve other pathogens and/or environmental factors [16, 17]. The prevalence of LSNV infection can vary significantly in different populations of P. monodon and, as stress is commonly a stimulus for the onset of disease. More work is required to elucidate possible associations with loose shell, slow growth or other pathologies. The best way to prevent MSGS is through adapting better management practices during culture [52].
Pathogenesis
The LO is suggested to be a major site for viral predilection as indicated by formation of LO spheroids as reported in a number of viral diseases of penaeid species [2, 3, 44, 45]. In LSNV-infected P. monodon, viral-like particles could be observed by TEM only in the LO tubules which were ISH positive [55]. Further work suggested that slow growth in the small shrimp from the MSGS ponds may be due to a specific infection of LSNV in the fasciculated zone and onion bodies of organ of Bellonci of the eyes. It is thought that progression of infection in the optic nerve may be the cause of slow growth. It is yet unclear whether damage to the fasciculated zone and onion body of the organ of bellonci is due to LSNV infection or other pathogens that finally lead to slow growth in P. monodon. Also, it could be possible that shrimp may suffer from growth retardation caused by retinopathy resulting from interaction between LSNV and some other agent or factor(s) like ICE or other mechanisms which may predispose their fasciculated zones and organs of Bellonci to infection and damage [38]. A comparative study on moulting frequency and the transcription level of moult-inhibiting hormone (MIH) in small and large shrimp from MSGS pond with normal shrimp showed that the moult frequency of small shrimps was higher than other groups. The transcription of MIH in small shrimps was lower than other groups in all stages of moulting suggesting that retinopathy may be involved in the reduction of MIH levels [37].
Histopathology
Histopathological studies of growth-retarded shrimp from MSGS ponds have revealed pathognomonic lesions in eyestalk [5, 33, 34, 36, 38, 41]. The striking difference between the small and large LSNV-positive shrimp was that retinopathy was observed exclusively in the small shrimp from the MSGS ponds. Retinopathy comprised of abnormally enlarged haemolymphatic vessels, haemocytic infiltration in the fasciculated zone of the small shrimp [33, 36, 38, 41] and rupture of the membrane that separated the fasciculated zone from the overlying row of retinular cells [36, 38]. There was no degeneration of nerve fibers or neurons, abnormal inclusions except haemocytic infiltration in the crystalline tracts in a few small shrimp. Lymphoid organs spheroids (LOS) of affected shrimp show unusually large, magenta cytoplasmic inclusions [55, 58]. It was suggested that slow growth in the small shrimp from the MSGS ponds may be due to specific infection of LSNV in the fasciculated zone and onion bodies of organ of Bellonci of the eyes [36]. A fatal disease, ‘peripheral neuropathy and retinopathy’ (PNR), associated with minor to heavy mortalities was reported from eastern Australia in shrimp infected with yellow head-like virus. However, the histopathological symptoms differed, in that, these shrimp showed mild to severe, focal to diffuse degeneration and necrosis of axons and their sheaths, together with associated glial cell apoptosis, in peripheral nerve fibres [9].
LSNV Diagnostics
Nucleic acid based diagnostic methods like RT-PCR, nested RT-PCR, loop-mediated isothermal amplification combined with a lateral flow dipstick (RT-LAMP-LFD) have been used for diagnosis of LSNV with improved sensitivity and specificity [4, 29, 32–36, 40, 51, 52, 55] using various primers (Tables 3, 4, 5, and 6). Methods of quantification of LSNV by real-time quantitative RT-PCR (qRT-PCR) has been also developed and the authors also reported variation in LSNV loads in different shrimp species tested [51].
Table 3.
Details of RT-PCR primers used in different studies for detection of LSNV
| Primer | Sequence 5′–3′ | Expected product (bp) | Reference |
|---|---|---|---|
| LLV-F | ttgccttctcccgagtggtc | 200 | [33, 35, 36, 40, 55] |
| LLV-R | ccggctgaggtagctgcttg | ||
| BLF | cgttgccttctcccgagtggt | 357 | [51, 52] |
| LR1 | aatctcaccatgaagctcctcac | ||
| LSNVF | gctctttgcgcctatgaatg | 850 | [29] |
| LSNVR | gccccagaaacgtattggcac | ||
| LSNVF | gttgccttctcccgagtg | 597 | [33] |
| LSNVR | gccccagaaacgtattgg | ||
| LSNVF1 | ttctcccgagtggtcaggttta | 588 | [4] |
| LSNVR1 | ccagaaacgtattggcacacg | ||
| LSNV-Fr | cgttcgcttctcccgagtggt | 600 | [32] |
| LSNV-Rv | ttgccccagaaacgtattggca | ||
| Nested primers | |||
| LSNVnF | gcgcaagagttctcaggctt | 140 | [33, 35, 40] |
| LSNVnR | atcaccgcaggctaatatag | ||
| LF2 | agatcatgctgcatatgcttgc | 205 | [51, 52] |
| LR2 | gtgtagattggttgcatggcg | ||
| LSNVFPn | acgcgcaagagttctcag | 340 | [33] |
| LSNVRPn | aggggtggtgagcgtac | ||
| LSNV-NFr | ttgccttctcccgagtggtc | 195 | [32] |
| LSNV-NRv | ccggctgaggtagctgcttg | ||
Table 4.
| Probe | Labelling of probe | Oligonucleotide probe sequences (5′–3′) |
|---|---|---|
| 20AF | DIG-labelled probe | ttgccttctcccgagtggtc |
| 20AR | ccggctgaggtagctgcttg |
Table 5.
Details of oligonucleotide sets used for SYBR Green qRT-PCR assay [51]
| Primer | Gene | Sequence [5′–3′] |
|---|---|---|
| LSNVQPR1 | RdRp | gcttgcgatcgacactcttaac |
| LSNVQPF1 | tagcctgcggtgatgacacta |
Table 6.
Primers and probe used for LSNV RT-LAMP detection [4]
| Primer and probe | Genome position | Sequences (5′–3′) |
|---|---|---|
| LSNV-F3 | 101–120 | tcatgctgcatatgcttgct |
| LSNV-B3 | 318–299 | tgcgatgtgtttcatggtgt |
| LSNV-FIP-biotin | 196–176/tttt/134–151 | biotin-cggctgaggtagctgcttgaattttgtgagcccgtgactccta |
| LSNV-BIP | 214–233/tttt/284–265 | gcgaaggcagggtgcattgttttt gcgccctcaaagttaaaacc |
| LSNV-LF | 155–172 | tgtcatcaccgcaggcta |
| LSNV-LB | 238–255 | agtgtcgatcgcaagcta |
| FITC-probe | 197–211 | fitc-gttatattgaagagc |
In Situ Hybridisation (ISH)
A number of studies have demonstrated LSNV in various tissues of MSGS affected shrimp using ISH [15, 36, 38, 49, 50, 55]. In situ hybridization tests revealed the presence of LSNV in the cytoplasm of cells in the LO, heart, hepatopancreatic, interstitial cells [20, 55], neural tissues including optic lobe, supra-esophageal ganglion, thoracic ganglion, abdominal ganglion, ventral nerve cord and connective tissue of the brain. LSNV was detected in the fasciculated zone and onion bodies of organ of Bellonci in the small shrimp, but not in the normal shrimp of both the MSGS and normal ponds [36, 38]. Also in situ hybridization tests with experimentally challenged shrimp showed positive reactions in LO, eyes and gills of the test shrimp only for both ICE and LSNV [32] supporting the link between retinopathy and LSNV [36].
Therapeutics
RNA interference (RNAi) based technology targeting RdRp gene involved in the replication of LSNV was exploited to inhibit replication of LSNV [29, 48]. It was observed that interference mediated by viral sequence-specific dsRNA appeared to be effective in a dose-dependent [48]. In another study, dsRNA targeting PmRab7 gene (dsRNA-PmRab7) was employed to investigate the inhibitory effect on LSNV replication in P. monodon. It was reported that dsRNA-PmRab7, could significantly knock-down PmRab7 gene resulting in the prevention of LSNV replication. Injection of dsRNA-PmRab7 24 h before challenge with the virus resulted in a drastic decrease of PmRab7 mRNA and complete inhibition of LSNV replication by 3 days post-challenge. However in a therapeutic mode, shrimp injected with dsRNAPmRab7 only one day but not at 3 or 5 days post-LSNV challenge resulted in inhibition of LSNV replication [29]. The reason for this was attributed to the possibility of prior infection of target cells by LSNV by day 3 or 5 or an increase in viral load before dsRNA injection, minimizing the protective effect of dsRNA. Also there may be loss of RNAi potency due to the virus produced RNAi suppressor [11]. However, whether some LSNV proteins act as suppressor needs to be investigated. These two studies suggested a promising use of RNAi in therapeutic intervention. Hence, RNAi may be developed as a tool for cleaning up domesticated or wild shrimp stocks found to be infected with LSNV [29, 48].
Strategies for the Control of LSNV
Shrimp farmers have been reporting slow growth of black tiger shrimp since 2002. The fifth meeting of the Asia Regional Advisory Group (AG) on Aquatic Animal Health (29) suggested that MSGS seriously affects cultured P. monodon. The specific cause of MSGS has yet to be determined unequivocally, and whilst its occurrence is strongly associated with infection by LSNV [17, 55], there is no evidence that LSNV infection alone can cause the syndrome [36]. Since the discovery of LSNV in 2006, it has been added to the list of viruses to be excluded from domesticated SPF stocks of P. monodon in Thailand and it has been recommended that shrimp farmers avoid stocking LSNV positive post larvae to avoid MSGS [4, 25, 32]. It is recommended that in countries where L. vannamei has already been introduced, L. vannamei and P. monodon should be reared separately, particularly at the maturation and hatchery phases [16, 42, 43] (http://www.biotec.or.th/rdereport/prjbioteceng.asp?Id=882). The reason is not only to prevent disease spreading but the cultivation method of the two species is quite different [58]. Incidence of MSGS has been reported to be low in farms adopting GMP, GAqP or using SPF PL [56]. It would be prudent to limit the potential spread of MSGS by initiating quarantine safeguards in the movement of living shrimp stocks for aquaculture and to exercise due caution to prevent its spread by live broodstock or PL for aquaculture [2, 15]. Further, the National authorities should increase surveillance for slow growth syndrome in P. monodon. These measures will help reduce the risk of importing exotic viral pathogens that may damage local aquaculture [20].
Concluding Remarks
MSGS an emerging new syndrome in farmed P. monodon is a serious problem that reduced the farm profitability and made farmers to switch over to cultivation of American white shrimp. The anecdotal reports of unusual slow growth in cultivated P. monodon revealed common occurrence of LSNV in South and South East Asian countries and probably P. monodon a primary host for LSNV. Extensive surveys are needed to explore the range of natural hosts of LSNV as other crustacean species might be sources of infection. In view of the severity of the loss in production, the specific cause of MSGS yet to be determined unequivocally. Although MSGS occurrence is strongly associated with infection by LSNV, it is essential to understand pathogenesis causing retinopathy and effect on moulting. The other cause(s) that lead to LSNV-associated stunting of shrimp in MSGS ponds is still unknown but may involve other pathogens and/or environmental factors. More genome information is needed for proper classification of LSNV. The sources of LSNV and the cause of MSGS agent are unknown, but it is possible that they might have been originated from exotic crustaceans that have been imported for aquaculture and for the ornamental aquarium trade [18]. The problem is further complicated by the fact that it is transmitted by both vertical and horizontal routes. Also detailed studies that address the epizootiology of MSGS infection among cultured species to assess the risk of potential danger of transfer from the wild or asymptomatic carriers to the cultured species needs to be taken up. To date, no effective therapeutic measures are available to cure LSNV infection. Despite the fact that the actual etiology of MSGS is unknown, it is recommended that farmers avoid stocking ponds with LSNV-infected post larvae. Further, L. vannamei and P. monodon should be reared separately, particularly at the maturation and hatchery phases, and LSNV should be added to the list of pathogens for exclusion. The RT-PCR methods will be useful tools to select and maintain virus free penaeid shrimp stocks. Further, information on survival and stability of the agent outside the host and possible role of vectors needs to be elucidated which would help in formulating control strategies of MSGS. Also, large-scale outbreaks of emerging viral diseases among shrimp illustrate the need for new approaches towards disease surveillance and control.
References
- 1.Anantasomboon G, Akrajamorn A, Panphut W, Saeng-Oum W, Sritunyaluksana K, Withyachumnarnkul B. Evidence for the presence of monodon-slow growth agent in the pacific white shrimp Penaeus vannamei. In: World aquaculture meeting, May 9–13. Indonesia: Bali; 2005 (Abstract 609).
- 2.Anantasomboon G, Sriurairatana S, Flegel TW, Withyachumnarnkul B. Unique lesions and viral like particles found in growth retarded black tiger shrimp Penaeus monodon from East Africa. Aquaculture. 2006;253:197–203. doi: 10.1016/j.aquaculture.2005.08.021. [DOI] [Google Scholar]
- 3.Anggraeni MS, Owens L. The haemocytic origin of lymphoid organ spheroid cells in the penaeid prawn Penaeus monodon. Dis Aquat Org. 2000;40:85–92. doi: 10.3354/dao040085. [DOI] [PubMed] [Google Scholar]
- 4.Arunrut N, Seetang-Nun Y, Phromjai J, Panphut W, Kiatpathomchai W. Rapid and sensitive detection of Laem-Singh virus by reverse transcription loop mediated isothermal amplificatin combined with a lateral flow dipstick. J Virol Meth. 2011;177:71–74. doi: 10.1016/j.jviromet.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 5.Ayyappan S, Kalaimani N, Ponniah AG. Disease status in Indian shrimp aquaculture and research efforts for better health management. Fish Chim. 2009;29:13–21. [Google Scholar]
- 6.Balke I, Resevica G, Zeltins A. The ryegrass mottle virus genome codes for a sobemovirus 3C-like serine protease and RNA-dependent RNA polymerase translated via ribosomal frame shifting. Virus Genes. 2007;35:395–398. doi: 10.1007/s11262-007-0087-y. [DOI] [PubMed] [Google Scholar]
- 7.Boeke JD, Eickbush T, Sandmeyer SB, Voytas DF. Family Pseudoviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus taxonomy eighth report of the international committee on taxonomy of viruses. Amsterdam: Elsevier; 2005. pp. 397–407. [Google Scholar]
- 8.Bruenn JA. Relationships among the positive strand and double-strand RNA viruses as viewed through their RNA-dependent RNA polymerases. Nucleic Acids Res. 1991;19:217–226. doi: 10.1093/nar/19.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callinan RB, Jiang L, Smith PT, Soowannayan C. Fatal, virus-associated peripheral neuropathy and retinopathy in farmed Penaeus monodon in eastern Australia I. Pathol Dis Aquat Org. 2003;53:181–193. doi: 10.3354/dao053181. [DOI] [PubMed] [Google Scholar]
- 10.Chayaburakul K, Nash G, Pratanpipat P, Sriurairatana S, Withyachumnarnkul B. Multiple pathogens found in growth-retarded black tiger shrimp Penaeus monodon cultivated in Thailand. Dis Aquat Org. 2004;60:89–96. doi: 10.3354/dao060089. [DOI] [PubMed] [Google Scholar]
- 11.de Vries W, Haasnoot J, van der Velden J, van Montfort T, Zorgdrager F, Paxton W, Cornelissen M, van Kuppeveld F, de Haan P, Berkhout B. Increased virus replication in mammalian cells by blocking intracellular innate defence responses. Gene Ther. 2008;15:545–552. doi: 10.1038/gt.2008.12. [DOI] [PubMed] [Google Scholar]
- 12.Eickbush T, Boeke JD, Sandmeyer SB, Voytas DF. Family Metaviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus taxonomy eighth report of the international committee on taxonomy of viruses. Amsterdam: Elsevier; 2005. pp. 409–420. [Google Scholar]
- 13.FAO. Fishstat Plus. Rome: Food and Agricultural Organisation of the United Nations; 2009.
- 14.Flegel TW. The shrimp response to viral pathogens. In: Browdy CL, Jory DE, editors. The new wave. Proceedings of the special session on sustainable shrimp aquaculture. Orlando: World Aquaculture, World Aquaculture Society; 2001. pp. 190–194. [Google Scholar]
- 15.Flegel TW. Detection of major penaeid shrimp viruses in Asia, a historical perspective with emphasis on Thailand. Aquaculture. 2006;258:1–33. doi: 10.1016/j.aquaculture.2006.05.013. [DOI] [Google Scholar]
- 16.Flegel TW. Monodon slow growth syndrome and Laem Singh virus retinopathy. Bangkok: Disease card. NACA; 2008. p. 2. [Google Scholar]
- 17.Flegel TW. Current status of viral diseases in asian shrimp aquaculture. Isr J Aquac Bamidgeh. 2009;61:229–239. [Google Scholar]
- 18.Flegel TW. Emerging viral diseases of cultivated shrimp in Asia. Asian-Pacific Aquaculture meeting. 2007 (Abstract 377).
- 19.Flegel TW. Important diseases of the giant tiger shrimp P. monodon in Asia in world aquaculture meeting Busan, Korea May 19–23; 2008, p. 173.
- 20.Flegel TW, Withyachumnarnkul B. Research progress on monodon slow growth syndrome (MSGS) in Thailand. World Aquaculture Meeting. 2005 (Abstract 561).
- 21.Kalaimani N, Ravisankar T. Final report of AP cess fund project. Chennai: Central Institute of Brackishwater Aquaculture; 2009. p. 96. [Google Scholar]
- 22.Kwom HJ, Tirumalai R, Landy A, Ellenberger T. Flexibility in DNA recombination: structure of the Lamda integrase catalytic core. Science. 1997;276:126–131. doi: 10.1126/science.276.5309.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lightner DV. A handbook of pathology and diagnostic procedures for diseases of penaeid shrimp. World Aquaculture Society, Baton Rouge. 1996.
- 24.Lightner DV, Redman RM. Shrimp diseases and current diagnostic methods. Aquaculture. 1998;164:201–220. doi: 10.1016/S0044-8486(98)00187-2. [DOI] [Google Scholar]
- 25.Limsuwan C. How to overcome disease problems in shrimp culture. Aquac Asia Pac. 2006;2:17–19. [Google Scholar]
- 26.Linial ML, Fan H, Hahn B, Lwer R, Neil J, Quackenbush S, Rethwilm A, Sonigo P, Stoye J, Tristem M. Family retroviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus taxonomy eighth report of the international committee on taxonomy of viruses. Amsterdam: Elsevier; 2005. pp. 421–440. [Google Scholar]
- 27.Loh PC, Tapay LM, Lu Y, Nadala EC. Viral pathogens of the penaeid shrimp. Adv Virus Res. 1997;48:263–312. doi: 10.1016/S0065-3527(08)60290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayo MA, Leibowitz MJ, Palukaitis P, Scholthof K-BG, Simon AE, Stanley J, Taliansky M. Satellites. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus taxonomy eighth report of the international committee on taxonomy of viruses. Amsterdam: Elsevier; 2005. pp. 1163–1169. [Google Scholar]
- 29.Ongvarrasopone C, Chomchaya E, Panyima S. Antiviral effect of PmRab7 knock-down on inhibition of Laem-Singh virus replication in black tiger shrimp. Antivir Res. 2010;88:116–18. [DOI] [PubMed]
- 30.Oram M, Woolston JE, Jacobson AD, Holmes RK, Oram DM. Bacteriophage base vectors for site-specific insertion of DNA in the chromosome of Corynebacteria. Gene. 2007;391:53–100. doi: 10.1016/j.gene.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Reilly EK, Kao CC. Analysis of RNA-dependent RNA polymerase structure and function as guided by known polymerase structures and computer predictions of secondary structure. Virology. 1998;252:287–300. doi: 10.1006/viro.1998.9463. [DOI] [PubMed] [Google Scholar]
- 32.Panphut W, Senapin S, Sriurairatana S, Withyachumnarnkul B, Flegel TW. A novel integrase-containing element may interact with Laem-Singh virus (LSNV) to cause slow growth in giant tiger shrimp. BMC Vet Res. 2011;7:18. doi: 10.1186/1746-6148-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poornima M, Alavandi SV and Kalaimani N (2012). Laem-Singh virus (LSNV) and monodon slow growth syndrome in farmed black tiger shrimp (Penaeus monodon): An update. In: Pillai SM et al., editors. National conference on new vistas in indian aquaculture—book of abstracts. Chennai: Coastal Aquaculture Society of India, Chennai and Central Institute of Brackishwater Aquaculture; 2012, p. 49–50 (23–24th February, Abstract # HM O-10).
- 34.Poornima M, Alavandi SV and Kalaimani N, Muralidhar M, Gopikrishna G, Vinay kumar K, Rajan JJS and Sivakumar S (2012). Molecular characterization, histopathology and experimental transmission studies on Laem-Singh virus (LSNV) from shrimp in India. Ibid, p. 65 (Abstract # HM P-11).
- 35.Prakasha BK, Ramakrishna RP, Karunasagar I, Karunasagar I. Detection of Laem-Singhvirus (LSNV) in cultured Penaeus monodon from India. Dis Aquat Org. 2007;77:83–86. doi: 10.3354/dao01835. [DOI] [PubMed] [Google Scholar]
- 36.Prathoomthai B, Sakaew W, Sriurairatana S, Wongprasert K, Withyachumnarnkul B. Retinopathy in stunted black tiger shrimp Penaeus monodon and possible association with Laem-Singh virus (LSNV) Aquaculture. 2008;284:53–58. doi: 10.1016/j.aquaculture.2008.07.040. [DOI] [Google Scholar]
- 37.Pratoomthai B, Sakaew W, Udomkit A, Wongprasert K, Withyachumnarnkul B. The level of molt-inhibiting hormone (MIH) in small growth and normal growth shrimp Penaeus Monodon. J Anat Thail. 2009;32:125 (32nd AAT Annual Conference Abstract 2009).
- 38.Pratoomthai B, Wongprasert K, Flegel WT, Withyachumnarnkul B. Infection by Laem- Singh virus in the fasciculated zone and organ of Bellonci of the eyes of small Penaeusmonodon from Monodon slow-growth syndrome pond. In: 7th symposium on diseases in asian aquaculture at Taipei, Taiwan, June 22–26; 2008, p. 225.
- 39.Quarterly aquatic animal disease report (Asia and Pacific region), 2010/4, October–December 2010. Bangkok: NACA and FAO; 2011, p. 30.
- 40.Rai P, Pradeep B, Karunasagar I, Karunasagar I. Detection of viruses in Penaeusmonodon from India showing signs of slow growth syndrome. Aquaculture. 2009;289:231–235. doi: 10.1016/j.aquaculture.2008.12.035. [DOI] [Google Scholar]
- 41.Rajan JJS, Sivakumar S, Singaravel R, Poornima M, AlavandiSV, Kalaimani N, Santiago TC. Detection of slow growth syndrome disease in farmed shrimp in India. In: Indian youth science congress (IYSC 2009) at Rajiv Gandhi National Institute of youth development, Sriperumbudur. 2009. p. 23.
- 42.Report of the eighth meeting of the Asia regional advisory group on aquatic animal health. Bangkok: Network of Aquaculture Centres in Asia-Pacific (NACA); 2009, p. 8.
- 43.Report of the seventh meeting of the Asia regional advisory group on aquatic animal health. Bangkok: Network of Aquaculture Centres in Asia-Pacific (NACA); 2008, p. 10.
- 44.Rusaini, Owens L. Insight into the lymphoid organ of penaeid prawns: a review. Fish Shellfish Immunol. 2010; 29:367–77. [DOI] [PubMed]
- 45.Rusaini. The lymphoid organ and its interaction with moulting. M.Sc. Thesis, James Cook University, Townsville. 2006.
- 46.Sakaew W, Anantasomboon G, Asuvapongpatana S, Sriurairattana S, Withyachumnarnkul B. Abdominal segment deformity syndrome in the pacific white shrimp Penaeus vannamei in 7th symposium on diseases in asian aquaculture at Taipei, Taiwan. 2008, p. 220.
- 47.Sakaew W, Pratoomthai B, Wongprasert K, Withyachumnarnkul B. Vertical and horizontal transmission of laem-singh virus (LSNV) in the black tiger shrimp Penaeus monodon. J Anat Thail. 2009;32 (Abstract: 126).
- 48.Saksmerprome V, Wongtripop S, Noonin C, Withyachumnarnkul B. Double-stranded RNA induces interference with Lame-Singh virus (LSNV) replication in penaeid shrimp. In: 7th symposium on diseases in asian aquaculture at Taipei, Taiwan. 2008, p. 286.
- 49.Sang-oum W, Cheevadhanarak S, Sritunyalucksana K. Comparative genomics analysis of Laem Singh virus (LSNV) isolated from slow growing black tiger shrimp. Asian-Pacific Aquaculture Meeting 2008 (Abstract 202).
- 50.Sang-oum W, Sritunyalucksana K, Flegel TW,Cheevadhanarak S. Genomic characterization of Leam Singh virus (LSNV) isolated from slow growing black tiger shrimp. In: 7th symposium on diseases in asian aquaculture at Taipei, Taiwan. 2008, p. 221.
- 51.Sathish Kumar T, Krishnan P, Makesh M, Chaudhari A, Purushothaman C.S, Rajendran KV. Natural host-range and experimental transmission of Laem-Singh virus (LSNV). Dis Aquatic Org. 2011;96:21–7. [DOI] [PubMed]
- 52.Sittidilokratna N, Dangtip S, Sritunyalucksana K, Babu R, Pradeep B, Mohan CV, Gudkovs N, Walker PJ. Detection of Laem-Singh virus in cultured Penaeus monodon shrimp from several sites in the indo-pacific region. Dis Aquat Org. 2009;84:195–200. doi: 10.3354/dao02059. [DOI] [PubMed] [Google Scholar]
- 53.Srisala J, Flegel TW, Bhumiratana A, Sritunyalucksana K. Characterization of LaemSingh Virus, a new virus found in Penaeus monodon. In: Proceedings of the 48th Kasetsart University annual conference. 2010;4:36–3.
- 54.Srisala J. Genome Characterization of Laem Singh Virus (LSNV) in Penaeus monodon. M.Sc. Thesis, Mahidol University, Bangkok. 2010.
- 55.Sritunyalucksana K, Apisawetakan S, Boon-nat A, Withyachumnarnkul B, Flegel TW. A new RNA virus found in black tiger-shrimp Penaeus monodon from Thailand. Virus Res. 2006;118:31–38. doi: 10.1016/j.virusres.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 56.Subramaniam K. Marine shrimp in Malaysia: an opportunity to return to black tiger shrimp culture. Aquac Asia Pac Mag. 2008;4:12–13. [Google Scholar]
- 57.Walker PJ, Winton JR. Emerging viral diseases of fish and shrimp.Vet Res. 2010;41. doi:10.1051/vetres/2010022. [DOI] [PMC free article] [PubMed]
- 58.Withyachumnarnkul B. Search for solutions for MSGS in farmed black tiger shrimp. Aquac Asia Pac Mag. 2005;1:14–15. [Google Scholar]
- 59.Withyachumnarnkul B, Boon-Nad A, Anantasomboon G, Chayaburakul K, Sriurairatana S, Flegel TW. Lymphoid organ extracts of growth retarded Penaeus monodon contain a growth retardation agent. Honolulu: World Aquaculture Meeting Society (WAS). 2004, p. 1–5.