Abstract
Bacteriology of Panipuri was studied and the antibacterial effect of eight essential oils (EOs) was established on pathogens found in Panipuri. Samples were collected from twelve respective vendors from different locations in Baripada city, Orissa. Samples were fractionated into two parts viz. khatta pani and smashed potato masala used in Panipuri. Total plate count and isolation of pathogenic bacteria were done on both basal and selective media. Coliforms were detected primarily by presumptive test and confirmed subsequently, using Eosine Methylene Blue Agar. Selected colonies were pure cultured and identified through staining and an array of biochemical reactions. Antibiogram pattern of the pathogens and their susceptibility towards eight different EOs were performed. Antibacterial efficacy of four EOs in food sample was studied. Aerobic bacterial load of solid samples was observed to be more than in the liquid samples. Coliform-positive samples were found to be of 80.33%. Pathogenic bacteria like Escherichia coli, Klebsiella sp., Enterobactor sp., Bacillus sp., Enterococcus sp., Micrococcus tetragens, Salmonella paratyphi, Shigella dysenteriae and Vibrio sp. were detected. Antibiogram studies of the isolates showed multiple antibiotic resistance index (MRI;%) ranging from 15 to 92%. Among the EOs studied Cinnamon and Clove oils showed maximum antibacterial activity. Antibacterial efficacy showed that Clove and Cinnamon oils were comparatively of superior quality than Turmeric leaf and Japanese mint oils to kill food borne pathogens. Although it was a preliminary endeavor, the present study is a prerequisite in understanding the significance of pathogenic microorganisms in street foods and use of EOs as both antibacterial agents and food preservatives.
Keywords: Bacteriology, Street food, Panipuri, Essential oils, Pathogens, Antibacterial activity
Introduction
Food and Agriculture Organization, (FAO 1997) defines street foods as “ready-to-eat foods and beverages prepared and/or sold by vendors and hawkers especially in street and other similar public places”. Urban people of present time are being dependent on street foods or ready-to-eat food items because of the scarcity of time and money. Consequently, street foods are enjoying the top list in popularity. In developing countries, drinks, meals and snacks sold by street food vendors are widely consumed by millions of people (FAO 1988). The most popular street foods in India are Panipuri or Gol gappas and Papdi chaat among others. Although it is very popular, easily available and cheap, it is frequently associated with various food borne diseases. Food borne illness associated with the consumption of street foods has been reported in several places in India and elsewhere (FAO 1988; Estrada-Garcia et al. 2004; Chumber et al.2007; Ghosh et al. 2007). Selling the foods road side, unhygienic preparation and handling, insufficiency in water supply for cleaning purposes, make the street food one of the major sources of food borne diseases.
Though, various physical and chemical agents are used in different food industries for preservation of food materials, natural biological compounds are always in demand as these are eco-friendly, degradable, devoid of side effects and more precisely cheaper than chemical preservatives. Recently, the World Health Organization (WHO) has compiled a list of 20,000 plants that are used for phytotherapy in herbal systems of medicine all over the world (Manavalan and Manian 2001). In this regard, medicinal and aromatic plants and their essences are in the front-line of choice in the food industry. Foremost, among these are the volatile compounds and essential oils. Essential oils are secretory, secondary metabolites of plants. Different essential oils have been reported to have antibacterial activity with antioxidant properties (Rath 2007; Lis-Balchin et al. 1998). Use of essential oils as natural food preservatives is being discussed in literature too (Rath 2007).
Considering the aspects discussed above regarding street foods and use of natural biological product as food preservatives, an attempt is made in this investigation to study the bacteriology of a popular street food Panipuri in and around Baripada municipality area in Mayurbhanj district of Orissa, India and to know antibacterial effect of selective essential oils on pathogens found in Panipuri.
Materials and methods
Materials
Nutrient Broth (NB), Nutrient Agar (NA) and various selective media viz. MacConkey Broth (MB), Xylose Lysine Deoxycholate (XLD) Agar, Salmonella Shigella (SS) Agar, Thiosulphate Citrate Bilesalt Sucrose (TCBS) Agar and Eosine Methelene Blue (EMB) Agar, Triple Sugar Iron (TSI) Agar, Simon’s Citrate (SC) Agar, Peptone water (PW), Manitol Motility (MM) Medium and Urinary-tract Infection (UTI)- Agar were procured from Hi-Media, Mumbai and prepared as per manufacturer’s instructions. Antibiotic discs were also procured from Hi-Media, Mumbai. Hydro steam distilled essential oils viz. Turmeric leaf (Curcuma longa), Japanese mint (Mentha arvensis), Peppermint (Mentha piperata), Orange (Citrus aurantium), Lemon (Citrus limonum), Ginger (Zingiber officinalis), Clove (Syzygium aromaticum) and Cinnamon (Cinnamum zeylanicum) were procured from Auroshikha, Shri Aurobindo Ashram, Pondicherry; DLR College, Gollalimamidada and Oil Technological Research Institute, Anantpur, AP, India.
Sample collection
Total of 12 Panipuri samples were collected from different vendors in and around Baripada town in Mayurbhanj district of Orissa. The khatta pani (sour soup) is the liquid part served with panipuri and the potato masala, the solid part were collected separately in different pre-sterilized vials and analysed within 1 h of collection.
Determination of pH of the sample
The pH of both the sample-fractions i.e. khatta pani and potato masala were determined by the help of pH paper strip, directly at the collection-point and also through pH meter (Systronics-361) in the laboratory.
Determination of aerobic bacterial load
The total viable aerobic bacterial load in Panipuri was estimated by spread plate method (Cruickshank et al. 1975) on pre-sterilized solidified NA, MA, SS, XLD, TCBS and EMB agar media.
Isolation of bacteria
Selective bacterial colonies were isolated from streak and spread plates on to NA slants which were preserved at 4 °C for future use.
Detection of coliforms
Presence of coliform was studied both in liquid and solid parts (potato masala dissolving in sterilized distilled water at a final concentration of 1%) of the sample collected. Presumptive and confirmatory coliform tests were carried out by Most Probable Number (MPN 3-tube test) and sub-culturing the same onto EMB agar plates, respectively (Aneja 1996).
Identification of isolates
Characterization and identification of the isolates were made through standard microbiological methods (Cruickshank et al. 1975; Collins and Lyne 1970; Bhat and Myero 1962; Holt et al. 2000).
Antibiogram pattern study
Antibiotic sensitivity test was carried out for the representative isolates of all identified genus against 14 antibiotics [ampicillin (10 μg), bacitracin (10 U), carbencillin (100 μg), cefotaxim (30 μg), ciprofloxacin (30 μg), erythromycin (15 μg), gentamycin (10 μg), gatifloxacin (30 μg), norfloxacin (10 μg), penecillin G (10 U), polymyxin-B (300 U), tetracycline (30 μg), trimethoprim (10 μg), vancomycin (30 μg)] by disc diffusion method (Bauer et al. 1966) and multiple antibiotic resistant index (MRI;%) was determined, (Mahapatra et al.2006), using the formula:
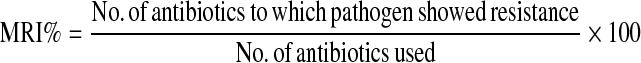 |
Screening of antimicrobial activity of essential oils
Antimicrobial efficiency of eight essential oils viz. Turmeric leaf, Japanese mint, Peppermint, Lemon, Orange, Ginger, Clove and Cinnamon were studied against representative isolates of all identified genus, by disc diffusion method. Freshly grown culture (overnight culture in NB) of the test pathogens was swabbed over the NA plates individually, using a sterile cotton swab as to make a lawn culture of the pathogen. Sterilized filter paper discs (5 mm diameter) were loaded with 10 μl of essential oil placed over the lawn culture at appropriate distance and the plates were incubated at 37 °C for 18–24 h. After the incubation period plates were observed for a clear zone around the discs which indicates a positive antibacterial activity of the respective oil (susceptibility of the pathogen towards the oil).
Study of antibacterial efficacy of essential oils in the samples
An experiment was designed to study the antibacterial efficacy of four essential oils (Clove, Cinnamon, Turmeric leaf and Japanese mint) that showed relatively better antibacterial activities (on the basis of the preliminary screening), by introducing them directly to the liquid and solid parts of Panipuri. In each of eight test tubes, 10 ml of khatta pani was taken. A set of four test tubes containing the sample were sterilized and regarded as ‘Set No. 1’ to which a bacterial consortium of 40 μl (10 μl each of Escherichia coli, Enterobacter sp., Staphylococcus sp., and Klebsiella sp. isolated previously) were inoculated. The other four tubes containing the unsterilized samples without the bacterial inoculum were labeled as ‘Set No. 2’. Different aliquots (125 μl, 250 μl and 500 μl) of essential oils were added separately to three test tubes of each set. The fourth tubes served as control. Similarly one gram of potato masala weighed aseptically and added separately to 9 ml of pre-sterilized distilled water taken in eight test tubes and divided into 2 sets (4-each). One set was sterilized to which 40 μl of bacterial consortium was added. Control and test sets were maintained by adding different aliquots of oils as described previously.
All the sets were incubated at 37 °C for 24 h, after which, these were subcultured by introducing one loop of sample onto NA plates and then kept under incubation at 37 °C for 24 h. After the incubation period plates were observed for growth and viability of pathogens. The subculturing was continued for 5 days at an interval of every 24 h and efficacy of essential oils was determined by comparing the growth of pathogens with control.
Results and discussion
In the present study total 12 Panipuri samples were collected from different public places of Baripada. Each sample was fragmented into two different segments (the liquid khatta pani and solid potato masala) and evaluated further. Vendors sell their samples on stands, carts or on improvised structures that are assembled every day on roadsides and share the area with several other street vendors. They cook/prepare these food items fully or partly at their homes and keep at ambient temperature, which stimulates the growth of the mesophilic organisms including food borne pathogenic bacteria. Further, none of the vendors use gloves or head caps during preparation and selling.
The pH of liquid samples observed to be highly acidic (pH 2.5–3.0) whereas, pH of solid samples ranged between 5.5–6.0. The high acidity of the khatta pani could be attributed to the addition of tamarind juice and other acidic ingredients to it.
The total aerobic bacterial load was determined separately for liquid and solid parts of the samples collected, through spread plate method. The aerobic bacterial load of khatta pani was more in NA and MA in comparison to the other media (Table 1). However, nine (66.7%) samples of potato masala showed viable aerobic bacterial load on all the media used. Highest bacterial load was recorded on NA (105–1010) followed by MA (106–107) (Table 1). The findings are in proper correlation with total aerobic bacterial loads reported earlier from varieties of street foods (Bryan 1988; Kumar et al. 2006; Tambekar et al.2008) on basal media like NA and MA and the specialized media used in this investigation. Above results revealed that the potato masala is more contaminated than the khatta pani. It could be attributed to high pH and more nutrient content of potato masala than khatta pani, as bacterial growth is regulated by nutrients and pH. The contamination in Panipuri is high because of the conditions under which it is prepared and vended. In most cases running water is not available at vending sites and thus hand and dish washing are usually done in buckets and sometimes without soap (Tambekar et al.2008). The serving plates or the containers made of plant leaves are not properly washed, waste water and garbage are discarded nearby in addition to unhygienic food handling increase the contamination. Vendors usually prepare and serve the food in bare and unwashed hands which is one of the most potable sources of contamination. Street foods are frequently associated with diarrhoeal diseases due to improper handling and serving practices (WHO 2002; Bhaskar et al.2004; Barro et al.2006). Cross contamination of street foods is also increased by unsanitary processing and preservation. The use of raw vegetables also contributes to the bacterial load (Terry and Overcast 1976).
Table 1.
Total aerobic bacterial load of Panipuri
| Sample No. | Viable count (log10 cfu/ml of Khatta pani, log10 cfu/g of Potato masala) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NA | MA | SS | XLD | TCBS | EMB | ||||||||
| Khatta pani | Potato masala | Khatta pani | Potato masala | Khatta pani | Potato masala | Khatta pani | Potato masala | Khatta pani | Potato masala | Khatta pani | Potato masala | ||
| 1 | 6.3 | 9.2 | 4.5 | 7.2 | 3.7 | 3.7 | 3.9 | 3.0 | – | 3.0 | 3.5 | 6.0 | |
| 2 | 3.9 | 9.3 | – | 7.4 | – | 2.9 | – | 4.0 | – | 3.9 | 4.1 | 2.3 | |
| 3 | 4.0 | 7.4 | 5.9 | 7.4 | 3.8 | 5.5 | 3.9 | 2.6 | 3.7 | 2.7 | 4.0 | 5.6 | |
| 4 | 5.3 | 6.9 | 4.5 | 6.7 | 3.8 | – | 4.3 | – | 3.5 | – | 4.1 | 3.5 | |
| 5 | 5.3 | 5.7 | – | – | – | – | – | – | – | – | – | 5.8 | |
| 6 | 4.3 | 9.4 | – | 7.3 | – | 3.5 | – | 2.6 | – | 3.5 | – | 3.9 | |
| 7 | 3.5 | 10.2 | – | 7.1 | – | 6.0 | – | 5.8 | – | 2.5 | – | 3.8 | |
| 8 | 3.5 | 10.2 | – | 7.4 | – | 2.3 | 3.5 | 3.4 | – | 3.4 | – | 5.6 | |
| 9 | 4.5 | 10.4 | – | 7.2 | – | 2.4 | – | 3.1 | – | 3.3 | 3.5 | 5.6 | |
| 10 | 3.5 | 5.7 | – | – | – | – | – | – | – | – | 3.5 | 3.5 | |
| 11 | 5.2 | 10.3 | 4.5 | 7.2 | 3.5 | 3.8 | 3.5 | – | – | – | 3.5 | 5.9 | |
| 12 | 3.8 | 10.5 | – | 7.1 | 3.6 | 5.9 | – | 6.6 | – | 3.0 | – | 5.8 | |
--No valid plate count, NA Nutrient Agar, MA MacConkey Agar, SS Salmonella Shigella, XLD Xylose Lysine Deoxycholate, TCBS Thiosulphate Citrate Bilesalt Sucrose, EMB Eosine Methelene Blue.
Presence of coliforms was reported in 80.33% of samples, indicating a high risk that other pathogenic organisms might have also contaminated the food (Hanashiro et al.2005). Our findings are in perfect correlation with the previous reports of high incidence of total coliform counts in street vended Fruit chaats in Patiala city, India (Kumar et al. 2006).
In toto 96 isolates were selected from 24 fragmented samples and identified. The isolates were characterized through colony characters on specific media, gram staining and via a battery of biochemical characters as described earlier and designated to different genera. The presence of Enterobacter sp. was highest (28.8%) in the samples followed by Escherichia coli (13.6%) and Klebsiella sp. (10.6%). However, Salmonella paratyphi (1.5%), Micrococcus (3.0%) and Bacillus sp. (3.0%) were present in low percentage (Table 2). The major occurrence of Enterobacter sp., Escherichia coli, Klebsiella sp., may be due to poor personal hygiene of the vendors, unhygienic handling of foods, poorly cleaned dishes and use of raw vegetables like cucumber, onion etc. Detection of Vibrio sp. in eight cases further indicates water borne contamination of these samples. In a few similar studies, Das-Mohapatra et al. (2002) reported presence of Escherichia coli, Shigella sp., Staphylococcus sp. and Bacillus sp. in four popular street foods (including Panipuri) of Bhubaneswar city, whereas, Das et al. (2010) showed that street foods such as Panipuri, Bhelpuri and Chaat in Bangalore city, were contaminated with high loads of pathogens viz. Streptococcus faecalis, Escherichia coli, Staphyloccus aureus, Bacullus sp., Klebsiella sp. and Pseudomonas sp., which corroborate with our findings. The detection of respiratory pathogens such as Klebsiella sp., in Panipuri water attributed to the bacterial aerosols generated due to sneezing and coughing in public places. The results of the present study are in agreement with those reported by Seth et al. (2005) which revealed the presence of high aerobic mesophilic colony count and detection of Staphylococcus and Escherichia coli in Bhelpuri samples in Vadodra city. Further, occurrence of Bacillus species in Panipuri implicated the ubiquitous nature of bacterial spores especially in dusty road side locations. Both Bacillus and Staphylococcus sp. normally exhibit tolerance to a wide range of temperature and pH which justifies their presence in Panipuri even at highly acidic conditions (Desai and Varadaraj 2010; de-Barros et al. 2009; Gibbons 2004).
Table 2.
Bacterial isolates recovered from different samples
| Isolates | Recovered from number of samples | Percentage (%) of recovery |
|---|---|---|
| Arizona sp. | 02 | 4.5 |
| Bacillus sp. | 04 | 3.0 |
| Enterobacter sp. | 24 | 28.8 |
| Enterococcus sp. | 04 | 6.1 |
| Escherichia coli | 20 | 13.6 |
| Klebsiella sp. | 08 | 10.6 |
| Micrococcus tetragens | 04 | 3.0 |
| Salmonella paratyphi B. | 02 | 1.5 |
| Shigella dysenteriae | 06 | 4.5 |
| Staphylococcus sp. | 04 | 3.0 |
| Vibrio sp. | 08 | 6.1 |
| Unidentified | 10 | 15.2 |
From the antibiogram pattern studies it was observed that all the isolates were sensitive to gatifloxacin, polymixin B, norfloxacin, tetracycline and erythromycin, except Micrococcus tetragens and Salmonella paratyphi. Micrococcus tetragens was only sensitive to polymixin B (Table 3). All the bacteria were resistant to carbenicillin, and ampicillin. The MRI;% of the isolates ranged between 15.4–92.3. Micrococcus tetragens showed the highest MRI;% (92.3), followed by Salmonella paratyphi (72.4), Shigella dysenteriae (57.1) and Bacillus sp. (50.0). Escherichia coli (15.4) showed the lowest MRI;% which in turn reflects its susceptibility towards the antibiotics. Development of multiple antibiotic resistance among bacteria is of global concern today in clinical studies, hence, presence of such pathogens in street food items as reported in this study is also alarming.
Table 3.
Antibiotic sensitivity pattern of the isolates
| Organism | Antibiotics | MRI;% | |
|---|---|---|---|
| Sensitivea to | Resistant to | ||
| Arizona sp. | Cf(32), B(11), E(15), T(17), Tr(25), G(21), Nx(32), Gf(29), Pb(10) | P, Va, A, Ce, Cb | 35.7 |
| Bacillus sp. | Cf(17), Va(17), T(24),G(12), Nx(25), Gf(27), Pb(11) | P, B, A,Ce, E, Tr, Cb | 50.0 |
| Enterobacter sp. | Cf(33),Va(15), E(18), T(20), G(17), Nx(23), Gf(22), Pb(12) | P, B, A, Ce, Tr, | 42.8 |
| Enterococcus sp. | Cf(33), B(9), Ce(11), E(12), T(15), Tr(22), Nx(27), Gf(24), Pb(12) | P, Va, A, G, Cb | 30.8 |
| Escherichia coli | Cf(29), B (9), Va(10), Ce(19), E(16), T(21), Tr(25), G(13), Nx(26), Gf(27), Pb(11) | P, A, Cb | 15.4 |
| Klebsiella sp. | Ce(17), E(16),T(20), Tr(24), G(18), Nx(15), Gf(23), Pb(11) | P, B, Va, A, Cb | 30.8 |
| Micrococcus tetragens | Pb(14) | Cf, B, Va, A, Ce, E, T, Tr, G, Nx, Gf, Cb, P | 92.3 |
| Salmonella paratyphi | Ce(12), E(14), T(28), G(22) | P, Cf, B, Va, A, Tr, Nx, Gf, Pb, Cb | 71.4 |
| Shigella dysenteriae | Cf(40), T(20), G(24), Nx(34), Gf(40), Pb(16) | P, B, Va, A, Ce, E, Tr, Cb | 57.1 |
| Staphylococcus sp. | Cf(12), B(9), Va(15), E(27), T(17), Tr(21), G(23), Nx(18), Gf(25), Pb(11), | A, Ce, Cb | 23.1 |
| Vibrio sp. | Cf(34), Va(12), E(17), T(18), Tr(28), G(21), Nx(21), Gf(22), Pb(10) | P, B, A, Ce, Cb | 38.5 |
aValues in parentheses represent zone of sensitivity in mm; MRI Multiple Antibiotic Resistance Index; A Ampicillin; B Bacitracin; Cb Carbencillin;
Ce Cefotaxime; Cf Ciprofloxacin; E Erythromycin; G Gentamycin; Gf Gatifloxacin; Nx Norfloxacin; P Penicillin; Pb Polymyxin B; T Tetracycline; Tr Trimethoprim, Va Vancomycin
While studying the antibacterial activity of the essential oils during the primary screening by disc diffusion method it was observed that Arizona sp., Bacillus sp., Enterococcus sp., Staphylococcus sp. and Vibrio sp., showed 100% susceptibility towards different essential oils (Table 4). Among the essential oils, Cinnamon and Clove oils showed 100% antibacterial efficacy towards the test pathogens followed by Japanese mint and Turmeric leaf oils, whereas, Orange oil showed the lowest activity. Antibacterial activity of Ginger, Turmeric leaf and Japanese mint essential oils against the above pathogens are also reported by Gupta et al. (2004). Rath et al. (2005) reported the antibacterial activity of Lemon oil against Staphylococcus sp. Considering the development of multiple antibiotic resistant microorganisms, the scientific communities in the recent past are relying on herbal medications all over the world. This prompted us to evaluate the antibacterial efficacy of some of these essential oils, directly supplementing to the Panipuri samples at varied concentrations to find their possible use as an alternate antibacterial agent and natural food preservatives.
Table 4.
Susceptibility of selected isolates towards different edible essential oils
| Isolates | Zone of inhibition (mm)a | % Susceptibility | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Source of essential oil | |||||||||
| Orange | Lemon | Peppermint | Japanese mint | Cinnamon | Ginger | Turmeric leaf | Clove | ||
| Arizona sp. | CI | CI | CI | CI | S(22) | S(19) | S(10) | S(25) | 100 |
| Bacillus sp. | CI | CI | CI | CI | S(16) | S(16) | S(16) | S(19) | 100 |
| Enterobacter sp. | S(28) | S(10) | S(13) | S(13) | S(15) | S(18) | S(14) | S(22) | 100 |
| Enterococcus sp. | R | R | R | S(10) | S(15) | R | S(12) | S(17) | 50 |
| Escherichia coli | R | S(8) | R | R | S(11) | S(15) | S(12) | S(20) | 62.5 |
| Klebsiella sp. | R | S(8) | S(15) | S(12) | S(13) | R | S(12) | S(18) | 75 |
| Micrococcus tetragens | R | R | S(10) | S(9) | S(30) | S(15) | R | S(30) | 62.5 |
| Salmonella paratyphi | R | R | S(10) | S(10) | S(13) | R | S(11) | S(18) | 62.5 |
| Shigella dysenteriae | R | R | S(10) | S(11) | S(13) | R | S(13) | S(28) | 62.5 |
| Staphylococcus sp. | S(20) | S(10) | S(30) | S(34) | S(12) | S(12) | S(12) | S(15) | 100 |
| Vibrio sp. | S(12) | S(12) | CI | CI | S(21) | S(12) | S(12) | S(26) | 100 |
Oil was loaded 10 μl in each disc
aValues in the parenthesis represents zones of inhibition, CI Complete Inhibition of the pathogen, R Resistant, S Sensitive
Antibacterial efficacy of the essential oils (viz. Turmeric leaf, Japanese mint, Clove and Cinnamon selected through screening) in varying concentrations revealed that all these oils were able to kill the pathogens of the liquid khatta pani within 24–72 h of incubation at a concentration of 125 μl/10 ml as well as 250 μl/10 ml irrespective of Set No. 1 (sterilized samples inoculated with 40 μl of bacterial consortium) and 2 (unsterilized samples containing pathogens) (Table 5). However, at a concentration of 500 μl/10 ml, this effect was observed within 24 h in case of Turmeric leaf, Clove and Cinnamon oils. On the other hand, when solid fraction of samples (potato masala) was studied, it was observed that efficiency of those essential oils varied with respect to the sets (Set No. 1 and Set No. 2) (Table 5). Clove and Cinnamon oils could kill the pathogens of Set No. 2 after 72 h of incubation at concentration of 125 μl/10 ml. At concentration of 500 μl/10 ml, Clove, Japanese mint and Turmeric leaf oils were effective after 48 h of incubation whereas, Cinnamon could show the efficacy after 24 h. In case of Set No. 1 pathogens were killed by Clove and Cinnamon oils within 24 h of incubation at concentration of 500 μl/10 ml. However, Japanese mint and Turmeric leaf oils were unable to exhibit any effect even after 5 days of incubation at 500 μl/10 ml concentration. Invariably, in all cases the killing time of pathogens was much quicker in case of liquid khatta pani in comparison to the solid potato masala sample. It may be due to low pH, better mixing, availability of less nutrients in liquid samples as discussed earlier. Further, in case of potato masala, the pathogens could have been coated with the potato paste (carbohydrate), which makes them non-available to the oils for reaction. Senhaji et al. (2007) observed the antibacterial activity of Cinnamon oil against Escherichia coli O157:H7 by outer membrane disintegration and increasing the permeability of ATP through cytoplasmic membrane indicating that physical contact of essential oil components to the bacterial cell surface is necessary for execution of their antibacterial properties. The antibacterial activity of essential oils through membrane inhibition could be attributable to the hydrophobicity of essential oils that enables them to make partitions in the membrane, rendering permeability and leading to leakage of cell contents resulting in death of microbial cells.
Table 5.
Antibacterial efficacy of essential oils in Panipuri
| Essential oils | Set No. | Killing Time (h) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Concentration of oil (μl/10 ml) | |||||||||
| 125 | 250 | 500 | |||||||
| Components of Panipuri | |||||||||
| Khatta pani | Potato masala | Khatta pani | Potato masala | Khatta pani | Potato masala | ||||
| Clove oil | 1 | 72 | 48 | 24 | 24 | 24 | 24 | ||
| 2 | 72 | 72 | 24 | 72 | 24 | 48 | |||
| Cinnamon oil | 1 | 48 | 24 | 24 | 24 | 24 | 24 | ||
| 2 | 24 | 72 | 24 | 72 | 24 | 24 | |||
| Turmeric leaf oil | 1 | 24 | NE | 24 | NE | 24 | NE | ||
| 2 | 48 | 48 | 24 | 48 | 24 | 48 | |||
| Japanese mint oil | 1 | 48 | NE | 48 | NE | 48 | NE | ||
| 2 | 24 | 72 | 24 | 72 | 24 | 48 | |||
Set No. 1=Sterilized sample inoculated with bacterial consortium; Set No. 2=Unsterilized sample containing pathogens;
NE No effect of essential oils even after 120 h (5 days) of incubation.
Lis-Balchin et al. (1998) studied the effect of Pelargonium oil on quiche filling on food borne pathogens that corroborates with our findings during this investigation. Tassou et al. (1995) also studied the effects of essential oils from mint (Mentha piperata) on Salmonella enteritidis and Listeria monocytogens in model food systems at different temperatures. In a study Rehman et al. (2007) determined the effect of citrus essential oil on microbial growth using bread as a model system. They reported the delayed and inhibitory microbial growth in bread as observed with potato masala during our investigation. High concentrations of essential oils or components would have a considerable odour effect when used in foods and the present study shows that low concentration of different essential oils used under adverse microbial conditions (low pH, adverse coating) have a beneficial effect. Research in the essential oils field has incredibly increased, chiefly with respect to the antimicrobials used to control food pathogens and food native microflora (Sabulal et al. 2006) and the knowledge of possible mechanism of action of these oils (Singh et al. 2002). These results revealed the potential of essential oils as natural preservatives in food products.
Conclusion
In this investigation we have reported the bacteriology of a commonly consumed street food, Panipuri and effect of essential oils on the isolated pathogens in in-vitro as well as in model food system. It is pertinent to be suggestive that these essential oils could be used in food items as antibacterial agents as well as natural food preservatives due to their edibility and nontoxic nature with antioxidant and potent antibacterial properties.
References
- Aneja KR. Experiments in microbiology, plant pathology, tissue culture and mushroom cultivation. New Delhi: New Age International Pvt. Ltd; 1996. [Google Scholar]
- Barro N, Bello AR, Savadogo A, Ouattara CAT, IIboudo AJ, Traore AS. Hygienic status assessment of dish washing waters, utensils, hands and pieces of money from street food processing sites in Ouagadougou (Burkina Faso) Am J Biotechnol. 2006;5(11):1107–1112. [Google Scholar]
- Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disc method. Am J Clin Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- Bhaskar J, Usman M, Smitha S, Bhat GK. Bacteriological profile of street foods in Mangalore. Indian J Med Micribiol. 2004;22:197–197. [PubMed] [Google Scholar]
- Bhat P, Myero RM. Standard methods and procedures used in the bacteriology laboratory of Vellore Christial Medical College Hospital for isolation and identification of organisms belonging to the family Enterobacteriaceae. Indian J Med Res. 1962;50(4):559–566. [Google Scholar]
- Bryan FL. Risk of practices, procedures and processes that lead to outbreaks of food borne diseases. J Food Prot. 1988;51:663–673. doi: 10.4315/0362-028X-51.8.663. [DOI] [PubMed] [Google Scholar]
- Chumber SK, Kaushik K, Savy S. Bacteriological analysis of street foods in Pune. Indian J Public Health. 2007;51(2):470–476. [PubMed] [Google Scholar]
- Collins CH, Lyne PM. Microbiological methods. London: Butterworths; 1970. [Google Scholar]
- Cruickshank R, Duguid JP, Marmion BP, Swain RHA. Medical microbiology. London: Churchill Livingstone; 1975. [Google Scholar]
- Das A, Nagananda GS, Bhatacharya S, Bharadwaj S. Microbiological quality of street vended Indian Chaats sold in Bangalore. J Biol Sci. 2010;10:255–260. doi: 10.3923/jbs.2010.255.260. [DOI] [Google Scholar]
- Das-Mohapatra A, Rath CC, Dash SK, Mishra RK. Microbiological evaluation of street foods in Bhubaneswar. J Food Sci Technol. 2002;39(1):59–61. [Google Scholar]
- de-Barros JC, da-Conceição ML, Neto NJG, da-Costa ACV, Júnior JPS, Junior IDB, de-Souza EL. Interference of Origanum vulgare L. essential oil on the growth and some physiological characteristics of Staphylococcus aureus strains isolated from foods. LWT Food Sci Technol. 2009;42(6):1139–1143. doi: 10.1016/j.lwt.2009.01.010. [DOI] [Google Scholar]
- Desai SV, Varadaraj MC. Behavioural pattern of vegetative cells and spores of Bacillus cereus as affected by time-temperature combinations used in processing of Indian traditional foods. J Food Sci Technol. 2010;47:549–556. doi: 10.1007/s13197-010-0099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Garcia T, Lopez-Sancedo C, Zamarripa-Ayala B, Thompson MR, Gutierrez L. Prevalence of Escherichia coli and Salmonella sp. in street vended food of open markets (tianguis) and general hygienic and trading practices in Mexico City. Epidemiol Infect. 2004;132:1181–1184. doi: 10.1017/S0950268804003036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street foods. Report of an FAO expert consultation, Yogyakarta, Indonesia. Rome: Food Agriculture Organization; 1988. [Google Scholar]
- Street foods. Report of an FAO technical meeting on street foods Calcutta. Rome: Food Agriculture Organization; 1997. [PubMed] [Google Scholar]
- Ghosh M, Wahi S, Ganguli KM. Prevalence of enterotoxigenic Staphylococcus aureus and Shigella sp. in some raw street vended Indian foods. Int J Environ Health Res. 2007;17(2):151–156. doi: 10.1080/09603120701219204. [DOI] [PubMed] [Google Scholar]
- Gibbons S. Anti-staphylococcal activity activity of natural plants. Nat Prod Rep. 2004;21:263–277. doi: 10.1039/b212695h. [DOI] [PubMed] [Google Scholar]
- Gupta R, Rath CC, Das SK, Mishra RK. In vitro antibacterial potential assessment of Carrot (Daucus carota) and Celery (Apium graveolens) seed essential oils against twenty one bacteria. JEOBP. 2004;7(1):79–86. [Google Scholar]
- Holt JG, Kreig NR, Sneath PHA, Steley JT, Williams ST. Bergey’s manual of determinative bacteriology. Philadelphia: Williams and Wilkins; 2000. [Google Scholar]
- Hanashiro A, Morita M, Matte GR, Matte MH, Torres EAFS. Microbiological quality of selected street foods from a restricted area of Sao Paulo city, Brazil. Food Control. 2005;16:439–444. doi: 10.1016/j.foodcont.2004.05.004. [DOI] [Google Scholar]
- Kumar M, Agarwal D, Ghosh M, Ganguli A. Microbiological safety of street vended fruit chats in patiala city. Indian J Med Microbiol. 2006;24:75–76. doi: 10.4103/0255-0857.19905. [DOI] [PubMed] [Google Scholar]
- Lis-Balchin M, Buchhauer G, Hirtenlehner T, Resch M. Antimicrobial activity of Pelargonium essential oils added to a quiche filling as a model food system. Lett Appl Microbiol. 1998;27:207–210. doi: 10.1046/j.1472-765x.1998.t01-1-00423.x. [DOI] [PubMed] [Google Scholar]
- Mahapatra S, Rath CC, Dash SK, Mishra RK. Microbial evaluation of wounds and their susceptibility to antibiotics and essential oils. J Microb World. 2006;8:101–109. [Google Scholar]
- Manavalan ARS, Manian K. Medicinal and aromatic plants– diversity and utility. New Delhi: Allied Publisher Ltd.; 2001. [Google Scholar]
- Rath CC. Prospects and challenges of essential oils as natural food preservatives—a review. Food. 2007;1(2):172–180. [Google Scholar]
- Rath CC, Dash SK, Mishra RK. Anti staphylococcal activity of lime and juniper essential oils against MRSA. Indian Drugs. 2005;42:797–801. [Google Scholar]
- Rehman S, Hussain S, Nawaz H, Ahmad MM, Murtaza MA, Rizvi AJ. Inhibitory effect of Citrus peel essential oils on the microbial growth of bread. Pakistan J Nutr. 2007;6(6):558–561. doi: 10.3923/pjn.2007.558.561. [DOI] [Google Scholar]
- Sabulal B, Dan M, John A, Kurup R. Caryophyllene—rich rhizome oil of Zingiber nimmonii from South India: chemical characterization and antimicrobial activity. Phytochemistry. 2006;67:2469–2471. doi: 10.1016/j.phytochem.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Senhaji O, Mohmad F, Ichraq K. Inactivation of Escherichia coli O157:H7 by essential oil from Cinnamomum zeylanicum. Braz J Infect Dis. 2007;11:234–236. doi: 10.1590/S1413-86702007000200013. [DOI] [PubMed] [Google Scholar]
- Seth M, Gurudasani M, Mudbidri R. Screening for pathogenic microorganisms in street vended Bhelpuri in urban Vadodra: A HACCP approach. J Food Sci Technol. 2005;42:395–399. [Google Scholar]
- Singh N, Singh RK, Bhunia AK, Stroshine RL. Efficacy of chlorine dioxide, ozone and thyme essential oil or a sequential washing in killing E.coli O157: H7 on lettuce and baby carrot. LWT Food Sci Technol. 2002;35:720–729. doi: 10.1006/fstl.2002.0933. [DOI] [Google Scholar]
- Tambekar DH, Jaiswal VJ, Dhanorkar DV, Gulhane PB, Dudhane MN. Identification of microbiological hazards and safety of ready-to-eat food vended in streets of Amravati city. Indian J Appl Biosci. 2008;7:195–201a. [Google Scholar]
- Tassou CC, Drosinos EH, Nychas GJE. Effect of essential oil from Mint (Mentha piperata) on Salmonella enteriditis and Listeria monocytogenes in model food at 40 °C and 100 °C. J Appl Bacteriol. 1995;78:593–600. doi: 10.1111/j.1365-2672.1995.tb03104.x. [DOI] [PubMed] [Google Scholar]
- Terry RC, Overcast WW. A microbiological profile of commercially prepared salads. J Food Sci. 1976;41:211. doi: 10.1111/j.1365-2621.1976.tb01141.x. [DOI] [Google Scholar]
- Food safety and food borne illness. Geneva: World Health Organization Fact sheet 237; 2002. [Google Scholar]


