Abstract
Canine parvovirus 2 (CPV-2) has been considered to be an important pathogen of domestic and wild canids and has spread worldwide since its emergence in 1978. It has been reported from Asia, Australia, New Zealand, the Americas and Europe. Two distinct parvoviruses are now known to infect dogs—the pathogenic CPV-2 and CPV-1 or the minute virus of canine (MVC). CPV-2, the causative agent of acute hemorrhagic enteritis and myocarditis in dogs, is one of the most important pathogenic viruses with high morbidity (100%) and frequent mortality up to 10% in adult dogs and 91% in pups. The disease condition has been complicated further due to emergence of a number of variants namely CPV-2a, CPV-2b and CPV-2c over the years and involvement of domestic and wild canines. There are a number of different serological and molecular tests available for prompt, specific and accurate diagnosis of the disease. Further, both live attenuated and inactivated vaccines are available to control the disease in animals. Besides, new generation vaccines namely recombinant vaccine, peptide vaccine and DNA vaccine are in different stages of development and offer hope for better management of the disease in canines. However, new generation vaccines have not been issued license to be used in the field condition. Again, the presence of maternal antibodies often interferes with the active immunization with live attenuated vaccine and there always exists a window of susceptibility in spite of following proper immunization regimen. Lastly, judicious use of the vaccines in pet dogs, stray dogs and wild canids keeping in mind the new variants of the CPV-2 along with the proper sanitation and disinfection practices must be implemented for the successful control the disease.
Keywords: Canine parvovirus, Hemorrhagic enteritis, Myocarditis, Minute virus of canine, Vaccination
Introduction
Canine parvovirus 2, the causative agent of acute hemorrhagic enteritis and myocarditis in dogs, is one of the most important pathogenic viruses. It is a highly contagious and often fatal disease. CPV-2 was first recognized in 1977 and since then it has been well established as an enteric pathogen of dogs throughout the world with high morbidity (100%) and frequent mortality up to 10% [2, 6]. CPV is believed to have originated as a host range variant from feline panleucopenia virus (FPV), include a direct mutation from FPV, a mutation from a FPV vaccine virus and the adaptation to the new host dog via non-domestic carnivores, like mink and foxes. The disease is characterized by two prominent clinical forms (i) enteritis with vomition and diarrhea in dogs of all ages [1, 99] (ii) myocarditis and subsequent heart failure in pups of less than 3 months of age [30]. The virus was named CPV-2 in order to differentiate it from a closely related parvovirus of canine known as CPV-1 or minute virus of canine (MVC). MVC, a completely different parvovirus, had not been associated with natural disease until 1992. MVC may cause pneumonia, myocarditis and enteritis in young pups or transplacental infections in pregnant dams, with embryo resorptions and fetal death [10]. About 30 confirmed cases of CPV-1 have been reported in USA, Sweden, Italy, Germany and more recently in Japan [52, 74]. The CPV-2 infections have been emerged to be a problem in dogs in recent times around the world. The disease has also been reported in high proportions in dogs in India with high level of casualties even in vaccinated populations. The disease is highly infectious and is spread from dog to dog by direct or indirect contact with their faeces. Over the years, a number of diagnostic assays both serological and molecular have been developed for prompt, precise and sensitive diagnosis of the disease. Again, both inactivated and live attenuated CPV vaccines both as monovalent and along with vaccines against other diseases have been developed and used for the control of the disease. However, in spite of proper vaccination of animals, vaccines failures have been reported due to presence of maternal antibodies and emergence of new variants. So, this review on CPV is aimed to provide detailed informations about the disease including diagnosis, immunoprophylaxis, treatment, etc. for the scientific fraternity, students, teachers, diagnosticians, practitioners, pet owners, kennel club owners, pet shop owners, defense personnel and lastly the general public so that it can be managed and controlled in a highly scientific and efficient manner [7].
Etiology
‘Parvo’ means small (Latin), canine parvovirus belongs to genus Parvovirus and family Parvoviridae. The genome is a single stranded negative sense DNA having size of 5.2 Kb [54] in length which has two promoters resulting in the expression of three structural (VP1, VP2 and VP3) and two non-structural proteins (NS1 and NS2) through alternate splicing of the viral mRNAs. VP2 (64 kDa) is an NH2-terminally truncated form of VP1 (84 kDa) and is the major component of the capsid. VP3 is derived from VP2 by posttranslational proteolytic cleavage and is present only in complete (DNA-containing) virions. Empty particles do not contain VP3 protein. Trypsin treatment of full particles cleaves VP2 to VP3 protein. CPV-2 has icosahedral symmetry, 25 nm in diameter and nonenveloped with a linear, single stranded DNA genome. The crystal structures of CPV-2 have been determined and their basic capsid organizations are similar. The 60 protein subunits, of which about 5–6 copies of VP1 and 54–55 copies of VP2 that make up the capsid have a common structure, arranged with T = 1 icosahedral symmetry [90]. There is some evidence that the VP1 terminus is internal and may help neutralize the DNA. The main structural motif is an eight-stranded, antiparallel β-barrel, which also has been found in most other viral capsid structures. The β-barrel motif contains only approximately one-third of the amino acid composition of VP2, the major structural protein in most parvovirus that comprises about 90% of the capsid [12]. The remaining two-thirds is present as large loops connecting the strands of the β-barrel. The loops form much of the capsid surface, onto which a number of biologic features, such as host species and tissue tropism, receptor binding and antigenic properties have been structurally and genetically mapped [66, 70]. Replication occurs in the nucleus of dividing cells and infection leads to large intranuclear inclusion bodies. Other characteristic features of the parvoviral capsid include spike like protrusions at the icosahedral threefold axes, a 15-Å canyon-like depression about the fivefold axes and a dimple-like depression at the icosahedral twofold axes. Antigenic regions have been mapped to the threefold protrusion whereas the twofold depression has been implicated in the attachment of host cell factors [54]. The particle has a molecular weight (MW) of 5.5 to 6.2 × 106 Da. Approximately 50% of the mass is protein, and the remainder is DNA. Because of the relatively high DNA-to-protein ratio, the buoyant density of the intact virion in cesium chloride (CsCl) is 1.39–1.42 g/cm3. Finally, the sedimentation coefficient of the virion in neutral sucrose gradients is 110–122 S [54, 69].
Emergence of Canine Parvovirus Strains and its Distribution
During the early 1970s, a new infectious disease of pups, characterized by either gastroenteritis or myocarditis, was observed worldwide. A small, round, non-enveloped virus was observed by electron microscopy in stool specimens and in tissues of affected animals. Subsequently, a novel parvovirus was isolated both in canine and feline cell cultures [1, 8, 41]. The virus was named CPV-2. It was speculated that CPV-2 might have emerged at least 10 years before the clinical disease was recognized [84]. It was deduced that beneficial mutations (to the virus) had accumulated during that period until a virus emerged from an unknown source that infected a new host (the dog) and acquired the ability to spread [84]. After a period of adaptation, the virus became highly infectious for dogs, resulting in the pandemic that became evident in 1978–1979. The rapid rate at which parvoviruses accumulate mutations in vivo similar to observations made in studies on CPV-2 vaccinal virus, where mutations were found to accumulate rapidly during passage in tissue culture [3, 84].
CPV-2 and FPV are significant pathogens for domestic dogs and cats as well as for various wild carnivore species. CPV-2 and FPV are grouped along with other viruses such as mink enteritis virus (MEV), raccoon parvovirus (RPV), raccoon dog parvovirus (RDPV) and blue fox parvovirus (BFPV) in the so-called feline parvovirus subgroup [89]. Phylogenetic analysis revealed that all CPV variants were descended from a single ancestor which emerged during the mid-1970s, which was closely related to the long-known feline panleukopenia virus (FPV) which infects cats, minks, and raccoons but not dogs or cultured dog cells [91]. There is more than 98% sequence homology and as few as six coding nucleotide differences in the VP2 gene at positions 3025, 3065, 3094, 3753, 4477 and 4498 [93]. The biological effects of these few genomic changes were sufficient for CPV-2 to acquire canine host range, but lost the ability to replicate in feline host [92]. Two differences at VP2 residues 93 from Lys to Asn and 323 from Asp to Asn between FPV and CPV could introduce the canine host range, a CPV-specific antigenic epitope [13]. Despite the close relationship to FPV, CPV type 2 isolates did not replicate in cats, and this host range was determined at least in part by VP2 residues 80, 564, and 568 which are in close proximity in the capsid structure [94].
Nucleotide substitutions in CPV-2 continued to be observed, but their biological significance is not known. In 1979, a CPV variant (CPV type 2a) emerged that spread worldwide within 1 year due to antigenic drift and replaced the CPV type 2 strains. CPV type 2a contained five substitutions in the capsid sequence compared to CPV type 2, including changes of VP2 residues 87 from Met to Leu, 300 from Ala to Gly, and 305 from Asp to Tyr [70]. CPV type 2a isolates were antigenically different from CPV type 2 and also infected and caused disease in cats [94]. An antigenic variant of CPV type 2a (CPV type 2b) was recognized in 1984, and it differed in an antigenic epitope as a result of the substitution of VP2 at residue 426 from Asn to Asp and at residue 555 from Ile to Val [68]. These CPV-2a and CPV-2b are the predominant strains currently circulating in the different dog population, and have completely replaced the original CPV-2 virus worldwide [70, 94]. Both the antigenic types coexist in different ratio in dog populations around the world. The regaining of feline host range by CPV-2a and CPV-2b was likely to be a selective advantage of the virus [94].
In 2000, another mutant called CPV-2c was reported in dogs from Italy and it differs from CPV-2b by one amino acid at 426 position from Asp to Glu [7] and subsequently from Vietnam, Spain, United Kingdom, South America, North America, Portugal and India [20, 55, 59]. The mutation Glu-426 affects the major antigenic region located over the three-fold spike of CPV-2 capsid. Monoclonal antibodies have been developed and used for detection of different novel mutants of CPV-2 [55]. In addition, sequence analysis of recent CPV-2a isolates has revealed a reversion at position 555 to the sequence of FPV/CPV-2, Ile to Val. This mutation restricts the differences among the antigenic variants CPV-2a, 2b and 2c to only one amino acid at position 426, which are Asn in CPV-2a, Asp in CPV-2b and Glu in the CPV-2c. Most CPV-2 strains spreading currently in Italy differ only in this residue [20]. There is no evidence that CPV-2c is a more serious threat to either shelter or owned dogs than the other CPV strains. It is not possible to distinguish CPV-2c from CPV-2b or 2a isolates based on clinical signs. CPV-2c causes similar clinical signs as the previously known strains, including mucoid or hemorrhagic diarrhea, leukopenia, and lymphopenia [33, 40]. Although a few reports suggest that CPV-2c may cause more severe clinical signs and mortality particularly in adult dogs than type 2a and 2b, others describe less-severe disease and lower mortality rates in CPV-2c infected dogs [39].
Incidence
Canine parvovirus infection occurs worldwide in domestic dogs and other members of the dog family. Incidence is higher in animal shelters, pet stores, and breeding kennels. CPV can affect dogs at any age. Severe infection is most common in puppies between 6 weeks and 4 months old. All breeds of dogs are susceptible. The crossbreds are less susceptible in comparison to pure breeds like Rottweilers, Doberman Pinchers, English Springer Spaniels and German Shepherd, the exception to this being Toy Poodles and Cocker Spaniels [35]. CPV affects only dogs, and cannot be transmitted to humans or other species. If a dog survives the first 4 days, they will usually recover rapidly and become immune to the virus for life. Most puppies die without medical treatment. The CPV infection is more severe in young puppies especially those younger than 3 months of age [2, 38]. All infected dogs may not necessarily exhibit clinical manifestations but they may shed the virus in feces during the acute phase of enteric fever and show significant rise in the serum antibody titers [86].
The different antigenic variants of CPV-2 are prevalent in varying proportion in different countries. The prevalence of CPV-2b has been reported by various authors in several countries namely Brazil [71], USA [69], Japan [32], Switzerland [95] and South Africa [87]. Contrastingly, CPV-2a was found to be the prevalent antigenic type in France, Taiwan and Italy [13, 48]. However both CPV-2a and CPV-2b have been found to be distributed in equal proportion in Spain [18] and U.K. [28]. CPV-2c has also been found in Vietnam [55], Spain [20], United Kingdom [22], South America [72], North America [40].
CPV-2 for the first time was isolated in India in 1982 [76]. After that, a large number of CPV outbreaks have been reported from different parts of India. The incidence of CPV-2 variants in dogs were reported from different states viz. Kerala [24], Assam [73], Tamil Nadu [78], Orissa [4], West Bengal [5], Pondicherry [65], Haryana [79] and Uttar Pradesh [59, 60, 54]. The prevalence of CPV-2a has been documented in 2001 in India [62]. It was also found that CPV-2b variants are more common in Northern India especially in Bareilly region compared to CPV-2a [57, 60, 64]. However, these observations were in contrast with the findings of a researcher [14] who reported that CPV-2a is the major antigenic variant prevalent in Southern and Central India, based on VP2 gene sequences. Further, based on VP2 gene sequences, it was revealed that the Indian isolates formed a separate lineage distinct from the South East Asian isolates and the canine parvovirus isolates in India appear to have evolved independently without any distinct geographical patterns of evolution [14]. Occurrence of CPV-2c was first reported in India in 2010 [59] based on the sequence analysis of CPV-2b positive sample. Its presence in India supports the assumption that CPV-2c is reaching a worldwide distribution and provides new information to understand the evolution of antigenic variants of CPV-2 [59].
Transmission
Canine parvovirus spreads through oral contact with infected faeces or contaminated surfaces (e.g., soil, shoes, dog toys etc.). The source of CPV infection is faecal waste from infected dogs. It has been diagnosed wherever groups of dogs are found: dog shows, obedience trials, breeding and boarding kennels, pet shops, animal shelters, parks and playgrounds [6]. Dogs that are confined to a house or yard and are not in contact with other dogs have much less chance of exposure to CPV. It’s easily transmitted via the hair or feet of infected dogs and also by contaminated objects such as cages or shoes. CPV is hardy and can remain in faeces-contaminated ground for 5 months or more if conditions are favorable. The faeces of infected dogs contaminate the places such as Veterinary hospitals, pet shops, boarding kennels and commercial breeding establishments. These contaminated premises serve as source of secondary infection to the susceptible canine population [38].
Pathogenesis
The virus enters the body through the mouth as the puppy cleans itself or eats food off the ground or floor. There is a 3–7 day incubation period before the puppy seems obviously ill. Upon entering into the body, it replicates to large numbers in the lymph nodes [86]. After a couple of days, significant amounts of virus have been released free into the bloodstream. Over the next 3–4 days, the viruses go to new organs containing the rapidly dividing cells like the bone marrow and the delicate intestinal cells and form large eosinophilic intranuclear inclusion bodies. Within the bone marrow, the virus is responsible for destruction of young cells of the immune system and then knocking out the body’s best defense mechanism. The virus causes most devastating effects in the gastro-intestinal tract. Canine parvoviral infections are characterized by a drop in white blood cell count due to the bone marrow infection.
It is in the GI tract where the heaviest damage occurs. The normal intestine possesses little finger-like protrusions called “villi.” Having these tiny fingers greatly increases the surface area available for the absorption of fluid and nutrients. To make the surface area available for absorption, the villi possess “microvilli” which are microscopic protrusions. The cells of the villi are relatively short-lived and are readily replaced by new cells. The source of the new cells is the rapidly dividing area at the foot of the villi called the Crypts of Lieberkuhn [50, 67]. It is right at the crypt where the parvovirus strikes. Without new cells coming from the crypt, the villus becomes blunted and unable to absorb nutrients and diarrhea results. The barrier separating the digestive bacteria from the blood stream breaks down. The diarrhea becomes bloody and bacteria can enter the body causing widespread infection. The virus kills one of two ways, diarrhea and vomiting lead to extreme fluid loss and dehydration until shock and death result. Loss of the intestinal barrier allows bacterial invasion of potentially the entire body.
Symptoms of Canine Parvovirus
Canine parvovirus (CPV) is the most dangerous and contagious virus that affects unprotected dogs. When it was first discovered in 1978, most of the puppies under 5 months old and 2–3% of older dogs died from CPV. CPV infection is now considered most threatening to puppies between the time of weaning and 6 months of age. Adult dogs can also contract the virus, although it’s relatively uncommon.
Diarrhoea occurs in dogs of any age but appears in serious proportions in pups. Dogs with enteritis act like they are in extreme pain. Early symptoms are depression, loss of appetite, vomiting, high fever and severe diarrhea (Fig. 1). There is slight rise of temperature in the initial stage of the disease but gradually turn to subnormal level with advancement of vomiting and diarrhoea [42]. There is no consistent character of the stool, it may be watery, yellow in color or tinged with frank blood in severe cases. Rapid dehydration is a danger, and dogs may continue to vomit and have diarrhoea until they die, usually 3 days after onset of symptoms. The course of illness is also highly variable depending on the infectious dose of the virus and clinical signs usually develop from 3 to 5 days following infection and typically persist for 5–7 days [25]. The morbidity and mortality vary according to the age of the animals, the severity of challenge and the presence of intercurrent disease problems. Puppies can die suddenly of shock as early as 2 days into the illness [86].
Fig. 1.

A case of canine parvovirus infection with severe diarrhea and vomiting undergoing treatment
The second form of CPV is cardiac syndrome, or myocarditis, which can affect puppies under 3 months old [2]. Within an infected litter, 70% pups will die in heart failure by 8 weeks of age and the remaining 30% will have pathological changes which may result in death many months or even years later. The most dramatic manifestation of CPV-2 myocarditis is the sudden death in young pups usually about 4 weeks of age [51]. The collapsed dying pup may have cold extremities, pale mucosae and show gasping respiration or terminal convulsions. Acute heart failure with respiratory distress occurs in pups between 4 and 8 weeks of age. Subacute heart failure occurs in older pups usually 8 weeks or more. They are tachypnoeic or dyspnoeic especially on exercise. The abdomen is swollen with hepatomegaly and ascitic fluid is blood tinged [11]. There is tachycardia, sometimes with arrhythmias and a weak pulse. Most animals die due to cardiogenic shock. However, if the animal survives it will suffer from chronic myocardial and circulatory complications [30, 77]. There is no diarrhoea because the virus multiplies rapidly in muscle cells of the immature heart.
Pathological Changes
The pathological changes produced by CPV reflect the requirement of the virus for dividing cells. The macroscopic lesions of CPV infection are highly variable and relatively non-specific. In the enteric disease, lesions may be distributed segmentally in the gastrointestinal tract. The lesions usually affect the jejunum and ileum but not the duodenum and colon. Affected segments may be somewhat flaccid with subserosal hemorrhage or congestion [77]. The lumen of the intestine is often empty but may contain variable watery ingesta. The mucosal surface is often congested but devoid of exudates. Mesenteric lymph nodes are frequently enlarged and edematous. Multifocal petechial hemorrhages are often seen within the cortex of a cut section of affected lymph nodes during acute stage of the disease and leucopenia is also common. Thymic cortical necrosis and atrophy are common findings in young dogs [16, 30, 77].
In cases of parvoviral myocarditis, gross lesions include cardiac enlargement with prominent dilatation of the left atrium and ventricle. The lungs often do not collapse when cut although white frothy fluid may be present in the trachea and bronchi. Evidence of pulmonary edema and passive congestion of the liver is often present, with the variable degree of ascites and pleural effusion. The ventricular myocardium frequently contains visible white streaks associated with the presence of a cellular infiltrate. Some pups may die from chronic decompensating left sided heart failure weeks or months after some of their littermates died suddenly with acute myocarditis. Pulmonary hypertension and myocardial dilation with scarring is often regarded as the cause of delayed death [30].
Histopathology
Microscopic lesions associated with CPV infection are initially confined to areas of proliferating cell population. In the enteric form of the disease, the early lesions consist of necrosis of the crypt epithelial cells [16]. Crypt lumenae are often dilated, lined by attenuated epithelium and filled with necrotic debris. There may be occasional intranuclear eosinophilic inclusion bodies in intact crypt epithelial cells. The villi and lamina propria may collapse completely as a result of the loss of crypt epithelium and the failure to replace sloughed villous epithelial cells. These lesions may be extensive or diffuse. Loss of digestive epithelium and absorptive surface area presumably results in diarrhoea caused by combined effect of maldigestion and malabsorption. Death may follow as a result of dehydration, electrolyte imbalance, endotoxic shock or secondary septicemia.
The regeneration of intestinal epithelial cells has been reported even in fatal cases. The remaining intestinal crypts are elongated and lined by hyperplastic epithelium with a high mitotic index. The shortened villi are covered by immature epithelial cells and adjacent villi are often fused. Necrosis and depletion of small lymphocytes is seen in Peyer’s patches, the germinal centers of mesentric lymph nodes, and in splenic nodules early in the course of infection [16]. Diffuse cortical necrosis of the thymus occurs in young dogs, with an associated loss in thymic mass. Later in the disease, there is evidence of regenerative lymphoid hyperplasia.
Canine Parvovirus Variants in Wild Animals
CPV-2 is closely related to FPV with more than 98% genome homology, and as few as six coding nucleotide differences in the VP2 protein positions: 3025, 3065, 3094, 3753, 4477, 4498 [69, 70]. The biological effects of these few genomic changes were enormous, in that CPV-2 acquired the canine host range, but lost the ability to replicate in cats [93]. The host ranges of CPV-2 and FPV are complex and differ in vitro and in vivo. FPV replicates in feline cells in vitro and in cats in vivo, but does not infect canine cells in vitro and shows only a restricted tissue spectrum in vivo. CPV-2 does replicate in canine and feline cells in vitro, but the in vivo replication is restricted to canides [37, 94]. No feline host has ever been described to be susceptible to CPV-2, although it replicates to low titers in mink which is a mustelid, after experimental inoculation [68]. After its emergence CPV spread to most populations of domestic and wild carnivores. In 1976, reports from Belgium and the Netherlands indicated that the virus had spread throughout the world infecting wild and domestic canids [92, 94]. Clinical signs of parvovirus disease were observed in captive and free-ranging coyotes and DNA sequence analysis of the VP2 gene showed the virus to be CPV-2. Raccoons, in contrast, were shown to be resistant to CPV-2 infection [93].
Serologic prevalence, infection or clinical signs of disease due to CPV viruses were found in jackals (Canis aureus, Canis adustus, Canis mesomelas), grey foxes (Urocyon littoralis), the San Joaquin kit fox, Asiatic raccoon dogs (Nyctereutes procyonoides) and the crab eating fox (Cerdocyon thous) in the Kenya [25, 89]. Canine parvovirus infections were reported in farmed raccoon dogs and confirmed to be CPV-2 by DNA sequence analysis of the VP2 gene [93]. CPV-2a and CPV-2b DNA sequences were recovered from six of nine cheetahs, as well as from one Siberian tiger, all showing clinical symptoms of parvovirus disease [88]. The very high prevalence of CPV-2a/2b infections in large cats compared to domestic cats may suggest a higher susceptibility of the species for these virus types [88]. Since vaccination of domestic cats and dogs is very effective in preventing disease, parvovirus vaccination of all domestic and non-domestic carnivores at risk of infection is highly recommended. CPV-2c type viruses have been isolated from leopard cats but not from domestic cats in the same area. Phylogenetic analysis indicated that CPV-2c(a) and CPV-2c(b) have been evolved from CPV-2a and CPV-2b to adapt to leopard cats and lost neutralizing epitopes compared to former serotypes CPV-2a and CPV-2b [37].
Diagnosis
A presumptive diagnosis of CPV enteritis can be made based on the clinical signs such as depression, vomiting, diarrhoea, anorexia and fever. The tests should be performed on any dog with diarrhoea that is also exhibiting signs of systemic disease: vomiting, lethargy, fever, loss of appetite, dehydration or dogs with unusually copious, smelly/bloody diarrhoea, or any dog with known exposure to parvovirus within the preceding 14 days of developing diarrhoea.
The diagnostic tests which were employed earlier include HA (Haemagglutination) [9], Electron Microscopy (EM) [8], virus isolation using in MDCK, CRFK or A 72 cell line [2], Enzyme Linked Immunosorbent Assay (ELISA) [53], Latex Agglutination Test (LAT) [1], Fluorescent Antibody Test (FAT), CIE test [76], Virus neutralization test, PCR and RE digestion [56, 71], real time PCR [19], loop-mediated isothermal amplification (LAMP) [34], nucleic acid hybridization or dot blot, in situ hybridization, nucleic acid sequencing etc. [15, 59], but they have varying degree of sensitivity and specificity and sometimes yielding false positive cases.
Haemagglutination (HA) Assay
The HA is simple and rapid test for detecting CPV in faeces and this test was performed using porcine, rhesus monkey or feline RBC’s [9]. The viral HA titer commonly ranges between 128 and 10,240, between PI days 4 and 7, or when the signs of enteritis commence. The HA activity generally ceases between PI days 7 and 9 [9]. Though it is less sensitive than virus isolation in A-72 cell line, HA test on stool samples is rapid and simple to perform. The nonspecific HA titer (<32) is common but it may be reduced by brief treatment of samples with fluorocarbon (Genetron, Freon 113) or CHCl3 (10% V/V). A modification of the HA test involves the adsorption of the CPV in faecal samples onto RBCs at 4°C. Antigen is eluted from cells at 37°C and tested for HA activity [63]. Although HA test is sensitive, relatively simple and inexpensive to perform, it has several disadvantages, including requirement of a continuous source of RBC, and the need to monitor the specificity of the low titred reactions with HA inhibition assay [17, 53]. HI test has also been used most frequently for the detection of CPV [43]. The antibody can be detected by HI after oral infection on PI day 3 or 4 and with a high titre (>640) by PI day 7 [17].
HA test can be performed by employing erythrocyte from various species as swine, sheep, goat, poultry and dogs. Among the erythrocyte of different species, pig RBCs showed the characteristic haemagglutination. Erythrocyte from other species does not give specific haemagglutination [17, 43]. The HA test can be performed by incubating the plates at various temperature such as 4°C, 25 and 37°C and the best results were found at 4°C followed by at 25°C and least titre at 37°C [43]. Apart from this, various buffer system have been evaluated for HA test such as normal saline solution (0.9% NSS), phosphate buffer solution with BSA (15 mM PBS + 0.1% BSA) and phosphate buffer saline solution (PBSS) (15 mM PBS + 0.9% NSS) etc. The optimum results were obtained with PBS followed by PBS with BSA and PBSS in a pH range of 4–6 but the results of all three systems were comparable [17, 43].
Electron Microscopy
During acute illness, parvoviral virions are readily demonstrated in faeces by negative staining and use of electron microscopy [8, 50]. Specific identification of CPV may be made using IEM, employing antibodies to CPV or FPV [8].
Isolation of CPV
A number of primary cell cultures and cell lines like MDCK (Madin-Darby Canine Kidney) or CRFK (Crandell Feline Kidney) support replication of CPV and virus could be isolated from the cases of CPV induced myocarditis and enteritis. The cell culture adapted virus will enable the biochemical and molecular characterization of the CPV isolates [2, 6]. A canine cell line (A-72) deserves special mention because it has proved to be particularly useful for CPV isolation from field materials. The A-72 cell line was established from a canine S/C tumour and it has maintained a fibroblastic appearance for more than 135 serial passages. This line proved to be particularly useful for isolation and growth of CPV because CPE were pronounced on initial culture or after one additional passage. The sizes of the plaques produced by CPV under methyl cellulose or agarose overlay media vary from 0.4 to 1.5 mm in diameter. Since the original tissues for culture were derived from an uncharacterized tumour, A-72 cells should not be used for vaccine virus production [9].
ELISA
This test is based on the antigen–antibody reactions with specific MAbs fixed on plastic, nitrocellulose membranes, latex or gold particles [96]. The tests are rapid, relatively cheap and can be performed in any veterinary clinic. Recently an ELISA test, using monoclonal antibodies was reported for the detection of CPV antigen in faeces as little as 1.5 ng of virus [53]. The double sandwich ELISA is a rapid, simple, sensitive and suitable test over ELISA for routine diagnostic use for detection of CPV antigen in canine faeces. The ELISA test has become the most common test for parvovirus in puppies [97].
Polymerase Chain Reaction
Recently the PCR technique has been increasingly used as a tool for the diagnosis of canine parvoviral infection [45, 51]. It has been widely applied to provide rapid, sensitive and accurate diagnosis of the disease. The PCR has been found to detect fewer particles of CPV-2 than other tests like HA and ELISA (Fig. 2). The PCR can now be used to differentiate the different mutants of CPV-2 using the primers specific for particular mutants [71]. To increase the sensitivity and specificity of the reaction, the nested PCR has been employed [31]. The conventional PCR could detect 10 fg of viral replicative form (RF) DNA on agarose gel electrophoresis, whereas as little as 100 ag of the RF DNA was detected by the nested PCR, which was shown to be 100 times more sensitive than the single PCR [31]. The number of the genome copy in positive samples was estimated about 109–1011/g of faeces by the conventional PCR and 1011–1013/g of faeces by the nested PCR. Thus, the nested PCR seems to be a sensitive, specific and practical method for the detection of CPV in faecal samples [31, 71].
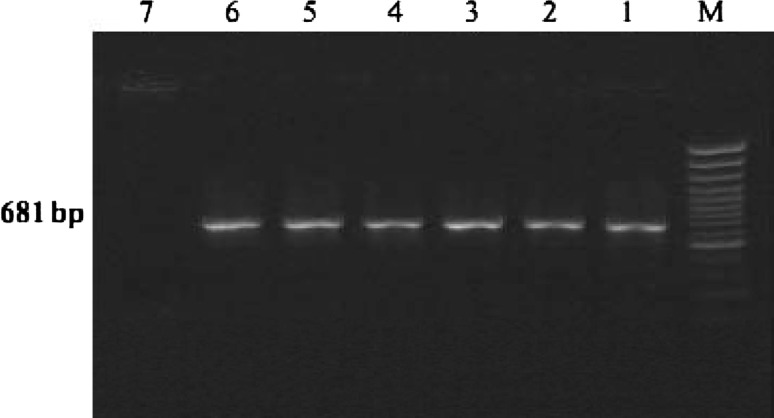
Fig. 2.
Amplification of part of the VP2 gene of the CPV-2 variants by PCR employing primers pCPV-2 (F) 5′-GAA GAG TGG TTG TAA ATA ATA-3′ (21 mer) and pCPV-2 (R) 5′-CCT ATA TCA CCA AAG TTA GTA G-3′ (22 mer) [57]. M Marker, 1–6 = 681 bp amplicon amplified by PCR, 7 = negative control
A touch-down protocol was used which enabled the specific amplification of virion DNA from faeces after a fast and simple boiling pretreatment. The sensitivity of PCR was as high as 10 infectious particles per reaction which corresponds, to a titer of about 10 infectious particles per gram of unprocessed feces. This renders the PCR about 10 to 100-fold more sensitive than electron microscopy [82].
The PCR followed by RFLP and sequencing have been used for typing the CPV strains [44]. On amplifying VP1/VP2 gene (~2.2 kb) and its RE digestion with HpaI and RsaI, it can differentiate between original CPV-2 type and CPV-2a/2b type. Also, RE digestion of amplicon employing AluI can differentiate between CPV-2a and CPV-2b type [44]. The typing of field samples using PCR followed by RsaI based RFLP showed that the vaccine strain used in India are CPV-2 type while field isolates are either of CPV-2a/2b type [79]. The results are in accordance with the other workers who found the same difference between field and vaccine strain of CPV employing PCR based differentiation [57, 71]. CPV-2c variant can be identified by MboII digestion of PCR products of CPV-2b positive samples [7].
Real Time PCR
Real time PCR (RT-PCR) employing the TaqMan assay has been used for the detection of CPV-2 DNA in the sample [19]. The minor groove binder (MGB) probe technology was applied to obtain rapid and unambiguous identification of the viral type [21]. MGB probes are short TaqMan probes conjugated with molecules that form hyper-stabilized duplexes with complementary DNA, allowing reduction in length of the probe and an increase in specificity [21]. MGB probes are, therefore, an attractive tool for revealing single nucleotide polymorphisms in the capsid protein gene between CPV types 2a and 2b and CPV types 2b and 2c. Recently, SYBR Green based real time PCR has been developed for detection and quantitation of CPV-2 variants in faecal samples of dogs employing primer set pCPV-2RT (forward 5′-CAT TGG GCT TAC CAC CAT TT-3′ and reverse 5′-CCA ACC TCA GCT GGT CTC AT-3′) based on the sequences of VP2 gene and produce a PCR product 160 bp [46]. The advantage of the real time PCR is that there is no need to analyse the PCR product by agarose gel electrophoresis. Everything will be graphically shown on the monitor of the computer. Another advantage is that amount of the DNA present in the sample can be quantitated [19].
Detection of CPV in Fecal Samples Using LAMP
The Loop Mediated Isothermal Amplification of DNA (LAMP) method was applied for the detection of CPV genomic DNA. A set of four primers, two outer and two inner, were designed from CPV genomic DNA targeting the VP2 gene. The optimal reaction time and temperature for LAMP were determined to be 60 min and 63.8°C respectively. The relative sensitivity of LAMP was 100% and the relative specificity was 76.9%. The detection limit of the LAMP method was 10−1 median tissue culture infective doses (TCID50)/ml [34].
Nucleic Acid Hybridization/Dot Blot
In this process the DNA is extracted from the stool samples or cell culture supernatant inoculated with the sample or stool sample suspected for canine parvovirus and charged on the nitrocellulose paper or nylon membrane. The DNA is then subjected to hybridization with CPV-specific probe either radio-labelled or biotin labeled. In the positive case there will be development of band in the X-ray film after autoradiography in case of radio-labelled probe or colour in the nitrocellulose paper in case of non-radio-labelled probe [15].
Detection of Canine Parvovirus by In situ Hybridization
This technique was developed to detect viral replication in tissue sections obtained from CPV-infected animals. In this method identification of CPV-specific nucleic acid was done. A CPV-specific DNA probe was produced by PCR amplification of a genome segment encoding capsid proteins VP-1 and VP-2 and was used for knowing the distribution of CPV specific nucleic acid in tissue specimens obtained from infected dogs [98].
Nucleic Acid Sequencing
The PCR product as it is or cloned in the suitable cloning vector can be sequenced using the suitable primer with the help of automated DNA sequencer for typing of CPV strains. The sequence is analysed using the appropriate software. This is an important technique to know with certainty the particular variant of the CPV present in the field sample. Both the nucleotide and amino acid sequence data can also be used to know the percent homology and for phylogenetic analysis of CPV-2 isolates from different geographical regions [58]. Based on sequence analysis CPV-2a and CPV-2b type could be differentiated and none of the isolates were belonging to original CPV-2 type [14]. In a further study, field isolate as well as vaccine strain of CPV were sequenced and it was found that vaccine strains are of CPV-2 type and field isolate of CPV-2b type [61] (Fig. 3). CPV-2c variants have been reported from various countries based on the nucleotide sequence analysis [40, 59].
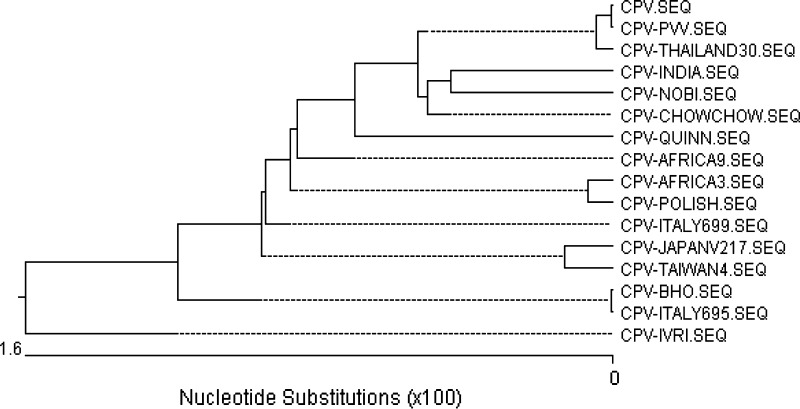
Fig. 3.
Phylogenetic analysis based on nucleotide sequence of VP1/VP2 gene of two Indian isolates [CPV-Bhopal (BHO) and CPV-IVRI] and two vaccine strain [Nobivac (NOBI) and Parvovirus vaccine (PVV)] and their comparison with several published nucleotide sequences of canine parvovirus employing using MegAlign programme of DNASTAR Inc, USA, Software. Both the isolates are of CPV-2b type and both the vaccine strains are found to be CPV-2 type [61]
Immunization
The biggest problem in protecting a puppy against canine parvovirus infection ironically stems from the natural mechanism of protection that has evolved. Puppies obtain their immunity from their mother’s first milk, the colostrum, on the first day of life. There is a strong correlation between HI or serum neutralizing antibody titers and resistance to infection with CPV. The HI test has been useful to measure antibodies which correlated well with immunity. The HI titre 1:80 or more is considered protective but HI titre of 1:40 is not protective but interferes with active immunization against CPV-2 in dogs. The highest rate of infection is reported in pups older than 6 weeks of age. As with other infectious diseases of dogs, puppies from immune bitches are protected for the first week of life by maternal antibodies which are acquired via the colostrums. Successful immunization with most vaccines can be accomplished with a high degree of confidence only in seronegative pups, or in pups with very low antibody titers. Maternal antibodies are acquired during the initial 2–3 days of life and then decline, with an average half life of about 9–10 days. There is a critical period where maternal antibodies are no longer present in sufficient quantity to confer protection. But 90% of the pups from vaccinated populations respond to vaccines at 12 weeks of age [75, 91].
Vaccination of dogs is generally performed using multivalent vaccines, which contain CDV, CPV, leptospira bacterin and inactivated rabies virus. Monovalent CPV-2 vaccines are also available, some of them containing very high titer virus (107 TCID50) and widely recommended for initial vaccination of pups. About 60% of all puppies seroconverted after a single vaccination either at 6 weeks of age with a CPV monovalent vaccine or at 8 weeks of age with a multivalent vaccine. At 12 weeks of age another shot is given when all pups had received 2–3 inoculation at this age but nearly 10% pups still had not been sero-converted. The principal reason for the non-responders was the persistence of interfering levels of maternal antibodies. None of the vaccines tested were capable of breaking through a maternal antibody titer of 1:160 or higher, regardless whether the vaccines were high tittered or not [75, 91]. If it is necessary to develop an individual vaccination schedule, determination of the antibody titer of one or two pups in the litter could be determined at 5 or 6 weeks of age, then vaccination of the litter may be calculated on the basis of titer. Vaccination is likely to be successful when the maternal antibody titer has declined to less than 1:10 [80].
There have been concerns expressed over the efficacy of canine parvovirus vaccines which are based on the original type 2 strain [49, 91]. The reports of gastroenteritis subsequent to vaccination are related to infection with CPV field strains shortly before or after the vaccine administration [23]. It has previously been demonstrated that a type 2 vaccine is able to provide protection against type 2a and 2b field isolates [27]. The emergence of the 2c variant naturally raises the question of whether the CPV-2 vaccines can provide protection against this new variant also. The research to date also showed that all currently available vaccines based on CPV-2 and CPV-2b protect against all known strains of CPV, including the newer CPV-2c strain [81, 85]. In India, most of the vaccines marketed are based on the CPV-2 isolated about 30 years ago [57, 61]. However, CPV-2a/2b/2c has recently replaced the CPV-2 incidence in dogs in most of the parts of the world including India. There are reports of gastroenteritis in vaccinated dogs and this may be due to CPV-2 is not capable enough to provide full protection against the new strains [79]. It is better to use homologous vaccines that use CPV-2a or CPV-2b mutants depending on the prevalence of disease in different places to control the disease.
Killed and Modified CPV Vaccine
First, a killed CPV vaccine and more recently live and recombinant vaccine have been developed in the search of a product of improved potency. However, no vaccine has proved to be of high efficacy in the face of maternally derived antibodies (MDA), hence a pup’s primary vaccination cannot be completed before 16 weeks [50]. In CPV infection live virus vaccines offer a longer duration of immunity than killed vaccines as in other diseases [80]. None of the currently available vaccines circumvent maternally derived immunity as effectively as does virulent CPV although MLV-CPV vaccines can overcome a higher concentration of maternally derived antibodies than vaccine containing inactivated virus [26]. MLV vaccine using highly attenuated CPV are more susceptible to maternal antibody induced suppression of active immunization than less attenuated strains. Another way of overcoming the interference of maternal antibodies with CPV vaccine is by using MLV-CPV of high antigenic mass [85].
Recombinant Vaccine
Recombinant vaccine containing the baculovirus expressed VP2 protein was found to be structurally and immunologically indistinguishable from authentic VP2. The recombinant VP2 also shows the capability to self assembles, forming virus like particles similar in size and appearance to CPV virions. The VP2 protein at the rate of 10 μg was able to elicit good protective response as measured by ELISA and shown to be better than commercially available inactivated CPV vaccine in terms of immune response. The expressed VP2 was used along with the Quil A (50 μg/animal), alumina or both adjuvants on 0 and 28 days to improve the immunogenicity of the vaccine at different doses (10, 25, 50 and 100 μg). All the vaccinated dogs maintained the protective antibody response up to 6 days observation period and withstood challenge virus infection 42 days after the booster doses [47].
DNA Vaccine
The prokaryotic vector harboring the genes coding for the structural proteins of the canine parvovirus have shown the encouraging results. The dogs immunized with the DNA vaccines withstood the challenge with virulent canine parvovirus. However, the DNA vaccines still is in the experimental stage and not yet licensed to be used in the field condition [29].
Peptide Vaccine
The N-terminal domain of the major capsid protein VP2 of canine parvovirus was shown to be an excellent target for development of a synthetic peptide vaccine. Several peptides based on this N-terminus were synthesized to establish conditions for optimal and reproducible induction of neutralizing antibodies in rabbits. Within the N-terminal 23 residues of VP2, two sub sites able to induce neutralizing antibodies. The shortest sequence sufficient for neutralization induction was nine residues. Peptides longer than 13 residues consistently induced neutralization, provided that their N-termini were located between positions 1 and 11 of VP2. The orientation of the peptides at the carrier protein was also of importance, being more effective when coupled through the N-terminus than through the C-terminus to keyhole limpet hemocyanin. This means that the presence of amino acid residues 2–21 (and probably 3–17) of VP2 in a single peptide is preferable for a synthetic peptide vaccine [36].
Therapy
The restoration of the electrolyte and fluid balance is the most important goal of therapy [99]. The affected dog should be put under broad spectrum antibiotic umbrella (ampicillin, chloramphenicol, erythromycin, gentamycin, etc.) Norfloxacin and nalidixic acid have been proved to be effective against canine haemorrhagic gastroenteritis [41]. Symptomatic treatment with steroid, broad spectrum antibiotic, fluid and electrolyte may save the life of the animal. As soon as the problem is recognized, fluid therapy should be started. Supplementation of these fluids with bicarbonate may be recommended. Metabolic acidosis develops if the diarrhoea is severe and potassium supplementation in the form of KCl may be necessary to maintain electrolyte balance. All oral intakes must be withheld in case of severe vomiting and should be given parenterally [99]. During the early phase of the disease, the application of hyperimmune serum may help to reduce the virus load and render the infection less dramatic. Such treatment has been shown to reduce the mortality and shorten the length of the disease however hyperimmune serum is difficult to obtain. In case of vomiting, chlorpromazine or metaclopromide (Reglan), out of which Reglan can be given at 0.5 mg/kg body weight parenterally at 8 h interval. To correct the gastric problem cimetidine, ranitidine, famotidine and to check diarrhoea, loparamide or bismuth subnitrate or other astringent preparations may be given [42]. A dog with persistent vomiting should not be given any food until the diarrhoea and vomiting subsides.
Prevention and Control
As the canine parvovirus is not enveloped, it is especially hardy in the environment. It is able to overcome winter freezing temperatures in the ground outdoors and many household disinfectants are not capable of killing it indoors. Infected dogs shed virus in their stool in gigantic amounts during the 2 weeks following exposure. A typical/average infectious dose for an unvaccinated dog is 1000 viral particles. An infected dog sheds 35 million viral particles (35,000 times the typical infectious dose) per ounce of stool [83]. Indoor decontamination: Indoors, virus loses its infectivity within 1 month; therefore, it should be safe to introduce a new puppy indoors 1 month after the active infection has ended. Outdoor decontamination: freezing is completely protective to the virus. If the outdoor is contaminated and is frozen, one must wait for it to thaw out before safely introducing a new puppy. Shaded areas should be considered contaminated for 7 months. Areas with good sunlight exposure should be considered contaminated for 5 months. Although most disinfectants cannot kill it, chlorine bleach is quite effective in the ratio of 1 part bleach and 30 parts water. There is no way to completely disinfect contaminated dirt and grass, although sunlight and drying has some effect [35]. Mechanical decontamination through irrigation may also be helpful, but the area must be allowed to dry thoroughly between applications. Potassium peroxymonosulfate has relatively good activity in the face of organic matter, and can be sprayed on contaminated areas using a pesticide sprayer or other applicator [83].
Conclusion
In summary, parvovirus is a very common problem of canines and is a huge killer of puppies. Due to its ability to be transmitted through hands, clothes, and most likely rodents and insects, it is virtually impossible to have a kennel that will not eventually be exposed to the disease. Modified live vaccines are safe and effective, but despite the best vaccination protocol, all puppies will have a window of susceptibility of at least several days where they will be at risk. In addition, the newer CPV-2c strain presents new challenges as the current vaccines may not be as effective in providing protection against it. Again, commercially available FPV or CPV-2 based vaccines might also protect animals from the new virus infection. However, if the new virus gains wider host ranges, deadly outbreaks could be observed like first emergence of CPV-2 in dogs. In that case, recent isolates need to be investigated to anticipate and assess the risk caused by newly emerging viruses. Further the homologous vaccine based on current or newer variant should be made ready to tackle the disease. Also, zoo sanitary measures should be employed to prevent the disease in wild animals.
Although potent and efficacious live attenuated and inactivated vaccines are available in India but large numbers of cases are diagnosed by HA, HI, ELISA or PCR, mostly from the unvaccinated dogs as the stray dogs usually are not vaccinated against the disease and they remain carrier of the virus and source of infection to other susceptible dogs. Extensive studies must be undertaken to know the molecular epidemiology of the canine parvovirus infections in different canine species and the variants of the CPV involved in the outbreak of the disease. The necessary preventive measures must be undertaken to immunize the susceptible dogs including the stray dogs with the potent and efficacious vaccines against the disease to check the spread of the disease. Prompt symptomatic treatment, restoration of fluid and antibiotics to prevent bacterial infection by a veterinarian will increase survivability in infected puppies but vaccination program should be considered the best way to control the disease in dog.
Contributor Information
S. Nandi, Email: snandi1901@yahoo.com
Manoj Kumar, Email: manojvet1901@yahoo.com.
References
- 1.Appel MJG, Cooper BJ, Greisen H, Carmichael LE. Status report: canine viral enteritis. J Am Vet Med Assoc. 1978;173:1516–1518. [Google Scholar]
- 2.Appel MJG, Scott FW, Carmichael LE. Isolation and immunization studies of a canine parvo-like virus from dogs with hemorrhagic enteritis. Vet Res. 1979;105:156–159. doi: 10.1136/vr.105.8.156. [DOI] [PubMed] [Google Scholar]
- 3.Badgett MR, Auer A, Carmichael LE, Parrish CR, Bull JJ. Evolutionary dynamics of viral attenuation. J Virol. 2002;76:10524–10529. doi: 10.1128/JVI.76.20.10524-10529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banja BK, Sahoo N, Das PK, Swain P, Panda HK. Comparison of different laboratory tests for diagnosis of parvo and corona viral infections in dogs. Indian Vet J. 2002;79:425–428. [Google Scholar]
- 5.Biswas S, Das PJ, Ghosh SK, Pradhan NR. Detection of canine parvovirus (CPV) DNA by polymerase chain reaction and its prevalence in dogs in and around Kolkata, West Bengal. Indian J Anim Sci. 2006;76(4):324–325. [Google Scholar]
- 6.Black JW, Holscher MA, Powell HS, Byerly CS. Parvoviral enteritis and panleucopenia in dogs. J Med Small Anim Clin. 1979;74:47–50. [PubMed] [Google Scholar]
- 7.Buonavoglia CV, Martella A, Pratelli M, Tempesta A, Cavalli D, Bozzo G, Decaro N, Carmichael LE. Evidence for evolution of canine parvovirus type-2 in Italy. J Gen Virol. 2001;82:1555–1560. doi: 10.1099/0022-1317-82-12-3021. [DOI] [PubMed] [Google Scholar]
- 8.Burtonboy G, Goignoul F, Delferriere N, Pastoret PP. Canine hemorrhagic enteritis: detection of viral particles by electron microscopy. Arch Virol. 1979;61:1–11. doi: 10.1007/BF01320586. [DOI] [PubMed] [Google Scholar]
- 9.Carmichael LM, Joubert JC, Pollock RV. Hemagglutination by canine parvovirus: serologic studies and diagnostic application. Am J Vet Res. 1980;40:784–791. [PubMed] [Google Scholar]
- 10.Carmichael LE, Schlafer DH, Hashimoto A. Minute virus of canines (MVC, canine parvovirus type-1): pathogenicity for pups, canine parvovirus type): pathogenicity for pups and seroprevalence estimate. J Vet Diagn Invest. 1994;6:165–174. doi: 10.1177/104063879400600206. [DOI] [PubMed] [Google Scholar]
- 11.Carpenter JL, Roberts RM, Harpster NK, King NW., Jr Intestinal and cardiopulmonary forms of parvovirus infection in a litter of pups. J Am Vet Med Assoc. 1980;176:1269–1273. [PubMed] [Google Scholar]
- 12.Cavalli A, Martella V, Costantina D, Michele C, Anna LB, Pasquale DP, Nicola D, Gabriella E, Buonavoglia C. Evaluation of the antigenic relationships among canine parvovirus type 2 variants. Clin Vaccine Immunol. 2008;15(3):534–539. doi: 10.1128/CVI.00444-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang SF, Sgro JY, Parrish CR. Multiple amino acids in the capsid structure of canine parvovirus coordinately determine the canine host range and specific antigenic and haemagglutination properties. J Virol. 1992;66:6858–6867. doi: 10.1128/jvi.66.12.6858-6867.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chinchkar SR, Mohan SB, Hanumantha RN, Rangarajan PN, Thiagarajan D, Srinivasan VA. Analysis of VP2 gene sequences of canine parvovirus isolates in India. Arch Virol. 2006;151:1881–1887. doi: 10.1007/s00705-006-0753-8. [DOI] [PubMed] [Google Scholar]
- 15.Cho HS, Song JE, Park YS, Park NY. Diagnosis of the canine parvovirus in faecal samples by in situ hybridization. Int Vet J. 2004;81:855–859. [Google Scholar]
- 16.Cooper BJ, Carmichael LE, Appel MJG, Greisen H. Canine viral enteritis. II. Morphological lesions in naturally occurring parvovirus infection. Cornell Vet. 1979;69:134–144. [PubMed] [Google Scholar]
- 17.Dahiya SS, Kulkarni DD. Optimization of haemagglutination test for the detection of canine Parvovirus infection. Indian J Comp Microbiol Immunol Infect Dis. 2004;25(2):119–220. [Google Scholar]
- 18.de Ybanez RR, Vela C, Cortes E, Simarro I, Casal JI. Identification of types of canine parvovirus circulating in Spain. Vet Rec. 1995;136:174–175. doi: 10.1136/vr.136.7.174. [DOI] [PubMed] [Google Scholar]
- 19.Decaro N, Elia G, Campolo M, Desario C, Lucente MS, Bellacicco AL. New approaches for molecular characterization of canine parvovirus type-2 strains. J Vet Med B. 2005;52:316–319. doi: 10.1111/j.1439-0450.2005.00869.x. [DOI] [PubMed] [Google Scholar]
- 20.Decaro N, Elia G, Martella V, Campolo M, Desario C, Camero M. First detection of canine parvovirus type 2c in pups with haemorrhagic enteritis in Spain. J Vet Med B. 2006;53:468–472. doi: 10.1111/j.1439-0450.2006.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Decaro N, Elia G, Martella V, Campolo M, Desario C, Camero M. Characterization of the canine parvovirus type 2 variants using minor groove binder probe technology. J Virol Methods. 2006;133:92–99. doi: 10.1016/j.jviromet.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Decaro N, Desario C, Addie DD, Martella V, Vieira MJ, Elia G, Davis C, Thompson G, Truyen U, Buonavoglia C. The study of molecular epidemiology of canine parvovirus, Europe. Emerg Infect Dis. 2007;13:1222–1224. doi: 10.3201/eid1308.070505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Decaro N, Elia G, Martella V, Campolo M, Lorusso A, Desario C, Mari V, Camero M, Buonavoglia C. Occurrence of severe gastroenteritis in pups after canine parvovirus vaccine administration: a clinical and laboratory diagnostic dilemma. Vaccine. 2007;25:1161–1166. doi: 10.1016/j.vaccine.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deepa PM, Saseendrannath MR. Serological studies on canine parvoviral infection. Indian Vet J. 2000;79:643–644. [Google Scholar]
- 25.Fletcher KC, Bugster AK, Schmidt RE, Hubbard GB. Parvovirus infection in maned wolves. J Am Vet Med Assoc. 1979;175:897–900. [PubMed] [Google Scholar]
- 26.Gamoh K, Senda M, Inoue Y, Itoh O. Efficacy of an inactivated feline panleucopenia virus vaccine against a canine parvovirus isolated from a domestic cat. Vet Rec. 2005;157:285–287. doi: 10.1136/vr.157.10.285. [DOI] [PubMed] [Google Scholar]
- 27.Greenwood NM, Chalmers SK, Baxendale W, Thompson H. Comparison of isolates of canine parvovirus by restriction enzyme analysis and vaccine efficacy against field strains. Vet Rec. 1995;136:63–67. doi: 10.1136/vr.136.3.63. [DOI] [PubMed] [Google Scholar]
- 28.Greenwood NM, Chalmer WSK, Baxendale W, Tampson H. Comparison of isolates of canine parvovirus by monoclonal antibody and restriction enzyme analysis. Vet Rec. 1996;138:495–496. doi: 10.1136/vr.138.20.495. [DOI] [PubMed] [Google Scholar]
- 29.Gupta PK, Rai A, Rai N, Raut AA, Chauhan RS. Cloning of canine parvovirus VP2 gene and its use as DNA vaccine in dogs. Curr Sci. 2005;88:778–782. [Google Scholar]
- 30.Hayes MA, Russell RG, Mueller RW, Lewis RJ. Myocarditis in young dogs associated with a parvovirus-like agent. Can Vet J. 1979;20:126–132. [PMC free article] [PubMed] [Google Scholar]
- 31.Hirasawa T, Kaneshige T, Mikazuki K. Sensitive detection of canine parvovirus DNA by the nested polymerase chain reaction. Vet Microbiol. 1994;41:135–145. doi: 10.1016/0378-1135(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 32.Hirasawa T, Yono K, Mikazuki K. Detection and genomic analysis of canine parvovirus by the polymerase chain reaction. J Vet Med B. 1996;43:545–554. doi: 10.1111/j.1439-0450.1996.tb00352.x. [DOI] [PubMed] [Google Scholar]
- 33.Hong C, Decaro N, Desario C, Tanner P, Pardo MC, Sanchez S, Buonavoglia C, Saliki JT. Occurrence of canine parvovirus type 2c in the United States. J Vet Diagn Invest. 2007;19:535–539. doi: 10.1177/104063870701900512. [DOI] [PubMed] [Google Scholar]
- 34.Ho-Seong C, Jong K, Nam-Yong P. Detection of canine parvovirus in fecal samples using loop-mediated isothermal amplification. J Vet Diagn Investig. 2006;18:81–84. doi: 10.1177/104063870601800111. [DOI] [PubMed] [Google Scholar]
- 35.Houston DM, Ribble CS, Head LL. Risk factors associated with parvovirus enteritis in dogs. J Am Vet Med Assoc. 1996;208:542–548. [PubMed] [Google Scholar]
- 36.Ignacio JC, Langeveld JPM, Elena CS, Schaaper WWM, Kamstrup S, Meloen RH. Peptide vaccine against canine parvovirus: identification of two neutralization subsites in the N terminus of VP2 and optimization of the amino acid sequence. J Virol. 1995;69(11):7274–7277. doi: 10.1128/jvi.69.11.7274-7277.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ikeda Y, Nakamura K, Miyazawa T, Tohya Y, Takahashi E, Mochizuki M. Feline host range of canine parvovirus: recent emergence of new antigenic types in cats. Emerg Infect Dis. 2002;6:341–346. doi: 10.3201/eid0804.010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacob RM, Weiser MG, Hall RL, Kowalski JJ. Clinic pathogenic features of canine parvoviral enteritis. J Am Vet Med Assoc. 1980;16:809–813. [Google Scholar]
- 39.João Vieira M, Silva E, Oliveira J, Luísa Vieira A, Decaro N, Desario C, Muller A, Carvalheira J, Buonavoglia C, Thompson G. Canine parvovirus 2c infection in central Portugal. J Vet Diagn Invest. 2008;20(4):488–491. doi: 10.1177/104063870802000412. [DOI] [PubMed] [Google Scholar]
- 40.Kapil S, Cooper E, Lame C, Brandy M, Grunt K, Johnson W, Campbell G, Johnson B. Canine parvovirus type 2c and 2b circulating in North American dogs in 2006–2007. J Clin Microbiol. 2007;45(12):4044–4047. doi: 10.1128/JCM.01300-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly WR, Atwell RB. Diffuse subacute myocarditis of possible viral etiology: a cause of sudden death in pups. Aust Vet J. 1979;55:36–37. doi: 10.1111/j.1751-0813.1979.tb09549.x. [DOI] [PubMed] [Google Scholar]
- 42.Kramer JM, Meunter PC, Pollock RVH. Canine parvovirus: update. Vet Med Small Anim Clin. 1980;175:1541–1555. [PubMed] [Google Scholar]
- 43.Kumar P, Garg SK, Babdhopadhyay SK, Singh R, Srivastava S. Haemagglutinating activity of canine parvovirus. Indian J Anim Sci. 2004;73(2):123–125. [Google Scholar]
- 44.Kumar A, Dharmadheeran JS, Kumar S, Thakral SS. Strain identification and characterization of VP1 and VP2 gene of canine parvovirus of Indian origin. J Appl Anim Res. 2004;25:57–60. [Google Scholar]
- 45.Kumar M, Chidri S, Nandi S. Molecular cloning and restriction endonuclease analysis of canine parvovirus DNA amplified by polymerase chain reaction. Glob Vet. 2010;4(2):125–129. [Google Scholar]
- 46.Kumar M, Nandi S, Verma R Development of SYBR Green based Real-Time PCR assay for quantitation of canine parvovirus in faecal samples. In: 7th Annual convention of ISACP on “Novel approaches in companion animal practice” held at Veterinary College, KVAFSU, Hebbal, Bangalore, 21–23; 2010, 146 pp.
- 47.Lopez JA, Turiso D, Elena C, Concepcion M, Rocto RY, Isabel S, Carmen V, Ignacio CI, Hermanos GN. Recombinant vaccine for canine parvovirus in dogs. J Virol. 1992;66:2748–2753. doi: 10.1128/jvi.66.5.2748-2753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martella V, Cavalli A, Pratelli A, Bozzo G, Camero M, Buonavoglia D, Narcisi D, Tempesta M, Buonavoglia C. A canine parvovirus mutant is spreading in Italy. J Clin Microbiol. 2004;42:1333–1336. doi: 10.1128/JCM.42.3.1333-1336.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martella V, Cavalli A, Decaro N, Elia G, Desario C, Campolo M, Bozzo G, Tarsitano E, Buonavoglia C. Immunogenicity of an intranasally administered modified live canine parvovirus type 2b vaccine in pups with maternally derived antibodies. Clin Diagn Lab Immunol. 2005;12:1243–1245. doi: 10.1128/CDLI.12.10.1243-1245.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCandlish IAP, Thompson H, Fisher EW, Cornwell HJC, Macartney J, Walton IA. Canine parvovirus infection. In Pract. 1981;3:5–14. doi: 10.1136/inpract.3.3.5. [DOI] [PubMed] [Google Scholar]
- 51.Mochizuki M, Horiuchi M, Hiragi H, San Gabriel MC, Yasuda N, Uno T. Isolation of canine parvovirus from a cat manifesting clinical signs of feline panleucopenia. J Clin Microbiol. 1996;34:2101–2105. doi: 10.1128/jcm.34.9.2101-2105.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mochizuki M, Hashimoto M, Hajima T, Takiguchi M, Hashimoto A, Yumi U, Roerink F, Ohshima T, Parrish CR, Carmichael LE. Virologic and serologic identification of minute virus of canines (canine parvovirus type 1) from dogs in Japan. J Clin Microbiol. 2002;40(11):3993–3998. doi: 10.1128/JCM.40.11.3993-3998.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohan R, Nauriyal DC, Singh KB. Detection of canine parvovirus in feces, using a parvovirus ELISA test kit. Indian Vet J. 1993;70:301–303. [Google Scholar]
- 54.Murphy FA, Gibbs EPJ, Horzinek M, Studdert MJ. Veterinary Virology. 3. New York: Academic Press; 1999. pp. 169–170. [Google Scholar]
- 55.Nakamura M, Tohya Y, Miyazawa T, Mochizuki M, Phung HT, Nguyen NH, Huynh LM, Nguyen LT, Nguyen PN, Nguyen PV, Nguyen NP, Akashi H. A novel antigenic variant of canine Parvo virus from a Vietnamese dog. Arch Virol. 2004;149:2261–2269. doi: 10.1007/s00705-004-0367-y. [DOI] [PubMed] [Google Scholar]
- 56.Nandi S, Kumar M, Anbazhagan R, Chidri S, Chauhan RS. A sensitive method to detect canine parvoviral DNA in the stool samples by polymerase chain reaction. Indian J Comp Microbiol Immunol Infect Dis. 2007;27:56–57. [Google Scholar]
- 57.Nandi S, Anbazhagan R, Kumar M, Chauhan RS. Molecular characterization of canine parvovirus strains in vaccines by polymerase chain reaction and restriction endonuclease analysis. Indian J Virol. 2009;20(1):12–15. [Google Scholar]
- 58.Nandi S, Chidri S, Kumar M. Molecular characterization and phylogenetic analysis of a canine parvovirus isolate in India. Vet Med. 2009;54(10):483–490. [Google Scholar]
- 59.Nandi S, Chidri S, Kumar M, Chauhan RS. Occurrence of canine parvovirus type 2c in the dogs with haemorrhagic enteritis in India. Res Vet Sci. 2010;88:169–171. doi: 10.1016/j.rvsc.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 60.Nandi S, Anbuzhagan R, Kumar M. Strain differentiation and characterization of canine parvovirus by PCR and RE mapping. Indian J Biotechnol. 2010;9:38–42. [Google Scholar]
- 61.Nandi S, Ambazhagan R, Kumar M. Molecular characterization and nucleotide sequence analysis of canine parvovirus strains in vaccine in India. Vet Ital. 2010;46(1):69–81. [PubMed] [Google Scholar]
- 62.Narayanan S, Kumar A, Chug PK, Bhawn S, Yadav RK, Sharma ML. Strain characterization of Indian isolates of canine parvovirus by PCR-RFLP. J Remount Vet Corps. 2001;40:67–74. [Google Scholar]
- 63.Osterhaus ADME, Steenis G, Kreek P. Isolation of a virus closely related to feline panleucopenia virus from dogs with diarrhoea. Zent Vet Med. 1980;27:11–21. doi: 10.1111/j.1439-0450.1980.tb01632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Panda D, Patra RC, Nandi S, Swarup D. Antigenic characterization of canine parvovirus by polymerase chain reaction. Indian J Anim Sci. 2009;79(9):876–879. [Google Scholar]
- 65.Panneer D, Mukhopadhyay HK, Antony PX, Pillai RM. Comparison of diagnostic tests and antigenic typing of Canine parvovirus. Indian J Virol. 2008;19(2):150–154. [Google Scholar]
- 66.Parker JS, Murphy WJ, Wang D, O’Brien SJ, Parrish CR. Canine and feline parvoviruses can use human or feline transferrin receptors to bind, enter, and infect cells. J Virol. 2001;75:3896–3902. doi: 10.1128/JVI.75.8.3896-3902.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parrish CR. Pathogenesis of feline panleucopenia virus and canine parvovirus. Baillieres Clin Haematol. 1995;8:57–71. doi: 10.1016/S0950-3536(05)80232-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parrish CR, O’Connell PH, Evermann JF, Carrmichael LE. Natural variation of canine parvovirus. Science. 1985;230:1046–1048. doi: 10.1126/science.4059921. [DOI] [PubMed] [Google Scholar]
- 69.Parrish CR, Aquadro CF, Carmichael LE. Canine host range and a specific epitope map along with variant sequences in the capsid protein gene of canine parvovirus and related feline, mink and racoon parvoviruses. Virology. 1988;166:293–307. doi: 10.1016/0042-6822(88)90500-4. [DOI] [PubMed] [Google Scholar]
- 70.Parrish CR, Aquadro CF, Strassheim ML, Evermann JF, Sgro JY, Mohammed HO. Rapid antigenic type replacement and DNA sequence evolution of canine parvovirus. J Virol. 1991;65:6544–6552. doi: 10.1128/jvi.65.12.6544-6552.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pereira CA, Monezi TA, Mehnert DU, D’Angelo M, Durigon EL. Molecular characterization of canine parvovirus in Brazil by PCR. Vet Microbiol. 2000;75:127–133. doi: 10.1016/S0378-1135(00)00214-5. [DOI] [PubMed] [Google Scholar]
- 72.Perez R, Francia L, Romero V, Maya L, Lopez I, Hernandez M. First detection of canine parvovirus type 2c in South America. Vet Microbiol. 2007;124:147–152. doi: 10.1016/j.vetmic.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 73.Phukan A, Deka D, Boro PK. Occurrence of canine parvovirus infection in and around Guwahati. Indian J Anim Sci. 2004;74(4):930–931. [Google Scholar]
- 74.Pratelli AA, Buonavoglia D, Tempesta M, Guarda F, Carmichael LE, Buonavoglia C. Fatal canine parvovirus type-1 infection in pups from Italy. J Vet Diagn Invest. 1999;11:365–367. doi: 10.1177/104063879901100413. [DOI] [PubMed] [Google Scholar]
- 75.Pratelli AA, Cavalli G, Normanno MG, Palma GD, Pastorelli AA, Buonavoglia C, Martella V. Immunization of pups with maternally derived antibodies to canine parvovirus (CPV) using a modified-live variant (CPV-2b) J Vet Med. 2000;47:273–276. doi: 10.1046/j.1439-0450.2000.00340.x. [DOI] [PubMed] [Google Scholar]
- 76.Ramadass P, Khadher TGA. Diagnosis of canine parvovirus infection by agar gel precipitation test and fluorescent antibody techniques. Cherion. 1982;11:323–326. [Google Scholar]
- 77.Robinson WF, Huxtable CR, Pass DA. Canine parvoviral myocarditis: a morphological description of the natural disease. Vet Pathol. 1980;17:282–293. doi: 10.1177/030098588001700508. [DOI] [PubMed] [Google Scholar]
- 78.Sanjukta R, Maheshkumar L, Nagappa B, Joken B, Karuna I, Mandakini (2008) Molecular epidemiological study of canine parvovirus in some parts of India. In: International conference on emerging infectious diseases of animals and biotechnological applications, held at TANUVAS, Chennai, 28–29 July 2008, 65 pp.
- 79.Savi J, Minakshi P, Prasad G. Genotyping of field strain of canine parvovirus in Haryana using PCR and RFPL. Indian J Anim Sci. 2009;79(10):971–973. [Google Scholar]
- 80.Schultz R. Duration of immunity for canine and feline vaccines: a review. Vet Microbiol. 2006;117(1):75–79. doi: 10.1016/j.vetmic.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 81.Schultz RL. Current Canine Parvovirus Type 2 (CPV-2) vaccines provide excellent immunity to all genotypes of CPV-2 (e.g. CPV-2a, 2b, and 2c) Vet Ther. 2008;9(2):94–101. [PubMed] [Google Scholar]
- 82.Schunck B, Kraft W, Truyen U. A simple touch-down polymerase chain reaction for the detection of canine parvovirus and feline panleukopenia virus in feces. J Virol Methods. 1995;55:427–433. doi: 10.1016/0166-0934(95)00069-3. [DOI] [PubMed] [Google Scholar]
- 83.Scott FW. Virucidal disinfectants and feline viruses. Am J Vet Res. 1980;41:410–414. [PubMed] [Google Scholar]
- 84.Shackelton LA, Parrish CR, Truyen U, Holmes EC. High rate of viral evolution associated with the emergence of carnivore parvovirus. Proc Natl Acad Sci USA. 2005;102:379–384. doi: 10.1073/pnas.0406765102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Spibey N, Greenwood NM, Sutton D, Chalmers WS, Tarpey I. Canine parvovirus type 2 vaccine protects against virulent challenge with type 2c virus. Vet Microbiol. 2008;128(1–2):48–55. doi: 10.1016/j.vetmic.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 86.Stann SE, DiGiacomo RF, Giddens WE, Evermann JF. Clinical and pathological features of parvoviral diarrhoea in dogs. J Am Vet Med Assoc. 1984;185:651–654. [PubMed] [Google Scholar]
- 87.Steinel A, Venter EH, Van Vuuren M, Truyen U. Antigenic and genetic analysis of canine parvovirus in Southern Africa. Onderstepoort J Vet Res. 1998;65:239–242. [PubMed] [Google Scholar]
- 88.Steinel L, Munson M, Van Vuuren C, Truyen U. Genetic characterization of feline parvovirus sequences from various carnivores. J Gen Virol. 2000;81:345–350. doi: 10.1099/0022-1317-81-2-345. [DOI] [PubMed] [Google Scholar]
- 89.Steinel A, Parrish CR, Bloom ME, Truyen U. Parvovirus infections in wild carnivores. J Wildl Dis. 2001;37(3):594–607. doi: 10.7589/0090-3558-37.3.594. [DOI] [PubMed] [Google Scholar]
- 90.Tratschin JD, McMaster GK, Kronauer G, Siegl G. Canine parvovirus relationship to wild-type and vaccine strains of feline panleucopenia virus and mink enteritis virus. J Gen Virol. 1982;61:33–41. doi: 10.1099/0022-1317-61-1-33. [DOI] [PubMed] [Google Scholar]
- 91.Truyen UA. Evolution of canine parvovirus—a need for new vaccines. Vet Microbiol. 2006;117:9–13. doi: 10.1016/j.vetmic.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 92.Truyen U, Parrish CR. Canine and feline host ranges of canine parvovirus and feline panleucopenia virus: distinct host cell tropisms of each virus in vitro and in vivo. J Virol. 1992;66:5399–5408. doi: 10.1128/jvi.66.9.5399-5408.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Truyen UA, Gruneberg SF, Chang B, Obermaier P, Parrish CR. Evolution of the feline-subgroup of parvoviruses and the control of canine host range in vivo. J Virol. 1995;69:4702–4710. doi: 10.1128/jvi.69.8.4702-4710.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Truyen U, Evermann JF, Vieler E, Parrish CR. Evolution of canine parvovirus involved loss and gain of feline host range. Virology. 1996;215:186–189. doi: 10.1006/viro.1996.0021. [DOI] [PubMed] [Google Scholar]
- 95.Truyen U, Steinel A, Bruckner L, Lutz H, Mostl K. Distribution of antigen types of canine parvovirus in Switzerland, Austria and Germany. Schweiz Arch Tierheilkd. 2000;142:115–119. [PubMed] [Google Scholar]
- 96.Waner T, Naveh A, Wudovsky I, Carmichael LE. Assessment of maternal antibody decay and response to canine parvovirus vaccination using a clinic-based enzyme linked immuno sorbent assay. J Vet Diagn Invest. 1996;8:427–432. doi: 10.1177/104063879600800404. [DOI] [PubMed] [Google Scholar]
- 97.Waner T, Mazar S, Nachmias E, Keren-Kornblatt E, Harrus S. Evaluation of a dot ELISA kit for measuring immunoglobulin M antibodies to canine parvovirus and distemper virus. Vet Rec. 2003;152:588–591. doi: 10.1136/vr.152.19.588. [DOI] [PubMed] [Google Scholar]
- 98.Whan-Gook N, Jung-Hyang S, Alan RD, Soon-Bok K. Detection of canine parvovirus in naturally infected dogs with enteritis and myocarditis by in situ hybridization. J Vet Diagn Investig. 1997;9:255–260. doi: 10.1177/104063879700900306. [DOI] [PubMed] [Google Scholar]
- 99.Woods CB, Pollock RVH, Carmichael LE. Canine parvoviral enteritis. J Am Anim Hosp Assoc. 1980;16:171–179. [Google Scholar]