Abstract
Chikungunya virus (CHIKV), a reemerging arboviral disease of public health concern is characterized by a triad of fever, rash and arthralgia. It was responsible for a number of epidemics in Asia and Africa. The severity of the current epidemic can be judged by the fact that an estimated 1.38 million people in India and one-third of the La Reunion population (by April 2006) were affected by CHIKV. Aedes aegypti and Aedes albopictus are the major mosquitoes transmitting CHIKV in Asia. Various neurological complications and CHIKV associated deaths were encountered during the current outbreak (2005–2010). The aggressive nature of the recent CHIKV epidemic was attributed to the mutations in the viral genome in addition to their adaptation and spread to vectors like Aedes albopictus. Proper diet, adequate rest and symptomatic treatment using non-salicylate analgesics and Non-steroidal anti inflammatory drugs (NSAIDS) helped the patients in recovering from CHIKV infections. In the absence of an effective vaccine, rapid implementation of mosquito control measures and establishment of a system for continuous surveillance of the disease seems to be the only possible solution to prevent any such outbreak in the near future.
Keywords: CHIKV, Aedes mosquito, Co-infection, Mutation, RT-PCR, Differential diagnosis, Vaccine
Introduction
Chikungunya virus (CHIKV), a positive sense single stranded RNA virus belongs to the genus Alphavirus, family Togaviridae. The alphavirus genus contains 29 viruses, six of which cause human joint disorders (Chikungunya virus, O’nyong-nyong virus, Ross River virus, Barmah Forest virus, Sindbis virus, Mayaro virus) [84]. CHIKV was first reported in 1952–1953 in Tanzania and since then has been responsible for numerous outbreaks in Africa, South East Asia and India. There has always been some linguistic confusion regarding the origin of the word Chikungunya. Some authors report it to be derived from Makonde language while some claim it to be derived from Swahili language and a recent report claimed Chikungunya to be derived from Bantu language. From the initial reports of Robinson and Lumsden and from the findings of Benjamin it is confirmed that the term Chikungunya is indeed derived from Makonde language [40]. Phylogenetic analysis based on partial E1gene sequences showed the presence of three distinct CHIKV phylogroups. The first phylogroup contained all isolates from West Africa; the second phylogroup involved all East, Central and South African strains (ECSA); and the third phylogroup contained Asian isolates. CHIKV isolates causing epidemic in 1960s and 1970s in India are of Asian genotype while the Yawat isolate (2000) and the current epidemic isolates belong to Central African genotype. CHIKV reemerged in India after a gap of 32 years and an estimated 1.38 million people were affected by the end of 2006 and which further declined to an estimated 59 thousand, 95 thousand and 68 thousand cases by the end of 2007, 2008 and 2009, respectively [44, 45]. CHIKV has been declared as a high priority pathogen by NIH [28]. The 2006 CHIKV epidemic accounted for nearly 391 million rupees productivity loss, in India [27]. Infected viremic travelers from Kenya (2004) are held responsible for introduction of CHIKV to South Western Indian ocean islands and subsequently it spread in Asian countries [72].
The role of Ae. albopictus in the current CHIKV outbreak was experimentally proved and established. Prominent mutation (E1A226V) in CHIKV, susceptibility to the infection among pediatric population, increasing trade and travel, successful establishment of virus into new vector species and difficulties in implementation of mosquito control measures may be some of the contributing factors in the rapid spread of CHIKV.
Origin and Geographical Distribution
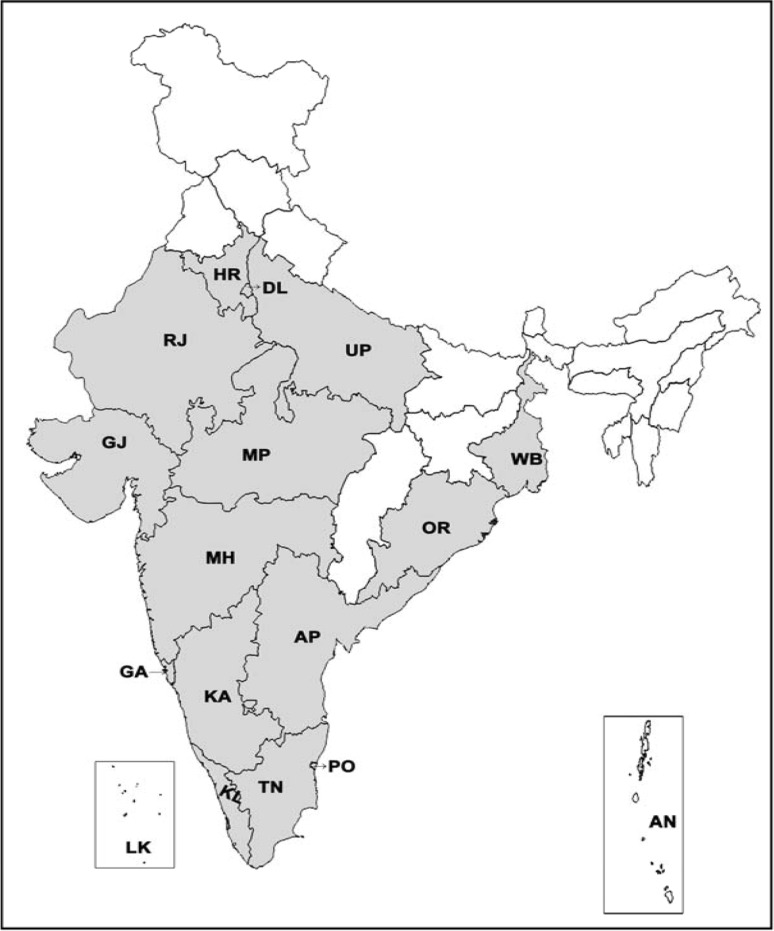
CHIKV epidemic was first reported from Makonde plateau, Tanzania during 1952–1953, followed by numerous outbreaks in South Africa, Congo, Zimbabwe, Uganda, Zambia, Senegal, Nigeria, Angola [25, 54]. It was responsible for explosive epidemics in Africa, India and Southeast Asia [18, 54]. Retrospective studies suggest that the occurrence of CHIKV epidemics in 1779 were erroneously documented as dengue outbreaks [78]. The first Asian outbreak was reported from Bangkok, Thailand in 1958, followed by other Asian countries which include India, Cambodia, Vietnam, Philippines, Srilanka, Indonesia and Malaysia [25]. In India the CHIKV outbreak was first reported in 1963 in Kolkata which accounted nearly 200 deaths [78]. Existence of CHIKV antibody in human sera collected in 1954–1956 suggested that CHIKV existed in India prior to 1963 [3, 51]. CHIKV outbreaks were recorded in Chennai, Pondicherry and Vellore in 1964; Visakhapatnam, Rajahmundry, Kakinada and Nagpur in 1965; and Barsi in 1973 [85]. CHIKV reemerged in democratic republic of Congo (1999–2000), Indonesia (2001–2003), Comoros islands (2005), Mauritius, Reunion islands (2005–2006) and reached India wherein 1.4 million people were affected [30, 49, 69]. CHIKV infection confirmed states/union territories in India during the current outbreak (2006–2009) is shown in Fig. 1. There are reports on CHIKV infections from tropical areas like Islands of Andaman and Nicobar, Lakshadweep and Singapore. Interestingly, certain European countries like France, Switzerland, Belgium, Netherlands, Spain, Greece, Croatia, Bosnia and other parts of the world (Israel, Taiwan, Central America, Brazil and USA) have reported imported cases of CHIKV.
Fig. 1.
Map of CHIKV confirmed states/Union territories in India from 2006 to 2009 (adapted from NVBDCP, Delhi. as on 31-12-2009). Shaded areas represent the affected states/UT. KL-Kerala; TN-Tamilnadu; PO-Pondicherry; KA-Karnataka; AP-Andhra Pradesh; GA-Goa; MH-Maharastra; OR-Orissa; MP-Madhya Pradesh; GJ-Gujarat; RJ-Rajasthan; WB-West Bengal; UP-Uttar Pradesh; DL-Delhi; HR-Harayana; LK-Lakshadweep Islands and AN*-Andaman and Nicobar Islands (* [34])
Genome Structure and Organization
Chikungunya is an enveloped virus which contains positive sense single stranded RNA as its genetic material. CHIKV particles reveal a characteristic Alphavirus morphology in green monkey kidney (Vero) cells under electron microscopy. It is about 70–100 nm in diameter by electron microscopy and atomic force microscopy [23]. The complete genome is about 11,805 nucleotides in length containing two open reading frames of about 7422 (non-structural genes: nsP1-nsP4) and 3744(Structural genes: C, E3, E2, 6K, E1) nucleotides in length (Fig. 2). The 5′ end contains 7-methyl guanosine cap while the 3′ end is polyadenylated. The genomic RNA acts as cellular messenger RNA. The nonstructural proteins are required for transcription and replication of the viral RNA and the structural proteins are involved in the replication of the virus and particularly in the interaction with the host. The junction region promotes transcription of an intracellular subgenomic 26S RNA. Antibodies directed against the E2 protein are usually virus-specific and sequence conservation in C protein and E1 glycoprotein leads to antigenic cross reactivity between alpha viruses [21]. E3 is a secreted polypeptide of unknown functions [11]. The 5′ UTR is required for plus strand RNA synthesis [47, 76] and 3′ UTR is involved in translation of viral proteins [31].
Fig. 2.
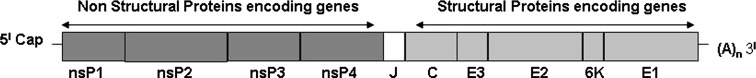
Genome structure of Chikungunya virus. nsP1, nsP2, nsP3 and nsP4: Non-structural genes 1–4. C: Capsid gene (structural gene). E3, E2, E1: Envelope. genes (structural genes). 6K: (structural gene). J: Junction region. (A)n: Poly A tail
CHIKV Replication
The CHIKV attaches to host receptors and enters the target cell by endocytosis (Fig. 3). After the delivery of the nucleocapsid into the cytoplasm, the CHIKV replication (Class IV) proceeds along parallel pathways. The Positive sense single stranded RNA genome (Viral genome) acts as an mRNA for the translation of the P1234 precursor and mature nonstructural proteins. RNA replication then proceeds through the synthesis of a full length minus strand RNA intermediate which is used both as a template for synthesis of genomic RNA and for the transcription of the 26S sub genomic plus strand RNA. The proteolytic processing of the P1234 nonstructural precursor regulates the transcription of positive and negative strand RNA to some extent. The cleavage product of P1234 is NSP4 which further associates with nsP123 polyprotein and with some host cellular machinery which synergistically acts as a negative strand RNA replicase. During replication when the cell concentration of nsP123 is raised sufficiently the precursor is further processed into mature proteins. The 26S sub genomic RNA serves as mRNA for the production of structural proteins. Autoproteolytic Serine Proteinase activity releases the capsid from the nascent polypeptide. The envelope polyprotein precursor is processed into pE2 and E1 which later gets associated in the Golgi complex and is exported to plasma membrane. Maturation of E1–E2 heterodimer and diffusion of mature nucleocapsids in cytoplasm occurs in parallel. The assembled virus particle consisting of an icosahedral nucleocapsid finally buds through the cell membrane and becomes an enveloped virion [11].
Fig. 3.
Chikungunya virus replication cycle
CHIKV Mouse Model Studies
CHIKV pathophysiology based on a mouse model developed by Couderc et al. [13] infers fibroblasts to be a predominant target cell. CHIKV replicates first in liver and targets muscle, joints and skin, closely resembling the cell/tissue tropism observed in biopsy samples of CHIK infected humans. In case of severe infections CHIKV also disseminates to other tissues including the CNS, where it specifically targets the choroids plexuses and the leptomeninges. Severity of CHIKV infection depends on two host factors: age and Type-I IFN signaling. Deep joint, bone tissues, brain parenchyma, micro vascular endothelial cells, and placenta are uninfected by CHIKV [13].
CHIKV Vectors and Virus Transmission
CHIKV Vectors and transmission cycle differs in Asia and Africa. CHIKV is maintained in a mosquito-human-mosquito cycle in Asia. Ae. aegypti is a well known vector for CHIKV transmission and the role of Ae albopictus (Asian tiger mosquito) has been experimentally proved and established during the recent outbreak [59, 64, 69, 82]. In Africa CHIKV is maintained in a sylvatic cycle involving wild primates and forest dwelling Aedes mosquitoes (Ae. africanus, Ae. furcifer, Ae. taylori, Ae. luteocephalus, Ae. neoafricanus, Ae. vittatus, Ae. fulgens, Ae. dalzieli, Ae. vigilax, Ae. camptorhynchites) [16, 37, 53]. Laboratory mice, rodents, African vervet, Asian monkeys, primates, prosimians and bats are susceptible to Chikungunya virus and develop high titre viremia [9]. The role of cattle in CHIKV transmission has also been reported [16]. Viremic human beings act as CHIKV reservoirs during the epidemics and as a precautionary measure blood donation was temporarily suspended in France to prevent transfusion transmitted CHIKV infection during the epidemic period. Ae. albopictus played a major role in CHIKV transmission in Lakshadweep [61].
Risk Groups
In humans the virus has no age or sex specificities but it has been seen that children, elderly and immune compromised are the most severely affected. It has been reported that people living in the geographic location where the CHIKV is imported are at a major risk.
Clinical Manifestations and Complications
CHIKV infection is characterized by high fever, headache, rashes, arthralgia and myalgia. Usually the disease is self limiting and the symptoms disappear within a week. Swelling of joints, retro orbital pain, vomiting, nausea, chills are some of the other well known symptoms observed in the affected patients. Swelling of feet and rashes encountered in CHIKV infected patients from Tirupati are shown in Figs. 4 and 5. Cases with lymphodenopathy and oral ulcers were also observed [79]. The severity and presence of various symptoms varied from individual to individual. Joint involvement is usually symmetrical, polyarticular, migratory and mainly affects small joints of hand, wrist, ankle and feet [26].Cases with hemorrhagic manifestations has also been described [43, 65, 66, 71]. Maculopapular rashes on face, trunk and limbs were observed. The severity of symptoms was more in patients suffering from diabetes, alcoholic hepatopathy and impaired renal functions [13]. Complications with involvement of the neurological and renal system were encountered [35, 36, 74]. Mother to child transmission [60] and CHIKV associated deaths were observed [17, 36, 79]. CHIKV causes encephalopathy in neonates and incidence of vertical maternal to fetal CHIKV transmission was at the peak during intra partum period and exceptional in case of ante partum fetal contamination [12]. The joint pains lasting for as long as 3 years after the onset of disease had been reported in the past [6]. Severe thrombocytopenia and neutropenia complicated by septicemia have also been reported in a few patients. Meningo-encephalitis, meningo encephalomyeloradiculitis, myeloradiculitis, myelitis, myeloneuropathy, Guillain–Barre syndrome, external opthalmoplegia, facial palsy, sensorineural hearing loss and optic neuritis are the various neurological complications observed during the current outbreak [38]. Asymptomatic CHIKV cases were encountered in the current outbreak [40]. A study in Mayotte showed that one out of every four CHIKV affected patient was asymptomatic [73]. This shows that the estimated risk of Viremic blood donation was high during CHIKV outbreaks [8] and hence proper screening of blood during the epidemics is necessary to prevent transfusion transmitted infection. To assess the severity of various symptoms prevalent in CHIKV affected patients; a questionnaire (enquiring details regarding the prevalence and severity of various symptoms) based survey was carried out. A total of 1388 subjects from 8 different areas in Tirupati region were interviewed, data collected using the questionnaire developed and analyzed using SPSS 13.0 package. Fever, arthralgia, headache, swelling of joints and retro orbital pain were the major symptoms encountered during the present study. Our epidemiological survey suggests that irrespective of gender, CHIKV affected all age groups [39].
Fig. 4.
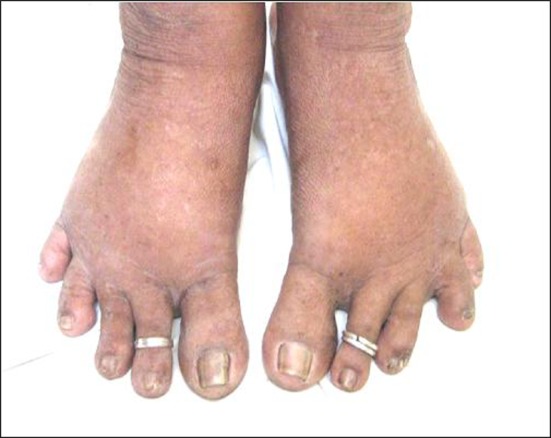
Swelling of feet, a major symptom observed in Chikungunya infected female patients during the recent CHIKV epidemic in Tirupati
Fig. 5.
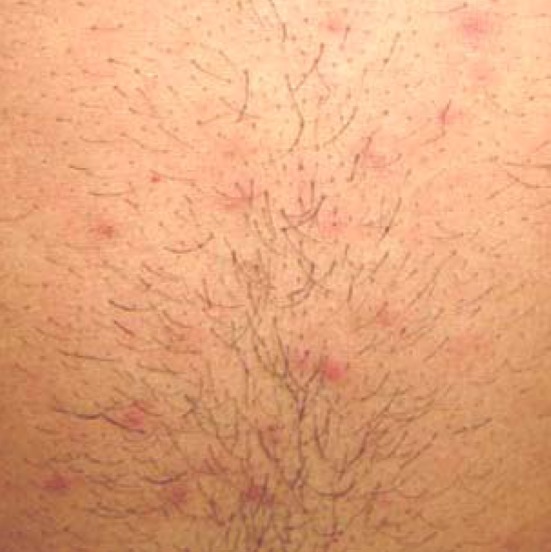
Rashes on upper abdominal area of a CHIKV infected patient. Rashes along with fever and arthralgia are characteristic symptom of Chikungunya
Diagnosis
During the epidemic, clinical triad of fever, rashes and arthralgia is suggestive of CHIKV infection. Serum amino transferase elevation, leucopenia and anaemia are associated with the onset of CHIKV but, due to the lack in their specificity it has become necessary to rely on molecular and serological diagnostics for detection of the causative agent [25]. Intracerebral inoculation of mice, Mosquito inoculation technique, use of mosquitoe cell lines (C6/36) and mammalian cell lines (Vero E6, BHK) are used for virus isolation. Recently a new cell line from the neonate larvae of Ae. aegypti was developed and it showed a greater yield of CHIKV than Vero E6 and C6/36 cell lines [77]. These techniques are expensive, time consuming and require expertise and hence cannot be used in clinical settings. Time of sample collection after the onset of disease, storage and transport of sample play a key role in the identification of any pathogen. CHIKV can be detected by RT-PCR up to 7 days [22, 41, 52]. A primer pair DVRChkF/DVRChkR (targeting 330 bp E1gene of CHIKV) detected CHIKV from total RNA of infected patient up to 60 ng (Fig. 6) and was used for rapid screening of CHIKV suspected samples in Andhra Pradesh, India as well as Southern Thailand [41, 80]. A real time RT-PCR test has been developed for CHIKV diagnosis [19, 50, 63]. RT-LAMP assay is highly sensitive and cost-effective tool, and can be used effectively during disease surveillance [48]. The convalascent phase samples (>7 days) are used for the detection of IgM and IgG antibodies. IgM capture ELISA is used as a reliable technique for detecting anti CHIK antibodies (Suggested cut-off levels: IgM > 0.15 and IgG > 0.10) [4]. Monoclonal antibody based antigen capture ELISA for detection of CHIKV antigens in mosquitoes and indirect ELISA for detection of IgM antibodies in CHIKV suspected patients have been described previously [24, 81]. Hemagglutination Inhibition test (HI) lacks specificity and identifies group rather than the individual virus and hence cannot be used as an accurate diagnostic test for CHIKV [29]. Indirect immunofluoroscent test (IIFT) is highly specific and sensitive tool [33]. Scientists at Singapore identified biomarkers and their association with disease severity and showed that an increase in IL-1 beta, IL-6 and a decrease in RANTES were associated with CHIKV disease severity [42].
Fig. 6.
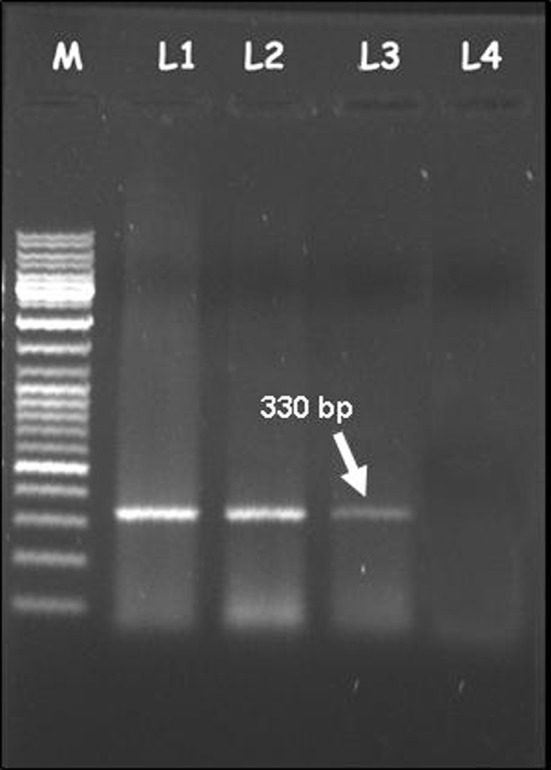
CHIKV detection by using the primer pair DVRChk-F/DVRChk-R which could amplify 330 bp E1 gene product. M: 1 Kb marker, Total RNA-L1: 180 ng; L2: 120 ng; L3: 60 ng; L4: 30 ng. CHIKV was detected from total RNA of infected patient up to 60 ng
Differential Diagnosis and CHIKV Coinfections
Due to similarity in symptoms with other arboviral (Dengue, Sindbis, RossRiver), viral(Rubella, Parvovirus B19, alphaviruses, mumps, VZV, EBV, CMV, Measles, HTLV-1, HIV, Hepatitis), bacterial (Meningococcemia, scarlet fever, Typhoid) and parasitic diseases(Leptospirosis, malaria) differential diagnosis of CHIKV plays an important role [67]. Cases of CHIKV and dengue co infections have been reported earlier [55]. During the current outbreak CHIKV and dengue co infections were again encountered [10, 32, 68]. CHIKV co infection with amoebiasis and RSV has been observed [20, 62].
E1:A226V Mutation and Its Importance
A single mutation in E1 gene of CHIKV was responsible for the severity of the current outbreak. Substitution of Alanine with Valine at position 226 of E1 envelope protein was observed and they speculated that this mutation reduced their cholesterol dependence to infect mosquito vectors which was later experimentally proved [69, 82]. Experiments were carried out to test the importance of A226V mutation and its role in infecting different vector species and it was observed that CHIKV isolates containing A226V mutation replicated and disseminated more efficiently in Ae. albopictus mosquitoes [83]. It was demonstrated that E1A226V mutation directly influenced vector specificity by enhancing CHIKV replication and transmission efficiency in Ae. Albopictus [82]. A226V mutation which was absent in all 2006 Indian isolates [2, 56] was found to be present in 2007 isolates from Kerala [57, 64]. Ae. albopictus vector has effectively displaced Ae. aegypti on Reunion islands and human activities such as commercial transportation of car tyres and plants further provided as a effective means for their dispersal into newer regions.
Therapeutics and Vaccines
There is no specific treatment for CHIKV infections. Adequate rest, proper diet and symptomatic treatment using non-salicylate analgesics and NSAIDS will help the patients in recovering from CHIKV infections. Paracetamol dosage of more than 3 gm/day was thought to be associated with severe liver afflictions and needs further confirmation [75]. Chloroquine is effective in treating chronic arthralgia due to its anti-inflamatory properties [5] and its use as an effective antiviral agent is under investigation. Possible use of Hydroxy chloroquine in CHIKV treatment needs further investigations in response to its extensive usage in rheumatological practices [70]. Synergistic efficacy was reported between interferon-α and ribavirin on CHIKV replication in invitro conditions [7]. Human polyvalent immunoglobulins, obtained from CHIKV infection convalescent donors (CHIKVIg) protected the experimental animals against CHIKV severity. Such a mode of treatment would be preferred for treating neonates born to viremic mothers as well as immuno compromised patients [14]. Inspite of the promising results shown during the phase II trials, the CHIKV vaccine (TSI-GSD-218) project had to be interrupted to focus on other infectious agents posing bioterrorism threat. Currently the phase III trials of the US army candidate vaccine is under progress [53]. RNA interference mediated inhibition of viral replication has emerged as a promising antiviral strategy and has been shown to inhibit partial to complete viral replication of several human and animal viruses like Polio virus, CMV, EBV, RSV, Influeza virus, FMDV, West Nile virus, HIV [58]. The effectiveness of siRNA mediated invitro replication of CHIKV was also demonstrated and it offered a potential new therapeutic approach [15]. Virus like particle (VLP) vaccine for viruses such as Hepatitis B and human Papillomavirus has already been approved by the Food and Drug Administration. An experimental vaccine developed using non-infectious VLP has shown promising results on macaques and mice against Chikungunya virus and its further evaluation on humans will be done in the near future [1].
Prevention and Control
Protective Measures Against Mosquito Bites
Ae. aegypti lives in close association with humans and A. albopictus prefers both domestic and peri domestic areas for breeding [46]. Ae Aegypti is active during early morning and the last 3–4 h of day light. Hence protective measures against mosquito bites such as usage of Mosquito nets (indoor protection); wearing full sleeve clothes (Outdoor protection) and usage of repellant containing 30–50% DEET as recommended by CDC are the important protection measures against mosquito bites.
Control of Mosquito Breeding Sites
Mechanical destruction of Mosquito breeding sites by elimination of all potential vector breeding sites near the domestic (or) peridomestic areas by removal, disposal or burning of all unused articles that can collect and hold water.
Use of Larvicide’s
For treatment of drinking water Temephos and methoprene at dosages not exceeding 1 mg of active ingredient (ai) per litre (1 ppm), pyriproxyfen at dosages not exceeding 0.01 mg ai per litre(0.01 ppm) can be used.
Indoor space spraying: Pyrethrum extract (0.1% ready to use emulsion) can be sprayed in rooms to kill the adult mosquitoes hiding in the houses.
Outdoor space spraying: Malathion is used for cold fogging and Malathion/Pyrethrum is used for Thermal fogging
Biological control: Use of larvivorous fish namely Gambusia and guppy or local species of cyclopoid copepods can be introduced to water tanks and cisterns as a control measure. Bacillus thuringiensis israelensis, is also used as a biological larvicide.
Surveillance
Epidemiological and entomological surveillance needs to be intensified. Reporting of fever cases should be monitored closely. This will help in identifying area for initiating control measure. Medical and Health Institution, professional association, private practitioners, NGOs should be involved for fever reporting and proper case management.
IEC Activities
IEC activities are crucial for community sensitization and participation. People need to be educated about the disease, mode of transmission, availability of treatment and adoption of control measures. The activities have to be intensified particularly to effect changes in practice of storage of water and personal protection. Special campaigns may be carried out with the involvement of mass media including local vernacular newspaper/magazines, radio and TV as well outdoor publicity like hoardings, miking, drum beating, rallies, etc. Health education materials should be developed and widely disseminated in the form of posters, pamphlets, handbills, hoardings, inter-personal communication through group meetings; traditional/folk media particularly must be optimally utilized.
Concluding Remarks and Challenges Ahead
To understand the survival nature of CHIKV during inter epidemic periods
To study the possible role of seasonal migratory birds and vertebrate reservoir hosts such as rodents in the maintenance of CHIKV.
To study the differential role of different species of mosquitoes in CHIKV maintenance and transmission.
Proper arrangement for supply of diagnostic kits during the epidemics.
To carry out epidemiological studies and proper implementation of mosquito control measures during the epidemics.
Acknowledgements
Naresh Kumar greatly acknowledges, University Grants Commission, New Delhi, Government of India, for financial assistance in the form of UGC-JRF in Research Fellowship in Sciences for Meritorious Students Scheme (RFSMS).
References
- 1.Akahata W, Yang ZY, Andersen H, Sun S, Holdaway HA, Kong WP, Lewis MG, Higgs S, Rossmann MG, Rao S, Nabel GJ. A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat Med. 2010;16:334–338. doi: 10.1038/nm.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arankalle VA, Shrivastava S, Cherian S, Gunjikar RS, Walimbe AM, Jadhav SM, Sudeep AB, Mishra AC. Genetic divergence of Chikungunya viruses in India (1963–2006) with special reference to the 2005–2006 explosive epidemics. J Gen Virol. 2007;88:1967–1976. doi: 10.1099/vir.0.82714-0. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee KA. Note on antibodies to Chikungunya virus in human sera collected in Madras State in 1956. Indian J Med Res. 1965;53:715. [PubMed] [Google Scholar]
- 4.Bodenmann P, Genton B. Chikungunya: an epidemic in real time. Lancet. 2006;368:258. doi: 10.1016/S0140-6736(06)69046-6. [DOI] [PubMed] [Google Scholar]
- 5.Brighton SW. Chloroquine phosphate treatment of chronic Chikungunya arthritis. An open pilot study. S Afr Med J. 1984;66:217–218. [PubMed] [Google Scholar]
- 6.Brighton SW, Prozesky OW, de la Harpe AL. Chikungunya virus infection. A retrospective study of 107 cases. S Afr Med J. 1983;63:313–315. [PubMed] [Google Scholar]
- 7.Briolant S, Garin D, Scaramozzino N, Jouan A, Crance JM. Invitro inhibition of Chikungunya and semiliki forest viruses replication by antiviral compounds: Synergistic effect of interferon-α and ribavirin combination. Antiviral Res. 2004;61:111–117. doi: 10.1016/j.antiviral.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Brouard C, Bernillion P, Quatresous I, Pillonel J, Assal A, De Valk H, Desenclos JD (2008). Estimated risk of Chikungunya viremic blood donation during an epidemic on Reunion Island in the Indian Ocean, 2005 to 2007. Transfusion. doi:10.1111/j.1537-2995.2008.01646.x. [DOI] [PubMed]
- 9.Calisher CH. In: Webster RG, Granoff A, editors. Chikungunya, O’nyong nyong and Mayaro viruses. In: Encyclopedia of virology, vol. 1. London: Academic Press; 1999, p. 261–265.
- 10.Chahar HS, Bharaj P, Dar L, Guleria R, Kabra SK, Broor S. Co-infections with Chikungunya virus and dengue virus in Delhi, India. Emerg Infect Dis. 2009;7:1077–1080. doi: 10.3201/eid1507.080638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chevillion C, Laurence B, Renaud F, Devaux C. The Chikungunya threat: an ecological and evolutionary perspective. Trends Microbiol. 2008;16:80–88. doi: 10.1016/j.tim.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Couderc T, Lecuit M. Focus on Chikungunya pathophysiology in human and animal models. Microbes Infect. 2009;11:1197–1205. doi: 10.1016/j.micinf.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Couderc T, Chretien F, Schilte C, Disson O, Brigitte M, GuivelBenhassine F, Touret Y, Barau G, Cayet N, Schuffenecker I, Desprès P, Arenzana-Seisdedos F, Michault A, Albert ML, Lecuit M. A mouse model for Chikungunya: young age and inefficient type-1 interferon signaling are risk factors for severe disease. PloS Pathog. 2008;4:e29. doi: 10.1371/journal.ppat.0040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couderc T, Khandoudi N, Grandadam M, Visse C, Gangneux N, Bagot S, Prost JF, Lecuit M. Prophylaxis and therapy for Chikungunya virus infection. J Infect Dis. 2009;200:516–523. doi: 10.1086/600381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dash PK, Tiwari M, Santhosh SR, Parida M, Lakshmana Rao PV. RNA interference mediated inhibition of Chikungunya virus replication in mammalian cells. Biochem Biophys Res Commun. 2008;376:718–722. doi: 10.1016/j.bbrc.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 16.Diallo M, Thonnon J, Traore Lamizana M, Fontenille D. Vectors of Chikungunya virus in Senegal: current data and transmission cycles. Am J Trop Med Hyg. 1999;60:281–286. doi: 10.4269/ajtmh.1999.60.281. [DOI] [PubMed] [Google Scholar]
- 17.Economopoulou A, Dominquez M, Helynck B, Sissoko D, Wichmann O, Ouenel P, Germonneau P, Ouatresous I. Atypical Chikungunya virus infections: clinical manifestations, mortality and risk factors for severe disease during the 2005–2006 outbreak on reunion. Epidemiol Infect. 2009;137:534–541. doi: 10.1017/S0950268808001167. [DOI] [PubMed] [Google Scholar]
- 18.Edelman R, Tacket CO, Wasserman SS, Bodison SA, Perry JG, Mangiafico JA. Phase II safety and immunogenicity study of live Chikungunya virus vaccine TSI-GSD-218. Am J Trop Med Hyg. 2000;62:681–685. doi: 10.4269/ajtmh.2000.62.681. [DOI] [PubMed] [Google Scholar]
- 19.Edwards CJ, Welch SR, Chamberlain J, Hewson R, Tolley H, Cane PA, Llyod G. Molecular diagnosis and analysis of Chikungunya virus. J Clin Virol. 2007;39:271–275. doi: 10.1016/j.jcv.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Ezzedine K, Cazanave C, Pistone T, Receveur MC, Neau D, Ragnaud JM, Malvy D. Dual infection by Chikungunya virus and other imported infectious agent in a traveller returning from India. Travel Med Infect Dis. 2008. doi:10.1016/j.tmaid.2008.02.002. [DOI] [PubMed]
- 21.Griffin DE. Alphaviruses. In: Knipe DM, Howley PM, editors. Fields virology. 4. Philadelphia: Lippincott Williams and Wilkins; 2001. pp. 917–962. [Google Scholar]
- 22.Hasebe F, Paraquet MC, Pandey BD, Mathenge EGM, Morita K, Balasubramaniam V, Saat Z, Yusop A, Sinniah M, Natkunam S, Igarshi A. Combined detection and genotyping of Chikungunya virus by a specific reverse transcriptase polymerase chain reaction. J Med Virol. 2002;67:370–374. doi: 10.1002/jmv.10085. [DOI] [PubMed] [Google Scholar]
- 23.Her Z, Kam YW, Lin RTP, Ng LFP. Chikungunya: a bending reality. Microb Infect. 2009;11:1165–76. [DOI] [PubMed]
- 24.Hundekar SL, Takare JP, Gokhale MD, Barde PV, Argade SV, Mourya DT. Development of monoclonal antibody based antigen capture ELISA to detect Chikungunya virus antigen in mosquitoes. Indian J Med Res. 2002;115:144–148. [PubMed] [Google Scholar]
- 25.Kalantri SP, Joshi R, Riley LW. Chikungunya epidemic: an Indian perspective. Natl Med J India. 2006;19:315–322. [PubMed] [Google Scholar]
- 26.Kennedy AC, Fleming J, Solomon L. Chikungunya viral arthropathy: a clinical description. J Rheumatol. 1980;7:231–236. [PubMed] [Google Scholar]
- 27.Krishnamoorthy K, Harichandrakumar KT, Krishna Kumari A, Das LK. Burden of Chikungunya in India: estimates of disability adjusted life years (DALY) lost in 2006 epidemic. J Vector Borne Dis. 2009;46:26–35. [PubMed] [Google Scholar]
- 28.Labadie K, Larcher T, Joubert C, Mannioui A, Delache B, Brochard P, Guigand L, Dubreil L, Lebon P, Verrier B, Lamballerie XD, Suhrbier A, Cherel Y, Grand RL, Roques P. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J Clin Invest. 2010;120:894–906. doi: 10.1172/JCI40104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laharyia C, Pradhan SK. Emergence of Chikungunya virus in Indian subcontinent after 32 years: a review. J Vector Borne Dis. 2006;43:151–160. [PubMed] [Google Scholar]
- 30.Laras K, Sukri NC, Larasati RP, Banjs MJ, Kosim R, Djauzi, Wandra T, Master J, Kosasih H, Hartati S, Beckett C, Sedyaningsih ER, Beecham HJ III, Corwin AL. Tracking the re-emergence of epidemic Chikungunya virus in Indonesia. Trans R Soc Trop Med Hyg. 2005;99:128–41. [DOI] [PubMed]
- 31.Leathers V, Tanguay R, Kobayashi M, Gallie DR. A phylogenetically conserved sequence within viral 3′ untranslated RNA pseudo knots regulates translation. Mol Cell Biol. 1993;13:5331–5347. doi: 10.1128/mcb.13.9.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leroy EM, Nkoghe D, Ollomo B, Nze-Nkoque C, Becquart P, Grard G, Pourrut X, Charrel R, Moureau G, Ndjoyi-Mbiquino A, De-Lamballerie X. Concurrent Chikungunya and dengue virus infections during simultaneous outbreaks. Gabon, 2007. Emerg Infect Dis. 2009;15:591–593. doi: 10.3201/eid1504.080664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Litzba N, Schuffenecker I, Zeller H, Drosten C, Emmerich P, Charrel R, Kreher P, Niedrig M. Evaluation of first commercial Chikungunya virus indirect immunofluoroscent test. J Virol Methods. 2008;149:175–179. doi: 10.1016/j.jviromet.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Manimunda SP, Singh SS, Sugunan AP, Singh O, Roy S, Shriram AN, Bharadwaj AP, Shah WA, Vijayachari P. Chikungunya fever, Andaman and Nicobar Islands, India. Emerg Infect Dis. 2007;13:1259–1260. doi: 10.3201/eid1308.070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mavalankar D, Shastri P, Raman P. Chikungunya epidemic in India: a major public health disaster. Lancet Infect Dis. 2007;7:306–307. doi: 10.1016/S1473-3099(07)70091-9. [DOI] [PubMed] [Google Scholar]
- 36.Mavalankar D, Shastri P, Bandyopadhyay T, Parmar J, Ramani KV. Increased mortality rate associated with Chikungunya epidemic, Ahmedabad, India. Emerg Infect Dis. 2008;14:412–415. doi: 10.3201/eid1403.070720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McIntosh BM, Jupp PG, Dos SI. Rural epidemic of Chikungunya in South Africa with involvement of Aedes (Diceromyia) furcifer (Edwards) and baboons. S Afr J Sci. 1977;73:267–269. [Google Scholar]
- 38.Murthy JMK. Chikungunya virus: the neurology. Neurol India. 2009;57:113–115. doi: 10.4103/0028-3886.51275. [DOI] [PubMed] [Google Scholar]
- 39.Naresh Kumar CVM, Afsar S, Sai Gopal DVR. Retrospective survey of Chikungunya disease in Tirupati region of Andhra Pradesh, India. Abstract of the paper presented at “International Symposium on Environmental Pollution, Ecology and Human Health” at Sri Venkateswara University, Tirupati, India, July 25–27, 2009, p. 20–1.
- 40.Naresh Kumar CVM, Sai Gopal DVR. Possible transmission of Chikungunya virus through blood transfusion. Curr Sci. 2008;94:1548–1549. [Google Scholar]
- 41.Naresh Kumar CVM, Anthony Johnson AM, Sai Gopal DVR. Molecular characterization of Chikungunya virus from Andhra Pradesh, India and phylogenetic relationship with Central African isolates. Indian J Med Res. 2007;126:534–540. [PubMed] [Google Scholar]
- 42.Ng LFP, Chow A, Sun Y-J, Kwek DJC, Lim P-L, Dimatatac F, Ng L-C, Ooi EE, Choo KH, Her Z, Kourilsky P, Leo Y. IL-1β, IL-6, and RANTES as biomarkers of Chikungunya Severity. PLoS One. 2009;4:e4261. doi: 10.1371/journal.pone.0004261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nimmannitiya S, Halstead SB, Cohen SN, Margiotta MR. Dengue and Chikungunya virus infections in man in Thailand, 1962–64. I. Observations on hospitalized patients with hemorrhagic fever. Am J Trop Med Hyg. 1969;18:954–971. doi: 10.4269/ajtmh.1969.18.954. [DOI] [PubMed] [Google Scholar]
- 44.NVBDCP. Epidemiological profile of Chikungunya virus fever in the country (prov.). http://nvbdcp.gov.in/Doc/chikun-update07.pdf. Accessed 28 December 2009.
- 45.NVBDCP. Status report on Dengue and Chikungunya as on 31.12.09. Dengue and Chikungunya virus update 2010. http://nvbdcp.gov.in/Doc/Den_Chik_Dec09.pdf. (2010). Accessed 16 April 2010.
- 46.NVBDCP. National Vector Borne Disease Control Programme, New Delhi. http://nvbdcp.gov.in (2010). Accessed 16 April 2010.
- 47.Ou JH, Strauss EG, Strauss JH. The 5′-terminal sequences of the genomic RNAs of several alphaviruses. J Mol Biol. 1983;168:1–15. doi: 10.1016/S0022-2836(83)80319-2. [DOI] [PubMed] [Google Scholar]
- 48.Parida MM. Rapid and real-time detection technologies for emerging viruses of biomedical importance. J Biosci. 2008;33:617–628. doi: 10.1007/s12038-008-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pastorino B, Muyembe-Tamfum JJ, Bessaud M, Tock F, Tolou H, Durand JP, Peyrefitte CN. Epidemic resurgence of Chikungunya virus in Democratic Republic of the Congo: identification of a new central African strain. J Med Virol. 2004;74:277–282. doi: 10.1002/jmv.20168. [DOI] [PubMed] [Google Scholar]
- 50.Pastorino B, Bessaud M, Grandadam M, Murri S, Tolou HJ, Peyrefitte CN. Development of a TaqMan RTPCR assay without RNA extraction step for the detection and quantification of African Chikungunya viruses. J Virol Methods. 2005;124:65–71. doi: 10.1016/j.jviromet.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 51.Pavri KM. Presence of Chikungunya antibodies in human sera collected from Calcutta, Jamshedpur before 1963. Indian J Med Res. 1963;52:698. [PubMed] [Google Scholar]
- 52.Pfeffer M, Linssen B, Parke MD, Kinney RM. Specific detection of Chikungunya virus using a RT-PCR/nested PCR combination. J Vet Med Ser B. 2002;49:49–54. doi: 10.1046/j.1439-0450.2002.00535.x. [DOI] [PubMed] [Google Scholar]
- 53.Pialoux G, Gauzere BA, Jaureguiberry S, Strobel M. Chikungunya: an epidemic arbovirus. Lancet Infect Dis. 2007;7:319–327. doi: 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- 54.Powers AM, Logue CH. Changing patterns of Chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol. 2007;88:2363–2377. doi: 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- 55.Powers AM, Brault AC, Tesh RB, Weaver SC. Re-emergence of Chikungunya virus and O’nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J Gen Virol. 2000;81:471–479. doi: 10.1099/0022-1317-81-2-471. [DOI] [PubMed] [Google Scholar]
- 56.Pradeep Kumar N, Madhu Mitha M, Krishnamoorthy N, Kamaraj T, Joseph R, Jambulingam P. Genotyping of virus involved in the 2006 Chikungunya outbreak in South India (Kerala and Puducherry) Curr Sci. 2007;93:1412–1416. [Google Scholar]
- 57.Pradeep Kumar N, Joseph R, Kamaraj T, Jambulingam P. A226V mutation in virus during the 2007 Chikungunya virus outbreak in Kerala, India. J Gen Virol. 2008;89:1945–1948. doi: 10.1099/vir.0.83628-0. [DOI] [PubMed] [Google Scholar]
- 58.Raut AA, Narutte P, Patial S, Rai A, Gupta PK. Small interfering RNA (siRNA) as antiviral therapeutics. Indian J Virol. 2006;17:1–7. [Google Scholar]
- 59.Reiskind MH, Pestko K, Westbrook CJ, Mores CN. Susceptibility of Florida mosquitoes to infection with Chikungunya virus. Am J Trop Med Hyg. 2008;78:422–425. [PMC free article] [PubMed] [Google Scholar]
- 60.Robillard PY, Boumahni B, Geradin P, Michault A, Fourmaintraux A, Schuffenecker I, Carbonnier M, Djémili S, Choker G, Roge-Wolter M, Barau G. Vertical maternal fetal transmission of the Chikungunya virus. Ten cases among 84 pregnant women. Presse Med. 2006;35:785–788. doi: 10.1016/S0755-4982(06)74690-5. [DOI] [PubMed] [Google Scholar]
- 61.Samuel PP, Krishnamoorthi R, Hamzakova KK, Aggarwal CS. Entomo-epidemiological investigations on Chikungunya outbreak in the Lakshadweep islands, Indian Ocean. Indian J Med Res. 2009;129:442–445. [PubMed] [Google Scholar]
- 62.Sankari T, Holti SL, Govindraj V, Das PK. Chikungunya and respiratory viral infections. Lancet. 2008;8:3–4. doi: 10.1016/S1473-3099(07)70295-5. [DOI] [PubMed] [Google Scholar]
- 63.Santhosh SR, Parida MM, Dash PK, Pateriya A, Pattnaik B, Pradhan HK, Tripathi NK, Ambuj S, Gupta N, Saxena P, Lakshmana Rao PV. Development and evaluation of SYBR green I-based one-step real-time RT-PCR assay for detection and quantification of Chikungunya virus. J Clin Virol. 2007;39:188–193. doi: 10.1016/j.jcv.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 64.Santhosh SR, Dash PK, Parida MM, Khan M, Tiwari M, Lakshmana rao PV. Comparative full genome analysis revealed E1: A226V shift in 2007 Indian Chikungunya virus isolates. Virus Res. 2008;135:36–41. doi: 10.1016/j.virusres.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 65.Sarkar JK, Chatterjee SN, Chakravarty SK. Haemorrhagic fever in Calcutta: some epidemiological observations. Indian J Med Res. 1964;52:651–659. [PubMed] [Google Scholar]
- 66.Sarkar JK, Pavri KM, Chatterjee SN, Chakravarthy SK, Anderson CR. Virological and serological studies of cases of hemorrhagic fever in Calcutta. Material collected by the Calcutta school of tropical medicine. Indian J Med Res. 1964;52:684–691. [PubMed] [Google Scholar]
- 67.Saxena SK. Re-emergence of the knotty Chikungunya virus: facts, fear or fiction. Future Virol. 2007;2:121–126. doi: 10.2217/17460794.2.2.121. [DOI] [Google Scholar]
- 68.Schilling S, Emmerich P, Gunther S, Schmidt-Chanasit J. Dengue and Chikungunya virus co-infections in a German traveler. J Clin Virol. 2009;45:163–164. doi: 10.1016/j.jcv.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 69.Schuffenecker I, Iteman I, Michault A, Murri S, Frangeul L, Vaney MC, Lavenir R, Pardigon N, Reynes JM, Pettinelli F, Biscornet L, Diancourt L, Michel S, Duquerroy S, Guigon G, Frenkiel MP, Bréhin AC, Cubito N, Desprès P, Kunst F, Rey FA, Zeller H, Sylvain Brisse S. Genome microevolution of Chikungunya virus causing the Indian Ocean outbreak. PLOS Med. 2006;3:e263. doi: 10.1371/journal.pmed.0030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seneviratne SL, Gurugama P, Perera J. Chikungunya viral infections: an emerging problem. J Travel Med. 2007;14:320–325. doi: 10.1111/j.1708-8305.2007.00135.x. [DOI] [PubMed] [Google Scholar]
- 71.Shah KV, Gibbs CJ, Banerjee G. Virological investigation of the epidemic of haemorrhagic fever in Calcutta: isolation of three strains of Chikungunya virus. Indian J Med Res. 1964;52:676–683. [PubMed] [Google Scholar]
- 72.Sissoko D, Malvy D, Ezzedine K, Renault P, Moscetti F, Ledrans M, Pierre V. Post-epidemic Chikungunya virus disease on Reunion islands: Course of rheumatic manifestations and associated factors over a 15-month period. PLoS Negl Trop Dis. 3:e389. doi:10.1371/journal.pntd.0000389 (2009). [DOI] [PMC free article] [PubMed]
- 73.Sissoko D, Moendandze A, Malvy D, Giry C, Ezzedine K, Solet JL, Pierre V. Seroprevalence and risk factors of Chikungunya virus infection in Mayotte, IndianOcean, 2005–2006: a population-based survey. PLoS One. 2008;3:e3066. doi: 10.1371/journal.pone.0003066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Solanki BS, Arya SC, Maheswari P. Chikungunya disease with nephritic presentation. Int J Clin Pract. 2007;61:19–41. doi: 10.1111/j.1742-1241.2007.01329.x. [DOI] [PubMed] [Google Scholar]
- 75.Staikowsky F, Roux KLe, Schuffenecker I, Laurent P, Grivard P, Develay A, Michault A. Retrospective survey of Chikungunya disease in Reunion island staff. Epidemiol Infect. 2008;136:196–206. doi: 10.1017/S0950268807008424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Strauss JH, Strauss EG. The alpha viruses, gene expression, replication and evolution. Microbiol Mol Biol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sudeep AB, Parashar D, Jadi RS, Basu A, Mokashi C, Arankalle VA, Mishra AC. Establishment and characterization of a new Aedes aegypti (L.) (Diptera:Culicidae) cell line with special emphasis on virus susceptibility. In Vitro Cell Dev Biol Anim. 2009;45:491–5. doi:10.1007/s11626-009-9218-1. [DOI] [PubMed]
- 78.Sudeep AB, Parashar D. Chikungunya: an overview. J Biosci. 2008;33:443–449. doi: 10.1007/s12038-008-0063-2. [DOI] [PubMed] [Google Scholar]
- 79.Suryawanshi SD, Dube AH, Khadse RK, Jalgaonkar SV, Sathe PS, Zawar SD, Holay MP. Clinical profile of Chikungunya fever in patients in a tertiary care centre in Maharashtra, India. Indian J Med Res. 2009;129:438–441. [PubMed] [Google Scholar]
- 80.Theamboonlers A, Rianthavorn P, Praiananthavorn K, Wuttirattanakowit N, Poovorawan Y. Clinical and molecular characterization of Chikungunya virus in South Thailand. Jpn J Infect Dis. 2009;62:303–5. [PubMed]
- 81.Thein S, La Linn M, Aaskov J, Aung MM, Aye M, Zaw A, Myint A. Development of a simple indirect enzyme-linked immunosorbent assay for the detection of immunoglobulin M antibody in serum from patients following an outbreak of Chikungunya virus infection in Yangon, Myanmar. Trans R Soc Trop Med Hyg. 1992;86:438–442. doi: 10.1016/0035-9203(92)90260-J. [DOI] [PubMed] [Google Scholar]
- 82.Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in Chikungunya virus affects vector specificity and epidemic potential. PLOS Pathog. 2007;3:e201. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vazeille M, Moutailler S, Coudner D, Rousseaux C, Khun H, Huerre M, et al. Two Chikungunya virus isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquitoe, Aedes albopictus. PLoS ONE. 2007;2:e1168. doi: 10.1371/journal.pone.0001168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weaver SC, Frey TK, Huang HV, Kinney RM, Rice CM, Roehrig JT, Shope RE, Strauss EG. Togaviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus taxonomy. Eighth report of the international committee on taxonomy of viruses. London: Elsevier/Academic Press; 2005. pp. 999–1008. [Google Scholar]
- 85.Yergolkar PN, Tandale BV, Arankalle VA, Sathe PS, Sudeep AB, Swati SG, Gokhle MD, Jacob GP, Hundekar SL, Mishra AC. Chikungunya outbreaks caused by African genotype, India. Emerg Infect Dis. 2006;12:1580–1583. doi: 10.3201/eid1210.060529. [DOI] [PMC free article] [PubMed] [Google Scholar]