Abstract
During 2006, pumpkin leaf curl—a new disease was observed in the experimental field at Indian Agricultural Research Institute. The disease was characterized by upward leaf curl with chlorotic patches and stunting of plant. Polymerase chain reaction (PCR) with coat protein specific primers to Tomato leaf curl New Delhi virus (ToLCNDV) indicated association of a begomovirus with the disease. The sequence comparison and phylogenetic analysis of the complete DNA genome further revealed the identity of the virus as ToLCNDV. The study provides evidence that ToLCNDV is associated with the leaf curl of pumpkin (Cucurbita moschata) in northern India.
Keywords: Tomato leaf curl New Delhi virus, Pumpkin leaf curl, Begomovirus
Tomato leaf curl New Delhi virus (ToLCNDV) (genus Begomovirus, family Geminivirdae) is the most important viral pathogen in tomato in India [25]; Varma et al. 2011) [26]. ToLCNDV a bipartite begomovirus containing DNA-A and DNA-B as its genome is transmitted by whitefly (Bemisia tabaci) and are known to infect a large number of host plants [13]. In 1950s–1970s, begomovirus disease in cucurbits was of minor importance in India. During this time, only pumpkin yellow vein mosaic disease was known to occur in central-western India [24]. Begomovirus has emerged as serious problem in several cucurbits since 1980s [10]. So far, only two different begomovirus species Squash leaf curl China virus (SLCCNV) [12] and ToLCNDV [9, 18] are known to affect cucurbits in India.
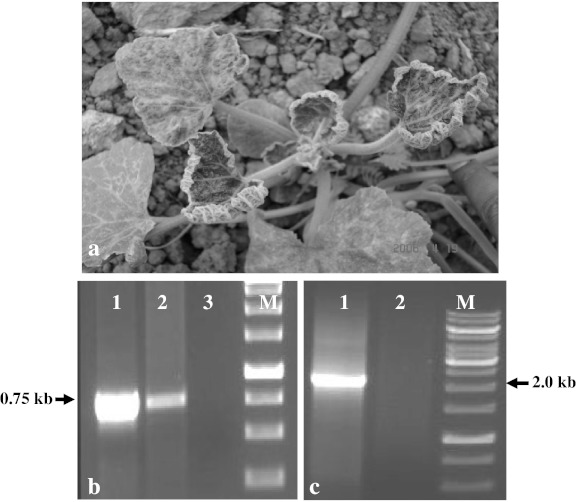
Pumpkin (Cucurbita moschata) is one of the important cucurbits widely cultivated in India under warm and humid conditions. Yellow vein mosaic was the only begomovirus associated disease known in pumpkin in India [12, 24]. SLCCNV has been shown to be associated with the yellow vein mosaic disease of pumpkin in both northern and southern India [11, 12, 15]. During 2006 cropping season (February-May), leaf curl, a new disease in pumpkin was observed in the experimental field at IARI, New Delhi. The disease was characterized by marginal rolling and curling of leaves and yellow patches on lamina (Fig. 1). The affected plants were stunted and produced less flowers and fruits. Electron microscopic study revealed association of geminate particles with the disease. The objective of this study was to characterize the causal virus associated with the pumpkin leaf curl disease.
Fig. 1.
Pumpkin plant showing leaf curl symptoms in the IARI experimental field. a Amplification of coat protein gene from pumpkin leaf curl sample (lane 1 +ve control ToLCNDV DNA-A clone, lane 2 pumpkin leaf curl, lane 3 negative control, lane M Marker). b Amplification of remaining part (2.0 kb) of the genome of DNA-A with the primers AV 111 and AV 112 from the leaf curl sample (lane 1 pumpkin leaf curl, lane 2 negative control, lane M marker)
Samples of pumpkin plants showing leaf curl were collected from the experimental field at IARI and total DNA was extracted using Plant Genomic DNA Miniprep Purification Spin Kit (Himedia, India). Based on symptoms and electron microscopic observations, begomovirus was suspected to be associated with the disease. Therefore, attempt was made to amplify the virus by polymerase chain reaction (PCR) with coat protein (CP) gene specific primers of ToLCNDV (AV30F:5′ttggatccatggcgaagcgacca3′ and AV31R:5′aagagctcttaatttgtgaccga3′). The PCR conditions for amplification were denaturation at 94°C for 5 min, followed by 30 cycles each consists of denaturation for 1 min at 94°C, annealing for 1 min at 48°C and synthesis for 1 min at 72°C with final extension for 10 min at 72°C. The PCR resulted in successful amplification of ~750 bp DNA fragment from the symptomatic samples, whereas no such amplification was obtained from healthy pumpkin seedlings. The 750 bp fragment was cloned into pGEM-T Easy vector and sequenced, which revealed close identity with the CP gene of ToLCNDV. Based on this sequence, new primers, AV111 5′ggccggcaagtatgagaa3′ and AV112 5′tgctggtcgcttcgccat3′ were designed to amplify the remaining portion (2.0 kb) of DNA-A genome (Fig. 1b, c). The sequences from both the amplicons (0.75 kb & 2.0 kb) were joined and the complete DNA-A sequence of the begomovirus associated with pumpkin leaf curl was determined to be 2740 bp. The genome structure was determined by analyzing using ORF Finder (www.ncbi.nlm.nih.gov/gorf/gorf.html). The nucleotide (nt) and amino acid (aa) sequence analysis was conducted using the clustalW program (www.ebi.ac.uk). Phylogenetic tree was constructed using MEGA 4.0 software (http://www.megasoftware.net/features.html). The evolutionary history was inferred using the Maximum Parsimony method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) are shown next to the branches. The MP tree was obtained using the Close-Neighbor-Interchange algorithm with search level 3 in which the initial trees were obtained with the random addition of sequences (10 replicates). All positions containing gaps and missing data were eliminated from the dataset (Complete Deletion option). There were a total of 2,620 positions in the final dataset, out of which 1,145 were parsimony informative.
The genome organization of the DNA-A was similar to that of all other begomoviruses containing four ORFs on the complementary strand (AC1; 1,499–2,674, AC2; 1,177–1,596, AC3; 1,047–1,457, and AC4; 2,251–2,427) and two ORFs on the viral sense strand (AV1; 280–1,050 and AV2; 120–458). The AV1 (CP) ORF was 771 nt long encoding 28.5 kDa coat protein. AV2 was 339 nt long encoding 12.5 kDa protein. The AC1 was 1,176 nt long encoding 43.5 kDa replication associated protein (Rep). AC2 was 420 nt long encoding (15.5 kDa) transcription activator protein. The AC3 was 411 nt long encoding 15.2 kDa replication enhancer protein. The AC4 was 177 nt long encoding 6.5 kDa. The ORFs were separated by 195 nt long intergenic region (IR) that contained a predicted stem-loop sequence with conserved mononucleotides TAATATTAC in the loop, which can be found in all geminiviruses characterized to date and marks the origin of virion-strand DNA replication. Within the IR, incomplete direct repeats of an iteron sequence GGTGTC at nucleotides 2,631–2,637 were detected.
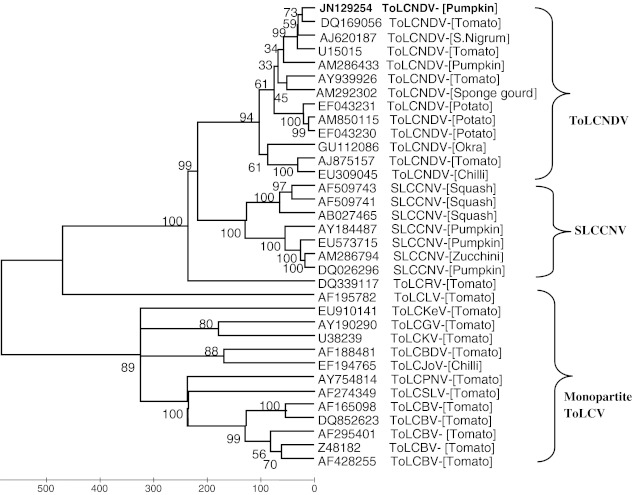
Sequence similarity were analysed by comparing the sequence of other begomoviruses in the database (http://www.ncbi.nlm.nih.gov/nucleotide). A total of 33 isolates of tomato and cucurbits infecting begomoviruses were used for the analysis. The complete DNA-A sequence shared maximum nucleotide sequence identity of 98.1% with ToLCNDV-Tai; (DQ 169056). The IR had the highest sequence identity of 99.6% with ToLNDV-Svr (U15015). When individual ORFs encoding proteins were compared, they were found to have sequence identity of 94.8- 99.2% with the isolate of ToLCNDV-ND. The Phylogenetic tree based on the complete nucleotide sequences of DNA-A showed that, the virus isolate from pumpkin clustered with 12 ToLCNDV isolates (Fig. 2). Based on the current convention [2] the present isolate description is ToLCNDV-[Pump: IARI: 06].
Fig. 2.
Parsimonious tree of ToLCNDV-[Pum: IARI: 06] and other related begomovirus isolates based on complete DNA-A sequence. The phylogenetic tree was constructed using MEGA 4.0 at 500 bootstrap values. The numbers at the nodes indicate bootstrap values (%). The begomovirus acronyms used are: ToLCNDV: Tomato leaf curl New Delhi virus, ToLCRV: Tomato leaf curl Rajasthan virus, ToLCGV: Tomato leaf curl Gujarat virus, ToLCBV: Tomato leaf curl Bangalore virus, ToLCKV: Tomato leaf curl Karnataka virus, ToLCBDV: Tomato leaf curl Bangladesh virus, ToLCLV: Tomato leaf curl Laos virus, ToLCSLV: Tomato leaf curl Sri Lanka virus, ToLCPNV: Tomato leaf curl Pune virus, ToLCKeV: Tomato leaf curl Kerala virus, ToLCJoV: Tomato leaf curl Joydebpur virus, SLCCNV: Squash leaf curl China virus
ToLCNDV is an emerging problem in numerous crops and widely distributed in India, Pakistan, Philippines and Thailand [8, 10, 21]. Although, ToLCNDV is a major viral pathogen in solanaceous vegetables [4, 7, 13, 14], it has emerged in several cucurbitaceous vegetables, bottle gourd, bitter gourd, cucumber, ivy gourd, long melon, pumpkin, ridge gourd and watermelon in northern India and chayote in north-western India [9, 19, 20, 22]. In Thailand, ToLCNDV has been reported to infect bottle gourd, cucumber and muskmelon [6]. In USA, leaf curl of squash (Cucurbita pepo) is caused by Squash leaf curl virus [1, 3]. In China and Philippines [5, 8], squash leaf curl was reported to be caused by another begomovirus species, Squash leaf curl China virus (SLCCNV). In India, SLCCNV was identified to cause yellow vein mosaic disease in pumpkin [16]. The present study for the first time established association of ToLCNDV with the pumpkin leaf curl based on complete DNA-A sequence. ToLCNDV (DNA A and B) was shown to cause severe leaf curl in tomato in association with betasatellite [17, 23]). In the present study, association of DNA-B or betasatellite with the disease was not demonstrated. Under field conditions, we have observed yellow vein mosaic and leaf curl symptoms in the same pumpkin plant resulting severe form of disease in the experimental field at IARI in successive years [26]. It is possible that two different begomoviruses (SLCCNV and ToLCNDV) along with betasatellite may cause severe disease in pumpkin which needs further investigation. The previous and present studies further show that ToLCNDV has emerged in several cucurbits in northern India. The leaf curl disease in pumpkin has been observed in the successive years in experimental field at IARI, however its prevalence in the farmers field needs further investigation.
Acknowledgment
Financial support by Indian Council of Agricultural Research (ICAR) is thankfully acknowledged.
References
- 1.Cohen S, Duffus JE, Larsen RC, Liu HY, Flock RA. Purification, serology, and vector relationships of squash leaf curl virus, a whitefly-transmitted geminivirus. J Phytopathol. 1983;73:1669–1673. doi: 10.1094/Phyto-73-1669. [DOI] [Google Scholar]
- 2.Fauquet CM, Briddon RW, Brown JK, Moriones E, Stanley J, Zerbini M, Zhou X. Geminivirus strain demarcation and nomenclature. Arch Virol. 2008;153:783–821. doi: 10.1007/s00705-008-0037-6. [DOI] [PubMed] [Google Scholar]
- 3.Flock RA, Mayhew DE. Squash leaf curl, a new disease of cucurbits in California. Plant Dis. 1981;65:75–77. doi: 10.1094/PD-65-75. [DOI] [Google Scholar]
- 4.Garg ID, Paul-Khurana SM, Kumar S, Lakra BS. Association of a geminivirus with potato apical leaf curl in India and its immuno-electron microscopic detection. J Indain Potato Assoc. 2001;28:227–232. [Google Scholar]
- 5.Hong Y, Wang X, Tian B, Cai J. Chinese squash leaf curl virus: a new whitefly-transmitted geminivirus. Sci China. 1995;B38:179–186. [PubMed] [Google Scholar]
- 6.Ito T, Sharma P, Kittipakorn K, Ikegami M. Complete nucleotide sequence of a new isolate of tomato leaf curl New Delhi virus infecting cucumber, bottle gourd and muskmelon in Thailand. Arch Virol. 2008;153:611–613. doi: 10.1007/s00705-007-0029-y. [DOI] [PubMed] [Google Scholar]
- 7.Khan MS, Raj SK, Singh R. First report of tomato leaf curl New Delhi virus infecteing chilli in India. Plant Pathol. 2006;55:289. doi: 10.1111/j.1365-3059.2006.01324.x. [DOI] [Google Scholar]
- 8.Kon T, Dolores M, Bajet NB, Hase S, Takahashi H, Ikegami M. Molecular characterization of a strain of squash leaf curl China virus from the Philippines. J Plant Pathol. 2003;15:535–539. [Google Scholar]
- 9.Mandal B, Mandal S, Sohrab SS, Pun KB, Varma A. A new yellow mosaic disease of chayote in India. Plant Pathol. 2004;53:797. doi: 10.1111/j.1365-3059.2004.01075.x. [DOI] [Google Scholar]
- 10.Mandal B. Emerging geminiviral diseases and their management. In: Sharma Pradeep, Gaur Rajarshi K, Ikegami Masato., editors. Emergence of begomovirus diseases in cucurbits in India. New York: Nova Science Publishers Inc; 2010. pp. 167–181. [Google Scholar]
- 11.Maruthi MN, Rekha AR, Muniyappa V. Pumpkin yellow vein mosaic disease is caused by two distinct begomoviruses: complete viral sequences and comparative transmission by an indegenoius Bemisia tabaci and the introduced B-biotype. Bull OEPP/EPPO Bull. 2007;37:412–419. doi: 10.1111/j.1365-2338.2007.01127.x. [DOI] [Google Scholar]
- 12.Muniyappa V, Maruthi MN, Babiha CR, Colvin J, Briddon RW, Rangaswamy KT. Characterisation of pumpkin yellow vein mosaic virus from India. Ann Appl Bio. 2003;142:323–331. doi: 10.1111/j.1744-7348.2003.tb00257.x. [DOI] [Google Scholar]
- 13.Padidam M, Beachy RN, Fauquet CM. Tomato leafcurl geminivirus from India has a bipartite genome and coat protein is not essential for infectivity. J GenVirol. 1995;76:249–263. doi: 10.1099/0022-1317-76-2-249. [DOI] [PubMed] [Google Scholar]
- 14.Reddy CRV, Colvin J, Munniyappa V, Seal S. Diversity and distribution of begomoviruses infecting tomato in India. Arch Virol. 2005;150:845–867. doi: 10.1007/s00705-004-0486-5. [DOI] [PubMed] [Google Scholar]
- 15.Singh AK, Mishra KK, Chattopadhyay B, Chakraborty S. Biological and molecular characterization of a begomovirus associated with yellow mosaic vein mosaic disease of pumpkin from Northern India. Virus Genes. 2009;39:359–370. doi: 10.1007/s11262-009-0396-4. [DOI] [PubMed] [Google Scholar]
- 16.Singh R, Raj SK, Prasad V. Molecular characterization of a strain of squash leaf curl China virus from North India. J Phytopathol. 2008;156:222–228. doi: 10.1111/j.1439-0434.2007.01347.x. [DOI] [Google Scholar]
- 17.Sivalingam PN. Characterization of begomoviruses affecting tomato in India. Ph.D. Thesis submitted to Indian Agricultural Research Institute, New Delhi-12; 2006. p. 142.
- 18.Sohrab SS, Mandal B, Pant RP, Varma A. First reports of association of Tomato leaf curl New Delhi virus with yellow mosaic disease of Luffa cylindrica in India. Plant Dis. 2003;87:1148. doi: 10.1094/PDIS.2003.87.9.1148A. [DOI] [PubMed] [Google Scholar]
- 19.Shorab SS, Mandal B, Ali A, Varma A. Molecular diagnosis of emerging begomovirus diseases in cucurbits occurring in northern India. Indian J Virol. 2006;17:88–95. [Google Scholar]
- 20.Sohrab SS, Mandal B, Ali A, Varma A. Chlorotic curly stunt: a severe begomovirus disease of bottle gourd in Northern India. Indian J Virol. 2010;21:56–63. doi: 10.1007/s13337-010-0002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tahir M, Haider MS. First report of Tomato leaf curl New Delhi virus infecting bitter gourd in Pakisatn. Plant Pathol. 2007;54:807. doi: 10.1111/j.1365-3059.2005.01215.x. [DOI] [Google Scholar]
- 22.Tiwari AK, Sharma PK, Khan MS, Snehi SK, Raj SK, Rao GP. Molecular detection and identification of Tomato leaf curl New Delhi virus isolate causing yellow mosaic disease in bitter gourd (Momordica charantia), a medicinally important plant in India. Med Plants. 2010;2:117–123. [Google Scholar]
- 23.Usahrani KS, Srivastava A, Padmalatha KV, Malathi VG. First report of the association of a defective satellite DNA β molecule with a bipartite genome begomovirus causing potato leaf curl disease in India. J Plant Pathol. 2004;86:177–180. [Google Scholar]
- 24.Varma PM. Ability of the whitefly to carry more than one virus simultaneously. Curr Sci. 1955;24:317–318. [Google Scholar]
- 25.Varma A, Malathi VG. Emerging geminivirus problems: a serious threat to crop production. Ann Appl Biol. 2003;142:145–164. doi: 10.1111/j.1744-7348.2003.tb00240.x. [DOI] [Google Scholar]
- 26.Varma A, Mandal B, Singh MK. Global emergence and spread of whitefly (Bemisia tabaci) transmitted geminiviruses. In: Thompson WMO, editors. The whitefly, Bemisiatabaci (Homoptera: Aleyrodidae) interaction with geminivirus-infected host plants. Springer; 2011. p. 205–292. doi:10.1007/978-94-007-1524-0_10.