Abstract
Rotavirus is the leading cause of acute gastroenteritis in worldwide young children. Effective vaccines to prevent rotavirus infection are currently available, although their clinical use is still limited, and rotavirus still causes many episodes of infantile gastroenteritis, mainly during the winter season. The aim of this study was to evaluate the prevalence of rotavirus infection in children aged <5-years-old who were hospitalised for gastroenteritis. One hundred and sixty-three stool samples from hospitalised children (<5-years-old) complicated with severe diarrhoea, in two hospitals in Jahrom City, Iran were collected from 2009 to 2010. Antigenic prevalence of rotavirus group A was distinguished by enzyme immunoassay. The antigen of group A rotavirus was diagnosed by EIA in 75 of 163 collected samples. The genotype of EIA-positive samples was determined by nested RT-PCR. The frequency of rotavirus genotypes G1, G2, G3, G4 and G9 was 17.33, 13.34, 2.67, 30.66 and 2.67 %, respectively. Also, the frequency of mixed and non-typable genotypes was detected in 2.67 and 30.66 %, respectively. G1/G8 mixed infection was the first of these rotavirus genotypes to be reported in Iran. Detection of high prevalence of group A rotavirus infection in hospitalised children with diarrhoea, and determination of circulating rotavirus genotypes in this region of Iran, provide useful data for formulating effective vaccines; especially for infants less than 5-years-old.
Keywords: Rotavirus, Prevalence, Molecular genotyping, RT-PCR
Introduction
Diarrhoea is still a major public health problem worldwide, especially in children. In developing countries, 12 or more diarrhoea episodes are estimated to occur per child per year within first 5 years of life. Different aetiological agents, including bacteria, parasites and especially viruses that have been intensively studied in recent years are related to acute diarrhoea [1]. Group A rotaviruses are the one of the most important causes of severe acute diarrhoea in young children worldwide.
Hospital-based studies have revealed that group A rotavirus infection is the cause of acute diarrhoea in 20–70 % of children <5-years-old in developed and developing countries, and leads to death in approximately 800,000 children annually in developing countries [2, 3]. Bishop et al. identified rotavirus for the first time in humans in 1973 when they observed characteristic rotavirus particles in the cytoplasm of duodenal epithelial cells from young children who had been admitted to hospital for acute diarrhoea [1].
The genus Rotavirus belongs to the family of Reoviridae and, according to the antigenic and genetic variants of the VP6 region, can be divided into seven groups named A–G. The majority of human infections are caused by viruses in group A, which can further be differentiated into 23G- and 32P-type, respectively, by VP7 and VP4 antigens [4, 5]. Knowledge of the distribution of G and P genotypes, including detection of emerging genotypes, is crucial to rotavirus vaccination programmes [6]. G1–G4 are the most common worldwide genotypes of rotaviruses [7]. The capacity of the first-generation of rotavirus vaccines to provide heterotypic protection against other G-types is unclear [8].
The epidemiology of rotavirus infection is complex and varies depending on laboratory setting. Use of newly optimised molecular techniques has increased sensitivity of detection and characterisation of unusual rotavirus strains apart from commonly described genotypes [9]. Results of earlier studies in Iran have shown that rotavirus is a major aetiological agent of acute diarrhoea in infants and young children [10]. Based on earlier findings, the prevalence of different genotypes of group A rotavirus that are circulating in Iran, and detection of uncommon and novel types of rotavirus were evaluated in this study.
Materials and Methods
Sampling
From November 2009 to October 2010, a total of 163 stool specimens were collected from children aged <5-years-old who were hospitalised with gastroenteric symptoms in Motaharri and Paimanie Hospitals in Fars Province, Iran.
Rotavirus antigen detection
Rotavirus antigens were detected in stool specimens by a solid-phase sandwich-type enzyme immunoassay method (IDEIA Rotavirus; Dako, Glostrup, Denmark).
RT-PCR
Rotavirus-positive specimens were categorised into strain G genotype by RT-PCR, as described by Gouvea et al. [11]. Rotavirus dsRNA was extracted from stool specimens by standard phenol–chloroform extraction method [11]. The extracted dsRNA was used in G typing by type-specific primers for RT-PCR. Consensus primers Beg9 and End9 were used in the first round of PCR (30 cycles) to amplify the full-length VP7 gene (1,062 bp). In the second round of PCR, cDNA was used for G typing (25 cycles) with primer sets including: aBT1 (G1), aCT2 (G2), aET3 (G3), aDT4 (G4), cFT5 (G5), aAT8 (G8) and aFT9 (G9) [11, 12]. All PCR products were analysed by electrophoresis in 1.2 % agarose gels that contained 0.5 μg/ml ethidium bromide, and visualised under UV illumination.
Statistical Analysis
Data were statistically analysed by SPSS version 14. P < 0.05 was considered statistically significant.
Results and Discussion
Serology of Rotavirus Infection
A total of 163 stool samples were collected from children aged < 5-years-old. All children had diarrhoea for a period of 1–5 days before hospitalization. Rotavirus was detected in 75 of 163 (46.02 %) stool specimens analysed for the presence of group A rotavirus antigen by EIA.
Rotavirus and Demographic Data
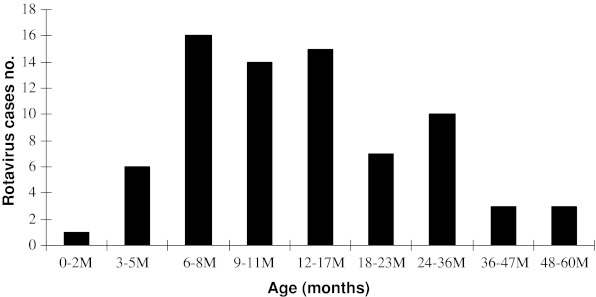
Rotavirus was detected more often in boys (28.83 %) than in girls (17.18 %). Forty-five of 75 (60 %) patients with rotavirus gastroenteritis were <2 years of age. Detection rate of rotavirus was the highest among infants aged 6–23 months old (Fig. 1).
FIG. 1.
Age distribution of rotavirus diarrhoea during study period from October 2009–November 2010
There was no significant sex difference (P > 0.05) between children with or without rotavirus gastroenteritis. Rotavirus was detected throughout the year but relative frequency of rotavirus gastroenteritis was highest in winter. The seasonal distribution of rotavirus infection was as follow: 6.13 % in spring, 4.29 % in summer, 12.88 % in autumn, and 22.69 % in winter. A significant relationship was also found between rotavirus infection and seasonal distribution (P < 0.05).
Rotavirus Genotyping
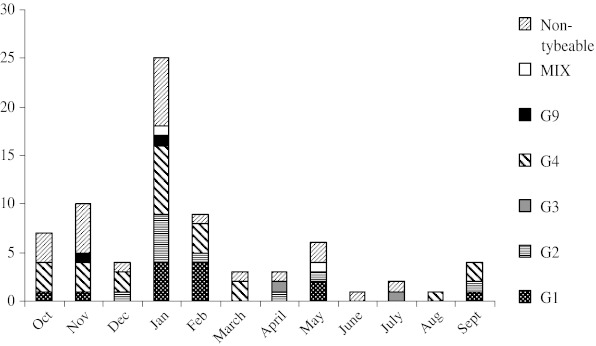
From different studied genotypes of VP7 gene of group A rotaviruses by nested-RT-PCR primer genotyping protocol, the G4 strain of group A rotavirus was the predominant genotype detected in 23 of 75 specimens (30.66 %). The prevalence of G1, G2, G3, G9 and non-typable genotypes of VP7 gene in children with rotavirus gastroenteritis was: 13 (17.33 %), 10 (13.34 %), two (2.67 %), two (2.67 %) and 23 (30.66 %), respectively. The G5 and G8 genotypes were not detected in all rotavirus-infected children. Mixed G1/G8 and G1/G2 genotypes of group A rotavirus were diagnosed in 1.33 % of these patients after confirmation of sequencing. The G1/G8 mixed genotype of rotavirus was reported for the first time in Iran. Detection rate of rotavirus genotypes was highest in January (33.34 %) and lowest in November (13.33 %), compared with other months (P > 0.05) (Fig. 2).
FIG. 2.
The seasonal distribution of different rotavirus genotypes
Many studies have shown that rotavirus is an important cause of diarrhoea in children in developed and developing countries [13]. Most cases occur in children less than 5 years of age. The prevalence of rotavirus in children with diarrhoea varies from 30 to 50 % [14]. From 2009 to 2010, consecutive surveillance of rotavirus diarrhoea in children under 5 years of age in Jahrom, Iran showed a prevalence of 46 %, which is similar to other reports [1, 15]. In the present study, a clear seasonal pattern in rotavirus diarrhoea was seen. Although rotavirus is detected throughout the year, there were obvious seasonal trends and regional variations in the incidence of rotavirus infection in the present study. In general, the incidence peak of rotavirus diarrhoea is in autumn and winter in different regions of countries evaluated [16]. These findings have been reported in Iran, Spain, America, Canada, Japan and Vietnam [1, 17–19]. In common with other investigations [20], the peak season of rotavirus diarrhoea was between November and January in the present study. Also, the highest rate of rotavirus infection was seen in children between 6 and 23 months of age, which was similar to other studies [16, 21]. In the present study, >60 % of human rotavirus infection was cumulatively found in infants younger than 2-years-old. Rotavirus age distribution was related to the peak incidence of infection, decline in maternal antibodies, and immaturity of new passive immune responses. Worldwide genetic diversity of circulating rotavirus strains is associated with presentation of new emerging strains, which are a cause of variability in the geographical distribution of the virus. The present study confirms the role of human rotavirus as an important enteropathogenic microbial agent and highlights the high prevalence of G1–G4 and G9 strains in Iranian paediatric patients. These findings are similar to epidemiological data described in Australia, Europe and North America, and differ from those observed in developing countries [7, 22, 23].
The G1 and G2 rotavirus genotypes have been detected in other Iranian studies [24]. The highest frequency of G1 strains has been reported in recent studies that have focused on genotyping of rotavirus VP7 gene in Iran and other countries [18]. Also the predominance of G4 strain of rotavirus was seen in the study period, which agrees with the results of VP7 gene typing in other geographical regions including Bangladesh, France and Malaysia [25, 26]. The G3 type of rotavirus was found in only 2.66 % of children with gastroenteritis, which is comparable with the results of previous studies in Iran [18].
In recent years, there has been an increase in research of the importance of G9 genotype in many countries including Iran, United States, Australia, Japan, France and Ireland [18, 27]. In the present study only, one G9 genotype isolate was detected. However, other investigations have demonstrated the need for new generations of rotavirus vaccines to include G9 strains, as a result of the increasing emergence of this type of group A rotavirus. In the present study, the G8 genotype that was first detected in Indonesia [28] was diagnosed in mixed infection with G1 genotype. Furthermore, we documented the first case of G1/G8 mixed infection in Iran. Also, the first reports of G1/G8 mixed infection have been documented in young children with gastroenteritis in Ireland and Nigeria [29, 30]. We found mixed infections with two different rotavirus genotypes in 2.66 % of children, which is similar to previous studies, with a range of 1–26.4 % [31]. These mixed infections occur more frequently in areas with high incidence of rotavirus infection.
The pattern of rotavirus mixed infections detected in the present investigation (G1/G2 and G1/G8) differed from other studies (G9/G3 and G9/G1) [28]. Mixed infection with different rotavirus strains might reflect frequent contamination of water resources with rotavirus strains, and could facilitate generation of novel rotavirus strains through reassortment. Therefore, the frequency of mixed infection of rotaviruses and its influence against efficacy of rotavirus vaccine should be completely investigated. Detection of non-typeable rotavirus strains might be due to the accumulation of segment mutation in the VP7 gene of rotavirus. These rotavirus strains are rarely detected in other investigations [16]. In the present study, the non-typable strains of rotavirus might represent other genotypes such as G10 and G12.
Conclusion
Diagnosis of high seroprevalence of group A rotavirus infection in hospitalised patients with diarrhoea, and genotyping of single and co-infection rotavirus isolates, provide useful data for designing new effective vaccines, especially for infants aged < 5-years-old.
Contributor Information
Mohammad Kargar, Phone: +989173149203, Email: mkargar@jia.ac.ir.
Amin Reza Akbarizadeh, Email: pharmicrob@sums.ac.ir.
References
- 1.Nguyen TV, Le Van Ph, Le Huy C, Weintraub A. Diarrhaea caused by rotavirus in children less than 5 years of age in Hanoi, Vietnam. J Clin Microbiol. 2004;42:5745–5750. doi: 10.1128/JCM.42.12.5745-5750.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim SY, Goldie SJ, Salomon JA. Cost-effectiveness of rotavirus vaccination in Vietnam. BMC Public Health. 2009;21(9):29. doi: 10.1186/1471-2458-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bioshop RF, Masendycz PJ, Bugg HC, Carlin JB, Baranes GL. Epidemiological patterns of rotavirus causing severe gastroenteritis in young children throughout Australia from 1993 to 1996. J Clin Microbiol. 2001;39:1085–1091. doi: 10.1128/JCM.39.3.1085-1091.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez-Laso J, Román A, Head J, Cervera I, Rodriguez M, Rodriguez-Avial I, Picazo JJ. Phylogeny of G9 rotavirus genotype: a possible explanation of its origin and evolution. J Clin Virol. 2009;44:52–57. doi: 10.1016/j.jcv.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 5.Adlhoch C, Kaiser M, Hoehne M, Mas Marques A, Stefas I, Ceas F, Ellebrok H. Highly sensitive detection of the group A rotavirus using apoliporotein H-coated ELISA plates compared to quantitative real-time PCR. Virol J. 2011;8:63. doi: 10.1186/1743-422X-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakagomi T, Cuevas LE, Gurgel RG, Elrokhsi SH, Belkhir YA, Abugalia M, Dove W, Montenegro FM. Apparent extinction of non-G2 rotavirus strains from circulation in Recife, Brazil, after the introduction of rotavirus vaccine. Arch Virol. 2008;153:591–593. doi: 10.1007/s00705-007-0028-z. [DOI] [PubMed] [Google Scholar]
- 7.Santos N, Hoshino Y. Global distribution rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev Med Virol. 2005;15:29–56. doi: 10.1002/rmv.448. [DOI] [PubMed] [Google Scholar]
- 8.Rahman M, Matthijnssens J, Goegebuer T, De Leener K, Vanderwegen L, Van Hoovels L, De Vos S, Azim T, Van Ranst M. Predominance of rotavirus G9 genotype in children hospitalized for rotavirus gastroenteritis in Belgium during 1999–2003. J Clin Virol. 2005;33:1–6. doi: 10.1016/j.jcv.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Romany S, Banerjee I, Gladstone B, Sarkar R, Selvapandian D, Le Fever AM, Jaffar Sh, Iturriza-Gomara M, Gray JJ, Estes MK. Geographic information systems and genotyping in identification of rotavirus G12 infections in residents of an urban slum with subsequent detection in hospitalized children: emergence of G12 genotype in South India. J Clin Virol. 2007;45:432–437. doi: 10.1128/JCM.01710-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samarbafzadeh A, Tehrani E, Makvandi M, Taremi M. Epidemiological aspects of rotavirus infection in Ahwaz, Iran. J Health Popul Nurt. 2005;23:245–249. [PubMed] [Google Scholar]
- 11.Gouvea V, Glass RI, Wood P, Taniguchi K, Clark HF, Forrester B, Fang ZH. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Virol. 1990;28:276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gouvea V, Santos N, Timenetsky MC. Identification of bovine and porcine rotavirus G types by PCR. J Clin Virol. 1994;32:1338–1340. doi: 10.1128/jcm.32.5.1338-1340.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bok K, Castagnaro N, Borsa A, Nates S, Espul C, Fay O, Fabri A, Grinstein S, Miceli I, Matson DO, Gomez JA. Surveillance for rotavirus in Argentina. J Med Virol. 2001;65:190–198. doi: 10.1002/jmv.2020. [DOI] [PubMed] [Google Scholar]
- 14.Staat MA, Azimi PH, Berke T, Roberts N, Bernstein DI, Ward RI, Pickering LK, Matson DO. Clinical presentations of rotavirus infection among hospitalized children. Pediatr Infect Dis J. 2002;21:221–227. doi: 10.1097/00006454-200203000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Kazemi A, Tabatabaie F, Agha-Ghazvini MR, Kelishadi R. The role of rotavirus in acute paediatric diarrhea in Isfahan, Iran. Pak J Med Sci. 2006;22:282–285. [Google Scholar]
- 16.Jin Y, Ye H, Fang ZY, Li YN, Yang XM, Dong QL, Huang X. Molecular epidemic feature and variation of rotavirus among children with diarrhaea in Lanzhou, China, 2001–2006. World J Pediatr. 2008;4:197–201. doi: 10.1007/s12519-008-0036-4. [DOI] [PubMed] [Google Scholar]
- 17.López-de-Andreś A, Jiménez-García R, Carrasco-Garrido P, Alvaro-Meca A, Galarza PG, de Miguel AG. Hospitalizations associated with rotavirus gastroenteritis in Spain, 2001–2005. BMC Public Health. 2008;8:109. doi: 10.1186/1471-2458-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kargar M, Zaree-Mahmood-abadi B, Tabatabaei H. Genotyping of VP7 protein with Nested RT-PCR in children hospitalized in Tehran. Iranian J Infect Dis Trop Med. 2008; 12:11–17 (Persian).
- 19.Suzuki H, Sakai T, Tanabe N, Okabe N. Peak rotavirus activity shifted from winter to early spring in Japan. Pediatr Infect Dis J. 2005;24:257–260. doi: 10.1097/01.inf.0000154327.00232.4d. [DOI] [PubMed] [Google Scholar]
- 20.Intusoma U, Sornsrivichai V, Jiraphongsa C, Varavithaya W. Epidemiology, clinical presentations and burden of rotavirus diarrhaea in children under five see at Ramathibodi hospital, Thailand. J Med Assoc Thai. 2008;91:1350–1355. [PubMed] [Google Scholar]
- 21.Zhang LJ, Zhang Q. Burden of hospitalizations attributable to rotavirus diarrhaea in the People, s Republic of China, August 2001–July 2003. J Infect Dis. 2005;192((Suppl1)):S94–S99. doi: 10.1086/431505. [DOI] [PubMed] [Google Scholar]
- 22.Iturriza-Gomara M, Kang G, Gray J. Rotavirus genotyping: keeping up with an evolving population of human rotaviruses. J Clin Virol. 2004;31:259–265. doi: 10.1016/j.jcv.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Desselberger U, Wolleswinkel-Van den Bosch J, Mrukowicz J, Mrukowicz J, Rodrigo C, Giaquinto C, Vesikari T. Rotavirus types in Europe and their significance for vaccination. Pediatr Infect Dis J. 2006;25:S30–S41. doi: 10.1097/01.inf.0000197707.70835.f3. [DOI] [PubMed] [Google Scholar]
- 24.Khalili B, Cuevas LE, Reisi N, Dove W, Cunliffe NA, Hart CA. Epidemiology of rotavirus diarrhoea in Iranian children. J Med Virol. 2004;73:309–312. doi: 10.1002/jmv.20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmed MU, Alam MM, Chowdhury NS, Haque MM, Shahid N, Kobayashi N, Taniguchi K, Urasawa T, Urasawa S. Analysis of human rotavirus G serotype in Bangladesh by enzyme-linked immunosorbent assay and polymerase chain reaction. J Diaprhoeal Dis Res. 1999;17:22–27. [PubMed] [Google Scholar]
- 26.Sravanan P, Ananthan S, Ananthasubramanian M. Rotavirus infection among infants and young children in Chennai, South India. Indian J Med Microbiol. 2004;22:212–221. [PubMed] [Google Scholar]
- 27.Van Damme P, Giaquinto C, Maxwell M, Todd P, Van der Wielen M. Distribution of rotavirus genotypes in Europe, 2004–2005: the REVEAL study. J Infect Dis. 2007;195:S17–S25. doi: 10.1086/516715. [DOI] [PubMed] [Google Scholar]
- 28.Santos N, Lima RCC, Pereira CFA, Gouvea V. Detection of rotavirus types G8 and G10 among Brazilian children with diarrhaea. J Clin Microbiol. 1998;36:2727–2729. doi: 10.1128/jcm.36.9.2727-2729.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reidy N, ÓHalloran F, Fanning S, Cryan B, ÓShea H. Emergence of G3 and G9 rotavirus and increased incidence mixed infections in the Southern region of Ireland 2001–2004. J Med Virol. 2005;27:571–578. doi: 10.1002/jmv.20494. [DOI] [PubMed] [Google Scholar]
- 30.Ahed MI, Wade A, Taniguchi K. Molecular epidemiology of rotavirus in Nigeria: detection of unusual strains with G2P[6] and G8P[1] specificities. J Clin Microbiol. 2001;39:3969–3975. doi: 10.1128/JCM.39.11.3969-3975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antunes H, Afonso A, Iturriza M, Martinho I, Ribeiro C, Magalhães C, Carvalho L, Branca F, Gray J. G2P[4] the most prevalent rotavirus genotype in 2007 winter season in European non-vaccinated population. J Clin Virol. 2009;45:76–78. doi: 10.1016/j.jcv.2009.03.010. [DOI] [PubMed] [Google Scholar]