Abstract
Nutraceutical is the hybrid of ‘nutrition’ and ‘pharmaceutical’. Nutraceuticals, in broad, are food or part of food playing a significant role in modifying and maintaining normal physiological function that maintains healthy human beings. The principal reasons for the growth of the nutraceutical market worldwide are the current population and the health trends. The food products used as nutraceuticals can be categorized as dietary fibre, prebiotics, probiotics, polyunsaturated fatty acids, antioxidants and other different types of herbal/ natural foods. These nutraceuticals help in combating some of the major health problems of the century such as obesity, cardiovascular diseases, cancer, osteoporosis, arthritis, diabetes, cholesterol etc. In whole, ‘nutraceutical’ has lead to the new era of medicine and health, in which the food industry has become a research oriented sector.
Keywords: Dietary fiber, Probiotics, Prebiotics, Polyphenols, Spices, Human diet
Introduction
In recent years, a new diet health paradigm is evolving which places more emphasis on the positive aspects of diet. The new lifestyle adopted by people today has changed the basic food habits of the latter. Consumption of the junk food has increased manifold leading to a number of diseases caused due to improper nutrition. Obesity is now recognized as a global issue. Heart disease continues to be a primary cause of death in most of the developing countries worldwide, followed by cancer, osteoporosis, arthritis and many others. Consumers being frustrated with the expensive, high-tech, disease-treatment approach in the modern medicines are seeking complementary or alternative beneficial products and the red tape of managed care makes nutraceuticals particularly appealing. “Let food be thy medicine and medicine be thy food”, quoted by Hippocrates about 2,500 years ago is certainly the tenet of today. Nutraceuticals are the emerging class of natural products that makes the line between food and drugs to fade (Adelaja and Schilling 1999). Although the use of nutraceuticals by people has a long history, only recently scientifically supported nutritional and medical evidence has allowed nutraceuticals to emerge as being potentially effective (Dillard and German 2000). The nutraceuticals of both plant and animal origin holds exciting opportunities for the food industries to create novel food products in future. Nutritional studies are now focusing on the examination of foods for their protective and disease preventing potential (Nicoli et al. 1999), instead of negative attributes such as micro-organism count, adulterants, fatty acids and inorganic pollutant concentration (Kaur and Kapoor 2001). The aim of this review is to focus on the general concept and the health-promoting effects of several nutraceuticals that have the potential of being incorporated into foods.
Nutraceutical
The concept of “nutraceutical” arose first in the survey from U.K., Germany and France, where diet was rated higher by the consumers, then exercise or hereditary factors to achieve a good health (Pandey et al. 2010). The term “nutraceutical” was coined from “nutrition” and “pharmaceutical” by Stephen De Felice, founder and chairman of the Foundation for Innovation in Medicine (FIM), Cranford, NJ in 1989 (Maddi et al. 2007; Brower 1998). According to De Felice, nutraceutical can be defined as, “a food (or a part of food) that provides medical or health benefits, including the prevention and or treatment of a disease”. On the other hand, Health Canada defines nutraceutical as “a product prepared from foods, but sold in the form of pills, or powder (potions) or in other medicinal forms, not usually associated with foods” (Wildman 2001; Bull 2000). Nutraceuticals are found in a mosaic of products emerging from (a) the food industry, (b) the herbal and dietary supplement market, (c) pharmaceutical industry, and (d) the newly merged pharmaceutical/agribusiness/nutrition conglomerates. It may range from isolated nutrients, herbal products, dietary supplements and diets to genetically engineered “designer” foods and processed products such as cereals, soups and beverages (Malik 2008; Dureja et al. 2003).
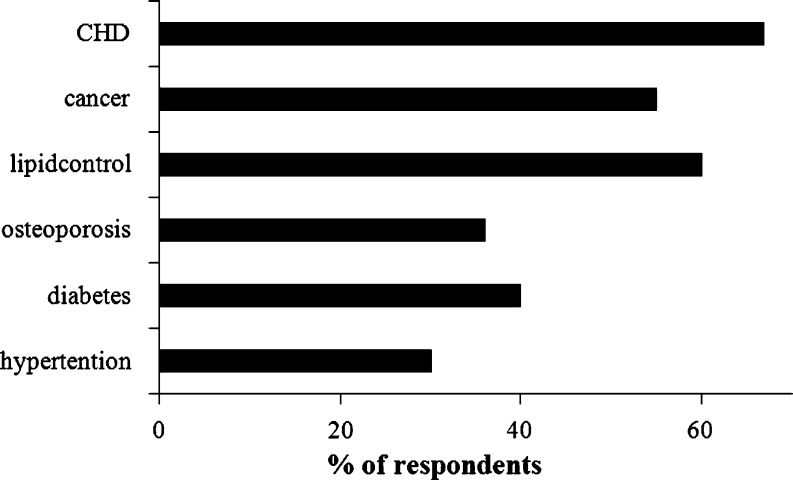
Nutraceuticals covers most of the therapeutics areas such as anti-arthritic, cold and cough, sleeping disorders, digestion and prevention of certain cancers, osteoporosis, blood pressure, cholesterol control, pain killers, depression and diabetes (Fig. 1) (Pandey et al. 2010; Sami Labs 2002).
Fig. 1.
Therapeutic areas covered by nutraceutical products
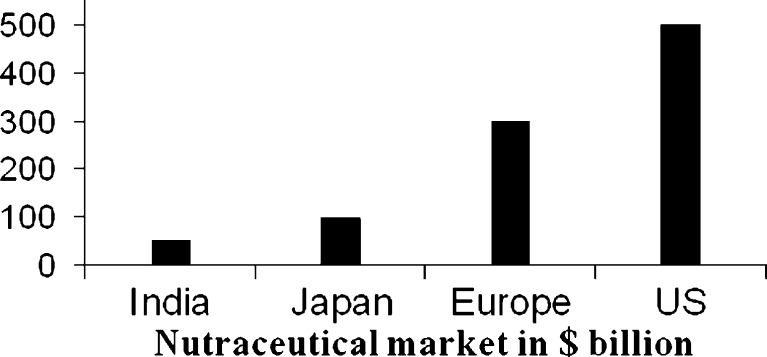
According to Rishi (2006) and Hathcock (2001), the nutraceutical industry’s three main segments include herbal/natural products, dietary supplements and functional foods. Among these, these most rapidly growing segments are the herbal/natural products and the dietary supplements (Nutrition Business Journal 2006). In 2007, the world nutraceutical market grew to reach $74.7 billion, compared to that of 2002, when it reached $46.7 billion (BCC Research). The leading countries having nutraceutical markets include USA, UK and Japan (Fig. 2) (BCC Research).
Fig. 2.
Nutraceutical market in different countries
Research and development is at the peak in this emerging nutraceutical field. The greatest scientific need pertains to standardization of the nutraceutical compounds or products carefully develop and execute clinical studies to provide the basis for health claims to produce an impact on the consumers as well as on the nutraceutical companies.
Categorizing nutraceuticals
Nutraceuticals can be organized in several ways depending upon its easier understanding and application, i.e. for academic instruction, clinical trial design, functional food development or dietary recommendations. Some of the most common ways of classifying nutraceuticals can be based on food sources, mechanism of action, chemical nature etc. The food sources used as nutraceuticals are all natural and can be categorized as (Kalia 2005; Kokate et al. 2002):
Dietary Fibre
Probiotics
Prebiotics
Polyunsaturated fatty acids
Antioxidant vitamins
Polyphenols
Spices
In the next part of the review, a brief description of the health and medical benefits of some such nutraceuticals are done.
More broadly, nutraceuticals can be classified in two groups (Pandey et al. 2010)
Potential nutraceuticals
Established nutraceuticals
A potential nutraceutical could become an established one only after efficient clinical data of its health and medical benefits are obtained. It is to be noted that much of the nutraceutical products are still lays in the ‘potential’ category.
Dietary fibre
Dietary fibre is the food material, more precisely the plant material that is not hydrolyzed by enzymes secreted by the digestive tract, but digested by microflora in the gut. Dietary fibres mostly include non-starch polysaccharides (NSP) such as celluloses, hemicelluloses, gums and pectins, lignin, resistant dextrins and resistant starches. Foods rich in soluble fibre include fruits, oats, barley and beans. The level of dietary fibre in certain foods has been illustrated in Table 1. Chemically dietary fibre means carbohydrate polymers with a degree of polymerization not lower than 3, which are neither digested nor absorbed in the small intestine. Based on their water solubility, dietary fibres may be divided into two forms: -
Insoluble dietary fibre (IDF), which includes celluloses, some hemicelluloses and lignins which is fermented to a limited extend in the colon.
Soluble dietary fibre (SDF), which includes β-glucans, pectins, gums, mucilages and hemicelluloses that are fermented in the colon.
The IDF and SDF compounds are collectively known as non-starch polysaccharides (NSP).
Table 1.
Level of dietary fibre in foods
| Product | AOAC (g/100 g)a |
|---|---|
| Apples (with skin) | 2.0 |
| Bananas | 1.9 |
| Carrots (boiled) | 3.1 |
| Baked beans | 4.2 |
| Cabbage | 2.0 |
| White Bread | 2.0 |
| Brown Bread | 4.5 |
| Wholemeal Bread | 7.4 |
aexcludes fructans
[Source-AOAC values CRC Handbook of Dietary Fibre in Human Nutrition, 2nd edition (1993)]
The soluble components of dietary fibre by virtue of their bulking and viscosity producing capabilities, retards the gastric emptying of the stomach (Leclere et al. 1994). This affects the rate of digestion and the uptake of nutrients and creates a feeling of satiety. Soluble fibre has been shown to lower selectively serum LDL cholesterol and to improve glucose tolerance (Glore et al. 1994). They also enhance insulin receptor binding and improve glycaemic response. In colon, dietary fibre increases faecal bulking due to increased water retention, increased transit time and increased faecal bacterial mass caused by soluble fibre fermentation. The fibre also promotes the growth of Bifidobacteria in the gut (especially fructooligosaccharides). Persons consuming generous amounts of dietary fibre, compared to those who have minimal fibre intake, are having low risk of CHR (Liu et al. 1999), stroke (Steffen et al. 2003), hypertension (Whelton et al. 2005), diabetes (Montonen et al. 2003), obesity (Lairon et al. 2005) and certain gastrointestinal disorders (Petruzziello et al. 2006). Again, increase in the intake of high fibre food improves serum lipoprotein values (Brown et al. 1999), lowers blood pressure level (Keenan et al. 2002), improves blood glucose control for diabetes (Anderson et al. 2004), aids weight loss (Birketvedt et al. 2005) and promotes regularity (Cummings 2001). Research reveals that certain soluble fibres enhance the immunity in humans (Watzl et al. 2005). Some potential negative effects of dietary fibre include reduced absorption of vitamins, minerals, proteins and calories. It is recommended that dietary fibre intake for adults generally fall in the range of 20–35 g/day (Pilch 1987). The recommended dietary fibre intake for children and adults are estimated to be 14 g/1,000 kCals (Anderson et al. 2009). Several case histories have reported that consumption of excessive amounts of dietary fibre causes diarrhea (Saibil 1989).
Polyunsaturated fatty acids (PUFA)
PUFAs are also called “essential fatty acids” as these are crucial to the body’s function and are introduced externally through the diet (Escott-Stump and Mahan 2000). PUFAs have two subdivisions: omega-3- (n-3) fatty acids and omega-6-(n-6) fatty acids. The major omega-3-fatty acids are α-linolenic acid (ALA), eicosapentanoic acid (EPA), docosahexanoic acid (DHA). ALA is the precursor of EPA and DHA. EPA and DHA are found mainly in fatty fishes such as mackerel, salmon, herring, trout, blue fin tuna and in fish-oils. Principal sources of ALA are mainly flaxseed, soybeans, canola, some nuts (e.g. walnuts) and red/black currant seeds (Institute of Medicine 2002). Omega-6-PUFAs mainly consist of linoleic acid (LA), γ-linolenic acid (GLA) and arachidonic acid (ARA). LA occurs mainly in vegetable oils e.g. corn, safflower, soyabean and sunflower. ARA is found in animal products such as meat, poultry and eggs.
Studies suggest that omega-3-fatty acids have three major effects as cardiovascular diseases anti-arrhythmic (preventing or alleviating irregularities in the force or rhythm of the heart) (Leray et al. 2001; Stoll et al. 1999), hypolipidemic (promoting the reduction of lipid concentrations in the serum) (Buchner et al. 2002; Nemets et al. 2002) and antithrombotic (decreased arteriosclerosis) (Hiroyasu et al. 2001; Buchner et al. 2002; Stoll et al. 1999; Albert et al. 2002).
Emerging research evidence shows the benefits of omega-3-oils in other areas of health including pre-mature infant health (Carlson 1999), asthma (Hodge et al. 1996; Broughton et al. 1997), bipolar and depressive disorders (Edwards et al. 1998; Hibbeln 1998; Stoll et al. 1999; Calabrese et al. 1999), dysmenorrhea and diabetes (Simopoulos 1991; Pepping 1999; Connor 2000). Omega-3-fatty acids have been shown to be beneficial at various stages of life. Infant formulas nowadays contain DHA along with ARA, which closely mimic the breast milk. FDA recommends a maximum of 3 g/day intake of EPA and DHA omega-3 fatty acids, with no more than 2 g per day from a dietary supplement (US FDA 2004).
Probiotics
The history of probiotics dates back as far as the first intake of fermented milks, over 2,000 years ago. The scientific interest in this area boosted from the work of Metchinkoff (1907) to transform the toxic flora of the large intestine into a host-friendly colony of Bacillus bulgaricus (Hord 2008). A probiotic can be defined as live microbial feed supplement, which when administered in adequate amounts beneficially affects the host animal by improving its intestinal microbial balance (Food and Agricultural Org. 2001; Fuller 1992). Probiotics generally include the following categories of bacteria: -
Lactobacilli such as L. acidophilus, L.casei, L.delbrueckii subsp. bulgaricus, L.brevis, L.cellobiosus.
Gram-positive cocci such as Lactococcus lactis, Streptococcus salivarius subsp. thermophilus, Enterococcus faecium
Bifidobacteria such as B.bifidun, B.adolescentis, B.infantis, B.longum, B. thermophilum.
Probiotics are available in various forms as powder form, liquid form, gel or paste or granule forms, capsule forms etc. (Suvarna and Boby 2005). Specific probiotics are generally used to treat gastrointestinal (GI) conditions such as lactose intolerance, acute diarrhea and antibiotic-associated GI side effects (Doron et al. 2005). Probiotic agents possess the properties of non-pathogenic, non-toxic, resistance to gastric acid, adherence to gut epithelial tissues producing antibacterial substances (Suvarna and Boby 2005). There are evidences that administration of probiotics decreases the risk of systemic conditions, such as allergy, asthma, cancer and several other infections of the ear, urinary tract (Lenoir-Wijnkoop et al. 2007).
Prebiotics
Prebiotics are dietary ingredients that beneficially affect the host by selectively altering the composition or metabolism of the gut microbiota (Macfarlane et al. 2006; Gibson and Roberfroid 1995). These are short-chain polysaccharides that have unique chemical structures that are not digested by humans; in particular fructose-based oligosaccharides that exist naturally in food or are added in the food. The prebiotic consumption generally promotes the Lactobacillus and Bifidobacterial growth in the gut, thus helping in metabolism (Hord 2008; Gibson 1999). Vegetables like chicory roots, banana, tomato, alliums are rich in fructo-oligosaccharides. Some other examples of these oligosaccharides are raffinose and stachyose, found in beans and peas. The health benefits of the prebiotics include improved lactose tolerance, antitumor properties, neutralization of toxins, and stimulation of intestinal immune system, reduction of constipation, blood lipids and blood cholesterol levels (Fuller 1992; Isolauri et al. 1991; Lin et al. 1989; Sanders 1994). A daily intake of 5–20 g of insulin and oligosachharides promote the growth of bifidobacteria (Schrezenmeir and De Vrese 2001). Again, consumption of large amounts of such oligosaccharides causes diarrhea, abdominal distension and flatulence (Gibson and Wang 1994; Guarner 2005; Nadeau 1999).
Selenium
Selenium is an essential trace element that is involved in the defense against the toxicity of reactive oxygen species, the regulation of the redox state of cells and in the regulation of thyroid hormone metabolism. Brazil nuts are the richest known source of selenium; one ounce contains approximately 200 mcg. Its deficiency has caused serious health effects in human, such as Keshan’s disease, a potentially fatal form of cardiomyopathy (disease of the heart muscle) that affects young women and child. The most important role of selenium is in the form of antioxidant selenoproteins or selenoenzymes such as glutathione peroxidase, thioredoxin reductase. Glutathione peroxidase plays a significant role in protecting cells against oxidative damage from reactive oxygen species (ROS) and reactive nitrogen species (RNS), which include superoxide, hydrogen peroxide, hydroxyl radicals and nitric oxide and peroxynitrite. The pentose phosphate pathway assists glutathione peroxidase, an enzyme that contains selenium as the trace element, in protecting erythrocytes against haemolysis. The antioxidant activity of selenium aids in prevention of cardiovascular diseases and helps in maintenance of proper immunity. It has been reported that the antioxidant activity of selenoenzymes may prevent the formation of oxidized LDL and hence reduce the incidence of heart diseases. However epidemiological studies do not firmly confirm the fact that low Se level is associated with increased risk of heart diseases. A recent study in USA also showed the same result (Stranges et al. 2006). But diet rich in Se has been found to reduce the effects of reperfusion and when Se becomes deficient, it can significantly impair intrinsic myocardial tolerance to ischemia. Selenium has also been found to act as a chemoprevention agent reducing oxidative stress, limiting DNA damage, inducing apoptosis, cell-cycle arrest. Epidemiological studies have increasingly indicated an inverse relationship between Se status and cancer risks in human populations. A clinical study by Clark and his colleagues revealed that participants who were given 200 μg of yeast-based selenium per day for four and half years had a 50% decrease in the cancer death rate compared with the placebo group (Clark et al. 1996). Se also plays an important role in the immune system by increasing the activity of natural killer (NK) cells, the production of interferonγ, and stimulating vaccine-induced immunity. Se plays a significant role in impairment of thyroid immunity involving the action of glutathione peroxidase and thioredoxin reductase thereby removing ROS and excess H2O2 produced by thyrocytes during thyroid hormone synthesis (Tinggi 2007). Recommended dietary allowances for Se varies from 20 μg per day for children to 55 μg per day for adults. On the other hand, the tolerable upper intake levels of selenium ranges from 90 μg per day to 400 μg per day for children and adults respectively (Institute of Medicine, Food and Nutrition Board 2000). High blood levels of selenium (>100 μg/dl) may lead to a condition called selenosis which has symptoms like gastrointestinal upsets, hair loss, white blotchy nails, fatigue, garlic breath odour, irritability, and mild nerve damage (Goldhaber 2003).
Antioxidant vitamins
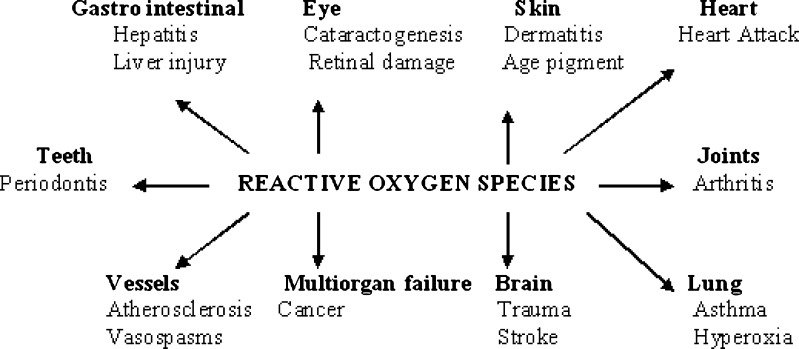
Vitamins like vitamin C, vitamin E and carotenoids are collectively known as antioxidant vitamins. These vitamins act both singly as well as synergistically for the prevention of oxidative reactions leading to several degenerative diseases including cancer, cardiovascular diseases, cataracts etc. (Elliot 1999) (Fig. 3). These vitamins are abundant in many fruits and vegetables and exert their protective action by free-radical scavenging mechanisms. Vitamin E which comprises of tocopherols together with tocotrienols transfer hydrogen atom and scavenge singlet oxygen and other reactive species thus protecting the peroxidation of PUFA within the biological membrane and LDL (Meydani 2000). Tocotrienols are more mobile within the biological membrane than tocopherols because of the presence of the unsaturated side-chain and hence penetrate tissues with saturated fatty layers, i.e. in brain and liver more efficiently. They have more recycling ability and are a better inhibitor of liver oxidation (Watkins et al. 1999). Vitamin E and selenium has a synergistic role against lipid peroxidation. Vitamin C, better known as ascorbic acid donates hydrogen atom to lipid radicals, quenches singlet oxygen radical and removes molecular oxygen. Scavenging of aqueous radicals by the synergistic effect of ascorbic acid along with tocopherol supplementation is a well known antioxidant mechanism (Lee et al. 2004). Carotenoids like lycopene, β-carotene, lutein, zeaxanthin are known to be the most efficient singlet oxygen quencher in the biological systems without the production of any oxidizing products. β-carotene traps peroxy free radicals in tissues at low oxygen concentrations. Hence β-carotene complements the antioxidant properties of vitamin E.
Fig. 3.
Clinical conditions involving reactive oxygen species
Polyphenols
Polyphenols form a large group of phytochemicals, which are produced by plants as secondary metabolites to protect them from photosynthetic stress, reactive oxygen species. There are approximately 8,000 different classes of polyphenols, the most important being flavonols, flavones, flavan-3-ols, flavanones and anthocyanins. The highly branched phenylpropanoid pathway synthesizes majority of polyphenols. The most commonly occurring polyphenols in food include flavonoids and phenolic acids. Dietary polyphenols are of current interest because substantial evidence in vitro have suggested that they can affect numerous cellular processes like, gene expression, apoptosis, platelet aggregation, intercellular signaling, that can have anti-carcinogenic and anti-atherogenic implications (Duthie et al. 2003) as illustrated in Fig. 4.
Fig. 4.
Proposed mechanistic scheme for prevention of cancer by dietary polyphenols
These apart, polyphenols also possess antioxidant, anti-inflammatory, anti-microbial, cardioprotective activities and play a role in the prevention of neurodegenerative diseases and diabetes mellitus (Scalbert et al. 2005). Polyphenols are mostly acknowledged for their antioxidant activities on the basis of their structural chemistry. Polyphenols have been shown to be more effective antioxidants in vitro than vitamin E and C on a molar basis. Bioavailablity of polyphenols is an important factor determining their biological activity. This depends on the chemical properties of polyphenol, conjugation and reconjugation in the intestines, intestinal absorption, and enzymes available for metabolism (Yang et al. 2001). Research has also focussed upon an interesting aspect of polyphenols. It has been found that flavonoids modulate the expression of γ-glutamylcysteine synthetase, an important rate-limiting enzyme involved in glutathione synthesis. Glutathione being important in redox regulation of transcription factors and enzymes for signal transduction, polyphenol mediated regulation of glutathione significantly alters cellular effects, as detoxification of xenobiotics, glutathionylation of proteins (Moskaug et al. 2005).
The age-old French Paradox refers to the fact that there is a relatively less incidence of coronary heart disease among the French, in spite of the fact that they consumed diets relatively rich in saturated fats. This trend was later found to be the result of France’s high consumption of red wine. The antioxidant and anti-inflammatory activities of red wine is due to the presence of reservatrol, a triphenolic stilbene present in the black skin of grapes and proanthocyanidins. Research has also showed that red wines strongly inhibit the synthesis of endothelin-1, a vasoactive peptide that is crucial in the development of coronary atherosclerosis (Corder et al. 2001). Moreover, studies have also proved that the oxidative stress induced by alcohol consumption has led to the expression of several cardioprotective oxidative stress-inducible proteins along with HSP 70 (heat shock protein) (Sato et al. 2002). Reservatrol is a phytoestrogen receptor agonist and research has suggested that this function may also serve a significant role in cardiovascular benefits (Dillard and German 2000). Even though the antioxidant activities of the wines vary over a factor of 2, the ratios of the activities to the total phenol content are approximately the same (about a factor of 10), indicating the direct relationship between the two (Rice-Evans et al. 1997). The inhibition of COX activity and suppression of COX-2 expression by reservatrol in different cell lines suggest that reservatrol is important in inhibiting carcinogenesis. A diet enriched with red wine solids (solid from 750 ml of red wine per kg diet), which contained catechins, gallic acid, and other polyphenols, delayed the onset of tumors in the HTLV-1 transgenic mouse (Yang et al. 2001). Moderate consumption of red wine (400 ml/day) for 2 weeks significantly increases antioxidant status and decreases oxidative stress in the circulation of humans (Micallef et al. 2007).
Tea (Camellia sinensis) is a rich source of polyphenols, such as catechins, which include (-)-epicatechin, (-)-epigallocatechin, (-)-epicatechin-3-gallate (ECGC), with ECGC being the major catechin. These apart, tea also constitutes of flavonols like quercetin and myricetin. Tea, mainly consumed in the form of black tea and green tea has been found to have cancer-preventing activities. Several studies have suggested that tea is effective in inhibiting 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) induced tumorigenesis in mice. Administration of black tea preparation to adenoma-bearing mice significantly inhibited tumor cell proliferation and the progression of adenoma to carcinoma (Lambert et al. 2005; Yang et al. 1997). These experiments indicate that tea has a broad inhibitory activity against lung carcinogenesis. Experimental evidence from animal models also suggest that tea plays a significant role in inhibition of carcinogenesis in organ sites in skin, lung, esophagus, stomach, liver, small intestine, pancreas, colon, bladder, and mammary gland ECGC has been considered by several authors as the active component of green tea and its anticarcinogenic activity has also been demonstrated (Yang et al. 2001). A case control study in Shanghai has reported that frequent consumption of green tea is associated with a lower incidence of oesophagal cancer, especially among nonsmokers and nonalcohol-drinkers (Gao et al. 1986). On the other hand, in the Netherlands Cohort Study on Diet and Cancer, consumption of black tea was not found to affect the risk for colorectal, stomach, lung, and breast cancers (Goldbohm et al. 1996). Such different results on tea and cancer suggest that the amount of tea consumed, lifestyle related factors such as smoking and diet and different etiological factors involved in different populations have an important bearing on the anticancer effect of tea. Green tea has also been found to be associated with lower risk of cardiovascular diseases through decreased serum cholesterol and triglyceride and provides protection against peroxidation of lipids in kidney.
Legumes are consumed worldwide as an alternative source of proteins, since they are rich in amino acids like lysine and tryptophan and they are much cheaper than animal proteins. Studies have revealed that in addition to complex carbohydrates, soluble fibers, essential vitamins, and metals, legumes also supply the diet with polyphenols like flavonoids, isoflavones and lignans (Oboh 2006). Of all legumes, soyabean has received most attention. Soyabean is most significant source of dietary isoflavones.
It has a relatively high concentration of genistein and diadzein, which are generally considered as phytoestrogens. These compounds have been shown to inhibit the growth of most hormone-dependent and independent cancer cells, especially breast, prostate and skin cancer in mouse models. In mice, dietary soyabean components inhibited the growth of experimental prostate cancer and altered tumor biomarkers associated with angiogenesis. The protective effect of soy isoflavones against colon cancer is unclear. Bioavailability of genistein is much superior with respect to green tea polyphenols (Dillard and German 2000; Yang et al. 2001; Lambert et al. 2005). Recent research has showed that there are several other legume components apart from soy isoflavones, which may have beneficial effects. For instance, kievitone, a potential breast-cancer fighting agent is found in hyacinth bean and antimicrobial agents, like agmatine and isovitexin are particularly found in winged bean, but not in common bean or soybean (Oboh 2006). Commonly consumed cowpea Vigna unguiculata (brown) and underutilized legumes Cajanus cajan (brown) and Sphenostylis sternocarpa also possess higher antioxidant activity due to their relative higher phenol content (Table 2). Hence they can play an active role in combating degenerating diseases along with their traditional role of preventing malnutrition.
Table 2.
Antioxidant phytochemicals of some tropical legumes
| Sample | Vitamin C (mg/100 g) | Phytate (%) | Total phenol (mg/g) |
|---|---|---|---|
| V.unigulata (white) | 0.5 ± 0.1b | 2.5 ± 0.4ab | 0.3 ± 0.0b |
| V.unigulata (IT8pD 997 white) | 0.9 ± 0.1a | 1.4 ± 0.1c | 0.3 ± 0.1b |
| V.unigulata (brown drum) | 0.9 ± 0.1a | 1.9 ± 0.3a | 0.4 ± 0.1b |
| V.unigulataI (brown) | 0.9 ± 0.2a | 2.3 ± 0.1ab | 1.0 ± 0.3a |
| V.unigulata(Ife brown) | 0.9 ± 0.1a | 2.0 ± 0.4b | 0.9 ± 0.1a |
| C.cajan (brown) | 0.9 ± 0.0a | 2.4 ± 0.2ab | 1.2 ± 0.2a |
| C.cajan (white) | 0.9 ± 0.1a | 2.0 ± 0.1b | 0.4 ± 0.1b |
| S.sternocarpa | 0.8 ± 0.2a | 2.4 ± 0.3ab | 0.7 ± 0.1ab |
Values represent means of triplicate. Values with the same alphabet along the same column are not significantly different (p > 0.05)
Source: Oboh (2006)
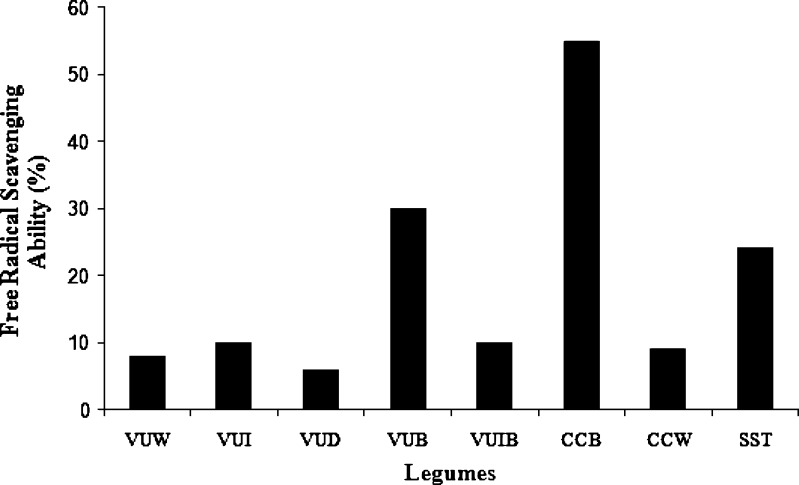
Although the free radical scavenging ability of C. cajan (brown), S. sternocarpa, and V. unguiculata (brown) were within the same range with the free radical scavenging ability of some commonly consumed green leafy vegetables, it was generally lower than that of fruits and non-leafy vegetables like broccoli and red pepper (Oboh 2006), shown in Fig. 5.
Fig. 5.
Free radical scavenging ability of some tropical legumes. VUW V. unguiculata (white); VUI V. unguiculata (IT8PD-997 (white)); VUD V. unguiculata (brown drum); VUB V. unguiculata (brown); VUIB V. unguiculata (Ifebrown); CCB C. cajan (brown); CCW C. cajan (white); SST S. sternocarpa (kokondo). Source: Oboh (2006)
The evidence that polyphenols are considered chemopreventive agents because of their antioxidative properties by some authors has been mostly circumstantial and hence more investigations are needed. For more precise information on the role of dietary polyphenols in cancer prevention in humans, reliable biomarkers for the consumption of specific polyphenols are needed, in addition to the use of dietary questionnaires. The association between the consumption of a specific type of polyphenol (or food item) and lowered cancer risk needs to be observed consistently in different studies, before dietary recommendations. High intake of polyphenols even from dietary sources can result in toxic effects. Flavonoids are reported to induce cleavage in the MLL gene, inhibit enzymes (such as topoisomerases) involved in DNA structure and replication and hence may predispose subjects to infant leukemia (Yang et al. 2001). Although the redox potentials of most flavonoid radicals are lower than those of the superoxide and peroxyl radicals, the effectiveness of the radicals in generating lipid peroxidation, DNA adducts, and mutations may still be significant in disease development. Flavonoid supplementation as a general recommendation to increase cellular glutathione concentrations may also be troublesome since the active compounds and the mechanisms involved in disease-preventing effects are still poorly understood. It remains to be determined whether dietary polyphenols modulate cellular glutathione concentrations among humans and whether they contribute to regulation of major cellular signaling pathways, which would explain the indisputable fact that fruits and vegetables protect against disease (Moskaug et al. 2005).
Spices
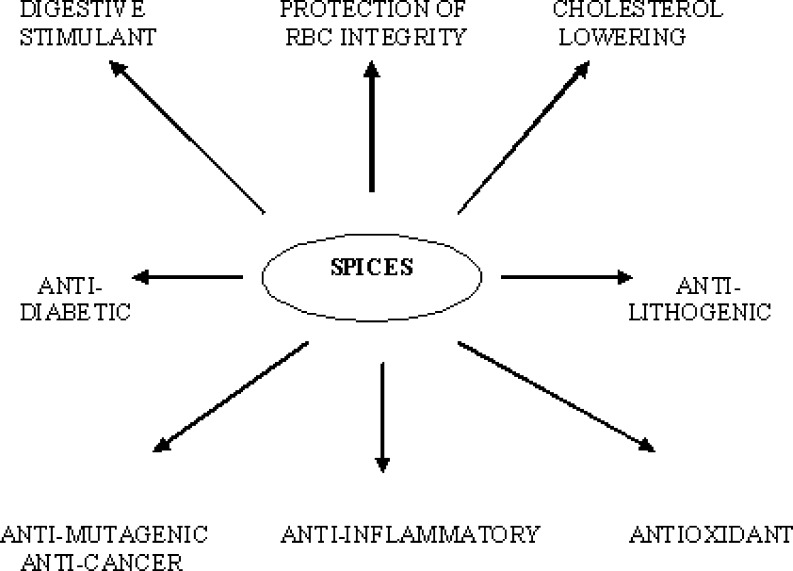
Spices are esoteric food adjuncts that are used for thousands of years to enhance the sensory quality of foods. The quantity and the variety of the spices consumed in the tropical countries are particularly extensive. These impart characteristic flavor, aroma, or piquancy and colour to foods, stimulating our appetite as well as modify the texture of food. Recent research reveals that dietary spices in their minute quantities has an immense influence on the human health by their antioxidative, chemopreventive, antimutagenic, anti-inflammatory, immune modulatory effects on cells and a wide range of beneficial effects on human health by the action of gastrointestinal, cardiovascular, respiratory, metabolic, reproductive, neural and other systems (Kochhar 2008; Lampe 2003; Kretchmer 1994; Kohlmeier et al. 1995; Hendrich et al. 1994; Rao 2003; John 2001). Some of these functional aspects of the spices are mentioned in the Fig. 6.
Fig. 6.
Summary of potential health benefits of spices
Most of the spice components are terpenes and other constituents of essential oils. They have been found to be effective in different forms. For instance, about 50 g of onion and 5–6 cloves of garlic in their raw form are adequate for lowering of cholesterol in human body. Recent studies on the lipid profile and blood pressure of moderately hypercholesterolemic subjects showed better beneficial effects of dietary supplement with aged garlic extract relative to the fresh ones (Steiner et al. 1996). Co-administration of garlic with fish oil had a better beneficial effect on serum lipid and lipoprotein concentrations by providing a combined lowering of total cholesterol, LDL cholesterol and triglyceride concentration. Spices and herbs are in most cases harmless, when used as food, but may exhibit toxicity, when used as medicine, because of their relative higher dose administered, or rather due to the possibilities of their interactions with other pharmaceutical medications (Ernst 2003; Argento et al. 2000). Again reports say that excessive consumption of garlic (4 ml/kg for raw garlic juice or of 100 mg/kg for garlic oil) lead to adverse effects on health such as anemia, weight loss, heart, liver, kidney toxicity (Bannerjee et al. 2003) and other dermatological problems (Sahu 2002). High doses of onion (500 mg/kg) as well causes lung and tissue damage in rats (Ali et al. 2000). Fenugreek seeds (25–50 g), garlic (5–6 cloves), onion (50 g) and turmeric powder (1 pinch) in the daily diet of diabetics prevent and manage long-term complications of diabetes. Regular intake of curcuminoids at about 0.5 g reduces blood lipid peroxide level upto about 33% due to their antioxidant activity (Sreejayan and Rao 1994). Table 3 depicts the beneficial functional aspects of various spices. The United States Code of Federal Regulations has considered spices and herbs as “GRAS”, i.e. generally recognized as safe for human consumption (Lai and Roy 2004; Kabara 1991).
Table 3.
Experimentally documented potential health benefits of spices
| Potential health benefits | Spices observed to exert |
|---|---|
| Lowering of blood cholesterol | Garlic, Onion, Fenugreek, Turmeric/Curcumin, Red pepper/Capsaicin |
| Prevention and dissolution of cholesterol gallstones | Curcumin, Capsaicin |
| Protection of erythrocyte integrity in hypercholesterolemic condition | Curcumin, Capsaicin, Garlic |
| Hypoglycaemic potential | Fenugreek, Garlic, Onion, Turmeric, Cumin |
| Amelioration of diabetic nephropathy | Curcumin, Onion |
| Antioxidant effect | Turmeric/Curcumin, Capsaicin, Eugenol |
| Anti-inflammatory and anti-arthritic effect | Turmeric/Curcumin, Capsaicin, Eugenol |
| Antimutagenic effect/Cancer preventing | Turmeric/Curcumin, garlic, Ginger/Gingerol, Mustard |
| Digestive stimulant action | Curcumin, Capsaicin, Piperine, Ginger, Cumin, Ajowan, Fennel, Coriander, Onion, Mint |
| Antimicrobial | Turmeric/Curcumin, Garlic, Asafoetida |
Conclusion
With the ever-changing lifestyle of humans, the antioxidant defense systems are often overloaded resulting in oxidative stress. Moreover, the levels of antioxidant defense mechanism decrease appreciably with age. These may result in the development of a great many diseases. Hence research over the past several decades have primarily focussed on different nutraceuticals. Antioxidant products may either function intrinsically to scavenge free radicals (e.g. vitamins, PUFA) or specifically stimulate the body’s defense system. This review reflects the potential merits and demerits of nutraceuticals among healthy individuals. However, an individual’s susceptibility to any particular disease predominantly depends upon genetic predisposition and lifestyle disorders like smoking, high alcohol consumption. So the response of nutraceuticals can vary from person to person. Nutraceuticals have proven health benefits and their consumption (within their acceptable Recommended Dietary Intakes) will keep diseases at bay and allow humans to maintain an overall good health.
Acknowledgement
This research work is financially supported by the Centre for Advanced Studies (CAS I) programme under University Grants Commission (UGC), India.
References
- Adelaja AO, Schilling BJ. Nutraceutical: blurring the line between food and drugs in the twenty-first century. Mag Food Farm Resour Issues. 1999;14:35–40. [Google Scholar]
- Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. New Engl J Med. 2002;346:1113–1118. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- Ali M, Thomson M, Afzal M. Garlic and onions: their effect on eicosanoid metabolism and its clinical relevance. Prostaglandins Leukot Essent Fatty Acids. 2000;6:55–73. doi: 10.1054/plef.1999.0124. [DOI] [PubMed] [Google Scholar]
- Anderson JW, Randles KM, Kendall CWC, Jenkins DJA. Carbohydrate and fiber recommendations for individuals with diabetes: a quantitative assessment and meta-analysis of the evidence. J Am Coll Nutr. 2004;23:5–17. doi: 10.1080/07315724.2004.10719338. [DOI] [PubMed] [Google Scholar]
- Anderson JW, Baird P, Davis RH, Jr, Ferreri S, Knutdson M, Koraym A, Waters V, Williams CL. Health benefits of dietary fibre. Nutr Rev. 2009;67:188–205. doi: 10.1111/j.1753-4887.2009.00189.x. [DOI] [PubMed] [Google Scholar]
- Argento A, Tiraferri E, Marzaloni M. Oral anticoagulants and medicinal plants: an emerging interaction. Ann Ital Med Interna. 2000;15:139–143. [PubMed] [Google Scholar]
- Bannerjee SK, Mukherjee PK, Maulik SK. Garlic as an antioxidant: the good, the bad and the ugly. Phytother Res. 2003;17:97–106. doi: 10.1002/ptr.1281. [DOI] [PubMed] [Google Scholar]
- Birketvedt GS, Shimshi M, Erling T, Florholmen J. Experiences with three different fiber supplements in weight reduction. Med Sci Monit. 2005;11:15–18. [PubMed] [Google Scholar]
- Broughton KS, Johnson CS, Pace BK, Liebman M, Kleppinger KM. Reduced asthma symptoms with n-3 fatty acid ingestion are related to 5-series leukotriene production. Am J Clin Nutr. 1997;65:1011–1017. doi: 10.1093/ajcn/65.4.1011. [DOI] [PubMed] [Google Scholar]
- Brower V. Nutraceuticals: poised for a healthy slice of the healthcare market? Nat Biotechnol. 1998;16:728–731. doi: 10.1038/nbt0898-728. [DOI] [PubMed] [Google Scholar]
- Brown L, Rosner B, Willett WW, Sacks FM. Cholesterollowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr. 1999;69:30–42. doi: 10.1093/ajcn/69.1.30. [DOI] [PubMed] [Google Scholar]
- Buchner HC, Hengstler P, Schindler C, Meier G. N-3 polyunsaturated fatty acids in coronary heart disease—a meta analysis of randomized controlled trials. Am J Med. 2002;112:298–304. doi: 10.1016/S0002-9343(01)01114-7. [DOI] [PubMed] [Google Scholar]
- Bull E. What is nutraceutical? Pharm J. 2000;265:57–58. [Google Scholar]
- Calabrese JR, Rappor DJ, Shelton MD. Fish oils and bipolar disorder. Arch Gen Psychiatry. 1999;56:413–414. doi: 10.1001/archpsyc.56.5.413. [DOI] [PubMed] [Google Scholar]
- Carlson SE. Long-chain polyunsaturated fatty acids and development of human infants. Acta Paediatr Suppl. 1999;88:72–77. doi: 10.1111/j.1651-2227.1999.tb01304.x. [DOI] [PubMed] [Google Scholar]
- Clark LC, Combs GF, Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, Kongrad A, Lesher JL, Park HK, Sanders BB, Smith CL, Taylor JR. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional prevention of cancer study group. J Am Med Assoc. 1996;276:1957–1963. doi: 10.1001/jama.1996.03540240035027. [DOI] [PubMed] [Google Scholar]
- Connor WE. Importance of n-3 fatty acids in health and disease. Am J Clin Nutr. 2000;71(suppl):171S–175S. doi: 10.1093/ajcn/71.1.171S. [DOI] [PubMed] [Google Scholar]
- Corder R, Douthwaite JA, Lees DM, Khan NQ, Santos AC, Wood EG, Carrier MJ. Health: endothelin-1 synthesis reduced by red wine. Nature. 2001;414:863–864. doi: 10.1038/414863a. [DOI] [PubMed] [Google Scholar]
- Cummings JH. The effect of dietary fiber on fecalweight and composition. In: Spiller G, editor. Dietary fiber in human nutrition. Boca Raton: CRC Press; 2001. pp. 183–252. [Google Scholar]
- Dillard CJ, German JB. Phytochemicals: nutraceuticals and human health. J Sci Food Agric. 2000;80:1744–1756. doi: 10.1002/1097-0010(20000915)80:12<1744::AID-JSFA725>3.0.CO;2-W. [DOI] [Google Scholar]
- Doron S, Snydman DR, Gorbach SL. Lactobacillus GG: bacteriology and clinical applications. Gastroenterol Clin North Am. 2005;34:483–498. doi: 10.1016/j.gtc.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Dureja H, Kaushik D, Kumar V. Developments in nutraceuticals. Indian J Pharmacol. 2003;35:363–372. [Google Scholar]
- Duthie GG, Gardner PT, Kyle JAM. Plant polyphenols: are they the new magic bullet? Proc Nutr Soc. 2003;62:599–603. doi: 10.1079/PNS2003275. [DOI] [PubMed] [Google Scholar]
- Edwards R, Peet M, Shay J, Horrobin D. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J Affect Disord. 1998;48:149–155. doi: 10.1016/S0165-0327(97)00166-3. [DOI] [PubMed] [Google Scholar]
- Elliot JG. Application of antioxidant vitamins in foods and beverages. Food Technol. 1999;53:46–48. [Google Scholar]
- Ernst E. Complementary medicine: where is the evidence? J Fam Pract. 2003;52:630–634. [PubMed] [Google Scholar]
- Escott-Stump E, Mahan LK. Krause’s food, nutrition and diet therapy. 10. Philadelphia: WB Saunders Company; 2000. pp. 553–559. [Google Scholar]
- Food and Agricultural Org World Health Org 2001. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. http://www.who.int/foodsafety/publications/fsmanagement/probiotics/en/index.html
- Fuller R, editor. Probiotics: the scientific basis. London: Chapman and Hall; 1992. [Google Scholar]
- Gibson GR. Dietary modulation of human gut microflora using the prebiotics Oligofructose and Inulin. J Nutr. 1999;129:1438S–1441S. doi: 10.1093/jn/129.7.1438S. [DOI] [PubMed] [Google Scholar]
- Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- Gibson GR, Wang X. Regulatory effects of bifidobacteria on other colonic bacteria. J Appl Bacteriol. 1994;77:412–420. doi: 10.1111/j.1365-2672.1994.tb03443.x. [DOI] [PubMed] [Google Scholar]
- Glore SR, Van Treeck D, Knehans A, et al. J Am Dent Assoc. 1994;94:425–436. doi: 10.1016/0002-8223(94)90099-X. [DOI] [PubMed] [Google Scholar]
- Goldbohm RA, Hertog MGL, Brants HAM, van Poppel G, van den Brandt PA. Consumption of black tea and cancer risk: a prospective cohort study. J Natl Cancer Inst. 1996;88:93–100. doi: 10.1093/jnci/88.2.93. [DOI] [PubMed] [Google Scholar]
- Goldhaber SB. Trace element risk assessment: essentiality vs. toxicity. Regul Toxicol Pharmacol. 2003;38:232–242. doi: 10.1016/S0273-2300(02)00020-X. [DOI] [PubMed] [Google Scholar]
- Guarner F. Inulin and oligofructose: impact on intestinal diseases and disorders. Br J Nutr. 2005;93(Suppl):S61–S65. doi: 10.1079/BJN20041345. [DOI] [PubMed] [Google Scholar]
- Hathcock J. Dietary supplements: how they are used and regulated. J Nutr. 2001;131:1114–1117. doi: 10.1093/jn/131.3.1114S. [DOI] [PubMed] [Google Scholar]
- Hendrich S, Lee K-W, Xu X, Wang HJ, Murphy PA. Defining food components as new nutrients. J Nutr. 1994;124:1789s–1792s. doi: 10.1093/jn/124.suppl_9.1789S. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351:1213. doi: 10.1016/S0140-6736(05)79168-6. [DOI] [PubMed] [Google Scholar]
- Hiroyasu I, Rexrode KM, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, Hennekens CH, Willett WC. Intake of fish and omega-3 fatty acids and risk of stroke in women. J Am Med Assoc. 2001;285:304–312. doi: 10.1001/jama.285.3.304. [DOI] [PubMed] [Google Scholar]
- Hodge L, Salome CM, Peak JK, Haby MM, Xuan W, Woodlock AJ. Consumption of oily fish and childhood asthma risk. Med J Aust. 1996;164:137–140. doi: 10.5694/j.1326-5377.1996.tb122010.x. [DOI] [PubMed] [Google Scholar]
- Hord NG. Eukaryotic microbiotic crosstalk: potential mechanisms for for health benefits of prebiotics and probiotics. Annu Rev Nutr. 2008;28:215–231. doi: 10.1146/annurev.nutr.28.061807.155402. [DOI] [PubMed] [Google Scholar]
- Food and Nutrition Board. Dietary reference intakes: vitamin C, vitamin E, selenium, and carotenoids. Washington DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- Institute of Medicine (2002) Dietary reference intakes for energy, carbohydrate, fibre, fat, fatty acids, cholesterol, protein and amino acids [DOI] [PubMed]
- Isolauri E, Juntunen M, Rautanen T, Sillanaukee P, Koivula T. A human Lactobacillus strain (Lactobacillus casei sp. Strain GG) promotes recovery from acute diarrhea in children. Pediatrics. 1991;88:90–97. [PubMed] [Google Scholar]
- John B. Natural compounds in cancer therapy. Princeton: Oregon Medical Press; 2001. [Google Scholar]
- Kabara JJ. Phenols and chelators. In: Russell NJ, Gould GW, editors. Food preservatives. Blackie: Glasgow & London; 1991. pp. 200–214. [Google Scholar]
- Kalia AN (2005) Textbook of Industrial Pharmacognocy, CBS publisher and distributor, New Delhi, pp 204–208
- Kaur C, Kapoor HC. Antioxidants in fruits and vegetables-the millenium’s health. Int J Food Sci Technol. 2001;36:703–725. doi: 10.1046/j.1365-2621.2001.00513.x. [DOI] [Google Scholar]
- Keenan JM, Pins JJ, Frazel C, Moran A, Turnquist L. Oat ingestion reduces systolic and diastolic blood pressure in patients with mild or borderline hypertension: a pilot trial. J Fam Pract. 2002;51:369–375. [PubMed] [Google Scholar]
- Kochhar KP. Dietary spices in health and diseases: I. Indian J Physiol Pharmacol. 2008;52:106–122. [PubMed] [Google Scholar]
- Kohlmeier L, Simonsen N, Mottus K. Dietary modifiers of Carcinogenesis. Environ Health Perspect. 1995;103:177–184. doi: 10.1289/ehp.95103s8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokate CK, Purohit AP, Gokhale SB (2002) Nutraceutical and Cosmaceutical. Pharmacognosy, 21st edition, Pune, India: Nirali Prakashan, pp 542–549
- Kretchmer N. Nutrition is the keystone of prevention (editorial) Am J Clin Nutr. 1994;60:1. doi: 10.1093/ajcn/60.1.1. [DOI] [PubMed] [Google Scholar]
- Lai PK, Roy J. Antimicrobial and chemopreventive properties of herbs and spices. Curr Med Chem. 2004;11:1451–1460. doi: 10.2174/0929867043365107. [DOI] [PubMed] [Google Scholar]
- Lairon D, Arnault N, Bertrais S, Planells R, Clero E, Hercberg S, Boutron-Ruault M-C. Dietary fiber intake and risk factors for cardiovascular disease in French adults. Am J Clin Nutr. 2005;82:1185–1194. doi: 10.1093/ajcn/82.6.1185. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Hong J, Yang G, Liao J, Yang CS. Inhibition of carcinogenesis by polyphenols: evidence from laboratory investigations. Am J Clin Nutr. 2005;81(suppl):284S–291S. doi: 10.1093/ajcn/81.1.284S. [DOI] [PubMed] [Google Scholar]
- Lampe JW. Spicing up a vegetarian diet: chemopreventive effects of phytochemicals. Am J Clin Nutr. 2003;78:579S–583S. doi: 10.1093/ajcn/78.3.579S. [DOI] [PubMed] [Google Scholar]
- Leclere CL, Champ M, Boillot J, Guille G, Lecannu G, Molis C, Bornet F, Krempff M, Delort-Laval J. Role of viscous guar guam in lowering the glycemic response after a solid meal. Am J Nutr. 1994;59:914–921. doi: 10.1093/ajcn/59.4.914. [DOI] [PubMed] [Google Scholar]
- Lee J, Koo N, Min DB. Reactive oxygen species, aging and antioxidative nutraceuticals. CRFSFS. 2004;3:21–33. doi: 10.1111/j.1541-4337.2004.tb00058.x. [DOI] [PubMed] [Google Scholar]
- Lenoir-Wijnkoop I, Sanders ME, Cabana MD, Caglar E, Corthier G, Rayes N, Sherman PM, Timmerman HM. Probiotic and prebiotic influence beyond the intestinal tract. Nutr Rev. 2007;65:469–89. doi: 10.1111/j.1753-4887.2007.tb00272.x. [DOI] [PubMed] [Google Scholar]
- Leray C, Wiesel ML, Freund M, Cazenave JP, Gachet J. Long chain n-3 fatty acids specifically affect rat coagulation factors dependent on vitamin K: relation to peroxidative stress. Arterioscler Thromb Vasc Biol. 2001;21:459–465. doi: 10.1161/01.ATV.21.3.459. [DOI] [PubMed] [Google Scholar]
- Lin SY, Ayres JW, Wrinkler W, Sandine WE. Lactobacillus effects on cholesterol: in-vitro and in-vivo results. J Dairy Sci. 1989;72:2885–2899. doi: 10.3168/jds.S0022-0302(89)79439-X. [DOI] [PubMed] [Google Scholar]
- Liu S, Stampfer MJ, Hu FB, et al. Whole-grain consumption and risk of coronary heart disease: results from the Nurses’ Health study. Am J Clin Nutr. 1999;70:412–419. doi: 10.1093/ajcn/70.3.412. [DOI] [PubMed] [Google Scholar]
- Macfarlane S, Macfarlane GT, Cummings JH. Review article: prebiotics in the gastrointestinal tract. Aliment Pharmacol Ther. 2006;24:701–714. doi: 10.1111/j.1365-2036.2006.03042.x. [DOI] [PubMed] [Google Scholar]
- Maddi VS, Aragade PD, Digge VG, Nitaliker MN. Importance of nutraceuticals in health management. Phcog Rev. 2007;1:377–379. [Google Scholar]
- Malik A (2008) The potentials of Nutraceuticals. Pharmainfo.net 6
- Metchinkoff E. The prolongation of life. New York: Putmans Sons; 1907. pp. 151–183. [Google Scholar]
- Meydani M. Effect of functional food ingredients: vitamin E modulation of cardiovascular diseases and immune status in the elderly. Am J Clin Nutr. 2000;71:1665S–1668S. doi: 10.1093/ajcn/71.6.1665S. [DOI] [PubMed] [Google Scholar]
- Micallef M, Lexis L, Lewandowski P. Red wine consumption increases antioxidant status and decreases oxidative stress in the circulation of both young and old humans. Nutrition J. 2007;6:27–34. doi: 10.1186/1475-2891-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montonen J, Knekt P, Jarvinen R, Aromaa A, Reunanen A. Whole-grain and fiber intake and the incidence of type 2 diabetes. Am J Clin Nutr. 2003;77:622–629. doi: 10.1093/ajcn/77.3.622. [DOI] [PubMed] [Google Scholar]
- Moskaug J, Carlsen H, Myhrstad Mari CW, Blomhoff R. Polyphenols and glutathione synthesis regulation. Am J Clin Nutr. 2005;81(suppl):277S–283S. doi: 10.1093/ajcn/81.1.277S. [DOI] [PubMed] [Google Scholar]
- Nadeau DA (1999) Oligosaccharides play important health role. The Soy Connection Interactive 7(3)
- Nemets B, Stahl Z, Bemaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatr. 2002;159:477–479. doi: 10.1176/appi.ajp.159.3.477. [DOI] [PubMed] [Google Scholar]
- Nicoli MC, Anese M, Parpinel M. Influence of processing on the antioxidant properties of fruits and vegetables. Trends Food Sci Technol. 1999;10:94–100. doi: 10.1016/S0924-2244(99)00023-0. [DOI] [Google Scholar]
- Nutrition Business Journal, 1:2, September 1996
- Oboh G. Antioxidant properties of some commonly consumed and underutilized tropical legumes. Eur Food Res Technol. 2006;224:61–65. doi: 10.1007/s00217-006-0289-x. [DOI] [Google Scholar]
- Pandey M, Verma RK, Saraf SA. Nutraceuticals:new era of medicine and health. Asian J Pharm Clin Res. 2010;3:11–15. [Google Scholar]
- Pepping J. Omega-3 essential fatty acids. Am J Health-Syst Pharm. 1999;56:719–724. [PubMed] [Google Scholar]
- Petruzziello L, Iacopini F, Bulajic M, Shah S, Costamagna G. Review article: uncomplicated diverticular disease of the colon. Aliment Pharmacol Ther. 2006;23:1379–1391. doi: 10.1111/j.1365-2036.2006.02896.x. [DOI] [PubMed] [Google Scholar]
- Pilch S (1987) Physiological effects and health consequences of dietary fibre. Bethesda, M.D.: Life Science Research Office. Federation of American Societies for Experimental Biology
- Rao BN. Bioactive Phytochemicals in Indian foods and their potential in health promotion and disease prevention. Asia Pac J Clin Nutr. 2003;12:9–22. [PubMed] [Google Scholar]
- Rice-Evans CA, Miller NJ, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci Rev. 1997;2:152–159. doi: 10.1016/S1360-1385(97)01018-2. [DOI] [Google Scholar]
- Rishi RK (2006) Nutraceuticals: borderline between food and drug? Pharma Rev 51–53
- Sahu SC. Dual role of organosulfur compounds in foods: a review. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2002;20:61–76. doi: 10.1081/GNC-120005388. [DOI] [PubMed] [Google Scholar]
- Saibil F. Diarrhea due to fiber overload. New Engl J Med. 1989;320:599. doi: 10.1056/NEJM198903023200920. [DOI] [PubMed] [Google Scholar]
- Sami Labs—pioneer in nutraceuticals Aug 05, 2002. The Hindu Newspaper; Avaliable from: http://www.hinduonnet.com/thehindu/biz/2002/08/05/stories/2002080500040200.htm
- Sanders ME. Lactic acid bacteria as promoters of human health. In: Goldberg I, editor. Functional foods: designer foods, pharmafoods and nutraceuticals. London: Chapman & Hall; 1994. pp. 294–322. [Google Scholar]
- Sato M, Maulik N, Das D. Cardioprotection with alcohol: role of both alcohol and polyphenolic antioxidants. Ann NY Acad Sci. 2002;957:122–135. doi: 10.1111/j.1749-6632.2002.tb02911.x. [DOI] [PubMed] [Google Scholar]
- Scalbert A, Johnson IT, Saltmarsh M. Polyphenols: antioxidants and beyond. Am J Clin Nutr. 2005;81(suppl):215S–217S. doi: 10.1093/ajcn/81.1.215S. [DOI] [PubMed] [Google Scholar]
- Schrezenmeir J, de Vrese M. Probiotics, prebiotics, and synbiotics—approaching a definition. Am J Clin Nutr. 2001;73:316S–364S. doi: 10.1093/ajcn/73.2.361s. [DOI] [PubMed] [Google Scholar]
- Simopoulos A. Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr. 1991;54:438–463. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- Sreejayan R, Rao MNA. Curcuminoids as potent inhibitors of lipid peroxidation. J Pharm Pharmacol. 1994;46:1013–1016. doi: 10.1111/j.2042-7158.1994.tb03258.x. [DOI] [PubMed] [Google Scholar]
- Steffen LM, Jacobs DR, Jr, Stevens J, Shahar E, Carithers T, Folsom AR. Associations of whole-grain, refined grain, and fruit and vegetable consumption with risks of all-cause mortality and incident coronary artery disease and ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2003;78:383–390. doi: 10.1093/ajcn/78.3.383. [DOI] [PubMed] [Google Scholar]
- Steiner M, Khan AH, Holbert D, Lin RIS. A double-blinded crossover study in moderately hypercholesterolemic men that compared the effect of aged garlic extracts and placebo administration on blood lipids. Am J Clin Nutr. 1996;64:866–870. doi: 10.1093/ajcn/64.6.866. [DOI] [PubMed] [Google Scholar]
- Stoll AL, Severus WE, Freeman MP, Rueter S, Zboyan HA, Diamond E, Cress KK, Marangell LB (1999) Omega 3 fatty acids in bipolar disorder. A preliminary double-blind, placebo-controlled trial. Arch Gen Psych 56:407–412 and pp 415–416 (commentary) [DOI] [PubMed]
- Stranges S, Marshall JR, Trevisan M, Natarajan R, Donahue RP, Combs GF, Farinaro E, Clark CL, Reid ME. Effects of selenium supplementation on cardiovascular disease incidence and mortality: secondary analyses in a randomized clinical trial. Am J Epidemiol. 2006;163:694–699. doi: 10.1093/aje/kwj097. [DOI] [PubMed] [Google Scholar]
- Suvarna VC, Boby VU. Probiotics in human health: a current assessment. Curr Sci. 2005;88:1744–1748. [Google Scholar]
- Tinggi U. Selenium: its role as antioxidant in human health. Environ Health Prev Med. 2007;13:102–108. doi: 10.1007/s12199-007-0019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Food and Drug Administration. September 8, 2004. FDA announces qualified health claims for omega-3-fatty acids. Press Release
- Watkins TR, Bierenbaurn MI, Giampalala A. Tocotrienols: biological and health effects. In: Papas AM, editor. Antioxidant status, diet, nutrition and health. Boca Raton: CRC Press; 1999. pp. 479–496. [Google Scholar]
- Watzl B, Girrbach S, Roller M. Inulin, oligofructose and immunomodulation. Br J Nutr. 2005;93(Suppl 1):S49–S55. doi: 10.1079/BJN20041357. [DOI] [PubMed] [Google Scholar]
- Whelton SP, Hyre AD, Pedersen B, Yi Y, Whelton PK, He J. Effect of dietary fiber intake on blood pressure: a metaanalysis of randomized, controlled clinical trials. J Hypertens. 2005;23:475–481. doi: 10.1097/01.hjh.0000160199.51158.cf. [DOI] [PubMed] [Google Scholar]
- Wildman REC, editor. Handbook of nutraceuticals and functional foods. Boca Raton: CRC Press; 2001. pp. 13–30. [Google Scholar]
- Yang G, Wang ZY, Kim S, Liao J, Seril DN, Chen X, Smith TJ, Yang CS (1997) Characterization of early pulmonary hyperproliferation and tumor progression and their inhibition by black tea in a 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis model with A/J mice. Cancer Res 57:1889–1894. http://www.bccresearch.com/food/GA085R.html. Evolving nutraceutical [PubMed]
- Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001;21:381–406. doi: 10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]