Abstract
An experiment was conducted on pear fruit (cv. ‘Lagoon’) to extend the shelf life by using different packaging materials. Fruits were packed in low density polyethylene (LDPE, 0.025 mm), polypropylene (PP, 0.025 mm), linear low density polyethylene (LLDPE, 0.0125 mm) and high density polyethylene (HDPE, 0.025 mm) with or without perforation and stored at ambient condition (25 ± 2 °C and 65.0 ± 5% RH). Periodical observations were recorded on CO2 & O2 concentration (%), physiological loss in weight (PLW, %), decay loss (%), firmness (kgf), colour value (colour difference and colour index), total soluble solid (TSS, °Brix), acidity (mg of malic acid/g), and ascorbic acid loss (%) at 3 days interval. Reduced rate of PLW and decay losses was recorded in pear fruits packed in PP non-perforated (8.04%) and PP perforated (12.5%), respectively as compared to other treatments. The maximum firmness (5.18 kgf) and minimum ascorbic acid loss (49.97%) were also recorded in PP non-perforated up to 12 and 15 days of storage, respectively. It could be inferred that the, PP non-perforated (0.025 mm) was the most suitable packaging materials for extending the shelf life of pear fruits up to 15 days at ambient condition.
Keywords: Pear fruit, Shelf life, Packaging materials, Storage, Quality, Firmness
Introduction
Pear (Pyrus communis L.) is popular with the consumers for its unique fragrance, subtle aroma, sweetness, and crispness. The fresh pear fruits are commonly used for table purposes as it has good eating quality with few stone cells. It matures in middle and late August, and is typically harvested at minimum soluble solids concentration of 10°–12°Brix. Pear fruits are harvested during a relatively narrow range of fruit maturity and it requires prompt cooling to remove field heat (Hansen and Mellenthin 1979). Pear fruits have very short shelf life of 7–10 days at room temperature (25–30 °C) without packaging. The shelf life of pear fruit is very short and it is susceptible to decay, mechanical damage, and moisture and nutritional losses during storage. In the North Eastern Hill Region of India, during harvest season, growers do not get remunerative prices at local markets due to glut and farmers are forced to sell their produce at throwaway prices.
Pear fruits are harvested in the month of August during which the temperature is comparatively high and therefore, it cannot be transported and stored for longer period. Moreover, rapid ripening process is also responsible for short shelf life of pear fruit and it represents a serious constraint for efficient handling and transportation (Zerbini 2002). Many storage techniques have been developed over the years to extend the storage life of fruits. Extension of shelf life has also been demonstrated using controlled atmosphere (CA) storage (Sun-XiSheng et al. 2000 and Ribeiro et al. 2003). However, CO2 injury, increased ethanol production and flavor problems due to anaerobic respiration have been reported (Sun-XiSheng et al. 2000; Saquet et al. 2003; Drake et al. 2004).
Among all the postharvest technologies available for the retention of overall quality of fruit at low temperatures, modified atmosphere packaging (MAP) has the advantage of low cost and easy implementation at the commercial level. The successful use of MAP is based on the specific permeation properties of polymer films to O2 and CO2 to generate atmospheres that are suitable for the postharvest life of many horticultural commodities (Sandhu and Singh 2000; Dou-ShiJuan et al. 2002, 2003). This technology also provides three advantages: it helps to reduce browning, to control postharvest diseases and it maintains a high humid environment inside the sealed plastic film (Dou-ShiJuan et al. 2002 and Kwon et al. 2003).
Little research has been reported in pear fruits storage in MAP at ambient conditions. Recently, the packaging of highly perishable fruits in polymeric films with specific gas permeabilities, in combination with low temperature storage has gained importance (Kader and Watkins 2000; Steward et al. 1999). Many types of plastic films are available for packaging, but relatively few have been used to wrap fresh produce. Low-density polyethylene, polyvinyl chloride, and polypropylene have been the main films used to package fruit and vegetables (Kader et al. 1989; Watkins 2000).
The purpose of the present study was to extend the shelf life of pear fruits (cv. Lagoon) at ambient condition using plastic packaging materials.
Materials and methods
Pear fruits (Pyrus communis L., Cv. Lagoon) were harvested at physiologically matured and ripen stage when fruit skin colour becomes dark green to light green and procured from the Horticulture farm, Govt. of Meghalaya during August, 2008. Fruits were washed thoroughly with tap water to remove dirt and other undesirable particles from the surface and also to reduce the microbial load causing contamination. After washing fruits were dried at room temperature for 2–3 h and thereafter, the fruits (450–500 g) containing 3–5 numbers having average weight of 85–110 g per fruit were packed in different plastic packaging materials (20 × 30 cm2) viz. T 1, control (kept open); T2, LDPE (0.025 mm, non-perforated); T3, LDPE (0.025 mm with 0.01% perforation), T4, PP (0.025 mm, non-perforated); T5, PP (0.025 mm with 0.01% perforation); T6, LLDPE (0.0125 mm non-perforated); T7, LLDPE (0.0125 mm with 0.01% perforation); T8, HDPE (0.025 mm non-perforated) and T9, HDPE (0.025 mm with 0.01% perforation). The total surface area of each film bag was 600 cm2, with film permeability of 3,000, 1,500, 4,100 and 3,500 cc/m2/mil/day at 1 atm for O2 and, 11,000, 6,000, 12,500 and 8,500 cc/m2/mil/day at 1 atm for CO2 respectively for all non perforated LDPE, PP, LLDPE and HDPE packaging materials. Each treatment was replicated five times. Packed fruits were kept at ambient condition (25 ± 2 °C and 65.0 ± 5% RH) and data were recorded at 3 days interval. Periodical observations were collected on headspace gases for CO2 and O2 concentration (%), physiological loss in weight (PLW, %), decay loss (%), firmness (kgf), colour (L, a, b value), total soluble solid (TSS, °Brix), acidity (mg of malic acid/g) and ascorbic acid loss (%).
Gas composition analysis
O2 and CO2 concentration were determined periodically by Gas analyzer (Model GS 3/P Gaspace Advance, Systech Instrument). Each of these packages had a gas sampling septum. On each sampling day, gas samples were withdrawn by inserting plastic syringe into the gas sampling septa and gas composition within the packages was determined.
Physiological loss in weight (PLW)
It was calculated as cumulative % loss in weight based on the initial fruit weight (before storage) and loss in weight was recorded at the time of periodical sampling during storage.
Decay loss
Decay loss was calculated from numbers fruits infected on each day of observation to the numbers of fruits initially taken. Then per cent of decay loss was worked out by the formula,
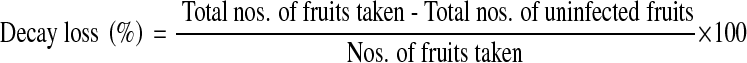 |
Fruit texture profile analysis
The texture characteristics of pear fruits in terms of firmness was measured using a Stable Micro System TA-XT2 texture analyzer (Texture Technologies Corp., UK) fitted with a 75 mm flat cutting blade probe. Firmness value was considered as mean peak cutting force and expressed in kgf. The studies were conducted at a pre test speed of 1.0 mm/s, test speed of 0.5 mm/s, distance of 30 mm, and load cell of 50.0 kg (Sirisomboon et al. 2000).
Fruit colour characteristics
The colour of fruit during initial and storage period was measured using Hunter L, a, b colour measuring system (Colour Quest XE model) and estimated as Hunter value L, a and b where ‘a’ (‘+’ value indicated redness and ‘−’ value indicated greenness), ‘b’ (‘+’ value indicated yellowness and ‘−’ value indicated blueness) and ‘L’ (varies from 0 to 100 where ‘100’ indicated white and ‘0’ indicated black) and colour index (a/b) and colour difference (ΔE) were calculated to study colour evolution. Hunter colour difference (ΔE) was calculated from the equation, 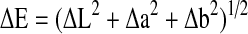 using initial colour values of pear as reference.
using initial colour values of pear as reference.
Chemical characteristics
The pulp of 3 fruits from each treatment was blended and the homogenized pulp was used for the estimation of total soluble solids (TSS), total acidity, TSS: acid ratio as per the methods of Ranganna (1995). TSS was determined with Erma hand refractometer (0–32°Brix). Total acidity was estimated by titrating against 0.1N sodium hydroxide using phenolphthalein as indicator and expressed as mg of malic acid per g. TSS: acid ratio was calculated by dividing the TSS value with acidity. Ascorbic acid content was determined by using 2, 6-Dichlorophenol-indophenol dye method of Freed (1966). 2.5 g of the sample were grounded with about 25 ml of 4% oxalic acid and filter through Whatman no. 4 filter paper. The filtrate was collected in a 50 ml volumetric flask and the volume was made up with 4% oxalic acid and titrated against the standard dye to a pink point. The amount of ascorbic acid was calculated using the formula given below and expressed as mg/ 100 g.
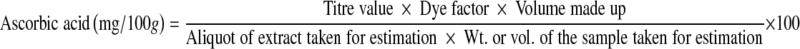 |
Statistical analysis
Data for all the quality parameters were subjected to analysis of variance (ANOVA), mean, standard deviation and standard error of mean. Sources of variation were storage time and treatments. All analysis was performed with a statistical software package SPSS v.11.0 for windows. Values of standard error of mean are showed in figures.
Results and discussion
Gaseous composition
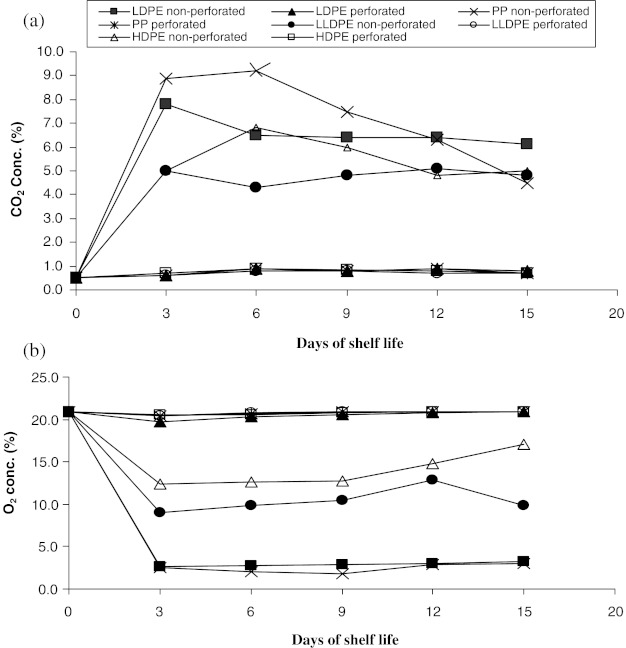
CO2 and O2 composition (%) inside the different plastic packaging materials during storage of pear fruits at ambient condition were presented in Fig. 1. From this Fig. 1, the pattern in the levels of CO2 and O2 gases in different non-perforated plastic packed fruits indicated that there was a rapid increase in CO2 levels in the initial storage period (up to 3 days) except LDPE perforated. While, rapid decrease in O2 gases was noticed in all non-perforated plastic packed fruits (up to 3 days). However, perforated plastic packed fruits during storage showed almost uniform levels of CO2 and O2 composition similar to open atmosphere. This rapid increase in CO2 levels and decrease in O2 levels inside the non-perforated plastic packed fruits during initial storage at ambient condition could be due to slower rate of respiration of fruits and also due to packaging materials low gas permeability. After initial storage, there was less availability of O2 inside the non-perforated packed fruits for respiration and therefore, reduction in the rate of respiration which ultimately caused decrease and increase in O2 and CO2 levels respectively as storage period advances. The balanced levels of CO2 and O2 in the package after initial storage could cause marked changes in the activities of specific enzymes of the respiratory metabolism and might have uncoupling effect on oxidative phosporylation (Kader 1986). Finally this might have led to the extension of shelf life of pear fruits in non-perforated plastic packaging materials as compared to control and perforated packed fruits. The present findings were in agreement with the previous findings of Drake et al. (2001), Dou-ShiJuan et al. (2002), Ribeiro et al. (2003) and Drake et al. (2004).
Fig. 1.
Effect of different packaging materials on gas concentration viz., a CO2 conc. (%) and b O2 conc. (%) of pear fruits during storage
Cumulative PLW
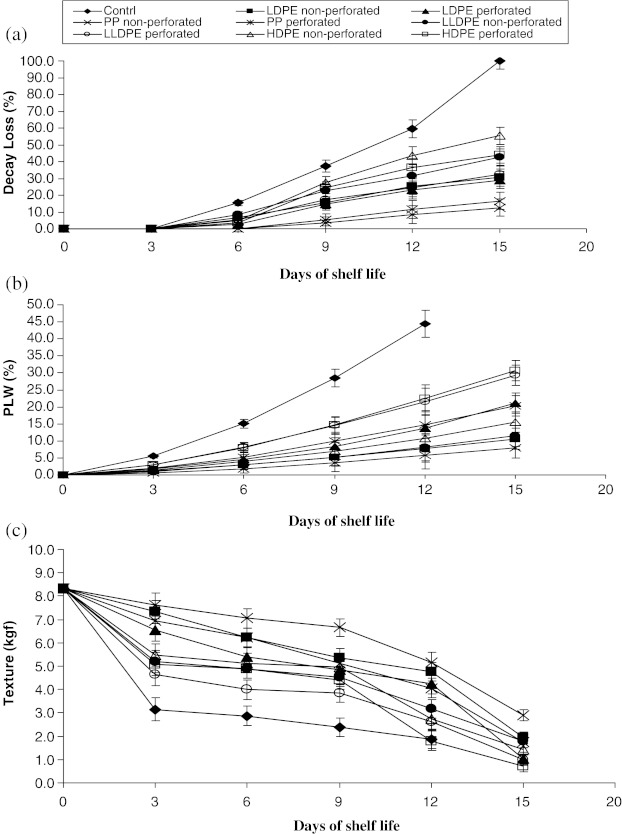
Results on PLW of pear fruits stored in different packages have indicated that weight loss was gradually increased in all the treatments. This could be mainly due to continuous loss of moisture due to transpiration from the fruit and respiration. The maximum weight loss (31.3%) was recorded in unpacked, control followed by HDPE perforated (22.4%) and LLDPE perforated (21.49%) on 12th day of storage (Fig. 2). However, among the different treatments on last day of storage (i.e. on 15th day), the minimum weight loss (8.04%) was observed in fruits packed in PP non-perforated followed by LDPE non-perforated (10.65%), LLDPE non-perforated (11.46%) and HDPE non-perforated (15.5%) respectively (Fig. 2). Weight loss was negligible (<1.0%) in PP non-perforated and LLDPE non-perforated up to 3 rd day of storage. Minimum weight loss in non-perforated polythene packed fruits could be due to less availability of oxygen for respiration, which ultimately retarded the rate of respiration and thereby lowering the moisture loss due to transpiration. The present findings were in agreement with the previous findings of Sandhu and Singh (2000), Baszczyk and Ysiak (2001), Calvo et al. (2002) and Tijskens and Vollebregt (2003).
Fig. 2.
Effect of different packaging materials on physical characteristics viz., a decay loss (%), b PLW (%) and c texture (kgf) of pear fruits during storage. Points represent the means of five replicates, vertical bars represent standard error of mean (±). Where error bars are not shown, the standard error was not greater than the size of the symbol
Decay loss
Fruit decay loss due to rotting also increased as the storage period advances irrespective of treatments (Fig. 2). This might be due to condensation of moisture in the surface of fruits during storage, anaerobic condition, and break down of enzymes etc., which helped in multiplication of micro flora. None of the treatment showed the fruits rotting up to 3rd day of storage. However, minimum cumulative decay loss was recorded in fruits packed in PP perforated (12.5%) followed by PP non-perforated (16.5%) and LDPE perforated (29.0%) on 15th day of storage, while, the maximum decay loss was recorded in control (100.0%). The reduced decay loss might be attributed to limited permeability of gases (CO2 and O2) and water vapour, which can interplay with physiological processes of fruit (Tijskens and Vollebregt 2003; Soliva and Martin 2003). The results of this finding were in agreement with the reports of Sandhu and Singh (2000), Drake and Gix (2000) and Dou-ShiJuan et al. (2002).
Fruit firmness
Firmness of pear fruits irrespective of treatments decreased during storage (Fig. 2). Among the treatments, the maximum firmness was recorded in PP non-perforated (5.18 kgf) followed by LDPE non-perforated (4.76 kgf) and LDPE perforated (4.23 kgf) respectively on 12th day of storage. However, the minimum firmness was recorded in HDPE perforated (1.8 kgf) on the same day. The reason for decrease in firmness during storage of pear fruits might be due to break down of enzymes, loss of water and degradation of pectic substances present in the fruits. Galvis Sanchez et al. (2003) and Drake et al. (2004) reported similar findings in pear fruits during storage.
Fruit colour characteristics
The changes in Hunter total colour difference (ΔE) compared to the initial colour before storage in respect to different treatment combinations are being shown in Fig. 3. The highest ΔE value (24.25) was found in control fruits on 12th day of storage. On the other hand, among the different treatments on last day of storage (i.e. on 15th day), the lower ΔE values of 11.99 and 13.55 were recorded in PP non-perforated and PP perforated respectively and this had indicated a delay in ripening. The less development of colour change in PP non-perforated and PP perforated might be due to less overall colour change which caused less deterioration or delay ripening of fruits in the said packaging material as compared with other treatments. Similar findings were also reported by Drake et al. (2001), Streif et al. (2001), Carrillo et al. (2003), Galvis Sanchez et al. (2003) and, Kwon et al. (2003) for colour change in pear fruits during storage. The color index (expressed as a*/b* ratio) irrespective of treatment increased during storage of pear fruit (Fig. 3). The maximum and minimum color index of 0.26 and 0.09 were recorded in HDPE non-perforated and PP non-perforated on 12th and 15th day of storage, respectively. In general, the color index increased with advancement in storage time, but it was delayed in pear fruits packed in PP non-perforated packaging materials. This might be due to low permeability of said film materials. Lee (2001) and Kwon et al. (2003) reported similar results in colour change for loquat fruit during storage period.
Fig. 3.
Effect of different packaging materials on colour characteristics viz., a colour difference and b colour index of pear fruits during storage. Points represent the means of five replicates, vertical bars represent standard error of mean (±). Where error bars are not shown, the standard error was not greater than the size of the symbol
Chemical characteristics
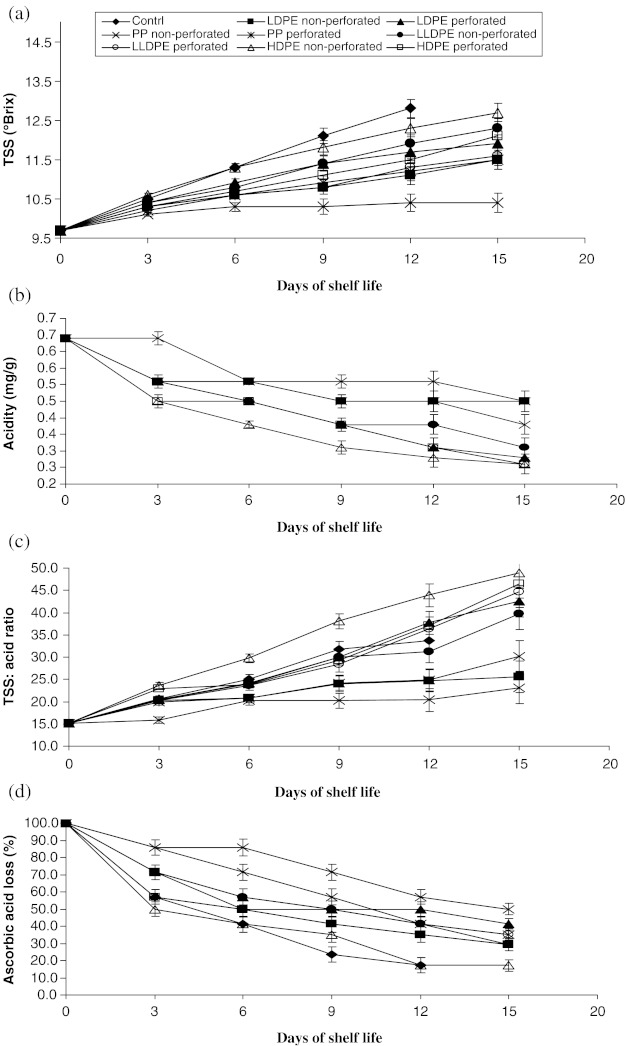
The titratable acidity and soluble solids are the best acceptable eating quality indicators for pear fruit during storage (Park 2002). Figure 4 showed the change in TSS content (°Brix) of pears fruits during storage. TSS content increased throughout the storage period in all treatments (Fig. 4), however, the minimum increment in TSS was recorded in PP non-perforated packed fruits during storage, while it was highest in control. The maximum and minimum TSS of 12.8°Brix and 10.4°Brix were recorded in control and PP non-perforated on 12th and 15th day of storage, respectively. Similar results in increase in TSS during storage period were found in ‘Buerre Bose’ and ‘Doyenne du Cornice’ pears (Elgar et al. 1997). Increase in TSS during storage might be associated with the transformation of pectic substances and starch hydrolysis and also with dehydration of fruits (Goncalves et al. 2000; Park 2002; Carrillo et al. 2003). Slow increment in TSS during storage in PP non-perforated packed pear fruits was due to production of higher levels of CO2, which may lead to less physiological processes of fruits for slow ripening.
Fig. 4.
Effect of different packaging materials on chemical characteristics viz., a TSS (°Brix), b acidity (mg of malic acid/g), c TSS: acid ratio and d ascorbic acid loss (%) of pear fruits during storage. Points represent the means of five replicates, vertical bars represent standard error of mean (±). Where error bars are not shown, the standard error was not greater than the size of the symbol
The titratable acidity of the samples irrespective of treatment decreased linearly throughout the storage period, however TSS: acid ratio was recorded to be increasing in trend (Fig. 4). The minimum acidity of 0.026 mg of malic acid per g was recorded in LLDPE perforated, HDPE non-perforated and HDPE perforated on the last day of storage, while, the maximum acidity of 0.045 mg of malic acid per g were recorded in LDPE non-perforated and PP non-perforated. Elgar et al. (1997), Baszczyk and Ysiak (2001) and Park (2002) reported similar findings in decrease in acidity in pear fruits during storage. The reduction in acidity during storage might be associated with the conversion of organic acids into sugars and their derivatives or their utilization in respiration (Zerbini 2002). LDPE non-perforated and PP non-perforated packed fruits could maintain a higher level of acidity up to 15 days of storage. It might be due to reduced respiration rate in the later stage of storage as affected by film permeability to atmospheric gas. The titratable acidity of fruits was an indicator of potential storage quality, and declined gradually over the storage period (Soliva and Martin 2003).
Initial ascorbic acid content of pear fruit was found to be 16.67 mg/100 g. Results on ascorbic acid retention of pear fruits stored in different packages have indicated that retention of ascorbic acid was gradually decreased in all treatments (Fig. 4). The maximum retention of ascorbic acid (49.97%) was recorded in PP non-perforated on the last day of storage, while, the minimum retention of ascorbic acid (17.16%) was recorded both in control and HDPE non-perforated packed fruits on 12th and 15th day of storage respectively. Similar findings in decrease in ascorbic acid during storage of fruits were also reported by Soliva and Martin (2003) in pear. PP non-perforated packed fruits could retain a higher level of ascorbic acid content up to 15 days of storage. Activities of oxidizing enzymes might be reduced in PP non-perforated packed fruits that resulted in higher retention of ascorbic acid up to last day of storage. Variation in ascorbic acid retention in different treatment might be due to different level of oxidation as affected by film permeability to atmospheric oxygen. The minimum loss of ascorbic acid content in PP non perforated packed fruits might be due to low O2 permeability (1,500 cc/m2/mil/day at 1 atm) of the said packaging materials as compared with other packaging materials (3,000, 4,100 and 3,500 cc/m2/mil/day at 1 atm respectively for LDPE, LLDPE and HDPE). During storage, oxidizing enzymes like ascorbic acid oxidase, peroxodase, catalase and polyphenol oxidase might help in reducing the ascorbic acid of the fruits (Mapson 1970).
Conclusion
Use of plastic packaging materials to extend shelf life of pear fruits may be considered as an economic and alternative method of fresh pear fruits storage at ambient condition. Plastic packaging materials have shown that besides reducing PLW and decay in pear fruits, they also effectively retained firmness, colour change and nutrient loss of fruits during storage. Out of four types of packaging films used, non-perforated PP (0.025 mm) packaging materials had more beneficial effect on shelf-life parameters of pear fruits, by maintaining the quality parameters close to those of fresh fruits.
References
- Baszczyk J, Ysiak G. Storage properties of Czech pear cultivars ‘Erica’ and ‘Dicolor’. J Fruit Ornam Plant Res. 2001;9:71–76. [Google Scholar]
- Calvo G, Salvador ME, Sanchez E. Control of superficial scald in ‘Beurre d’Anjou’ pears with low oxygen levels. Acta Hortic. 2002;596:879–882. [Google Scholar]
- Carrillo LA, Cruz Hernandez A, Guevara Lara F, Paredes Lopez O. Physico-chemical changes during ripening in storage of two varieties of prickly pear stored at 18 °C. J Food Sci Technol. 2003;40:461–464. [Google Scholar]
- Dou-ShiJuan, Chen-KunSong, LuJunLiang, Zheng JT. The storability and its regulatory mechanism of Huanghua pear (Pyrus pyrifolia Nakai.) fruit as influenced by postharvest treatments. Agric Sci China. 2002;1:1238–1245. [Google Scholar]
- Dou-ShiJuan, Chen-KunSong, LuJunLiang, Zheng JT. Effects of different postharvest treatments on storage of Huanghua pear fruit (Pyrus pyrifolia Nakai) and its physiological basis. Scientia Agricultura Sinica. 2003;36:82–88. [Google Scholar]
- Drake SR, Gix RD. Response of d’Anjou pears to controlled atmosphere storage in elevated temperature and carbon dioxide. Good Fruit Grower. 2000;51:55–57. [Google Scholar]
- Drake SR, Gix RD, Coureau C. Quality of ‘Anjou’ pears after different types of controlled atmosphere storage. J Food Qual. 2001;24:27–36. doi: 10.1111/j.1745-4557.2001.tb00588.x. [DOI] [Google Scholar]
- Drake SR, Mielke EA, Elfving DC. Maturity and storage quality of ‘Concorde’ pears. Hortic Technol. 2004;14:250–256. [Google Scholar]
- Elgar HJ, Watkins CB, Murray SHF, Gunson A. Quality of ‘Buerre Bose’ and ‘Doyenne du Cornice’ pears in relation to harvest date and storage period. Postharvest Biol Technol. 1997;10:29–37. doi: 10.1016/S0925-5214(96)00058-0. [DOI] [Google Scholar]
- Freed M. Method of vitamin assay. New York: Interscience Publication Inc.; 1966. [Google Scholar]
- Galvis Sanchez AC, Fonseca SC, Morais AMMB, Malcata FX. Physicochemical and sensory evaluation of ‘Rocha’ pear following controlled atmosphere storage. J Food Sci. 2003;68:318–327. doi: 10.1111/j.1365-2621.2003.tb14159.x. [DOI] [Google Scholar]
- Goncalves ED, Antunes PL, Brackmann A. Controlled atmosphere storage of Asian pears cv. Nijisseiki. Rev Bras Frutic. 2000;22:226–231. [Google Scholar]
- Hansen E, Mellenthin WM (1979) Commercial handling and storage practices for winter pears. Ore. State Univ. Agric Experimental Station, Special Report 550
- Kader AA. Biochemical and physiological basis for effects of controlled atmospheres on fruits and vegetables. Food Technol. 1986;40:99–104. [Google Scholar]
- Kader AA, Watkins CB. Modified atmosphere packaging-toward 2000 and beyond. Hortic Technol. 2000;10(3):483–486. [Google Scholar]
- Kader AA, Zagory D, Kerbel EL. Modified atmosphere packaging of fruits and vegetables. Crit Rev Food Sci Nutr. 1989;28:1–30. doi: 10.1080/10408398909527490. [DOI] [PubMed] [Google Scholar]
- Kwon YB, Park SK, Myunh SI, Hong SJ. Effect of postharvest treatments on fruit quality during storage of ‘Niitaka’ pear. Korean J of Hortic Sci Technol. 2003;21:114–119. [Google Scholar]
- Lee YJ. Discoloration disorder as influenced by sealing methods of PE film bag in MAP storage of ‘Fuyu’ persimmon fruit. J Korean Soc Hortic Sci. 2001;42:721–724. [Google Scholar]
- Mapson CW. Vitamins in fruits: stability of L-ascorbic acid. In: Biochemistry of fruits and their products. Academic Press, London; 1970. pp. 376–387. [Google Scholar]
- Park YM. Relationship between instrumental and sensory analysis of quality factors in apple and pear fruits. Korean J Hortic Sci Technol. 2002;20:394–398. [Google Scholar]
- Ranganna S. Handbook of analysis and quality control for fruits and vegetable products. 2. New Delhi, India: Tata McGraw Hill Publishing Co. Ltd; 1995. [Google Scholar]
- Ribeiro CJO, Nazare PA, Sobreiro J, Veltman RH. Influence of orchard, harvest date and controlled atmosphere, on storage quality of “Rocha” pear. Acta Hortic. 2003;599:639–645. [Google Scholar]
- Sandhu SS, Singh AP. Effect of harvesting dates and individual seal packaging on the pear fruit cv. Le Conte during cold storage. Haryana J Hortic Sci. 2000;29:48–52. [Google Scholar]
- Saquet A, Streif J, Bangerth F. Brown heart incidence in ‘Conference’ pears as affected by ATP and ADP levels and membrane lipid alterations during controlled atmosphere storage. Acta Hortic. 2003;600:839–842. [Google Scholar]
- Sirisomboon P, Tanaka M, Fujita S, Kojima T. Relationship between the texture and pectin constituents of Japanese pear. J Texture Stud. 2000;31:679–690. doi: 10.1111/j.1745-4603.2000.tb01028.x. [DOI] [Google Scholar]
- Soliva FRC, Martin BO. Microbiological and biochemical changes in minimally processed fresh-cut Conference pears. Eur Food Res Technol. 2003;217:4–9. doi: 10.1007/s00217-003-0701-8. [DOI] [Google Scholar]
- Steward D, Oparka J, Johnstone C, Iannetta, PPM, Davies HV (1999) Scottish crop research institute, annual report, effect of modified atmosphere packaging (MAP) on soft fruit quality. Plant Biochem Phytochem 119–124
- Streif J, Xuan H, Saquet AA, Rabus C. CA-storage related disorders in ‘Conference’ pears. Acta Hortic. 2001;553:635–638. [Google Scholar]
- Sun-XiSheng, Wang-WenHui, Li-ZhiQiang, Feng-XiaoYuan, Zhang ZY. Experiment of CA storage for Jinxiang pear variety. China Fruits. 2000;4:15–17. [Google Scholar]
- Tijskens LMM, Vollebregt HM. Passive and semi-active modified atmosphere packaging of prickly pear cactus stems (Opuntis spp.) Acta Hortic. 2003;604:665–668. [Google Scholar]
- Watkins CB. Responses of horticultural commodities to high carbon dioxide as related to modified atmosphere packaging. Hort Technol. 2000;10:501–506. [Google Scholar]
- Zerbini PE. The quality of pear fruit. Acta Hortic. 2002;596:805–810. [Google Scholar]