Abstract
Macrobrachium rosenbergii nodavirus along with a satellite virus, extra small virus (XSV) causes white tail disease (WTD) in the giant freshwater prawn M. rosenbergii. Infected M. rosenbergii postlarvae were collected from a hatchery in Kakinada, Andhra Pradesh. The gene coding the capsid protein of XSV was cloned in a bacterial expression vector pRSET A and the recombinant protein was expressed in Escherichia coli BL21(DE3)pLysS cells. The recombinant protein was purified by Nickel affinity chromatography. Polyclonal antibodies were produced in mice against the recombinant protein and the antibodies reacted specifically with the recombinant protein and XSV in WTD-infected tissues. This is the first report of detection of XSV using antibodies against recombinant capsid protein.
Keywords: Macrobrachium rosenbergii, White tail disease, XSV, Capsid protein, Polyclonal antibodies
The giant freshwater prawn Macrobrachium rosenbergii is one of the most economically important farmed palaemonids in the world. The culture of M. rosenbergii in India is declining due to disease outbreaks, especially white tail disease (WTD). WTD is caused by M. rosenbergii nodavirus (MrNV) and an associated satellite virus, extra small virus (XSV) [6, 10]. The disease affects larval, post-larval and early juvenile stages causing up to 100 % mortality. The affected animals exhibit lethargy and whitish discoloration of the abdominal muscles. The disease has been observed since 1994 and was first reported in 1999 in Guadeloupe in French West Indies [1]. Since then, the disease is reported in Taiwan [14], China [5], India [8], Thailand [17] and Australia [3].
MrNV is a small icosahedral, non-enveloped virus, of 25 nm in diameter. Its genome is composed of two fragments of linear single-stranded RNA (ss-RNA), of 2.9 and 1.3 kb, respectively and its capsid is made up of a single polypeptide of 43 kDa [2]. MrNV is associated with a small virus, XSV, a non-enveloped icosahedral virus, 15 nm in diameter [10]. The exact role of these viruses in the disease pathogenesis or their interaction is not completely understood. The XSV is classified as a satellite virus owing to its very small size, lack of genes encoding RNA polymerase for replication and therefore, is dependant on the helper virus (MrNV) and possesses a single gene encoding the structural protein [10]. Sequencing of the XSV genome showed that it consists of a linear single-stranded RNA of 796 nucleotides. The genome is in sense orientation, with a short poly (A) tail at the 3′-end. The genome has an ORF of 522 nucleotides coding for a 174 amino acid polypeptide of 17 kDa and a truncated protein of 16 kDa lacking the first 11 N-terminal amino acids [2, 10].
Several tests have been reported for detecting WTD which include dot-blot hybridization, in situ hybridization and reverse-transcriptase polymerase chain reaction (RT-PCR) [11], loop-mediated isothermal amplification (LAMP) [4], sandwich enzyme-linked immunosorbent assay (S-ELISA) [7] and triple antibody sandwich enzyme-linked immunosorbent assay (TAS-ELISA) [6] for MrNV; dot-blot hybridization and RT-PCR for XSV [12] and one-step multiplex RT-PCR [13, 16] for the detection of both MrNV and XSV. Sahul Hameed et al. [9] reported western blot and ELISA for the detection of both MrNV and XSV. The antibodies used in the immunological techniques were developed either against the whole virus particles or against the capsid proteins obtained by electroelution from SDS-PAGE separated viral proteins. Since there are problems associated with purifying the virus from the infected tissue samples, the present work aimed at producing antibodies against recombinant capsid protein of XSV and to use it as a diagnostic tool to detect the virus.
WTD-infected live M. rosenbergii postlarvae (PL) were collected from a hatchery in Kakinada, Andhra Pradesh and transported to the laboratory. Total RNA was extracted from infected PL using TRIzol® Reagent (Invitrogen, USA), following the manufacturer’s protocol. cDNA was synthesized from 1 μg of RNA using First Strand cDNA Synthesis Kit (Fermentas) with random hexamer primers. PCR was performed using specific primers (ATGGCTAGAGGTAAACAAAATTC and ACAACCTAATTATTGCCGAC for MrNV [11] and CCACGTCTAGCTGCTGAC and CGGAATAATGCCTAACCAAT for XSV [15]). The reaction mixture (25 μL) contained 12.5 μL PCR master mix (Fermentas), 25 pmol each of forward and reverse primers and 1 μL of cDNA. The thermal profile for MrNV was initial denaturation at 94 °C/2 min followed by 30 cycles of 94 °C/60 s, 55 °C/40 s and 72 °C/90 s and a final extension at 72 °C/5 min. The thermal profile for XSV was initial denaturation at 94 °C/5 min followed by 30 cycles of 94 °C/60 s, 52 °C/40 s and 72 °C/60 s and a final extension at 72 °C/5 min. The amplified products were analysed by electrophoresis on a 1.2 % agarose gel stained with ethidium bromide.
A 640 bp gene fragment encoding the capsid protein of XSV was amplified using specific primers (XSV-F1-5′-CGGGATCCCGTAGGGGACGTGGTAGGACA-3′ and XSV-R1-5′-GGAATTCCACAATTGGCCATAAGGGTTTTC-3′) with restriction sites, using the same thermal profile as described above. The purified PCR product was RE digested, ligated into pRSETA and transformed into DH5α competent cells. The recombinant plasmid was purified from positive clones and subsequently transformed into E.coli BL21(DE3)pLysS competent cells for expression of recombinant protein. The cloned insert was sequenced using commercial services (Bangalore Genei, India). The sequence was analysed for homology with other reported XSV sequences using Basic Local Alignment Search Tool (BLAST) of NCBI.
After a pilot expression, the recombinant capsid protein of XSV was expressed by inducing a 250 ml culture with 1 mM IPTG for 14 h. The recombinant protein was purified using Ni-NTA Fast Start kit (Qiagen) following the manufacturer’s protocol. The aliquots of the purified protein as well as the column wash fractions were boiled with sample buffer, electrophoresed on a 12 % polyacrylamide gel and stained with Coomassie brilliant blue. All the fractions having the recombinant protein were pooled and concentrated using a 3 kDa cutoff Centricon centrifugal filter device (Millipore) by centrifugation at 7500×g for 2 h at 4 °C. The purified protein was quantified using GeNei™ Protein Estimation Kit (BCA Method) (Bangalore Genei, India) following the manufacturer’s procedure.
The purified recombinant capsid protein of XSV was emulsified with Freund’s complete adjuvant and injected intraperitoneally into balb/c mice at a dose of 50 μg mouse−1. The mice were boosted twice with same quantity of antigen emulsified with Freund’s incomplete adjuvant at 2-week intervals. Eight days after the final booster the mice were anaesthetized and bled from the retro-orbital plexus using a capillary tube. The serum was separated by centrifugation and stored at −80 °C.
For western blot, the recombinant capsid protein of XSV was electrophoresed on a 12 % SDS-PAGE gel and transferred to a PVDF membrane by electroblotting. The membrane was blocked with 2 % bovine serum albumin, incubated with diluted (1:5,000) polyclonal antibodies for 1 h. After washing, the membrane was incubated for 45 min with anti-mouse HRP conjugate. The membrane was washed and incubated with diaminobenzidine with 0.1 % H2O2 in dark until the brown precipitate could be observed.
Healthy M. rosenbergii PLs obtained from the hatchery of Central Institute of Fisheries Education, Mumbai were experimentally infected with WTD. The inoculum was prepared by homogenizing the WTD-infected samples in a tissue homogenizer, clarified at 12,000×g for 20 min at 4 °C and filtered through a 0.22 μm syringe filter. The inoculum was added to the PLs kept in a 1 L glass beaker with vigourous aeration. After 2 h, all the PLs were transferred to an aquarium tank and observed daily for clinical signs. The infection was confirmed by RT-PCR for MrNV and XSV.
Immunohistochemistry assay was done with experimentally infected M. rosenbergii PLs. The infected and uninfected PLs were fixed in Davidson’s fixative and longitudinal sections were made following standard histology procedure. The deparaffinized and rehydrated sections on glass slides were blocked with 2 % skimmed milk powder. Following washing, the tissue sections were incubated with 1:2,000 dilution of polyclonal XSV antibodies for 2 h. The slides were washed and incubated with anti-mouse HRP conjugate for 1 h. After washing, the slides were incubated with diaminobenzidine with 0.1 % H2O2. After washing the slides were air-dried, mounted and observed under a light microscope.
PCR amplification of cDNA prepared from RNA extracted from infected animals showed specific products of 1.14 kb and 772 bp for MrNV and XSV, respectively (Fig. 1). PCR specific for XSV using primers with restriction sites yielded an expected product of 640 bp (Fig. 1). The recombinant colonies upon colony PCR yielded a 640 bp product. The purified recombinant plasmid upon double digestion with BamH1 and EcoR1 released a 640 bp insert. The insert in the recombinant plasmid was sequenced and the sequence was submitted to the Genbank (Accession No. HQ637180). The sequence showed 99 % homology with Indian and Taiwanese isolates and 98 % with Chinese and Thailand isolates.
Fig. 1.
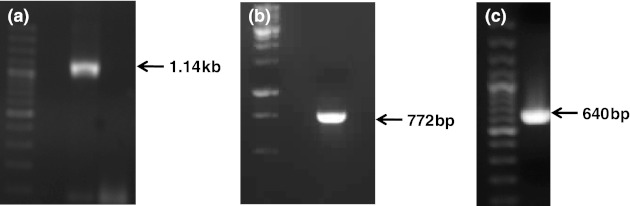
Reverse-transcription polymerase chain reaction for the detection of MrNV (a) and XSV (b, c) in freshwater prawn. The 640 bp product in panelc was used for cloning in pRSETA expression vector
Upon pilot expression, XSV capsid protein (His-tagged) was found to get expressed maximum after overnight incubation producing an approximately 21 kDa protein, as expected (Fig. 2). After eluting the recombinant protein from Ni-NTA columns, the 3rd, 4th and 5th elutes showed the presence of recombinant proteins (Fig. 2). The purified protein was concentrated and the concentration of the recombinant protein was estimated to be 450 μg mL−1. The polyclonal antibodies produced against the recombinant protein reacted specifically with the recombinant protein in western blot (Fig. 2).
Fig. 2.
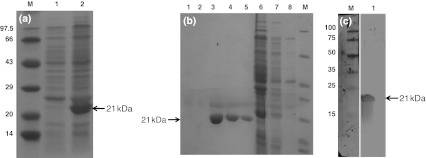
XSV recombinant capsid protein expression (a) (Lane M Marker, lane 1 uninduced BL21 (DE3) cell lysate, lane 2 BL21 (DE3) cell lysate after overnight IPTG induction. Purification of expressed protein (b) (Lane1–5 elutes 1–5, lanes6–8 washings 1–3, lane M protein marker). Western blot (c) (Lane M Protein marker stained with Ponceau S solution, lane 2 western blot of XSV recombinant protein with polyclonal antibody)
When healthy PLs of M. rosenbergii were exposed to the inoculum prepared from WTD-infected animals, the clinical signs such as whitish discolouration of the abdominal muscles (Fig. 3) and lethargy were observed 7 days post-infection. Immunohistochemistry revealed a positive reaction in most of the tissues except hepatopancreas of infected PLs while the uninfected animals did not show any reaction (Fig. 3).
Fig. 3.
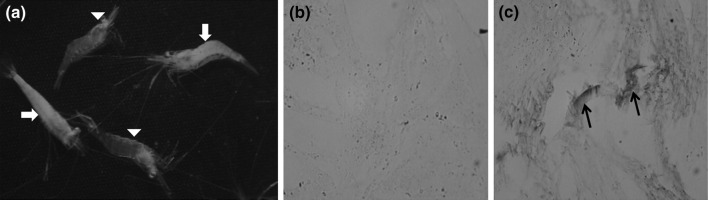
Experimentally infected postlarvae of M. rosenbergii showing whitish muscle discolouration (arrows); arrow heads indicate uninfected animals (a). Immunohistochemical staining of tissue section of whole PL using XSV antibodies; uninfected (b) and infected (c). Arrows indicate the dark immunostaining in the abdominal muscle
WTD is the most significant viral infection of M. rosenbergii. The disease which infects larval and PL stages in hatcheries has resulted in a sharp decline in the number of M. rosenbergii hatcheries and the PLs produced in India. As a result, the number of WTD outbreaks reported has also decreased significantly. However, the threat the disease poses still remains in the hatcheries being operated. Hence, a rapid, economical and high throughput diagnostic test is essential to screen the broodstock, larval and PL stages periodically to keep the infection under control. Antibody-based techniques like ELISA to detect MrNV and XSV [6, 7, 9] have been reported earlier. However, purification of the virus from infected PLs is laborious and it is not practical to purify the virus devoid of any host tissue protein. The hyperimmune serum produced using the viruses purified from infected tissues will also have antibodies against the host protein rendering it unsuitable as a diagnostic tool. Purification of XSV devoid of MrNV or vice versa from infected PLs is also not possible even after gradient centrifugations [18]. Purification of the virus from SDS-PAGE gel slices is time consuming and the amount of protein that can be purified is a limiting factor. Hence, in the present study, recombinant XSV capsid protein produced in prokaryotes was used to develop hyperimmune serum. The polyclonal serum reacted specifically with the recombinant viral protein in western blot and with infected tissue sections. Positive reaction was observed in most of the tissues except hepatopancreas indicating that the virus does not infect the hepatopancreas as also reported by Sahul Hameed et al. [9].
This is the first report of detection of XSV using antibodies produced against the recombinant capsid protein. The antibodies thus produced can be used to develop immunological techniques for diagnosis of WTD in M. rosenbergii. The antibody can also be used to neutralize the virus in the inoculum to elucidate its role in the pathogenesis of WTD.
Acknowledgments
We thank the Department of Biotechnology, Government of India, for funding this study. We also thank the Director, Dr. W. S. Lakra and the former Director Dr. Dilip Kumar, Central Institute of Fisheries Education, Mumbai, India, for providing necessary facilities and support for this study.
References
- 1.Arcier JM, Herman F, Lightner DV, Redman R, Mari J, Bonami JR. A viral disease associated with mortalities in hatchery-reared postlarvae of the giant freshwater prawn Macrobrachium rosenbergii. Dis Aquat Organ. 1999;38:177–181. doi: 10.3354/dao038177. [DOI] [Google Scholar]
- 2.Bonami JR, Shi Z, Qian D, Sri Widada J. White tail disease of the giant freshwater prawn, Macrobrachium rosenbergii: separation of the associated virions and characterization of MrNV as a new type of nodavirus. J Fish Dis. 2005;28:23–31. doi: 10.1111/j.1365-2761.2004.00595.x. [DOI] [PubMed] [Google Scholar]
- 3.Owens L, La Fauce K, Juntunen K, Hayakijkosol O, Zeng C. Macrobrachium rosenbergii nodavirus disease (white tail disease) in Australia. Dis Aquat Organ. 2009;85:175–180. doi: 10.3354/dao02086. [DOI] [PubMed] [Google Scholar]
- 4.Pillai D, Bonami JR, Sri Widada J. Rapid detection of Macrobrachium rosenbergii nodavirus (MrNV) and extra small virus (XSV), the pathogenic agents of white tail disease of Macrobrachium rosenbergii (De Man), by loop-mediated isothermal amplification. J Fish Dis. 2006;29:275–283. doi: 10.1111/j.1365-2761.2006.00718.x. [DOI] [PubMed] [Google Scholar]
- 5.Qian D, Shi Z, Zhang S, Cao Z, Liu W, Li L, Xie Y, Cambournac I, Bonami JR. Extra small virus-like particles (XSV) and nodavirus associated with white muscle disease in the giant freshwater prawn, Macrobrachiumrosenbergii. J Fish Dis. 2003;26:521–527. doi: 10.1046/j.1365-2761.2003.00486.x. [DOI] [PubMed] [Google Scholar]
- 6.Qian D, Liu W, Jianxiang W, Yu L. Preparation of monoclonal antibody against Macrobrachium rosenbergii Nodavirus and application of TAS-ELISA for virus diagnosis in post-larvae hatcheries in east China during 2000–2004. Aquaculture. 2006;261:1144–1150. doi: 10.1016/j.aquaculture.2006.05.022. [DOI] [Google Scholar]
- 7.Romestand B, Bonami JR. A sandwich enzyme linked immunosorbent assay (S-ELISA) for detection of MrNV in the giant freshwater prawn, Macrobrachium rosenbergii (de Man) J Fish Dis. 2003;26:71–75. doi: 10.1046/j.1365-2761.2003.00432.x. [DOI] [PubMed] [Google Scholar]
- 8.Sahul Hameed AS, Yoganandhan K, Sri Widada J, Bonami JR. Studies on the occurrence of Macrobrachium rosenbergii nodavirus (MrNV) and extra small virus-like particles (XSV) associated with white tail disease (WTD) of Macrobrachium rosenbergii in India by RT-PCR detection. Aquaculture. 2004;238:127–133. doi: 10.1016/j.aquaculture.2004.06.009. [DOI] [Google Scholar]
- 9.Sahul Hameed AS, Ravi M, Farook MA, Taju G, Hernandez-Herrera RI, Bonami JR. Screening the post-larvae of Macrobrachium rosenbergii for early detection of Macrobrachium rosenbergii nodavirus (MrNV) and extra small virus (XSV) by RT-PCR and immunological techniques. Aquaculture. 2011;317:42–47. doi: 10.1016/j.aquaculture.2011.04.022. [DOI] [Google Scholar]
- 10.Sri Widada J, Bonami JR. Characteristics of the monocistronic genome of extra small virus, a virus-like particle associated with Macrobrachium rosenbergii nodavirus: possible candidate for a new species of satellite virus. J Gen Virol. 2004;85:643–646. doi: 10.1099/vir.0.79777-0. [DOI] [PubMed] [Google Scholar]
- 11.Sri Widada J, Durand S, Cambournac I, Qian D, Shi Z, Dejonghe E, Richard V, Bonami JR. Genome-based detection methods of Macrobrachium rosenbergii nodavirus, a pathogen of the giant freshwater prawn, Macrobrachium rosenbergii: dot-blot, in situ hybridization and RT-PCR. J Fish Dis. 2003;26:583–590. doi: 10.1046/j.1365-2761.2003.00493.x. [DOI] [PubMed] [Google Scholar]
- 12.Sri Widada J, Richard V, Shi Z, Qian D, Bonami JR. Dot-blot hybridization and RT-PCR detection of extra small virus (XSV) associated with white tail disease of prawn Macrobrachium rosenbergii. Dis Aquat Organ. 2004;58:83–87. doi: 10.3354/dao058083. [DOI] [PubMed] [Google Scholar]
- 13.Tripathy S, Sahoo PK, Kumari J, Mishra BK, Sarangi N, Ayyappan S. Multiplex RT-PCR detection and sequence comparison of viruses MrNV and XSV associated with white tail disease in Macrobrachium rosenbergii. Aquaculture. 2006;258:134–139. doi: 10.1016/j.aquaculture.2006.04.016. [DOI] [Google Scholar]
- 14.Tung CW, Wang CS, Chen SN. Histological and electron microscopic study on Macrobrachium muscle virus (MMV) infection in the giant freshwater prawn, Macrobrachium rosenbergii (de Man), cultured in Taiwan. J Fish Dis. 1999;22:319–323. doi: 10.1046/j.1365-2761.1999.00172.x. [DOI] [Google Scholar]
- 15.Wang CS, Chang JS, Shih HH, Chen SN. RT-PCR amplification and sequence analysis of extra small virus associated with white tail disease of Macrobrachium rosenbergii (de Man) cultured in Taiwan. J Fish Dis. 2007;30:127–132. doi: 10.1111/j.1365-2761.2007.00793.x. [DOI] [PubMed] [Google Scholar]
- 16.Yoganandhan K, Sri Widada J, Bonami JR, Sahul Hameed AS. Simultaneous detection of Macrobrachium rosenbergii nodavirus and extra small virus by a single tube, one-step multiplex RT-PCR assay. J Fish Dis. 2005;28:65–69. doi: 10.1111/j.1365-2761.2004.00606.x. [DOI] [PubMed] [Google Scholar]
- 17.Yoganandhan K, Leartvibhas M, Sriwongpuk S, Limsuwan C. White tail disease of the giant freshwater prawn Macrobrachium rosenbergii in Thailand. Dis Aquat organ. 2006;69:255–258. doi: 10.3354/dao069255. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Wang J, Yuan J, Li L, Zhang J, Bonami JR, Shi Z. Quantitative relationship of two viruses (MrNV and XSV) in white-tail disease of Macrobrachium rosenbergii. Dis Aquat organ. 2006;71:11–17. doi: 10.3354/dao071011. [DOI] [PubMed] [Google Scholar]


