Abstract
The 50 % tissue culture infectious dose (TCID50) is still one of the most commonly used techniques for estimating virus titers. However, the traditional TCID50 assay is time consuming, susceptible to subjective errors and generates only quantal data. Here, we describe a colorimetric-based approach for the titration of Enterovirus 71 (EV71) using a modified method for making virus dilutions. In summary, the titration of EV71 using MTT or MTS staining with a modified virus dilution method decreased the time of the assay and eliminated the subjectivity of observational results, improving accuracy, reproducibility and reliability of virus titration, in comparison with the conventional TCID50 approach (p < 0.01). In addition, the results provided evidence that there was better correlation between a plaquing assay and our approach when compared to the traditional TCID50 approach. This increased accuracy also improved the ability to predict the number of virus plaque forming units present in a solution. These improvements could be of use for any virological experimentation, where a quick accurate titration of a virus capable of causing cell destruction is required or a sensible estimation of the number of viral plaques based on TCID50 of a virus is desired.
Keywords: Enterovirus 71, MTS, MTT, TCID50, Virus titration
Introduction
Titration of viruses is a crucial step in any virological study, particularly where exact amounts of a virus need to be applied in experimental procedures such as testing efficacy of a potential antiviral drug [8]. The most common approaches to quantify a virus titer are based on either finding the 50 % end-point where 50 % of the infected test cells or animals die [12], also well known as the 50 % tissue culture infectious dose (TCID50), or directly counting plaques caused by virus in infected cells [5]. Other approaches may utilize polymerase chain reaction (PCR) [2, 7], or reverse transcriptase (RT)-PCR [11]. Use of each method may be justified by defined circumstances; under the limitation of the virus and the cell type.
TCID50 assay is traditionally referred to the algorithm used by Reed and Muench [12], where they used a murine model to formulate a method for titration of a protective serum. Unlike the symptomatic animals in Reed and Muench’s in vivo experiment, however, results of a classic TCID50 assay are usually generated by microscopic observations of cytopathic effect (CPE), which is open to subjective errors, including misidentification of CPE within replications of a virus dilution and/or between different virus dilutions. In addition, the conventional TCID50 method is unable to measure viral CPE as a quantitative phenomenon to be contributed to cells and proportional to virus dilution [8]. Furthermore, there are certain cells and viruses that do not show clear CPE, resulting in limited usefulness of conventional observation-based TCID50 methods.
A number of solutions have been suggested to improve reliability and reproducibility of the traditional TCID50 method, including the use of colorimetric methods that can generate continuous data as well as eliminate the need for observation of CPE, resulting in reducing subjective errors. Among these examples, the reagent MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide, a yellow tetrazole] has commonly been used for virus titration [1, 9, 13, 16]. Another such compound with a similar action is MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS(a)], although its use for the estimation of virus titers appears to be limited [4].
Several viruses of the Picornaviridae family have been titrated using an MTT-based colorimetric method [1]; however, the use of colorimetric methods has not been reported for the viral titration of Enterovirus 71 (EV71). The major aim of this study was, therefore, to establish a rapid and accurate technique to quantify EV71 titers. In addition, other conceptual issues, such as virus dilution, incubation times and number of wells used in both the conventional and colorimetric-based methods of TCID50 were investigated. In addition, this study aimed to elucidate how the conventional and modified methods of TCID50 correlate to a viral plaque forming assay.
Materials and Methods
Cell and Virus
Two EV71 strains were used for this study: a cloned isolate of EV71 strain 6F/AUS/6/99 (GenBank ID: DQ381846.1) provided by Prof. Peter C. McMinn (Central Clinical School, University of Sydney, Australia), and a clinically isolated EV71 (isolate number 99018233) supplied from Dr. Julian Druce of the Victorian Infectious Disease Reference Laboratories (VIDRL, Parkville VIC, Australia). African Green Monkey Kidney (Vero) cells (provided by Prof. Peter C. McMinn) were maintained in Dulbecco’s Modified Eagle’s Medium with high glucose (DMEM, Invitrogen, Mulgrave, VIC, Australia) supplemented with 10 % heat in-activated fetal bovine serum (FBS, Invitrogen, Mulgrave, VIC, Australia). The viruses were propagated in serum-free DMEM.
Cell Seeding and Infection of Cell Cultures
Vero cells were seeded at 3 × 104 cells (150 μL/well) in 96-well plates or at 3.6 × 105 cells (3 mL/well) in 6-well plates (Becton–Dickinson, BD, North Ryde, NSW, Australia) followed by incubation at 37 °C in a humidified atmosphere containing 5 % CO2 until 80 % confluent. Before viral inoculation, or when quantifying the results, cell monolayers were thoroughly washed with phosphate buffered-saline (PBS, pH 7.4 at room temperature) three times. In all the experiments, the following controls were included: mock-infected control (cells that were not infected with the virus) and undiluted virus control (cells that were infected with the undiluted stock virus). The virus dilutions were made in DMEM + 5 % FBS and added to cells in a total volume of 50 μL/well mixed with 100 μL of the growth media (DMEM + 5 % FBS). Where stated, incubation was done at 37 °C in a humidified atmosphere containing 5 % CO2.
Conventional TCID50 Titration Assay
The conventional endpoint titration assay has been reported previously [12]. Briefly, virus was diluted 1:10 from which a further eight log10 dilutions were made and added to cells in six replicates. The plates were then incubated for 72 h. After incubation, the wells were observed by an inverted microscope (Olympus Imaging Corp, China) and marked as “positive CPE” if cells had developed observable CPE in 30 % or more of the surface of the well, compared to both the undiluted virus controls and the mock-infected controls. Cells that did not show such conditions were marked as “negative CPE”. Further calculations were conducted using the Reed-Muench formula [12]. In brief, a proportionate distance (PD) was calculated and then added to the log of the dilution factor at which the infection (dead cell percentage) was just over 50 %. The resulting value was considered as the TCID50 log. In addition to the Reed-Muench formula, the dead cell percentages were plotted against the virus dilutions on a log10 scale. Then, the TCID50 value was calculated using a regression analysis of the curve.
Modified Traditional TCID50 Titration Assay
The above mentioned method was modified as follows: Initially, a 1:10 dilution of the virus stock was prepared and a further thirteen dilutions were made based on log2 and added to cells in six replicates. Following incubation for 72 h, microscopic observations were preformed as stated above and the 50 % endpoint was calculated using a regression analysis of the curve.
The MTS-Based Endpoint Dilution Assay
Initially, a 1:10 dilution of the virus stock was prepared followed by a further eight log2 dilutions and added to cells in triplicate. Plates were incubated for 72 h at which microscopic observations and virus quantification were performed. In order to quantify virus titration, the cell supernatant was aspirated and the monolayer was washed with PBS three times. Twenty microlitre of MTS reagent (Promega, VIC, Australia) mixed in 150 μL of serum-free DMEM was added into each well. After 90 min of incubation, the color change was measured using a Microplate Reader (BioRad, Japan) at 490 nm. The average absorbance of the mock-infected controls was considered as 100 % to which cell death percentages of each virus dilution were calculated [14]. The results were then graphed by plotting dead cell percentages against the virus dilutions. The 50 % infectivity point was calculated through a linear regression analysis of the curve.
The MTT-Based Endpoint Dilution Assay
The method described above was used; 1:10 and a further thirteen dilutions were prepared, however, instead of MTS, 20 μL of MTT solution (Invitrogen, Mulgrave, VIC, Australia) (0.5 % w/v in PBS) was dissolved in 150 μL of serum-free DMEM and the mix was added into each well. The plates were then incubated for 3 h after which the formazane was dissolved with 50 μL/well of dimethyl sulphoxide (DMSO) followed by another 8 min incubation. Color change was then recorded using the Microplate Reader at 540 nm from which the background absorption at 670 nm was subtracted. Results were analyzed in the manner described above.
Virus Titration Via Plaquing Assay
The EV71 isolates were titrated through the typical plaquing assay [5, 10, 15]. Briefly, Vero cells were seeded in 6-well plates at 3.6 × 105 cells/well in 3 mL of the growth media and incubated until 80 % cell confluency was reached. Cells were then washed three times with PBS followed by infection with 1 mL/well of various virus dilutions in DMEM + 5 % FBS. The virus dilutions were made based on the prediction of the number of plaque forming units (PFU) using a conversion formula: PFU (mL)/TCID50 (mL) = 0.7 [3]. Then, the required dilution factor for producing 1,000 plaques per well from a 100 TCID50 virus suspension was estimated followed by serial twofold dilutions. After 1 h of incubation, the viral inocula were aspirated followed by washing cells three times with PBS. Three milliliters of overlay media containing the growth media mixed with 3 % agarose gel (Ultrapure™ Agarose, Mulgrave, VIC, Australia, Invitrogen) at 1:4 (agarose gel : medium) ratio was then added to each well. The plates were incubated until the virus plaques were formed; they were then fixed by 10 % formaldehyde for one and half hours and the agarose plugs were removed. The monolayer was stained with one mL/well of the staining solution (0.4 % crystal violet, 1.67 % ethanol in water) and incubated for 8 min. The wells were then gently rinsed with water and air dried. The viral plaques were then counted and virus titer was measured in PFU/mL and multiplicity of infection (MOI), according to the following formula:
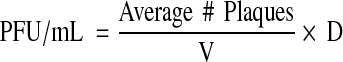 |
where D = dilution factor; V = volume of inoculum (per mL)
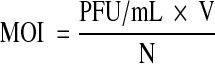 |
where V = volume of viral inoculum (per mL); N = number of the cells seeded in the well
Statistical Analysis
All of the treatments were applied in triplicate, and each experiment was independently repeated three times. Statistical analyses were performed using the unpaired independent two-sample t test (assuming equal variance).
Results
Traditional TCID50
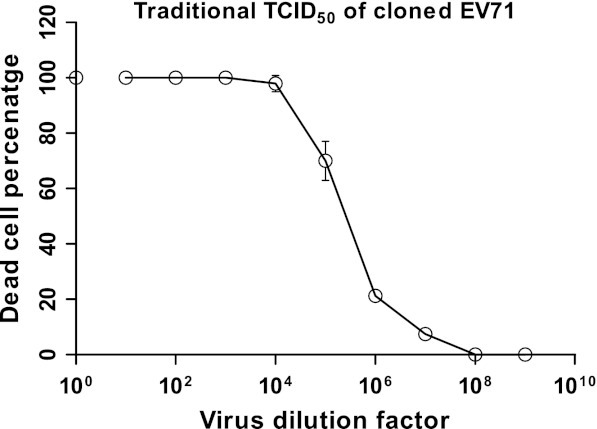
The cloned EV71 strain was titrated based on the traditional TCID50 method, where visible cell shrinkage and rounding were considered symptoms of CPE by which further calculations were conducted. A traditional algorithm of titration estimated the TCID50 to be 5.8 × 106 per mL (Table 1), whereas linear regression analysis of the curve (Fig. 1) generated a 50 % endpoint value of 5.3 × 106 per mL (Table 1). The results of both approaches were found not to be statistically different (p > 0.05).
Table 1.
Comparison of TCID50/mL amounts resulted from different titration assays
| Method of titration | |||||
|---|---|---|---|---|---|
| Virus | Traditionala | Modified traditionala | MTSa | MTTa | |
| Reed and Muench’s formula | Regression analysis | ||||
| Cloned EV71 | 5.8 × 106 ± 2,150,000 | 5.3 × 106 ± 970,000 | 5.5 × 104 ± 21000 | 6.5 × 103 ± 3400 | 8.1 × 103 ± 3700 |
| Clinically isolated EV71 | – | 4.8 × 104 ± 14000 | – | 8.7 × 103 ± 3200 | |
The values are expressed as mean ± SD. The mean are taken from three independent experiments from which standard deviations (SD) were calculated
aThere was a significant difference with a p < 0.01 between MTT or MTS-based TCID50 and the traditional methods of TCID50. Also, the modified traditional method significantly (p < 0.01) differed from the traditional methods
Fig. 1.
Depiction of the titer of the cloned EV71 virus using the conventional method of TCID50. The dead cell percentages are plotted against virus dilutions on a log10 scale. The dilution factor 100 represents undiluted virus control that was assumed to display 100 % cell death. The values are means of three independent experiments from which standard deviations were calculated
Modified Traditional TCID50
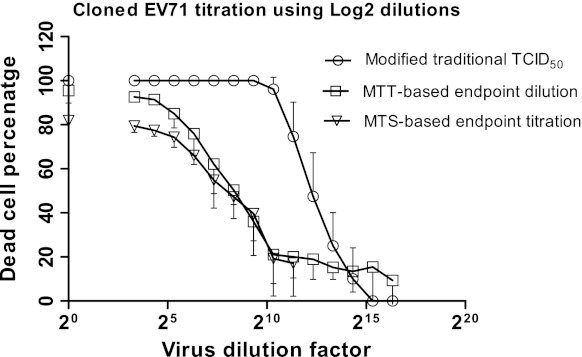
In order to improve the accuracy of the TCID50 calculated using the traditional TCID50 method, a lower magnitude dilution factor was used to carry out serial virus dilutions. The quantification of CPE was then done as described for the traditional TCID50, while the 50 % endpoint was measured only using the regression analysis of the curve (Figs. 2, 3). The 50 % endpoint value was 5.5 × 104 for the cloned EV71, which showed a statistically significant difference (p < 0.01) from that of traditional TCID50 (Table 1). The modified traditional TCID50 was then tested with clinical EV71. The TCID50 value was 4.8 × 104 showed no statistical difference (p > 0.05) from that of cloned EV71 (Table 1).
Fig. 2.
Depiction of the titer of the cloned EV71 virus using three methods based on log2 virus dilution. The dead cell percentages are plotted against virus dilutions on a log2 scale. The dilution factor 20 represents undiluted virus control, which was assumed to display 100 % cell death in the modified traditional TCID50 but was quantifiably calculated using MTT or MTS in the other methods. The values represent mean ± SD generated from three independent experiments
Fig. 3.
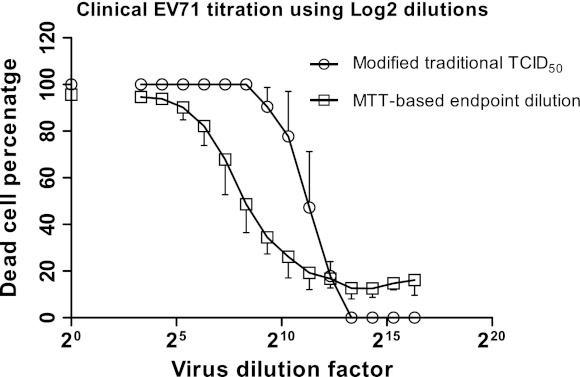
Depiction of the titer of the clinical EV71 virus using two methods based on log2 dilution. The dead cell percentages are plotted against virus dilutions on a log2 scale. The dilution factor 20 represents undiluted virus control, which was assumed to display 100 % cell death but was quantifiably calculated using MTT in the other method. The values are means of three independent experiments from which standard deviations were calculated
The Colorimetric-Based Virus Titration: Analyzing Microscopic Observations
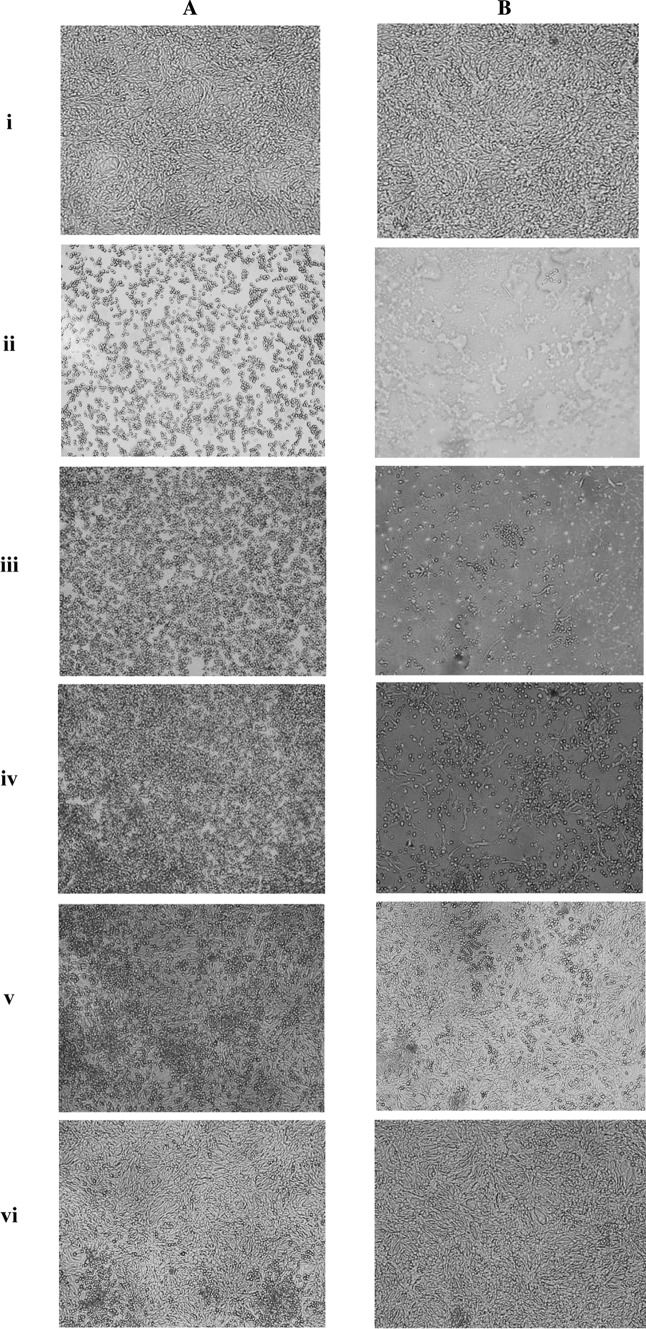
MTS and MTT reagents were used to generate continuous data of the virus titration. Before applying the MTS or MTT reagents, microscopic observations were performed both before removing supernatant (Fig. 4a) and after removing supernatant followed by washing the monolayer with PBS (Fig. 4b) to determine the amount of CPE in each well. For the observations prior to removing supernatant, indications of CPE such as cell rounding, shrinking or floating were seen in all wells in a virus dilution-dependent manner. Mock-infected controls appeared healthy, whereas the undiluted virus controls showed maximal CPE with few or no adhered cells. The microscopic analysis after removing the supernatant clearly showed the monolayer damage due to viral infection. The amount of damage to the monolayer in any sample corresponded to the amount of virus present; the undiluted virus controls showed the greatest damage to the monolayer, while the mock-infected controls displayed healthy monolayers. Both sets of images (before/after removing the supernatant) indicated that the degree of CPE or monolayer damage was associated with virus concentration. However, determination of the monolayer damage and associating it with virus dilution was obviously more practicable in the wells following removal of supernatant and washing with PBS (Fig. 4a, b).
Fig. 4.
Micrographs displaying the Vero cell monolayer at a MTT-based TCID50 assay. The photos in Panel A, show the wells before removing supernatant, while the photos in Panel B show the same wells after removing supernatant and washing with PBS. i Mock-infected control, ii undiluted virus control (1:1), iii viral dilution 1:101, iv viral dilution 1:10 × 23, v viral dilution 1:10 × 27, vi viral dilution 1:10 × 29. The photos are taken 72 h post infection using inverted microscope (×100 magnification, Olympus, CKX 41, Japan)
Determination of the 50 % Endpoint: MTS-Based Titration
The color change caused by the addition of MTS was proportional to the number of healthy living cells. It was assumed that the mock-infected cell controls maintained a viable cell percentage of 100 %; this allowed a comparative calculation of the percentage of live cells in all other samples to be made. Using the quantifiable data generated by the MTS-TCID50 assay, a graph was constructed by plotting the percentage of dead cells against the various virus dilutions (Fig. 2). As expected, the undiluted virus controls showed the highest dead cell percentage (but not 100 %); while the number was decreased as the virus became more diluted up to dilution factor 10 × 27. These findings were supported by microscopic observations. The MTS-generated results demonstrated a statistically significant difference (p < 0.01) compared to those from both the conventional TCID50 titration and the modified traditional TCID50 assays (Table 1).
Determination of the 50 % Endpoint: MTT-Based Titration
Similar to MTS, the color change caused by the addition of MTT was proportional to the number of healthy living cells. A graph was constructed by plotting the various virus dilutions against the percentage of dead cell (Figs. 2, 3). Using the regression analysis, the 50 % infectivity point was calculated. These findings were compatible with the microscopic observations (Fig. 4). There was no significant difference (p > 0.05) between the TCID50 values measured by either colorimetric method when investigating the cloned EV71 virus. Therefore, in all future tests only MTT was used to titrate the clinical EV71, as it was more cost effective when compared to MTS. Using the MTT method, the TCID50 values calculated for the clinical isolate of EV71 were not significantly different (p > 0.05) from the cloned EV71 virus (Table 1).
Titration Via Plaquing Assay
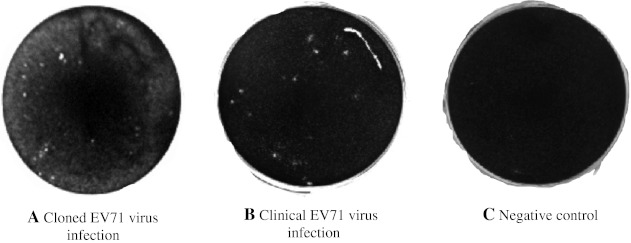
The EV71 plaques were extremely small yet detectable at day 2 post infection; in this test all plaques were counted at day 3 post infection. Plaque formation was noted at an earlier time-point when cells were infected with the cloned EV71 compared to the clinical EV71 infection, but there was no obvious difference between the size and the shape of the plaques noted (Fig. 5). In addition, there was no statistically significant difference (p > 0.05) between the total numbers of plaques counted when cells were infected with 100 TCID50 of either virus isolate (Table 2).
Fig. 5.
Plaque formation of both cloned and clinical EV71: A plaquing assay was performed on both isolates of EV71. The plaques were visualized with crystal violet 3 days post infection in Vero cells. The expected plaques were calculated based on the equation PFU/TCID50 (mL) = 0.7, while the actual number of plaques was counted using the image taken by gel document device (BioRad). The panels show photographs of plaque formation in the wells following infections with: a the cloned EV71 virus, b the clinical EV71 virus, c negative control
Table 2.
Comparison of TCID50, PFU and MOI amounts in clinical and cloned EV71
| Virus | 100 TCID50a (per mL) | PFUb (per mL) | MOIc in the plaquing assay | MOIc in the TCID50 assay | |||
|---|---|---|---|---|---|---|---|
| Actual | Predicted | Actual | Predicted | Actual | Predicted | ||
| Cloned EV71e | 4.5 × 101 (5.5 × 102)d | 33,180 ± 4,728 | 315,000 (3,850,000)d | 0.09 ± 0.01 | 0.9 (10.7)d | 0.04 ± 0.01 | 0.4 (5.1)d |
| Clinically isolated EV71e | 8 × 101 (4.8 × 102)d | 30,856 ± 13,546 | 560,000 (3,360,000)d | 0.09 ± 0.03 | 1.5 (9.3)d | 0.04 ± 0.02 | 0.7 (4.4)d |
aThe MTT-based virus titration was used to measure 100 TCID50 on samples of cloned EV71 and clinically isolated EV71 that were directly used in the plaquing assays mentioned in this table
bThe actual values of PFU were generated from the plaquing assays (mean ± SD) based on the 100 TCID50 virus samples, while the predicted PFUs were calculated using the conversion factor 0.7
cThe actual and the predicted values of MOI were calculated based on the actual and the predicted values of PFUs, respectively
dIndicated in the parentheses are values generated based on the modified traditional TCID50
eThere was no statistically significant difference in PFU or MOI between cloned EV71 and clinical EV71
A conventional method was used to predict the plaque numbers using the known TCID50 PFU/TCID50 = 0.7, based on the MTT-TCID50 results [3]. The current results showed that this conversion formula was not true for either the cloned or clinical EV71 virus isolates, where the ratio of PFU/TCID50 was found to be 0.04 for clinical EV71 and 0.07 for cloned EV71. These results showed that there was an obvious difference in the predicted number of plaques between the MTT-TCID50 assay and the traditional TCID50 assay, with the MTS assay being more accurate (Table 2). The MOI values for both clinical and cloned EV71 were also calculated (Table 2) based on PFU values and the conditions of each experiment (Table 2). There appeared to be no significant difference in the value calculated for either virus isolate (p > 0.05).
Discussion
In this study, a thorough colourimetric-based method was developed to quantify the titration of EV71 strains. There was a statistically significant difference (p < 0.01) between the TCID50 amounts that resulted from the traditional method and that of the MTT or MTS methods. In addition, the described MTS or MTT-TCID50 assay took only 5 days to reach completion. This allows for a more rapid analysis of large numbers of samples, as opposed to some of the previously described MTT methods, which took 7 days [6] or 8–10 days [4] from seeding the plates to completion. There was no significant difference in titration of the cloned EV71 strain between MTS and MTT methods (p > 0.05). This being said, MTS dye has the advantage in that the formazane does not require solubilization, making the analysis procedure quicker that may, in turn, reduce well-to-well and assay-to-assay variability. However, MTT is advantageous in that it is more cost effective and more commonly used. Moreover, in this study no significant difference was noticed in variability between MTS and MTT-based EV71 titration.
The improved methods of TCID50 reported here also have the advantage of being more sensitive, compared to the conventional method of TCID50. Most of the conventional, and even some colourimetric-based viral titration assays, utilize log10-based dilutions of virus. Use of log2 dilutions can increase sensitivity of the virus titration by generating more analyzable data. It also narrows the range of virus dilutions that surround the 50 % endpoint that, in turn, would increase accuracy of calculation of the 50 % endpoint. One pitfall with log10-virus titration can be that if the 50 % endpoint of the virus occurs somewhere between two log10 dilutions, the estimated 50 % point on the curve trendline would potentially be different from the actual value. This was well noted in this study while the TCID50 values calculated using the conventional method was placed between virus dilution factors 105–106, this point was somewhere between 10 × 28 and 10 × 29 using the log2-based modified traditional method. It is of note that in the original work of Reed and Muench [12], a log2-based dilution was used. Therefore, sensitivity and accuracy of a virus titration assay can be increased by making virus dilutions using a 1:10 starting dilution followed by log2 dilutions.
Another point with the traditional assay of TCID50 is the use of six replicates at each virus dilution. This would not necessarily increase accuracy of the assay; instead, it might even generate additional undefined errors. The reason lies in the fact that the conventional TCID50 relies on microscopic observations; and thus, the total subjective errors due to wrong CPE scoring can raise as the number of individual wells increases per sample. By contrast, this is not the case with the MTT or MTS methods where the virus is diluted using a log2 basis.
Previously, it was reported that the use of MTS in viral assays led to more variability in the results [4]. We would speculate that this might be due to some cells being in the early stages of infection, where they are damaged and detached but not yet dead, which would lead to false positive results and a low specificity of MTS. Due to this observation, removal of supernatant and thorough washing of the monolayer with PBS before addition of MTS reagent was performed in order to remove any unbound cells and eliminate this error. Thus, in the results presented here, MTS showed high specificity and produced results that were more consistent.
In this study, the relationship between PFU and TCID50 was found to be different from the one commonly used by researchers (PFU/TCID50 = 0.7), and this was found to be different between two strains of EV71 as well. This would possibly suggest that there is no accurate, fixed conversion factor to estimate PFU based on a TCID50 value of a virus sample. However, the plaquing assay demonstrated that both PFU and MOI amounts of both strains of EV71 virus were more compatible with the corresponding TCID50 amounts resulting from MTT or MTS than the traditional methods. Therefore, the plaquing assay results support the conclusion that MTT or MTS-based TCID50 is more accurate and reliable compared to traditional methods of TCID50. Given a virus produces both viral plaques and CPE, one could use either plaquing assay or the improved TCID50 assay, depending on the purpose of titration. However, when working with an unknown titer sample of virus, the results of this study suggest performing a MTT-based TCID50 in order to attain a general picture of the virus infectivity strength and the extent to which the virus should be diluted. The TCID50 information would enable the researcher to predict the number of plaques for any infective dilution of the virus.
In conclusion, use of the log2-based MTS or MTT end-point dilution reported here is concluded to increase reliability, sensitivity, and reproducibility of the EV71 titration analysis and reduce the time involved compared to the conventional method. Although established for titration of EV71, the improved method of TCID50 can also be used for other viruses to solve the common conceptual and mathematical problems of the traditional TCID50 and improve upon the colourimetric-based titration methods currently in use. Thus, it could play a key role in any viral experimentation that needs accurate, quick, and cost-effective titration of CPE-generating viruses. Notably, this improved method would be useful for viruses for which antiviral compounds are increasingly sought after, including EV71, as it is a requisite for researchers to ensure that a consistently accurate and quantified titre of the virus is used in any step of their research.
Acknowledgments
We wish to thank Professor Peter C. McMinn (Central Clinical School, University of Sydney, Australia) for providing the cloned virus and the cells and Dr Julian Druce (Polio reference laboratory, Victorian Infectious Disease Reference Laboratory, Parkville, VIC, Australia) for providing us with the clinical isolate used. We also thank Dr. Jillian Shaw for reading the manuscript.
References
- 1.Anderson P, Alm S, Edman K, Lindberg AM. A novel and rapid method to quantify cytolytic replication of picornaviruses in cell culture. J Virol Methods. 2005;130:117–123. doi: 10.1016/j.jviromet.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 2.Baylis AS, Shah N, Minor DP. Evaluation of different assays for the detection of parvovirus B19 DNA in human plasma. J Virol Methods. 2004;121:7–16. doi: 10.1016/j.jviromet.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Davis BD, Dulbecco R, Eisen HN, Ginsberg HS, Wood WB. Nature of viruses. In: Microbiology. New York: Harper and Row; 1972. p. 1044–1053.
- 4.Distefano DJ, Gould SL, Munshi S, Robinson DK. Titration of human-bovine rotavirus reassortants using a tetrazolium-based colorimetric end-point dilution assay. J Virol Methods. 1995;55:199–208. doi: 10.1016/0166-0934(95)00057-2. [DOI] [PubMed] [Google Scholar]
- 5.Dulbecco R, Vogt M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954;99:167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heldt CL, Hernandez R, Mudiganti U, Gurgel PV, Brown DT, Carbonell RG. A colorimetric assay for viral agents that produce cytopathic effects. J Virol Methods. 2006;135:56–65. doi: 10.1016/j.jviromet.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Huang C, Hung JJ, Wu CY, Chien MS. Multiplex PCR for rapid detection of pseudorabies virus, porcine parvovirus and porcine circoviruses. Vet Microbiol. 2004;101:209–214. doi: 10.1016/j.vetmic.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 8.LaBarre DD, Lowy RJ. Improvements in methods for calculating virus titer estimates from TCID50 and plaque assays. J Virol Methods. 2001;96:107–126. doi: 10.1016/S0166-0934(01)00316-0. [DOI] [PubMed] [Google Scholar]
- 9.Levi R, Beeor-Tzahar T, Arnon R. Microculture virus titration-a simple colourimetric assay for influenza virus titration. J Virol Methods. 1995;52:55–64. doi: 10.1016/0166-0934(94)00137-6. [DOI] [PubMed] [Google Scholar]
- 10.Lin YC, Wu CN, Shih SR, Ho MS. Characterization of a Vero cell-adapted virulent strain of enterovirus 71 suitable for use as a vaccine candidate. Vaccine. 2002;20:2485–2493. doi: 10.1016/S0264-410X(02)00182-2. [DOI] [PubMed] [Google Scholar]
- 11.Prikhod’ko GG, Vasilyeva I, Reyes H, Wong S, Brown KE, Jameson T, Busby TF. Evaluation of a new LightCycler reverse transcription-polymerase chain reaction infectivity assay for detection of human parvovirus B19 in dry-heat inactivation studies. Transfusion. 2005;45:1011–1019. doi: 10.1111/j.1537-2995.2005.04393.x. [DOI] [PubMed] [Google Scholar]
- 12.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Epidemiol. 1938;27:4931–4937. [Google Scholar]
- 13.Rubino KL, Nicholas JA. A novel, spectrophotometric microneutralization assay for respiratory syncytial virus. J Virol Methods. 1992;39:55–67. doi: 10.1016/0166-0934(92)90125-W. [DOI] [PubMed] [Google Scholar]
- 14.Schmidtke M, Schnittler U, Jahn B, Dahse HM, Stelzner A. A rapid assay for evaluation of antiviral activity against coxsackie virus B3, influenza virus A, and herpes simplex virus type 1. J Virol Methods. 2001;95:133–143. doi: 10.1016/S0166-0934(01)00305-6. [DOI] [PubMed] [Google Scholar]
- 15.Shih SR, Tsai MC, Tseng SN, Won KF, Shia KS, Li WT, Chern JH, Chen GW, Lee CC, Lee YC, Peng KC, Chao YS. Mutation in Enterovirus 71 capsid protein VP1 confers resistance to the inhibitory effects of pyridylimidazolidinone. Antimicrob Agents Chemother. 2004;48:3523–3529. doi: 10.1128/AAC.48.9.3523-3529.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe W, Konno K, Ijichi K, Inoue H, Yokota T, Shigeta S. MTT colorimetric assay system for the screening of anti-orthomyxo- and anti-paramyxoviral agents. J Virol Methods. 1994;48:257–265. doi: 10.1016/0166-0934(94)90124-4. [DOI] [PubMed] [Google Scholar]