Abstract
Chitosan oligomers (COS) were obtained by enzymatic hydrolysis and H2O2 oxidative treatment, and then separated into different fractions using ultra-filtration membranes. Each COSM fraction prepared using enzymatic hydrolysis retained its structure, especially the reduced end residue (−NH2 group), and had a peak for molecular weight. On the other hand, each COSH fraction prepared by oxidative treatment had partly damaged −NH2 groups and two peaks for molecular weight. These results indicate that the same COS fractions prepared by the two methods differ in their amino groups and in their molecular weights, though they can both pass through the same size ultra-filtration membrane. The effect of COS on the retrogradation of intermediate amylose rice starch (IA-RS) was also investigated. The 5 k < COSM < 10 k fraction had the best anti-retrogradation ability; the retrogradation ratio of IA-RS with this fraction was reduced by 14.5%, compared to the control, and its relative crystallinity was only 59.69%. 10 k < COSM < 30 k fraction was second best, while the COSM < 5 k fraction had no effect. Therefore, the molecular size of COS determined its anti-retrogradation capability. All COSH fractions from oxidative treatment had no effect on the retrogradation.
Keywords: Intermediate amylose rice starch (IA-RS), Retrogradation, Chitosan oligomers (COS), Enzymatic hydrolysis, H2O2 oxidative treatment
Introduction
Rice is not only considered a staple food in Asian countries (Chitra et al. 2010), rice-based products also have profound commercial potential because it is easy to digestible and hypoallergenic (Frederick and Kim 1997). However, these products are not better produced on an industrial scale due to the high rate of starch retrogradation, which, in turn, shortens the shelf life of rice products. The term “retrogradation” refers to the changes that occur in gelatinized starch upon cooling, which implies fully reversible recrystallization in the case of amylopectin and partially irreversible recrystallization in the case of amylose (Bjöck 1996). The multiplicity of inter-chain hydrogen bonds is the origin of the aggregation. Retrogradation is of greatly concern to the starch-based food industry because it usually causes deterioration of starch-containing systems including viscosity reduction, gel firming, enzyme-resistance, and syneresis (Englyst et al. 1992; Ibanez-carranza 2002). Therefore, retrogradation is important for improving the current starch processing methods and prolonging the shelf-life of starch-based foods.
Chitosan has important functional activities (Verma and Banerjee 2010). Its poor solubility, however, makes it difficult to use in food and bio-medical applications. Unlike chitosan, hydrolyzed products and chitosan oligomers (Chitosan oligosaccharides, COS) are readily soluble in water due to their short chain lengths and free amino groups in D-glucosamine units (Jeon and Kim 2000). The lower viscosity and better solubility of COS at neutral pH attracted researchers to use chitosan in its oligomer form. Especially, studies on COS in the areas of food and nutrition have emphasized their ability to improve food quality and human health progression (Kim and Rajapakse 2005).
Generally, COS can be prepared from chitosan by depolymerization methods, including acid hydrolysis, enzymatic hydrolysis, oxidative treatment, physical treatment, or a comprehensive method. The depolymerization outcome of physical treatment is undesirable; this makes the chemical and enzymatic methods the most widely used approaches for producing COS. Hydrogen peroxide (H2O2) is a strong oxidant, which produces free radicals in acidic, neutral, and basic reaction systems. The radicals can attack the β-D-(1-4) glycosidic bond and degrade chitosan. H2O2 is used to treat chitosan in preparing COS it is easy to handle, readily available, and environment friendly (Chang et al. 2001; Qin et al. 2002; Shao et al. 2003; Tian et al. 2003). The enzymatic processes of COS are generally carried out in batch reactors and are preferred over chemical methods because of minimized adverse chemical modifications of products during enzymatic hydrolysis and the promotion of COS biological activities. Using specific chitosanase in such hydrolysis is limited because of its cost and unavailability in bulk quantity (Yalpani and Pantaleone 1994). Chitosan is generally susceptible to a number of different enzymatic reactions; this indicates its broad substrate unspecificity (Aiba 1994a, b). Hence, the non-specific hydrolysis of chitosan by a complex enzymatic system is an alternative economical way of obtaining COS from chitosan.
Malto-oligosaccharides have positive effects on the retardation of starch retrogradation (León et al. 1997; Durán et al. 2001; Rojas et al. 2001; Smits et al. 2003). Compared to systems with equal amounts of plasticizers, these oligomers are known to reduce retrogradation by interfering with starch recrystallization (Katsuta et al. 1992a, b). Previous studies have focused mainly on the biological activities of COS. Few attempts, however, were made to evaluate the ability of COS to retard the retrogradation of starch-based foods. Wei (2004) reported that COS could retard the retrogradation of dextrin through a rough turbidimetric method. It would be interesting to determine whether COS can prevent the retrogradation in a manner similar to that of malto-oligosaccharides through accurate evaluations. Here, the goal is to prepare a series of COS through enzymatic hydrolysis and H2O2 oxidative treatment. The effect of COS prepared using the two methods on the retrogradation of intermediate amylose rice starch (IA-RS) is then further conducted. To study anti-retrogradation, gelatinized IA-RS gel with 66.7% (w/w) water content was stored at 4 °C storage for 7 days as easily retrograded model. The water content of rice starch was described as the percentage range of moisture content in which maximum retrogradation occurs (Ding et al. 2003). The storage temperature of gelatinized starch is 4 °C, which can accelerate retrogradation. When the rice starch has higher amylose content, the higher degree of retrogradation occurs (Baik et al. 1997).
Materials and methods
Chitosan was obtained from Jinan Haidebei Marline Bioengineering Co., Ltd., (Jinan, China). It has a 91.6% degree of deacetylation and its viscosity average molecular weight (Mv) was 3.2 × 105 Da. 30% H2O2 (w/w) reagent and all the other chemicals were analytical grade and were purchased from Shanghai Chemical Co. (Shanghai, China). The complex enzyme, including cellulase, pepsin, and lysozyme, was provided by Professor Xia Wenshui’s laboratory in Jiangnan University. Freshly harvested intermediate amylose content rice was purchased from a local market.
Extraction of IA-RS
IA-RS was extracted using the modified method described by Lin and Chang (2006). Rice kernels (400 g, dry basis), after cleaning, were steeped overnight in 2 L of 0.1% (w/w) NaOH solution. The supernatant was decanted; the kernels were milled with 2 L of 0.1% (w/w) NaOH solution by a colloid mill. The slurry was passed through a 100-mesh sieve and poured into a 5-L beaker. The breaker was then filled with deionized water and surface yellow foam was removed. The solution stood at 4 °C for 12 h, with the starch slowly precipitating at the bottom of the break; the water at the top was recovered by siphoning. Afterward, filling the water and removing the yellow foam, standing and siphoning were repeated four times until the protein was completely removed. The starch layer was collected, oven-dried at 40 °C, passed through a 100-mesh sieve, and stored. The isolated starch had 12% moisture content, 0.5% protein content (AOAC 2000), and 23% amylose content (Gunaratne and Hoover 2000).
Preparation of different COSM fractions by enzymatic hydrolysis
To prepare the different COSM fractions using enzymatic hydrolysis, 5 g chitosan was first mixed with 50 ml of a 0.1 mol/L sodium acetate-0.2 mol/L acetic acid buffer (pH 5.0), and then treated with the complex enzyme in an enzyme/substrate ratio of 1:100 (w/w). Chitosan hydrolysis was carried out at 45 °C with shaking for 3 days. During hydrolysis, the pH of the solution was readjusted to 5.0 using 0.2 mol/L acetic acid after reacting for 2 h. Once the reaction was complete, the solution was adjusted to pH 9.0 with 0.2 mol/L of NaOH and then filtrated. The solution was separated using an ultra-filter (molecular weight cut-off: 5 k, 10 k, and 30 k). The solutions from 10–30 k, 5–10 k, and <5 k ultra-filtration membranes were collected separately and concentrated to 50 ml. The precipitates were obtained by adding six volumes of ethanol to the concentrated solutions. Different COSM fractions were collected after the precipitates were dried at 40 °C by vacuum.
Preparation of different COSH fractions by H2O2 oxidative treatment
To prepare COS, 5 g chitosan was first mixed with 50 ml deionized water and 100 μl glacial acetic acid (anhydrous acetic acid). The pH value of solution could decrease as the oxidative reaction progresses; hence, chitosan was added into the subacid solution. This was to avoid using excess NaOH solution adjustment and produce more salts in the subsequent step. Therefore, this oxidative degradation of chitosan by H2O2 is heterogeneous. The reactor was kept in a 70 °C water bath and shaken for 2 h. In this process, 6 ml 30% H2O2 was added before the reaction and every 30 min thereafter, so that 24 ml of 30% H2O2 was used all in all. Once the reaction was complete, the solution was adjusted to pH 9.0 with 0.2 mol/L of NaOH and, then, filtrated. The preparation of different COSH fractions by H2O2 treatment was conducted through the enzymatic hydrolysis method as described above.
During the oxidative degradation of chitosan, the solution viscosity dropped sharply at the initial reaction, and then further decreased slowly. After 2 h of reaction, there was almost no water-insoluble chitosan precipitate was observed in the reaction solution which was adjusted to pH 9.0. Therefore, to effectively obtain COSH, the reaction time was limited to 2 h. Although the rate of reaction usually increased with temperature, the color of the COS product became dark when the temperature was over 70 °C. This may be due to the very high reaction temperature, which resulted in the rapid and severe depolymerization of chitosan that produces other by-products. Thus, from the preliminary experimental results, the most suitable depolymerization temperature is 70 °C (Tian et al. 2004).
Measurements of the molecular weights of the different COS fractions
The molecular weights of different COS fractions were measured using high-performance gel filtration chromatography (HPGFC). The HPGFC instrument (Waters 600) consists of a connected column (Ultrahydrogel™ Linear 300 mm × 7.8 mmid × 2) and a 2410 differential refraction detector. The concentration of a COS sample solution was 0.5 mg/ml. Sample solutions were allowed to pass through a 0.45 μm Millipore filter. The eluent was 0.2 mol/L CH3COOH + 0.15 mol/L CH3COONH4, at a flow rate of 0.9 ml/min at 45 °C. The dextran standards (Pharmacia & Upjohn Company, USA) were used to construct a standard curve. All data provided by the HPGFC system were collected and analyzed using an Empower workstation.
FT-IR analyses of the different COS fractions
Fourier transformed infrared spectroscopy (FT-IR) was conducted on an FT-IR Nicolet NEXUS470 spectrometer (Thermo Nicolet Corp., USA) in the range of 4000–400 cm−1. Thirty-two co-added scans were taken for each sample at a resolution of 2 cm−1. All powder samples of COS were compressed into KBr disks. The spectra were collected in triplicates and averaged to a spectrum using the Omnic 6.2 software.
Differential scanning calorimetry (DSC)
The DSC measurements were carried out using Pyris 1-DSC (Perkin-Elmer Corp., Norwalk, CT, USA). The gelatinization and retrogradation properties of the samples were determined from the DSC curves. The mixing ratio of IA-RS/COS was 5/0.4 (w/w), which is equal to an IA-RS containing 8% COS (based on the IA-RS weight). The calorimeter was calibrated using an indium standard. Samples of the mixtures (about 2 mg) were accurately weighed into aluminum DSC pans, and deionized water was added by micropipette in order to achieve a water-sample ratio of 2:1. Sample pans were sealed and equilibrated at room temperature for 24 h before the analysis. The samples were heated at a rate of 10 °C/min from 10 °C to 95 °C using an empty pan as reference. The onset temperature To, peak temperature Tp, and conclusion temperature Tc of gelatinization were determined from the DSC curves for the first run of heating. Gelatinization enthalpy (ΔHg) was evaluated based on the area of the main endothermic peak. The gelatinized samples were then stored at 4 °C for 7 days. To study retrogradation, these stored samples were again heated under the same conditions used for the gelatinization analysis, and the retrogradation enthalpy (ΔHr) was determined from the second run of heating. In addition, the percentage of retrogradation (R%) was calculated as the ratio of ΔHr to ΔHg in the heating run (Rodríguez-Sandoval et al. 2008). Analyses were performed in triplicates.
X-ray diffraction
The samples as same as DSC analysis were IA-RS with 8% different COS and adding double deionized water and gelatinized by steam heating for 20 min in a closed thermostat water bath. These samples were cooled to room temperature and then stored at 4 °C condition for 7 days to retrograde. The freeze-dried samples were ground and then passed through a 100-mesh sieve before testing. The recrystallization analysis was carried out using a Bruker D8 Advance speed X-ray diffractometer (Bruker AXS, Rheinfelden, Germany) equipped with a copper tube operating at 40 kV and 200 mA, producing CuKα radiation of 0.154 nm wavelength. Diffractograms were obtained by scanning from 4° (2θ) to 40° (2θ) at a rate of 4°/min, a step size of 0.02°, a divergence slit width (DS) of 1°, a receiving slit width (RS) of 0.02 mm, and a scatter slit width (SS) of 1°. Each sample was measured in triplicate. MDI Jade 5.0 software was used to analyze the diffractograms. The retrogradation crystallinity was quantified by integrating the area under the fitting crystalline peak at 17°. The relative crystallinity (XRC) was expressed as XRC = (Is/Ic) × 100, where Is and Ic are the integrated areas of the crystalline peak at 17° in the samples of IA-RS with different COS fractions and in the control, respectively. The integrated area was obtained after smoothing and fitting the original peak using the PeakFit v4.12 software with some modification as described in previous reports (Ribotta et al. 2004; Primo-Martín et al. 2007).
Statistical analysis
The mean, standard deviations, and the significant differences found in the collected data were calculated and reported using Origin (version 7.5). T-test statistical analyses were also conducted. For the differences reported significant, a confidence level of 95% was considered.
Results and discussion
Characterization of the molecular weights of the different COS fractions
The molecular weights of different COS fractions obtained from the two preparation methods are summarized in Table 1. The differences in molecular weights due to ultra-filtration separation can be observed for all fractions. The COSM synthesized by enzymatic method all have a peak for the molecular weight and these values decreased gradually in the order of 10 k < COSM < 30 k, 5 k < COSM < 10 k, and COSM < 5 k (Table 1). All molecular weights of the COSH produced by H2O2 treatment were all divided into two peaks (Table 1). The molecular weights for Peak 1 were at 1100–2500 and they also decreased in the order of 10 k < COSH < 30 k, 5 k < COSH < 10 k, and COSH < 5 k. On the other hand, the various molecular weights for Peak 2 were from 440–1000 and were particularly similar to that of the COSM prepared using the enzymatic method. These data provide information on the preparation of COS, indicating that the H2O2 treatment should destroy the structure of the COS to some extent, which resulted in all three fractions having two peaks. It could be assumed that Peak 1 of all the COSH fractions prepared by the H2O2 oxidative treatment represented “damaged COS”, and Peak 2 was the “normal COS” and is similar to the COS prepared using the enzymatic method.
Table 1.
The molecular weights of the different COS fractions prepared by enzymatic hydrolysis and H2O2 oxidative treatment
| Different COS fractionsa | Name | % Area | Mnb | Mwc | Mpd |
|---|---|---|---|---|---|
| 10 k < COSM < 30 k | Peak1 | 100.00 | 886 | 911 | 942 |
| 5 k < COSM < 10 k | Peak1 | 100.00 | 597 | 637 | 659 |
| COSM < 5 k | Peak1 | 100.00 | 448 | 461 | 495 |
| 10 k < COSH < 30 k | Peak1 | 73.03 | 1958 | 2428 | 2004 |
| Peak2 | 26.97 | 756 | 873 | 910 | |
| 5 k < COSH < 10 k | Peak1 | 54.16 | 1219 | 2024 | 1779 |
| Peak2 | 45.84 | 579 | 617 | 644 | |
| COSH < 5 k | Peak1 | 41.33 | 1162 | 1756 | 1590 |
| Peak2 | 58.67 | 485 | 495 | 523 |
aCOSM is represented as COS prepared by enzymatic hydrolysis; COSH is represented as COS prepared by H2O2 oxidative treatment
bThe number average molecular weight (Mn)
cThe weight average molecular weight (Mw)
dThe peak molecular weight (Mp)
FT-IR spectra of the different COS fractions
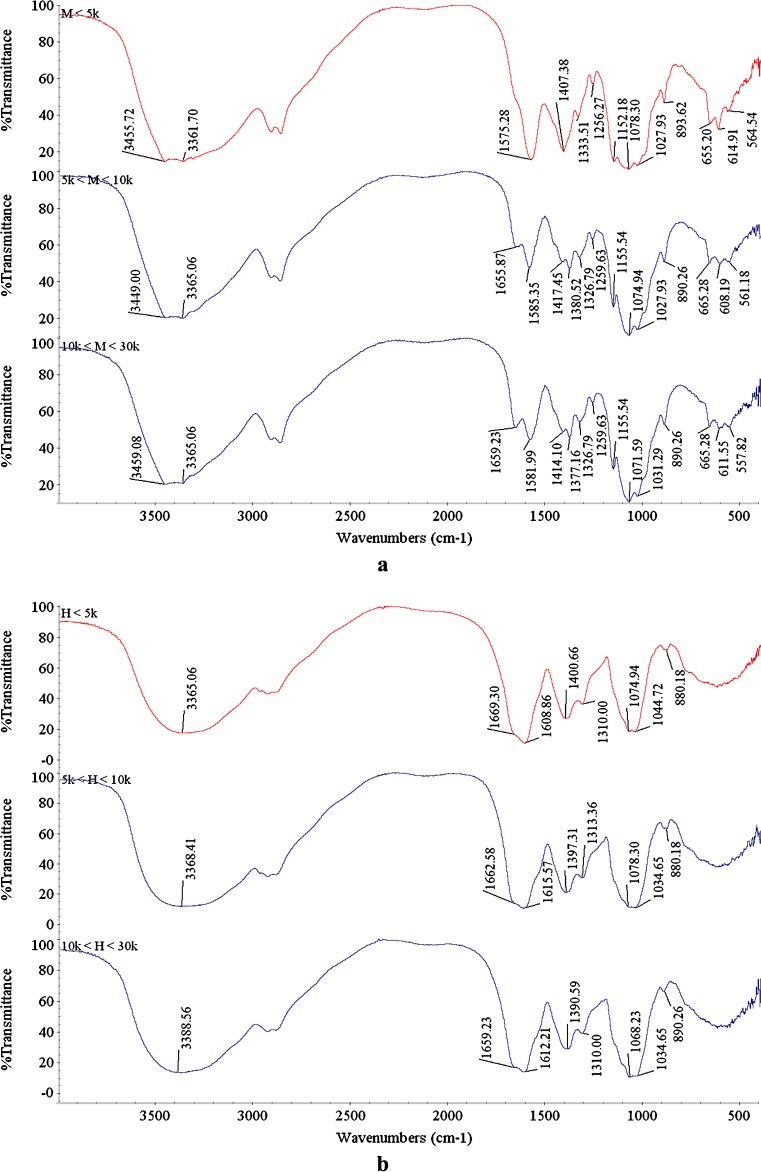
FT-IR is effective for characterizing COS. The FT-IR spectra of different COS fractions prepared through enzymatic hydrolysis and H2O2 oxidative treatment are shown in Fig. 1. The spectra of the COS prepared by the two methods had obvious differences. All COSM produced by the enzymatic hydrolysis had two peaks that noticeably split at 3500–3300 cm−1; this corresponds to the typical N-H stretching vibration of the primary amine (Guiver et al. 1995), though the O-H stretching vibration in this range should be covered up. All COSH obtained by H2O2 oxidative treatment had only one single strong, broad band centered at about 3370 cm−1. This is due to the O-H stretching vibration and the N-H stretching vibration, which should be less prominent. Further, for the COSH prepared by the H2O2 oxidative treatment, the intensities of amide I (C = O stretch) and amide II (N-H bend) bands at 1655 and 1560 cm−1 (Ngo et al. 2008), respectively, decreased compared to those of 5 k < COSM < 10 k and 10 k < COSM < 30 k, and moved towards higher wave numbers. The COSM < 5 k fraction also lacked clear amide I bands at 1655 cm−1. The absorption bands at 1155 cm−1 (anti-symmetric stretching of the C-O-C bridge), 1082 and 1032 cm−1 (skeletal vibrations involving the C-O stretching) are characteristic of its saccharide structure (Peniche et al. 1999). The vibrational band at about 1155 cm−1, corresponds to the ether bond in the pyranose ring, which almost disappeared in the COSH prepared using the H2O2 treatment; the rupture of the β-glycosidic bonds may have affected the amount and distribution of the glycosidic bonds in the molecular chains of COS (Wang et al. 2005). The information obtained from the FT-IR spectra of the COSH prepared by H2O2 treatment imply that the breaking of the C-O-C glycosidic bond leads to chain scission (Shao et al. 2003). Since the peak at about 660 cm−1 is assigned to out-of-plane -NH deformation (Sirdeshmukh and Puranik 1962). The spectra of the COSM prepared using enzymatic hydrolysis showed three slit peaks at 666–557 cm−1. In contrast, COSH prepared using H2O2 oxidative treatment had none of these peaks, which also indicated losing of the −NH groups.
Fig. 1.
The FT-IR spectrum of the different COS fractions prepared by enzymatic hydrolysis (a) and H2O2 oxidative treatment (b)
From the above results, although the molecular weights of all COSM fractions prepared by enzymatic hydrolysis decreased, their unit structure, particularly, the reduced end residue (−NH2 group), did not change. The H2O2 treatment under this condition broke the reduced end residue (−NH2 group) of the COSH, changed the glucoside bonds, and weakened the amide bands. As described by Qin et al. (2002), when chitosan was treated with H2O2, oxidative scission produced diverse effects, which may partially destroy the normal structure of the COS. The −NH2 group of the COSH prepared by the H2O2 oxidative treatment was destroyed, though −COOH was not formed as reported (Qin et al. 2002).
The FT-IR data also proved the analyses of the molecular weight as given above; all COSH fractions prepared by oxidative treatment included both the “damaged COSH” and “normal COSH” parts. The results from the two analyses indicated that the same COS fractions prepared from the two methods vary in structure and in molecular weight though they can both pass through the same size of ultra-filtration membranes.
Retrogradation analyses of IA-RS with the different COS fractions
DSC is most useful in measuring and providing the basic information on starch retrogradation. Table 2 shows the effects of the different COS fractions on the gelatinization and retrogradation properties of the IA-RS. The addition of COS affected the gelatinization temperatures of the IA-RS gel. There was a clear shift in the endotherms toward a higher temperature compared to the control (Table 2). In addition, IA-RS with 5 k < COSM < 10 k fraction had a slightly lower gelatinization enthalpy than the other samples, which could indicate that a certain molecular size of COS could facilitate easy hydration of the starch granules. However, the gelatinization enthalpies of the IA-RS with the other COS fractions do not significantly differ from the control.
Table 2.
The temperatures (To, Tp and Tc) and enthalpy (ΔHg) of gelatinization of intermediate amylose rice starch (IA-RS) with 8% (based on starch weight) the different COS fractions and the corresponding retrogradation enthalpy (ΔHr) and ratio (R%) after 7 days’ storage at 4 °C
| Samplesa | Gelatinizationb | Retrogradationb | ||||
|---|---|---|---|---|---|---|
| To (°C) | Tp (°C) | Tc (°C) | ΔHg (J/g dry starch) | ΔHr (J/g dry starch) | R% | |
| The control | 68.5 ± 0.27 a | 75.1 ± 0.10 a | 82.5 ± 0.15 a | 12.3 ± 0.20 a | 7.1 ± 0.01 a | 57.8 |
| IA-RS with 10 k < COSM < 30 k | 69.5 ± 0.23 b | 76.3 ± 0.12 b | 83.8 ± 0.06 b | 12.0 ± 0.23 a | 6.1 ± 0.14 b | 53.5 |
| IA-RS with 5 k < COSM < 10 k | 69.8 ± 0.20 b | 76.4 ± 0.17 b | 83.5 ± 0.36 b | 11.8 ± 0.14 b | 5.1 ± 0.21 c | 43.3 |
| IA-RS with COSM < 5 k | 72.1 ± 0.19 c | 78.8 ± 0.00 c | 85.8 ± 0.11 c | 12.5 ± 0.20 a | 7.0 ± 0.10 a | 55.8 |
| IA-RS with 10 k < COSH < 30 k | 70.9 ± 0.22 d | 77.6 ± 0.10 d | 84.5 ± 0.02 d | 11.9 ± 0.36 a | 7.2 ± 0.55 a | 60.7 |
| IA-RS with 5 k < COSH < 10 k | 71.4 ± 0.11 e | 78.0 ± 0.00 e | 84.9 ± 0.20 d | 12.7 ± 0.52 a | 7.1 ± 0.41 a | 56.1 |
| IA-RS with COSH < 5 k | 72.2 ± 0.23 f | 79.0 ± 0.26 f | 86.2 ± 0.70 e | 12.3 ± 0.40 a | 7.0 ± 0.09 a | 57.2 |
aCOSM is represented as COS prepared by enzymatic hydrolysis; COSH is represented as COS prepared by H2O2 oxidative treatment
bValues are means ± standard deviations (n = 3); Values followed by the same letter in the same column are not significantly different (p < 0.05)
The enthalpy value of the retrograded starch reflects the melting of the crystallites formed through the association between adjacent double helices during gel storage (Hoover and Senanayake 1996). This endotherm peak was caused by the melting of retrograded amylopectin (Fearn and Russell 1982; Karim et al. 2000) instead of amylose. Table 2 also lists the changes in retrogradation enthalpy and the R% of gelatinized IA-RS with different COS fractions after 7 days of storage. In the absence of COS, the ΔHr and R% of the gelatinized IA-RS significantly increased to 7.11 J/g and 57.8%, respectively. However, the ΔHr and R% of the gelatinized IA-RS sample with 5 k < COSM < 10 k fraction had an evident reduction at 5.10 J/g and 43.3%, respectively. The 10 k < COSM < 30 k fraction also showed slightly lower ΔHr (6.09 J/g) and R% (53.5%) values. When the addition of the COSM < 5 k fraction and all other COS fractions prepared using H2O2 method were complete, there was no effect seen on the anti-retrogradaition of the IA-RS.
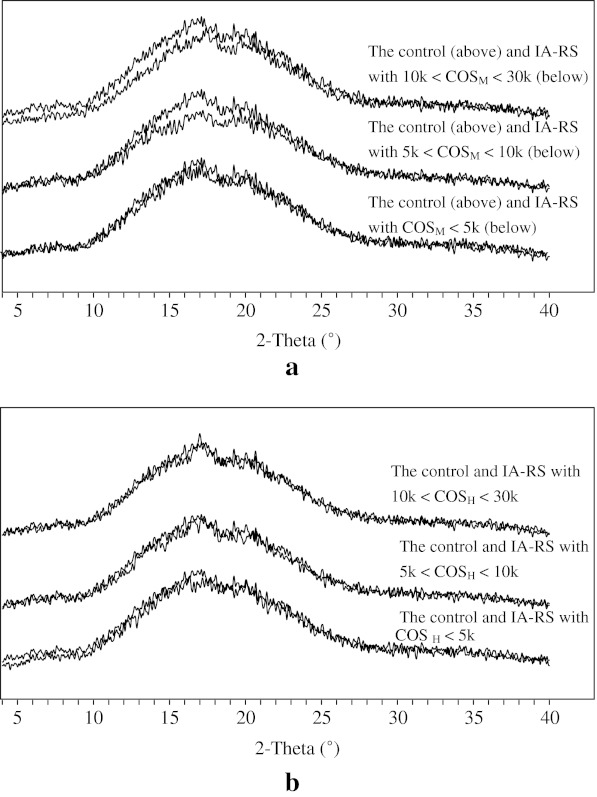
The final recrystallization was investigated using XRD to further prove the effect of COS on the retrogradation. Traditional powder XRD is the most sensitive to the recrystallization of the molecular association, which is the long-range order structure (Petkov 2008). The sensitivity of the powder XRD is relatively low compared with DSC in the terms of the retrogradation. The XRD patterns and corresponding crystallinity observed from the retrograded IA-RS systems with different COS fractions are present in Fig. 2, in which the overlapping separation method was used to distinguish the difference of the recrystallization between the samples and the control. The retrograded starch (B-type crystal) is characterized by a well-defined peak at 17° (2θ) (Kim et al. 1997), and is clearly distinct from the pattern of raw starch. This is also accompanied by the gradual increase in the rigidity and phase separation between the polymer and the solvent (syneresis). The formation of this peak was the result of the crystallization of the amorphous starch melt, mainly of the amylopectin fraction, and increased during storage (Thiré et al. 2003; Osella et al. 2005). Starch-lipid complexes, which exhibit V-type corresponding to this peak at 20°, have been found to be metastable and to transform gradually into the more stable B-type crystal on aging of cooked rice (Hibi et al. 1990; Zobel and Kulp 1996).
Fig. 2.
X-ray diffraction overlapping patterns between retrograded intermediate amylose rice starch (IA-RS) gels with the different COS fractions and the control, respectively: COSM prepared by enzymatic hydrolysis (a) and COSH prepared by H2O2 oxidative treatment (b) Legend: The overlapping separation method was used to distinguish the difference of recrystallization between the samples and the control in these XRD patterns
Two major broad peaks were identified in the all retrograded samples at 2θ angles of 17 and 20° (Fig. 2). In Fig. 2a, by observing the overlapping XRD patterns, the peak area close to 17° of the IA-RS sample with 5 k < COSM < 10 k fraction was evidently smaller than the control, which indicates the lack of the typical B-type crystal. This also implies that 5 k < COSM < 10 k part could retard the recrystallization of amylopection or the long-term retrogradation behavior of the gelatinized IA-RS. Likewise, the peak size at about 17° of the IA-RS with 10 k < COSM < 30 k fraction was also slightly smaller than the control. However, the XRD patterns of IA-RS with COSM < 5 k or all COSH fraction prepared by H2O2 oxidative treatment and the control appeared to all be consistent (Fig. 2b), suggesting their extent of retrogradation being almost the same.
The XRD pattern of retrograded starch was normally the dispersion broad peak (Kim et al. 1997; Primo-Martín et al. 2007), thus, the crystallinity of the samples was calculated to quantitatively compare the degree of recrystallization. In calculation, crystallinity was quantified based on the integrated area under the fitting crystalline peak at about 17° with PeakFit software. The relative crystallinity (XRC) is defined as the ratio of the integrated area of the fitting crystalline peak at 17° in the samples to this integrated area in the control, then these calculated values of retrograded samples with different COS fractions are listed in Table 3. Among the samples studied, XRC value of IA-RS with the 5 k < COSM < 10 k fraction was significantly decreased to 59.69% and next was the 10 k < COSM < 30 k (84.59%) sample as compared with the control (100%), which showed that 5 k < COSM < 10 k and 10 k < COSM < 30 k fractions could retard the recrystallization or retrogradation behavior of gelatinized IA-RS. But there was no evident discrepancy in the XRC values of the IA-RS among COSM < 5 k fraction (92.72%), 10 k < COSH < 30 k fraction (91.71%), 5 k < COSH < 10 k fraction (95.45%), COSH < 5 k (90.55%), and the control (100%) (Table 3), which also indicates that these COS fractions had no effect on the retrogradation of IA-RS.
Table 3.
Relative crystallinity (XRC) of retrograded sample with the different COS fraction as determined using X-ray diffraction
| Samplesa | The integrated area of fitting crystallinity peak at 17°b | XRC%c |
|---|---|---|
| The control | 723.2 ± 16.43 a | 100 |
| IA-RS with 10 k < COSM < 30 k | 611.8 ± 21.08 b | 84.6 |
| IA-RS with 5 k < COSM < 10 k | 431.7 ± 10.60 c | 59.7 |
| IA-RS with COSM < 5 k | 670.6 ± 11.34 d | 92.7 |
| IA-RS with 10 k < COSH < 30 k | 663.3 ± 15.64 d | 91.7 |
| IA-RS with 5 k < COSH < 10 k | 690.4 ± 22.59 a, d | 95.5 |
| IA-RS with COSH < 5 k | 654.9 ± 13.14 d | 90.6 |
aCOSM is represented as COS prepared by enzymatic hydrolysis; COSH is represented as COS prepared by H2O2 oxidative treatment
bValues are means ± standard deviations (n = 3); Values followed by the same letter in the same column are not significantly different (p < 0.05)
cXRC, the relative crystallinity, is calculatetd using: XRC = (Is/Ic) × 100, where Is and Ic are the integrated areas of the crystalline peak at 17° in the samples of IA-RS with different COS fractions and in the control, respectively
Hence, these XRD results from observation and calculation were in agreement with the ΔHr and R% assessed by DSC. Among the three COSM fractions obtained by enzymatic hydrolysis, the 5 k < COSM < 10 k fraction had the best anti-retrogradation ability, with the 10 k < COSM < 30 k fraction coming in second. However, the COSM < 5 k fraction did not have this ability. Thus, a special molecular weight of COS should be involved in preventing the retrogradation of starch. As some research were reported that malto-oligosaccharides of DP (degree of polymerization) 2–6 may hinder helix formation and reduce long-term retrogradation. In comparison, larger malto-oligosaccharides of DP >6 did not have this effect (León et al. 1997; Durán et al. 2001; Rojas et al. 2001; Smits et al. 2003). However, malto-oligosaccharides of DP >7 can increase starch retrogradation (León et al. 1997; Durán et al. 2001; Rojas et al. 2001). The molecular weight of glucosamine repeating unit is 160; thus, the DP of 5 k < COSM < 10 k fraction having the best anti-retrogradation ability in this study may be about 3 or 4. Hydrogen bonding between starch chains leading to recrystallization may be interrupted by a certain molecular size of COS, thereby reducing the retrogradation rate. Crosslink may occur between COS and the hydroxyl groups of adjacent amylopectin chains, retarding the conformational reordering of amylopectin chains. On the other hand, different COSH fractions prepared by H2O2 oxidative treatment did not exhibit anti-retrogradation activity. It may be due to the chemical structure of some COSH during H2O2 oxidative treatment being damaged, which results in the reduction of the effective part of the anti-retrogradation.
Conclusion
The results of this study demonstrate that although H2O2 oxidative treatment can be a quick and simple method to obtain COSH, the chemical structure of COSH could be destroyed, thus affecting its function of anti-retrogradation. On the other hand, the COSM prepared by enzymatic hydrolysis retained its active group with molecular weight reduction; however, this method is time-consuming. Thus, a more efficient method needs to be developed to obtain COS owning anti-retrogradation effect without damaging the unit structure itself.
Moreover, it is also found in this study that a specific molecular size of COSM prepared by enzymatic hydrolysis could clearly prevent the retrogradation of intermediate-content amylose rice starch. This is important in the application of COS to starch-based foods. It must be pointed out, however, that a relatively high-level of COS was added to starch at analyses. Therefore, further research should assess the possible effect of COS with individual DP 3 or 4 on the retrogradation of IA-RS, thereby effectively reducing the amount of its usage.
Acknowledgment
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (Grant No. 31050012) and Special Fund for Agro-scientific Research in the Public Interest (Grant No. 200903043).
References
- Aiba S. Preparation of N-acetylchitooligosaccharides by hydrolysis of chitosan with chitinase followed by N-acetylation. Carbohydr Res. 1994;256:323–328. doi: 10.1016/0008-6215(94)00243-6. [DOI] [PubMed] [Google Scholar]
- Aiba S. Preparation of N-acetylchitooligosaccharides by lysozymic hydrolysates of partially N-acetylated chitosans. Carbohydr Res. 1994;261:297–306. doi: 10.1016/0008-6215(94)84025-3. [DOI] [Google Scholar]
- Horwitz W, editor. Official methods of analysis of AOAC International. 17. Maryland-Gaithersburg: AOAC International; 2000. [Google Scholar]
- Baik MY, Kim KJ, Cheon KC, Ha YC, Kim WS. Recrystallization kinetics and glass transition of rice starch gel system. J Agric Food Chem. 1997;45:4242–4248. doi: 10.1021/jf960713u. [DOI] [Google Scholar]
- Bjöck I. In: Carbohydrates in food. Eliasson AC, editor. New York: Marcel Dekker; 1996. pp. 505–553. [Google Scholar]
- Chang KLB, Tai MC, Cheng FH. Kinetics and products of the treatment of chitosan by hydrogen peroxide. J Agric Food Chem. 2001;49:4845–4851. doi: 10.1021/jf001469g. [DOI] [PubMed] [Google Scholar]
- Chitra M, Singh V, Ali SZ. Effect of processing paddy on digestibility of rice starch by in vitro studies. J Food Sci Technol. 2010;47(4):414–419. doi: 10.1007/s13197-010-0068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WP, Tan YB, Ding XL. Effects of water content on the gelatinization and retrogradation of rice starch. Cereal Feed Ind. 2003;33:44–47. [Google Scholar]
- Durán E, León A, de Barber CB. Effect of low molecular weight dextrins on gelatinization and retrogradation of starch. Eur Food Res Technol. 2001;212:203–207. doi: 10.1007/s002170000205. [DOI] [Google Scholar]
- Englyst HN, Kingman SM, Cummings JH. Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr. 1992;46:33–50. [PubMed] [Google Scholar]
- Fearn T, Russell PL. A kinetic study of bread staling by differential scanning calorimetry: the effect of specific volume. J Sci Food Agric. 1982;33:537–541. doi: 10.1002/jsfa.2740330607. [DOI] [Google Scholar]
- Frederick FS, Kim D. Use of enzymes for the separation of protein from rice flour. Cereal Chem. 1997;74:437–441. doi: 10.1094/CCHEM.1997.74.4.437. [DOI] [Google Scholar]
- Guiver MD, Robertson GP, Foley S. Chemical modification of polysulfones II: an efficient method for introducing primary amine groups onto the aromatic chain. Macromolecules. 1995;28:7612–7621. doi: 10.1021/ma00127a005. [DOI] [Google Scholar]
- Gunaratne A, Hoover R. Effect of heat-moisture treatment of the structure and physicochemiscal properties of tuber and root starches. Carbohydr Polym. 2000;49:425–437. doi: 10.1016/S0144-8617(01)00354-X. [DOI] [Google Scholar]
- Hibi Y, Kitamura S, Kuge T. Effect of lipids in the retrogradation of cooked rice. Cereal Chem. 1990;67:7–10. [Google Scholar]
- Hoover R, Senanayake SPJN. Composition and physicochemical properties of oat starches. Food Res Int. 1996;29:15–26. doi: 10.1016/0963-9969(95)00060-7. [DOI] [Google Scholar]
- Ibanez-carranza AM (2002) A study of the pasting properties of rice flour and starch as affected by rice varietry and physicochemical properties. PhD Thesis, UC Davis, USA
- Jeon YJ, Kim SK. Continuous production of chitooligosaccharides using a dual reactor system. Process Biochem. 2000;35:623–632. doi: 10.1016/S0032-9592(99)00118-1. [DOI] [Google Scholar]
- Karim AA, Norziah MH, Seow CC. Methods for the study of starch retrogradation. Food Chem. 2000;71:9–36. doi: 10.1016/S0308-8146(00)00130-8. [DOI] [Google Scholar]
- Katsuta K, Miura M, Nishimura A. Kinetic treatment for rheological properties and effects of saccharides on retrogradation of rice starch gels. Food Hydrocoll. 1992;6:187–198. doi: 10.1016/S0268-005X(09)80359-7. [DOI] [Google Scholar]
- Katsuta K, Nishimura A, Miura M. Effect of saccharides of rice starch gels. 1. Mono- and disaccharides. Food Hydrocoll. 1992;6:387–398. doi: 10.1016/S0268-005X(09)80006-4. [DOI] [Google Scholar]
- Kim JO, Kim WS, Shin MS, Kwangju A comparative of rice starch and α-Amylase study on retrogradation gels by DSC, X-Ray methods. Starch/Stärke. 1997;49:71–75. doi: 10.1002/star.19970490207. [DOI] [Google Scholar]
- Kim SK, Rajapakse N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): a review. Carbohydr Polym. 2005;62:357–368. doi: 10.1016/j.carbpol.2005.08.012. [DOI] [Google Scholar]
- León A, Durán E, de Barber CB. Firming of starch gels and amylopectin retrogradation as related to dextrin production by α-amylase. Eur Food Res Technol. 1997;205:131–134. [Google Scholar]
- Lin JH, Chang YH. Molecular degradation rate of rice and corn starches during acid-methanol treatment and its relation to the molecular structure of starch. J Agric Food Chem. 2006;54:5880–5886. doi: 10.1021/jf060424y. [DOI] [PubMed] [Google Scholar]
- Ngo DN, Kim MM, Kim SK. Chitin oligosaccharides inhibit oxidative stress in live cells. Carbohydr Polym. 2008;74:228–234. doi: 10.1016/j.carbpol.2008.02.005. [DOI] [Google Scholar]
- Osella CA, Sánchez HD, Carrara CR, de la Torre MA, Buera MP. Water redistribution and structural changes of starch during storage of a gluten-free bread. Starch/Stäke. 2005;57:208–216. doi: 10.1002/star.200400330. [DOI] [Google Scholar]
- Peniche C, Arguelles-Monal W, Davidenko N, Sastre R, Gallardo A, Román J. Biomaterials Self-curing membranes of chitosan/PAA IPNs obtained by radical polymerization: preparation, characterization and interpolymer complexation. Biomaterials. 1999;20:1869–1878. doi: 10.1016/S0142-9612(99)00048-4. [DOI] [PubMed] [Google Scholar]
- Petkov V. Nanostructure by high energy X-ray diffraction. Mater Today. 2008;11:28–38. doi: 10.1016/S1369-7021(08)70236-0. [DOI] [Google Scholar]
- Primo-Martín C, van Nieuwenhuijzen NH, Hamer RJ, van Vliet T. Crystallinity changes in wheat starch during the bread-making process: starch crystallinity in the bread crust. J Cereal Sci. 2007;45:219–226. doi: 10.1016/j.jcs.2006.08.009. [DOI] [Google Scholar]
- Qin CQ, Du YM, Xiao L. Effect of hydrogen peroxide treatment on the molecular weight and structure of chitosan. Polym Degrad Stab. 2002;76:211–218. doi: 10.1016/S0141-3910(02)00016-2. [DOI] [Google Scholar]
- Ribotta PD, Cuffini S, León AE, Añón MC. The staling of bread: an X-ray diffraction study. Eur Food Res Technol. 2004;218:219–223. doi: 10.1007/s00217-003-0835-8. [DOI] [Google Scholar]
- Rodríguez-Sandoval E, Fernández-Quintero A, Cuvelier G, Relkin P, Bello-Pérez LA. Starch retrogradation in cassava flour from cooked parenchyma. Starch/Stäke. 2008;60:174–180. doi: 10.1002/star.200700683. [DOI] [Google Scholar]
- Rojas JA, Rosell CM, de Barber CB. Role of maltodextrins in the staling of starch gels. Eur Food Res Technol. 2001;212:364–368. doi: 10.1007/s002170000218. [DOI] [Google Scholar]
- Shao J, Yang YM, Zhong QQ. Studies on preparation of oligoglucosamine by oxidative treatment under microwave irradiation. Polym Degrad Stab. 2003;82:395–398. doi: 10.1016/S0141-3910(03)00177-0. [DOI] [Google Scholar]
- Sirdeshmukh L, Puranik PG. Structure of formamide and its force constants. P Math Sci. 1962;56:115–124. [Google Scholar]
- Smits ALM, Kruiskamp PH, van Soest JJG, Vliegenthart JFG. The influence of various small plasticisers and malto-oligosaccharides on the retrogradation of (partly) gelatinised starch. Carbohydr Polym. 2003;51:417–424. doi: 10.1016/S0144-8617(02)00206-0. [DOI] [Google Scholar]
- Thiré RMSM, Simáo RA, Andradeb CT. High resolution imaging of the microstructure of maize starch films. Carbohydr Polym. 2003;54:149–158. doi: 10.1016/S0144-8617(03)00167-X. [DOI] [Google Scholar]
- Tian F, Liu Y, Hu KA, Zhao BY. The depolymerization mechanism of chitosan by hydrogen peroxide. J Mater Sci. 2003;38:4709–4712. doi: 10.1023/A:1027466716950. [DOI] [Google Scholar]
- Tian F, Liu Y, Hu KA, Zhao BY. Study of the depolymerization behavior of chitosan by hydrogen peroxide. Carbohydr Polym. 2004;57:31–37. doi: 10.1016/j.carbpol.2004.03.016. [DOI] [Google Scholar]
- Verma AK, Banerjee R. Dietary fi bre as functional ingredient in meat products: a novel approach for healthy living-a review. J Food Sci Technol. 2010;47:247–257. doi: 10.1007/s13197-010-0039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SM, Huang QZ, Wang QS. Study on the synergetic treatment of chitosan with ultraviolet light and hydrogen peroxide. Carbohydr Res. 2005;340:1143–1147. doi: 10.1016/j.carres.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Wei XL 2004. Study on properties, fractionation, and immune activity of chitooligosaccharides. PhD thesis, School of Food Science and Technology, Jiangnan University, Wuxi, China
- Yalpani M, Pantaleone M. An examination of the unusual susceptibilities of aminoglycans to enzymatic hydrolysis. Carbohydr Res. 1994;256:159–175. doi: 10.1016/0008-6215(94)84235-3. [DOI] [Google Scholar]
- Zobel HF, Kulp K. The staling mechanism. In: Hebeda RE, Zobel HF, editors. Baked goods freshness: technology, evaluation, and inhibition of staling. New York: Marcel Dekker; 1996. pp. 1–64. [Google Scholar]