Abstract
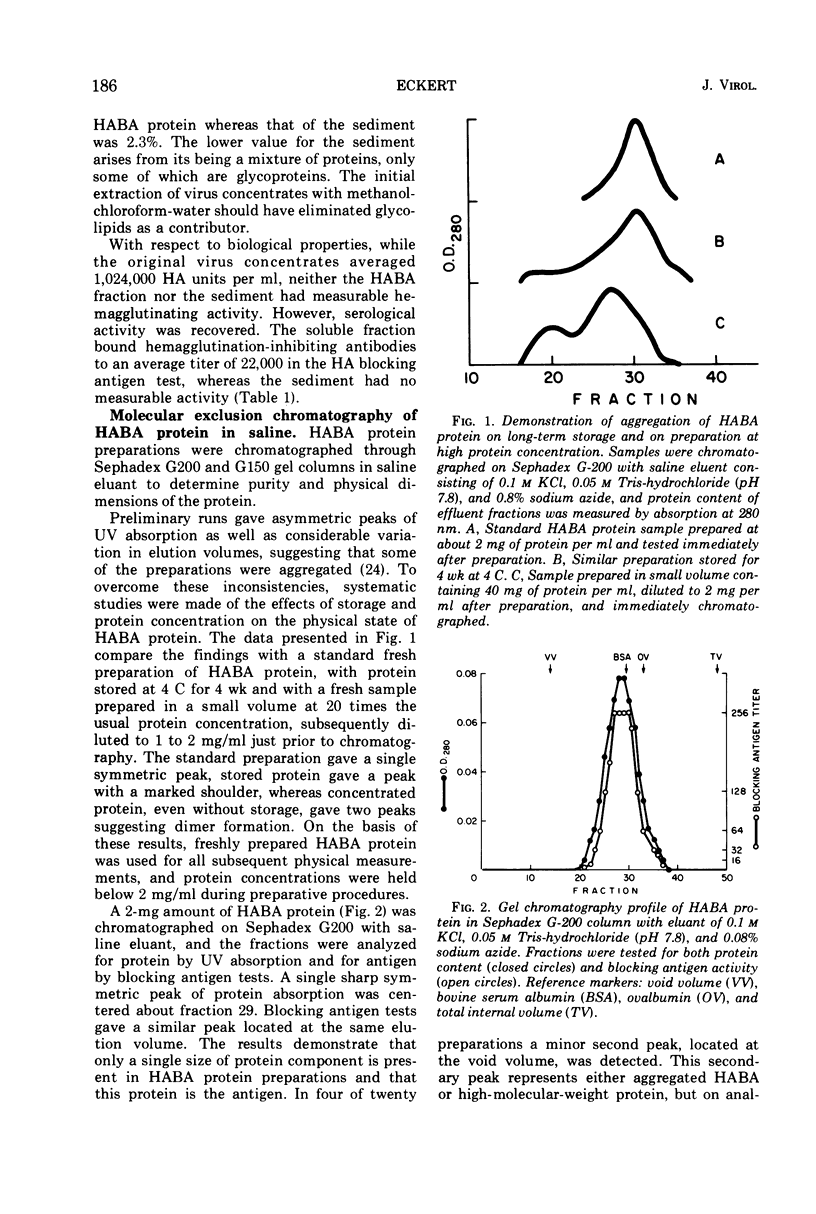
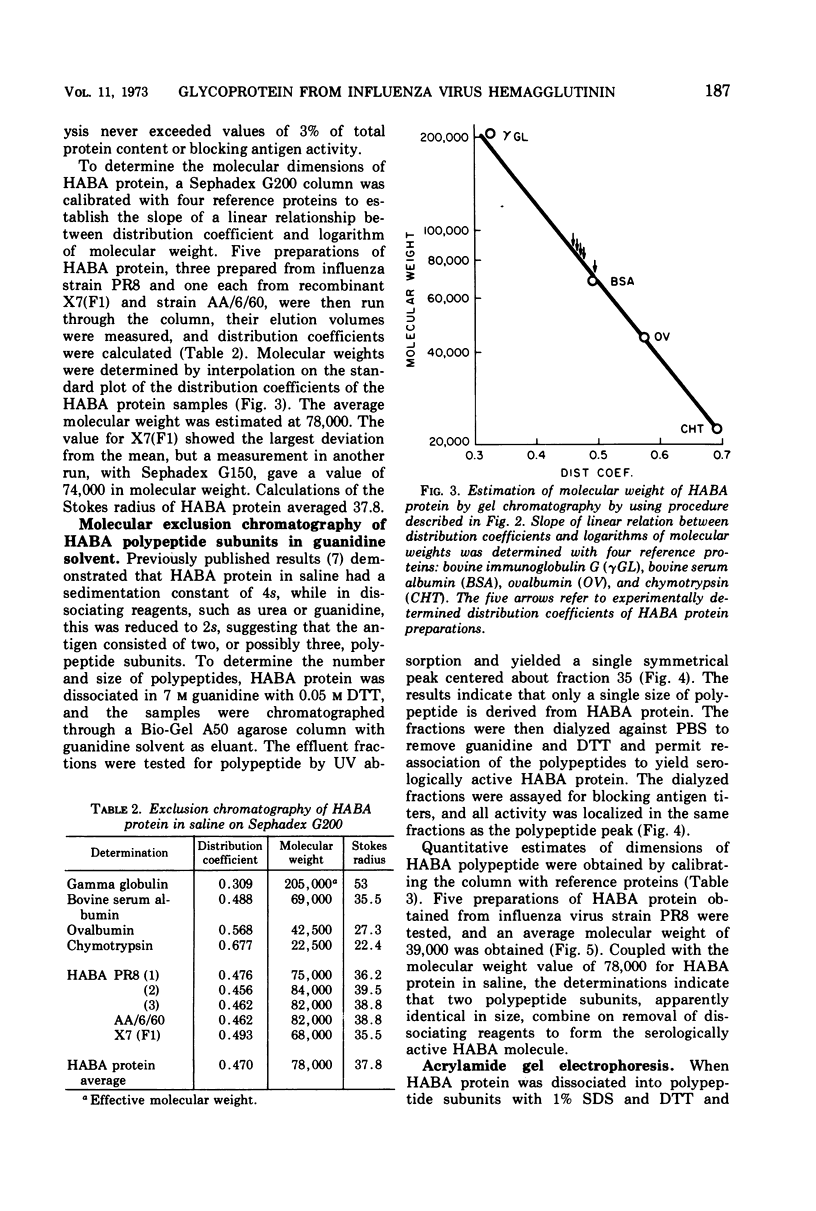
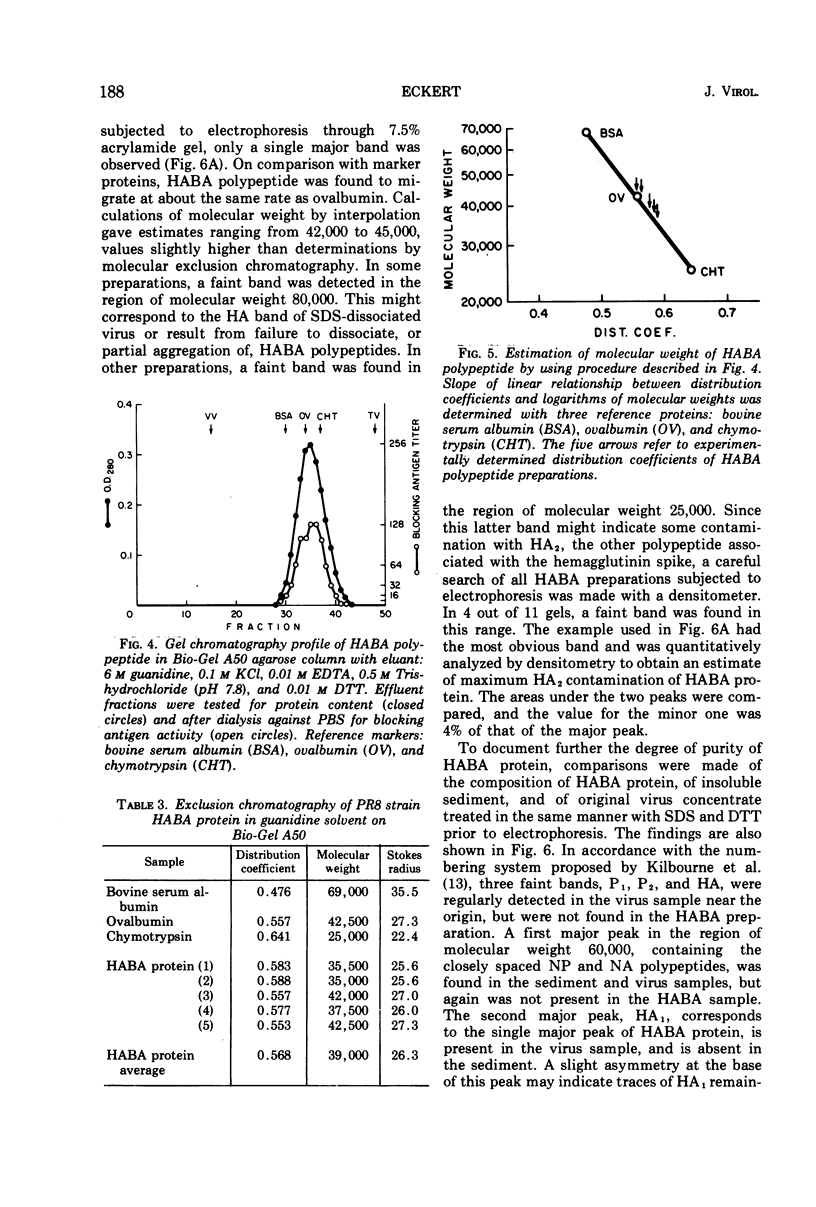
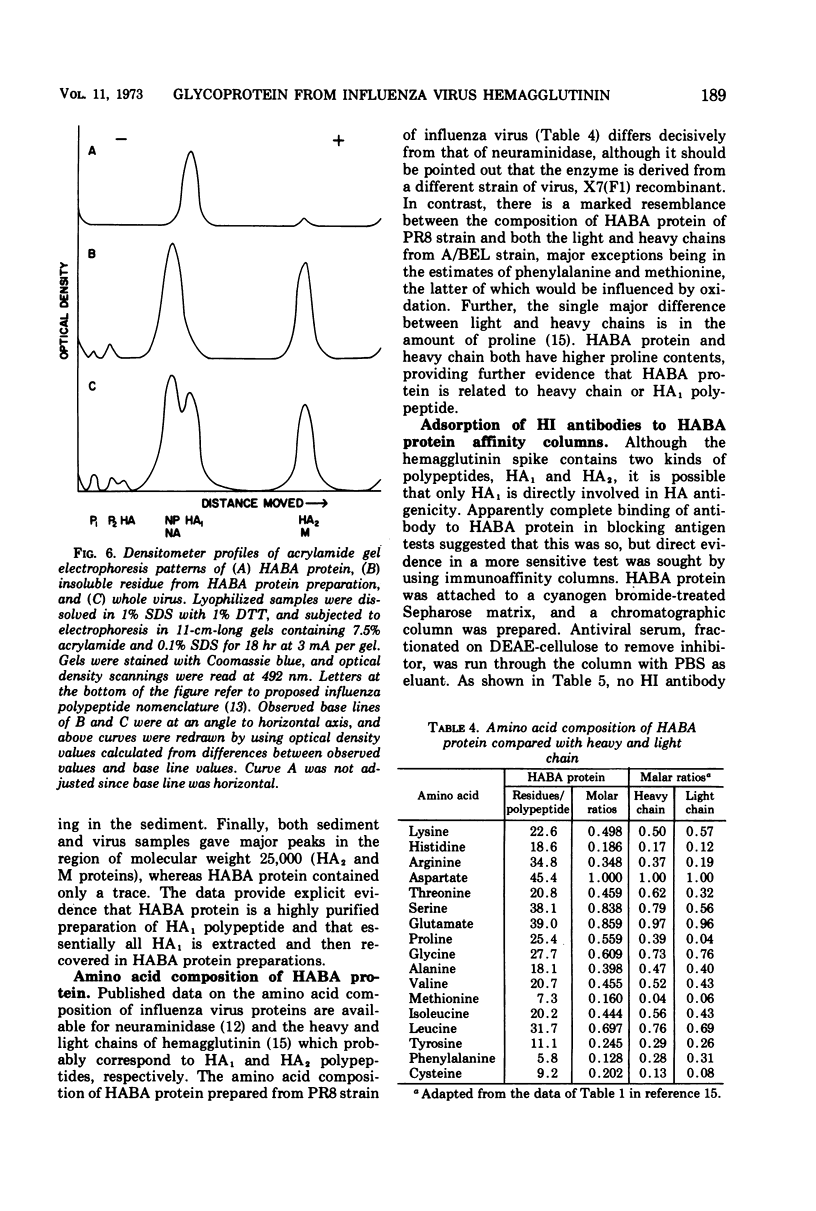
A purified antigen, HABA protein, has been derived from influenza virus concentrates by extraction with denaturing solvents. The protein lacks hemagglutinating activity but binds completely strain-specific, hemagglutination-inhibiting antibodies and induces neutralizing antibodies in experimental animals. Physicochemical characterization of HABA protein identifies it as a single homogeneous glycoprotein with a molecular weight of 78,000. On dissociation with guanidine or sodium dodecyl sulfate, in the presence of reducing agents, only one size of polypeptide with a molecular weight of the order of 40,000 is characteristic of the preparations. The data indicate that HABA protein is a dimer of HA1 polypeptide of the influenza virus hemagglutinin substructure, and that only trace amounts of other polypeptides are present.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Brand C. M., Skehel J. J. Crystalline antigen from the influenza virus envelope. Nat New Biol. 1972 Aug 2;238(83):145–147. doi: 10.1038/newbio238145a0. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Klenk H. D., Caliguiri L. A., Choppin P. W. Influenza virus proteins. I. Analysis of polypeptides of the virion and identification of spike glycoproteins. Virology. 1970 Dec;42(4):880–889. doi: 10.1016/0042-6822(70)90337-5. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Wilchek M., Anfinsen C. B. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A. 1968 Oct;61(2):636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert E. A. Characterization of a low molecular weight antigenic protein from the envelope of influenza virus. J Bacteriol. 1966 Nov;92(5):1430–1434. doi: 10.1128/jb.92.5.1430-1434.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert E. A. The subunit structure of influenza virus envelope protein. J Immunol. 1969 Apr;102(4):1105–1107. [PubMed] [Google Scholar]
- Fish W. W., Reynolds J. A., Tanford C. Gel chromatography of proteins in denaturing solvents. Comparison between sodium dodecyl sulfate and guanidine hydrochloride as denaturants. J Biol Chem. 1970 Oct 10;245(19):5166–5168. [PubMed] [Google Scholar]
- Kendal A. P., Eckert E. A. The preparation and properties of 14 C-carboxamidomethylated subunits from A 2 -1957 influenza neuraminidase. Biochim Biophys Acta. 1972 Feb 28;258(2):484–495. doi: 10.1016/0005-2744(72)90240-9. [DOI] [PubMed] [Google Scholar]
- LEVY H. B., SOBER H. A. A simple chromatographic method for preparation of gamma globulin. Proc Soc Exp Biol Med. 1960 Jan;103:250–252. doi: 10.3181/00379727-103-25476. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laver W. G. Separation of two polypeptide chains from the hemagglutinin subunit of influenza virus. Virology. 1971 Jul;45(1):275–288. doi: 10.1016/0042-6822(71)90134-6. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Webster R. G. Selection of antigenic mutants of influenza viruses. Isolation and peptide mapping of their hemagglutination proteins. Virology. 1968 Feb;34(2):193–202. doi: 10.1016/0042-6822(68)90230-4. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968 Sep;36(1):115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. Binding of dodecyl sulfate to proteins at high binding ratios. Possible implications for the state of proteins in biological membranes. Proc Natl Acad Sci U S A. 1970 Jul;66(3):1002–1007. doi: 10.1073/pnas.66.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Darnell J. E. Carbohydrate content of the membrane protein of Sindbis virus. J Mol Biol. 1970 Feb 14;47(3):437–448. doi: 10.1016/0022-2836(70)90313-x. [DOI] [PubMed] [Google Scholar]
- Teipel J. W., Koshland D. E., Jr Kineticsspects of conformational changes in proteins. II. Structural changes in renaturation of denatured proteins. Biochemistry. 1971 Mar 2;10(5):798–805. doi: 10.1021/bi00781a012. [DOI] [PubMed] [Google Scholar]
- Webster R. G. Estimation of the molecular weights of the polypeptide chains from the isolated hemagglutinin and neuraminidase subunits of influenza viruses. Virology. 1970 Mar;40(3):643–654. doi: 10.1016/0042-6822(70)90209-6. [DOI] [PubMed] [Google Scholar]



