Abstract
Phenolic compounds and colour stability of red wines produced from Vranec Vitis vinifera L. grape variety were investigated by means of different maceration times (3, 6 and 10 days), two doses of SO2 (30 and 70 mg/L SO2), two yeasts for fermentation (Vinalco and Levuline), temperature of storage and time of aging (3, 6 and 16 months). In general, maceration time influenced the phenolics extraction from the grapes into the wine. Highest concentrations of phenolic components were observed in the wines produced with 6 days of maceration, except for the flavan-3-ols which were present in highest amounts in the wines macerated for 10 days. Higher doses of SO2 increased the extraction of polyphenols, preventing the wines from oxidation, while the effect of yeast on phenolics extraction was not significant. Wine aging affected the phenolic content of wines produced with 3 days of maceration and caused intensive decrease of anthocyanins during the storage period. Wines aged at higher temperature showed lower anthocyanin levels and less intense coloration. Principal component analysis revealed that separation of the wines was performed according to the hue value in correlation with the maceration time and time of wine aging.
Keywords: Polyphenols, Flavonoids, Anthocyanins, Flavan-3-ols, Colour intensity, Hue, Vranec variety, Spectrophotometry
Introduction
Polyphenolic compounds are a large and complex group responsible for the characteristics, colour and quality of wines, especially for red wines. Proanthocyanidins, also called condensed tannins, as compounds responsible for bitterness and astringency (Arnold et al. 1980) and anthocyanins, as responsible for the color stability of red wines (Ribereau-Gayon 1965), have attracted the great interest of the scientists. Since the properties of proanthocyanidins depend on their structures, low molecular weight flavan-3-ols, such as catechins and procyanidin oligomers are responsible for the bitterness; and polymeric flavan-3-ols, which occur as galloylated species, either conjugated with anthocyanins or in free form, are largely responsible for the red wine astringency (Robichaud and Noble 1990). Anthocyanins originating from the grapes give the colour of the young wines. During winemaking and aging of wine, anthocyanins may be modified to create stable, C4-substituted pigments through reactions involving vinyl phenol derivatives (Fulcrand et al. 1996) and pyruvic acid (Bakker et al. 1997; Fulcrand et al. 1998). A number of oligomeric pigments resulting from a condensation reaction involving acetaldehyde and from direct reactions of anthocyanins with flavanols have also been described (Somers 1971; Berg and Akiyoshi 1975; Bakker and Timberlake 1986; Fulcrand et al. 1996). Recently, the existence of anthocyanins acylated with lactic acid, formed in wine, has been reported (Alcalde-Eon et al. 2006).
For red wine production, studies are primarily focused on the influence of maceration on the extraction of grape pigments and tannins. Anthocyanins are the first components to be extracted from the grape skins together with the skin tannins at the beginning of fermentation. Tannin extraction from seeds, which is more dependent on increasing of the ethanol content because of their lower solubility in water, starts towards the mid-point of alcoholic fermentation and continues until pressing during the post-fermentation phase (Canals et al. 2005). Addition of SO2 increases the transfer of polyphenols into the must (Mayen et al. 1995) and acts as a protector against enzymatic oxidative reactions. During maturation, aging and storage of wines, coloured and non-coloured phenolics have an important role on the colour and taste of wine and they undergo a number of reactions during aging that result in changes of the sensory characteristics. These changes are mostly due to conversion of grape anthocyanins to derived pigments (Somers 1971) through reactions of anthocyanins with flavan-3-ols, polymerization reactions, and reactions leading to pyranoanthocyanin formation (Glories 1984a; Glories 1984b, Bakker and Timberlake 1986, Fulcrand et al. 1996; Remy et al. 2000).
Spectrophotometric methods are affordable for routine analyses and particularly they are rapid and simple. These methods have been used for determination of polyphenols (Slinkard and Singleton 1977; Di Stefano et al. 1989; Ivanova et al. 2010), anthocyanins (Di Stefano et al. 1989; Burns et al. 2000; Ho et al. 2003), flavonoids (Zhishen et al. 1999; Mazza et al. 1999; Marinova et al. 2005), flavan-3-ols (Di Stefano et al. 1989), colour intensity and hue of wines (Glories 1984b). The Folin-Chiocalteu method (Slinkard and Singleton 1977) is widely used for determination of total phenolics in wine. It is based on redox reaction of phosphomolybdic-phosphotungstic acid (Folin-Chiocalteu reagent) to a blue-coloured complex in an alkaline solution in the presence of phenolic compounds and shows maximum absorbance wavelength of 765 nm. For determination of total flavonoids, colorimetric method with AlCl3 can be applied for analysis of wines (Zhishen et al. 1999). This method is based on formation of stable complexes with the C-4 carboxyl group and either the C-3 or C-5 hydroxyl group of flavones and flavonols, showing maximum absorbance at 510 nm. Determination of flavan-3-ols can be performed using p-DMACA (p-dimethylaminocinnamaldehyde) method, which was first reported by Thies and Fischer (1971). This method relies on the formation of coloured product formed from the reaction between tannins and the aldehyde reagent. Applying this method, monomeric procyanidins ((+)-catechin and (−)-epicatehicn)) are determined, reacting with the p-DMACA reagent and detection of the formed adducts at 640 nm. The important fact with this method is that it theoretically responds only to the units which are not substituted in the A-ring, and thus only one unit per chain, regardless of its length unless there is some branching. For anthocyanin analysis, the simple method proposed by Di Stefano et al. (1989) which is based on dillution of the sample in appropriate amounts with ethanol/water/HCl = 69/30/1 mixture can be used, expressing the concentrations of total anthocyanins as malvidin-3-glucoside equivalents.
“Vranec” is the most widely cultivated and the most important variety grown in Republic of Macedonia and used for production of high quality red wines. Outside Republic of Macedonia, it is primarily planted in Montenegro (autochthonous variety), Serbia and Croatia (Dalmatia). In R. Macedonia, it is planted in Povardarie wine region, the most famous region for vine growing and winemaking, where more than 80% of the Macedonian vineyards are located. It is high yielding, producing fruit that is deep coloured. Since there are not published results for the phenolic content of Vranec wines from R. Macedonia, the main purpose of this study was to apply spectrophotometric methods for determination of total phenolics, total flavonoids, total anthocyanins and total flavan-3-ols, as well as the colour intensity and hue of Vranec wines obtained under different vinifications, in order to study and compare the effects of different parameters, such as maceration time, SO2 and yeast on the extraction of grape polyphenolic compounds and to study the changes of phenolics during the wine aging.
Materials and methods
Winemaking
Grapes from Vranec variety L. cv, harvested at optimal maturity (22°Brix) (2007 vintage) were transported to the experimental cellar of the Department for Enology, Institute of Agriculture in Skopje, Republic of Macedonia and divided into 12 lots (12.5 kg for each lot). Using mechanical crusher/destemmer, grapes were separately processed (crushed and destemmed) in the same way, and crushed grapes were collected in twelve 25 L plastic fermentation tanks. Two doses of aqueous solution of potassium metabisulfite were applied to the grape mashes, to give six tanks having 30 mg/L SO2 (V30) and six tanks with 70 mg/L SO2 (V70) in order to suppress polyphenol oxidase enzymes and wild yeast activity and thus, to check its effect on grape phenolic extraction. Then, musts were inoculated with two yeasts (Saccharomyces cerevisiae): Vinalco (Bitola, R. Macedonia) and Levuline CHP (Bordeaux, France). Vinalco was isolated from the Factory for yeast and alcohol manufacture (Bitola), from Tikveš region, R. Macedonia and Levuline was isolated in the terroirs of Champagne and selected by CIVC 8130, France. Yeasts were prepared by rehydrating (20 g/100 L for Vinalco and 30 g/100 L for Levuline) in water (30 °C) and applied to the musts after 15 min. Macedonian yeast Vinalco (Mac) was applied to three lots of each set, i.e. Vranec with 30 mg/L SO2 (V30-Mac) and 70 mg/L SO2 (V70-Mac), respectively. French yeast Levuline (Fr) was applied to the other three lots of each set, Vranec with 30 mg/L SO2 (V30-Fr) and 70 mg/L SO2 (V70-Fr). After addition of SO2 and yeasts, maceration times of 3 days (3d), 6 (6d) and 10 days (10d) were applied in order to study the effect of time of contact between the grape mash and juice on the phenolics extraction and their content in the final wines.
The pomace from all lots was mechanically “pumped over” twice a day during the fermentation. After the period of maceration (3, 6 and 10 days), the wines were separated from the pomace by mechanically pressing. Pressed wines were stabilized at −4 °C for a period of two weeks for tartrate stabilization and bottled. After bottling, wines were separated and kept at two different temperatures. Thus, one set of wine bottles was stored in a temperature-controled room at ~15 °C in the cellar and another set of bottles were stored in a separate room at higher temperature (~25 °C). After period of 6 months of storage, wines stored at different temperatures were analysed and results were compared in order to check the influence of storage temperature on the polyphenolic concentration.
In order to follow the changes of polyphenols during aging, wines stored in a cellar, were analysed after maceration (3, 6 and 10 days, sampling after pressing), after 2 months (2 m), after 6 (6 m) and 16 months (16 m) of storage.
Chemicals and standards
The reagents p-(dimethylamino)cinnamaldehyde (p-DMACA)), gallic acid and (+)-catechin were from Fluka (Switzerland), and the Folin-Ciocalteu reagent was from Merck (Germany). All the other used reagents were of analytical purity grade.
Total phenolics determination
All spectrophotometric measurements were carried out with Agilent 8453 UV–vis spectrophotometer, using a 1 cm cuvette optical path.
The Folin-Ciocalteu method (Slinkard and Singleton 1977; Ivanova et al. 2010) was used for determination of total phenolics. In brief, an aliquot (1 mL) of appropriate diluted wine was added to a 10 mL volumetric flask, containing 5 mL of distilled water. 0.5 mL of Folin-Ciocalteu’s phenolic reagent was added and mixed. After 3 min, 1.5 mL solution of Na2CO3 (2 g/L) was added, followed with addition of distilled water making up the total volume to 10 mL. After 16 min incubation of the samples at 50 °C (water bath) in sealed flasks, samples were cooled and read their absorbencies at 765 nm against the blank prepared with distilled water. Calibration curve was constructed using gallic acid standard solution (0–100 mg/L) using the same procedure as above. The concentration of total phenolics was expressed as gallic acid equivalents (mg/L GAE).
Total anthocyanins determination
Determination of total anthocyanins was performed by the method proposed by Di Stefano et al. (1989). Samples were diluted with a solution consisting of C2H5OH/H2O/HCl = 69/30/1 (v/v/v) and the absorbance was measured at 540 nm. Because of the lack of standard, the total anthocyanins content was calculated using the following equation proposed by Di Stefano et al. (1989):
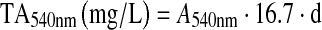 |
- A540 nm
absorbance at 540 nm
- d
dilution, expressed as malvidin-3-glucoside equivalents.
Total flavonoids determination
Total flavonoid content was evaluated according to a colourimetric assay with aluminium chloride (Zhishen et al. 1999, Ivanova et al. 2010). An aliquot of 1 mL of sample was added to a 10 mL volumetric flask containing 4 mL of distilled water, followed with addition of 0.3 mL of of solution of NaNO2 with concentration of 0.5 g/L. After 5 min, 0.3 mL of 1 g/L solution of AlCl3 was added and 6 min later, 2 mL NaOH (1 M) was added to the mixture. The total volume was made up to 10 mL with distilled water, the solution was mixed well and the absorbance was measured at 510 nm against the prepared water blank. Catechin was used as a standard for a calibration curve and the concentration was expressed as catechin equivalents (mg/g CE).
Total flavan-3-ols determination
The concentration of total flavan-3-ols was measured using the p-(dimethylamino)cinamaldehyde (p-DMACA) method (Di Stefano et al. 1989; Ivanova et al. 2010) and the content was expressed as catechin equivalents (CE mg/L) of the wines. An aliquot (1 mL) of appropriately diluted wine with water, was added to a 10 mL volumetric flask, followed by adding of 3 drops of glycerol and 5 mL p-DMACA. The total volume was made up to 10 mL with methanol, and after 7 min the absorbance was read at 640 nm against the blank-methanol. The DMACA reagent was prepared before use, containing 1% (w/v) p-DMACA in a cold mixture of methanol and HCl (4:1).
Colour intensity and hue of wines
The colour intensity determined with the content and structure of wine anthocyanins is defined as a sum of the absorbances at 420 nm, 520 nm and 620 nm (Glories 1984b). The hue of the wine that gives a measure of the ‘hue’ or redness of the wine is defined as the ratio of A420/A520 (Glories 1984b). A direct measurement of wine absorbance at 420, 520 and 620 nm was carried out using a 2 mm optical path and the colour intensity (CI) and hue (H) were calculated (Glories 1984b).
Statistical analysis
Statistical analysis, including means, standard deviations, ANOVA and Principal Component Analyses, was performed using the PC software package STATISTICA 6.0 (StatSoft, Tylsa, USA). Each wine was analyzed in triplicate.
Results and discussion
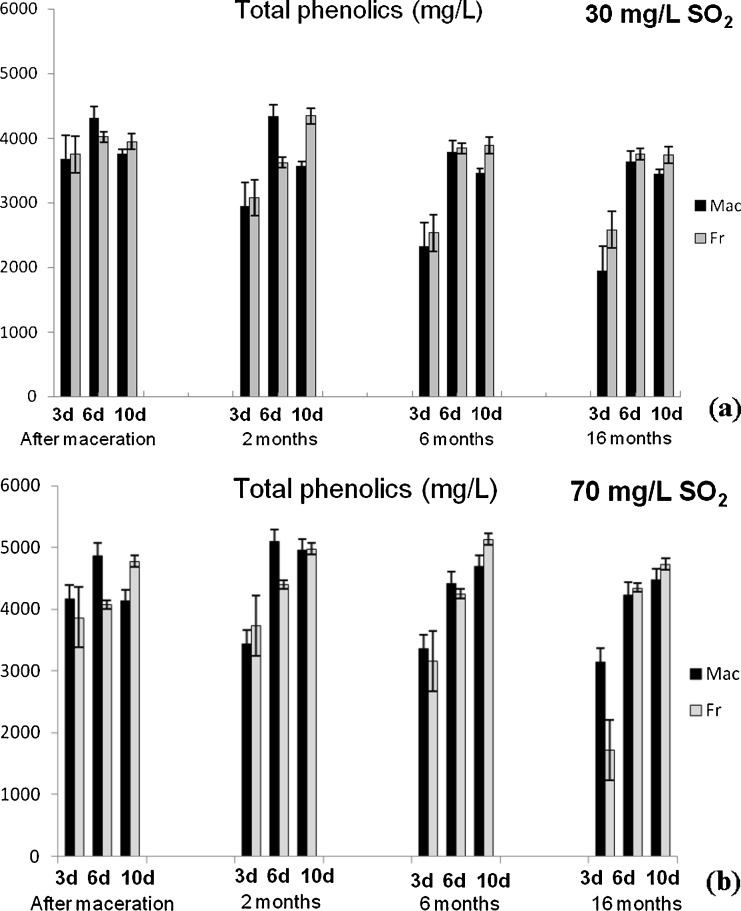
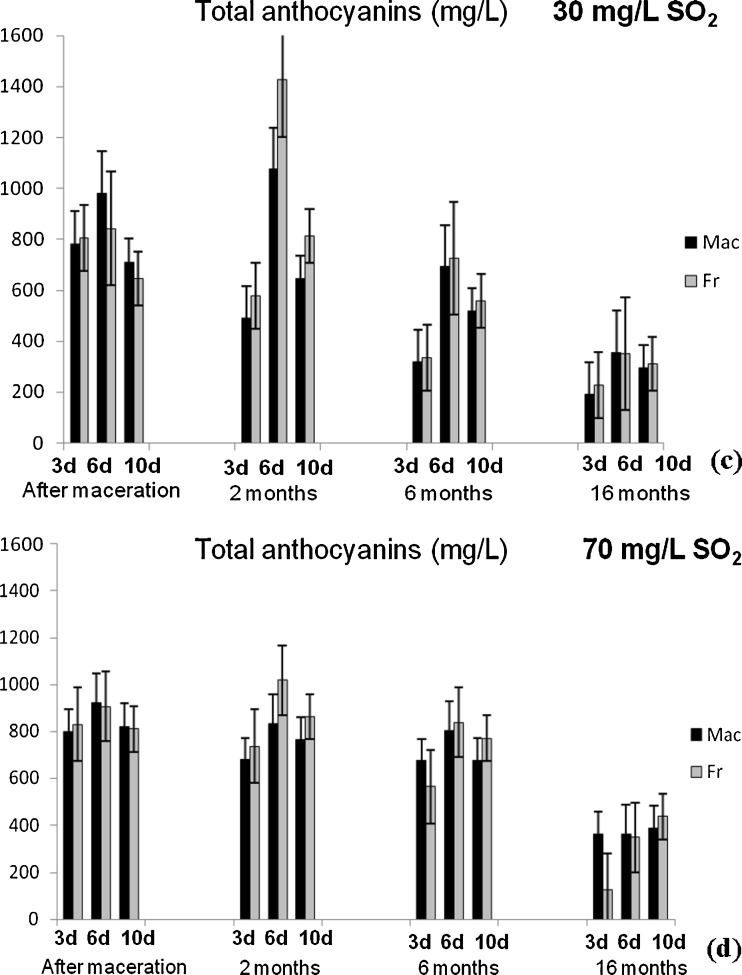
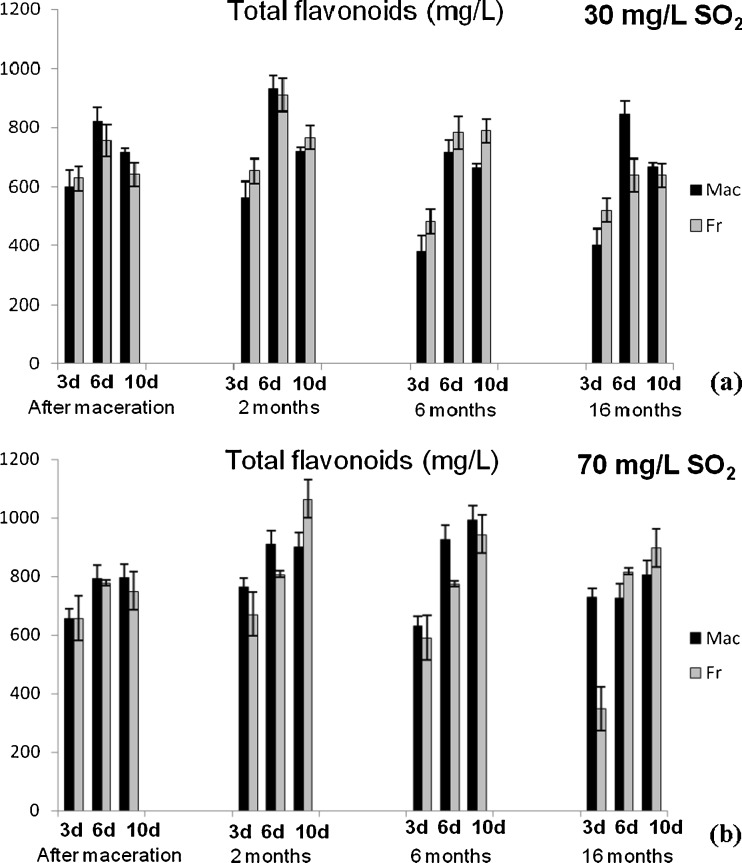
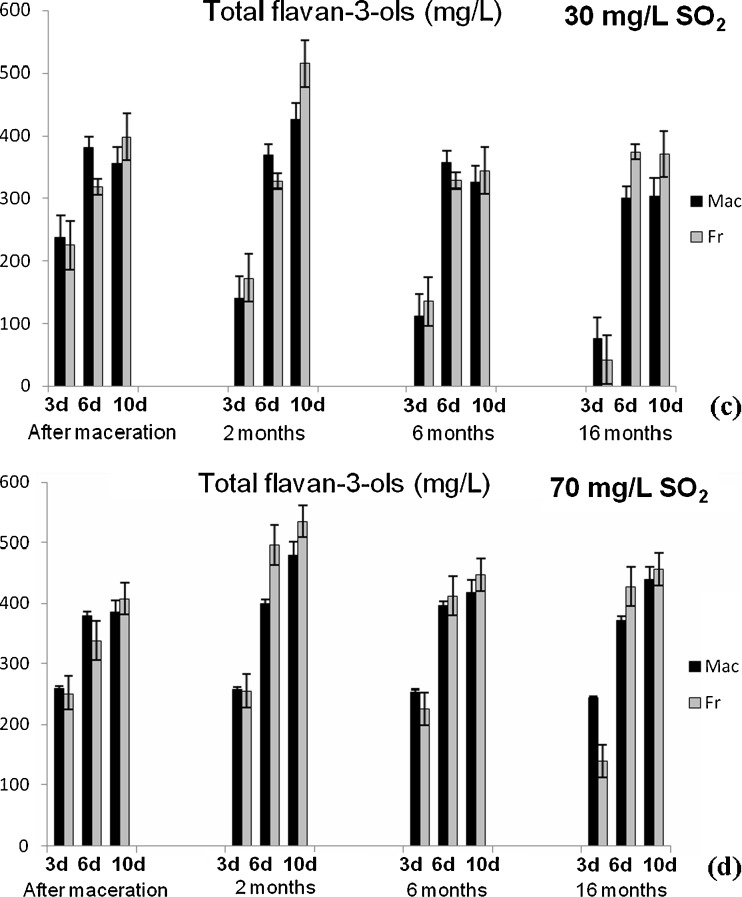
Content of total phenolics (TP) and anthocyanins (TA) (Fig. 1), flavonoids (TF) and flavan-3-ols (TF3-ols) (Fig. 2), colour intensity (CI) and hue (H) (Fig. 3) were determined applying spectrophotometric methods for analysis of the Vranec wines, in order to check the influence of time of maceration (3, 6 and 10 days), doses of SO2 (30 and 70 mg/L SO2) and yeasts (Macedonian, Vinalco and French, Levuline) on the extraction of phenolic components from grapes. Changes of polyphenols were followed at four stages: after maceration (3, 6 and 10 days, sampling after pressing); and after 2, 6 and 16 months of storage of the wines. Standard deviations of three replicate analyses are given in the figures.
Fig. 1.
Changes of total phenolics content in Vranec wines fermented with Macedonian and French yeast with: a 30 mg/L SO2 and b 70 mg/L SO2 and changes of total anthocyanins content in the wines fermented with same yeasts, containing: c 30 mg/L SO2 and d 70 mg/L SO2, followed in four phases (after maceration, after 2, 6 and 16 months of storage). Error bars represent standard deviations
Fig. 2.
Changes of total flavonoids content in Vranec wines fermented with Macedonian (Mac) and French (Fr) yeast with: a 30 mg/L SO2 and b 70 mg/L SO2 and changes of total flavan-3-ols content in the wines fermented with same yeasts, containing: c 30 mg/L SO2 and d 70 mg/L SO2 followed in four phases (after maceration, after 2, 6 and 16 months of storage). Error bars represent standard deviations
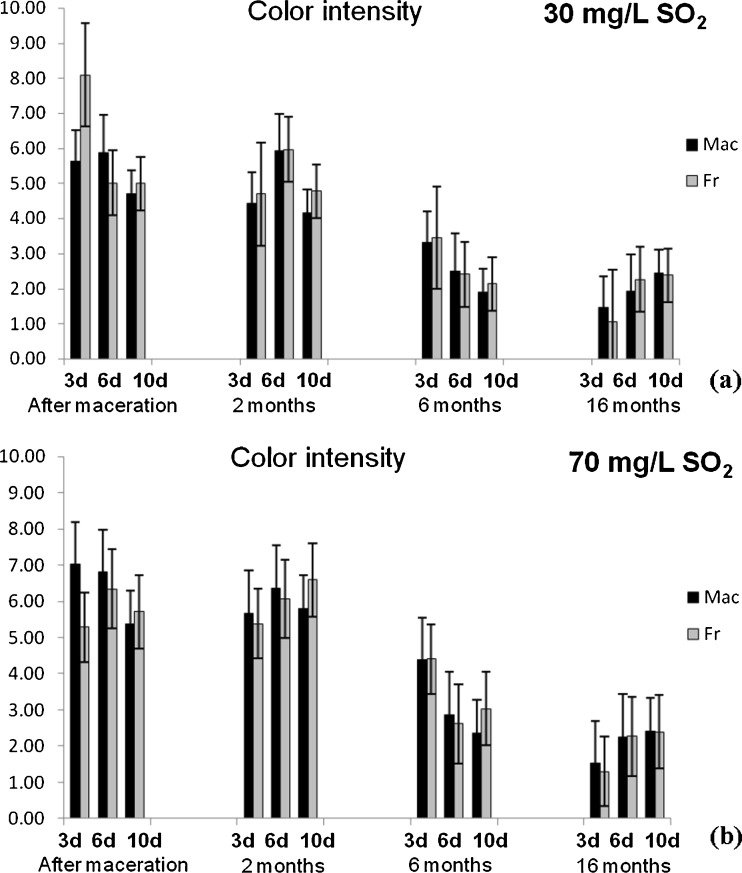
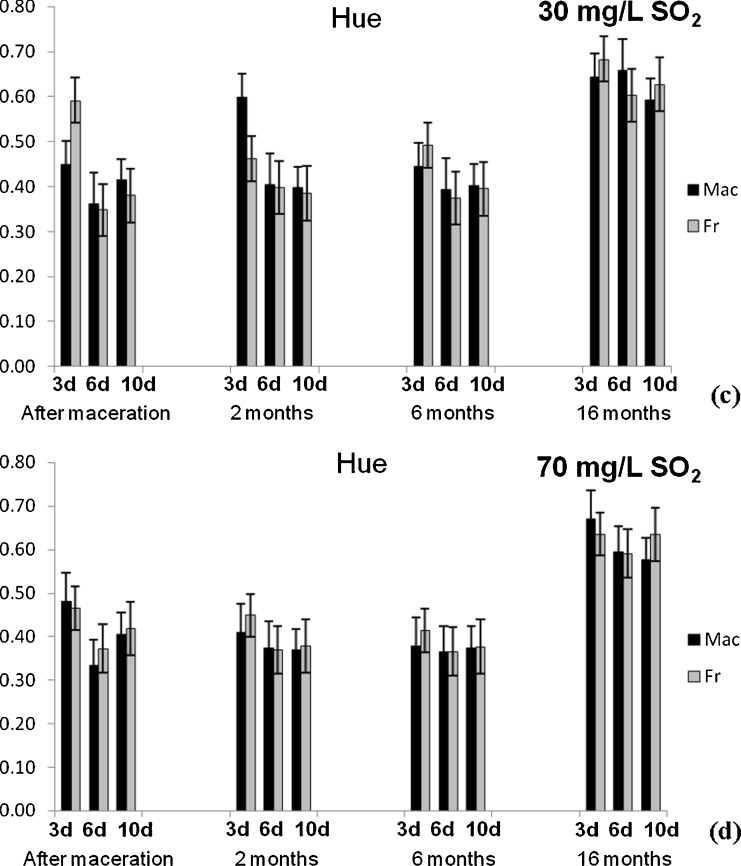
Fig. 3.
Changes of colour intenisty of Vranec wines fermented with Macedonian (Mac) and French (Fr) yeast with: a 30 mg/L SO2 and b 70 mg/L SO2 and f changes of hue in the wines fermented with same yeasts, containing: c 30 mg/L SO2 and d 70 mg/L SO2, followed in four phases (after maceration, after 2, 6 and 16 months of storage). Error bars represent standard deviations
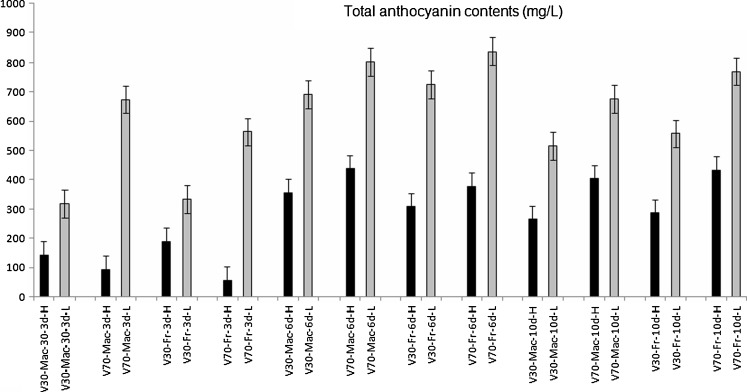
Results for the total anthocyanins determined in the wines stored at higher temperature (~25 °C), were compared to the results for the corresponding wines stored at lower temperature (~15 °C) (Fig. 4).
Fig. 4.
Changes of anthocyanin content in Vranec wines stored at high (H) and low (L) temperatures. Error bars represent standard deviations
Influence of maceration time on the polyphenolic content of Vranec wines
Process of maceration that occurs during the fermentation, has influence on the extraction of grape polyphenolics into the wine, increasing their content and diminishing the colour. In this investigation, three maceration times for Vranec wines production were applied, including 3, 6 and 10 days. It was observed that the total phenolic contant was highest in the wines obtained with 6 days of maceration and also, in one wine macerated for 10 days containing 70 mg/L SO2, even the difference between the wines macerated for 6 and 10 days was not statistically significant (p > 0.05).
Similar results were obtained for the content of anthocyanins and flavonoids, which reached highest values in the wines macerated for 6 days. Increased time of maceration (10 days) led to slight decrease of their content in the wines. These results were in accordance with previous investigations, confirming that the anthocyanins reached the maximal values during the first days of vinification (Gil-Muñoz et al. 1997; Gil-Muñoz et al. 1999; Bautista-Ortín et al. 2004) and decreasing till the end of malolactic fermentation. With regard to colour intensity and hue values, there was no statistically significant difference (p > 0.05) between the wines macerated for 3, 6 and 10 days. Only slightly higher value of the colour intensity was observed in the wines macerated for 3 days, followed by its loss during the maceartion, presumably due to the conversion of anthocyanins into non-pigmented species as a result of longer incubation times. Also, the lowest colour intensity in the wines macerated for 10 days probably is a result of anthocyanins precipitation or adsorption on the pomace. In addition, longer maceration time is very often accompanied by oxidative polymerization of monomeric anthocyanins and their complexation with other phenolics, followed by formation of oligomeric and polymeric pigments, that cause decrease of the red wine colour and enhance the brown colour of wine (Somers 1971).
With regard to flavan-3-ols, the highest content was measured for the wines macerated for 10 days, confirming that maceration time influences the extraction of grape tannins into the wine. In fact, flavan-3-ols in seeds are protected with a lipidic layer, which is disrupted when appropriate content of alcohol is formed, allowing their extraction from the seeds. Therefore, the extraction of seed tannins occurs at later phases of vinification, as a concern increasing of their concentrations during longer maceration time is expected which was in concordance with the previous published data for Macedonian Merlot wines (Ivanova et al. 2009).
According to the previous knowledge for anthocyanins and tannins in grape and wine, longer maceration time prompt higher extraction of tannins from skins and seeds (Gómez-Plaza et al. 2000; Sacchi et al. 2005) as Gómez-Plaza et al. (2000) measured higher concentrations of anthocyanins and tannins in wines aged in bottles, obtained with different times of maceration. The most intensive increase of anthocyanins was observed between the fourth and fifth day with slight decreasing after the tenth day of contact with the pomace.
Influence of the content of SO2
The use of SO2 in winemaking is due to its ability of an effective antioxidant, preventing the activity of the oxidases. Also, it has significant activity as antimicrobial agent, as well as potential for bleaching the pigments and elimination of unpleasant odours (as a result of oxidation). Because yeasts are very sensitive to SO2 (also, to other stress factors), it can selectively act against the wild yeasts, which come from the grape skin or equipment in the winery, and stop their activity.
In this research, two doses of SO2 were used, 30 and 70 mg/L, in order to check its influence on phenolics extraction during the maceration. From the results it can be concluded that higher concentrations of phenolic components, anthocyanins, flavonoids and flavan-3-ols were measured in the wines with higher dose of SO2, fermented with both yeasts, since SO2 prevented the phenolic oxidation and thus, allowed higher extraction of polyphenols.
Influence of the yeast
The potential of yeast to affect the extraction of phenolic compounds in red wines has already attracted the scientists’ attention. The first study of the influence of yeast selection on the phenolic profile of Burgundy wine (Cuinier 1988) presented small effects on colour intensity and total phenolics. Another study of the effect of two yeast strains used for vinification of Pinot noir wines showed that was no difference in total phenolics, anthocyanins and colour intensity in the wines fermented with both yeasts (Girard et al. 2001). Similar, in this research, comparing the results obtained after maceration of the Vranec wines with same amount of SO2, but fermented with different yeasts, it can be concluded that influence of the yeast on TP, TA, TF, TF3-ols, CI and H was not statistically significant (p > 0.05), probably because the used yeasts for fermentation were from the same Saccharomyces cerevisiae species. In addition, recent studies showed that anthocyanins can be adsorbed at the yeast cell walls (Morata et al. 2003; Mazauric and Salmon 2005) which led to a reduction of their content.
Influence of time of aging on the changes of polyphenolic components in Vranec wines
Wine aging in bottles has influence on the polyphenolic content. From the results for total phenolics in the analysed Vranec wines, it was noticed that slight decrease of phenolics occurred in the wines macerated for 3 days and almost no changes for the other wines were observed, which suggests that the other compounds, i.e. flavan-3-ols, extracted with longer maceration stabilise the other phenolic compounds extracted earlier in vinification.
Intensive decrease of total anthocyanins was observed for the wines macerated for 3 days after the first two months. During the time, decreasing of anthocyanins was noticed for all wines, which ranged from 54.55 to 84.15% for the wines macerated for 3 days; 58.33 to 63.68% for the 6 days macerated wines; and 46.11 to 58.67% for the wines macerated for 10 days. The highest decrease of anthocyanins was in the wines macerated for 3 days, as it was expected, taking into account that 3 days of maceration is not enough neither for extraction of skin anthocyanins, or for tannins extraction, and as a consequence of lack of tannins, anthocyanin content decreases very fast because of impossibility to form stable pigments. Also, anthocyanins can precipitate, leading to decrease of their concentration during the wine aging (Gómez-Plaza et al. 2000). For the wines macerated for 10 days, lower decrease of the anthocyanin content was noticed since they are more stabilized (involved in complexes, polymers with flavan-3-ols).
Similarly like anthocyanins, the colour intensity decreased during the wine storage, and the most intense decrease was registered for the wines macerated for 3 days (74.23 and 86.87%), while for the wines macerated for 6 and 10 days, the decrease ranged from 54.86 to 67.45% and 48.20 to 58.08%, respectively. The hue values increased during the aging time, observing highest values for the wines after 16 months of storage.
Total flavan-3-ols decreased for the wines macerated for 3 days, with 30 mg/L SO2. Their content remained stable for the other wines.
Influence of temperature of storage
A set of twelve wines, macerated for 3, 6 and 10 days, with 30 and 70 mg/L SO2, fermented with both yeasts, were stored at a room temperature of ~25 °C and obtained results were compared with the results for the same set of wines stored at lower temperature (~15 °C). The analyses were performed after the period of 6 months of storage, to check the effect of the temperature on the changes of polyphenolic content during aging. It was observed that the content of anthocyanins was lower in the wines stored at ~25 °C (Fig. 4) possibly because reactions occur faster at higher temperatures i.e. oxidation of anthocyanins occurs faster at higher temperatures causing their decrease. Analysis of variance confirmed statistically significant difference (p < 0.001) between the wines obtained at the same way, but stored at different temperatures. Contrary, no statistically significant differences were observed for the concentrations of total phenolics, flavonoids and flavan-3-ols in the wines stored at different temperature.
Principal component analysis (PCA)
Principal Component Analysis was applied on the parameters obtained from the analyses of Vranec wine samples:
Obtained with different winemaking technologies applying: different maceration times of 3, 6 and 10 days, two doses of SO2 and two yeasts for fermentation;
Analysed during the four phases of storage: after maceration (3, 6 and 10 days), after 2, 6 and 16 months of wine aging.
PCA was performed to evaluate the parameters: maceration time, time of aging, SO2 or yeast to distinguish the wines and for that purpose the spectrophotometric results for total phenolics, anthocyanins, flavonoids, flavan-3-ols, colour intensity and hue were used.
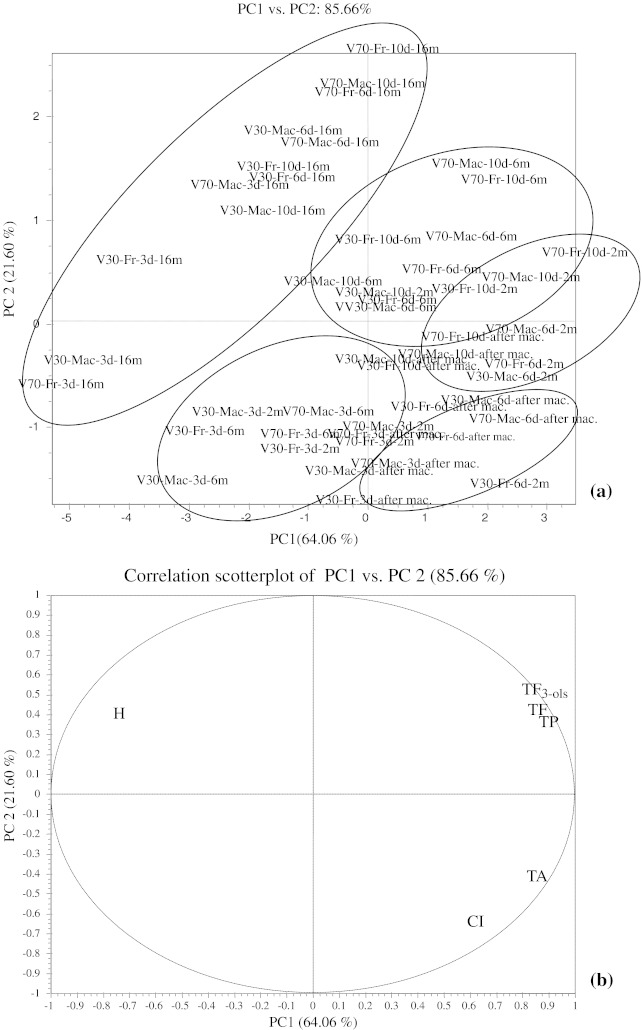
From Table 1 and Fig. 5a it can be seen that the first principal component (PC1) accounts for 64.09% of the variability and the second principal component (PC2) accounts for 21.06% from the variability, i.e. together, PC1 and PC2 account for 85.66% of the total variance.
Table 1.
Individual influence of the principal components
| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | |
|---|---|---|---|---|---|---|
| Eigen value | 3.8435 | 1.2961 | 0.4587 | 0.2310 | 0.1062 | 0.0642 |
| Explained (%) | 64.06 | 21.60 | 7.65 | 3.85 | 1.77 | 1.07 |
| Cumulated (%) | 64.06 | 85.66 | 93.31 | 97.16 | 98.93 | 100 |
Fig. 5.
a Principal Component score plot with PC1 and PC2 of the variables based on the spectrophotometric data for total phenolics, anthocyanins, flavonoids, flavan-3-ols, colour intensity and hue, and grouping of the Vranec wines according to maceration time and aging. Labels of the wine samples—30: 30 mg/L SO2; 70: 70 mg/L SO2; Mac: Macedonian yeast, Vinalco; Fr: French yeast, Levuline; 3d: 3 days of maceration; 6d: 6 days of maceration, 10d: 10 days of maceration; after mac.: after maceration; 2 m: 2 months, 6 m: 6 months, 16 m: 16 months. b Correlation scatterplot with PC1 and PC2 of the variables based of spectrophotometric data for total phenolics (TP), total anthocyanins (TA), total flavonoids (TF), total flavan-3-ols (TF3-ols), colour intensity (CI) and hue (H)
Grouping of the samples was observed according to the aging and maceration time. Thus, wines analysed after 16 months of storage, were clearly separated from the other samples and were mainly located in the negative part of PC1 and all other wines were located in the positive part of PC1 which means that PC1 is mostly related to aging of the wines. Further differences in the wines appeared in correlation with the maceration time. Thus, grouping in the positive part of PC1 was noticed: wines macerated for 6 and 10 days were located around the values 2 and 1, while the wines macerated for 3 days were located around the value 0 at the score plot. PC1 contrasts high levels of all phenolic compounds with high hue value, which means that more phenolics have been extracted with longer maceration and they decrease throughout aging in all samples.
With regard to PC2, separation of the wines was made according to the maceration time. Thus, the wines macerated for 6 and 10 days were located in the positive part of PC2, while the most of the wines macerated for 3 days were located in the negative part of PC2 and separated from the other wines (6 and 10 days macerated).
As can be seen from the correlation scatterplot in Fig. 5b all parameters contribute positively to PC1, except hue that contributes to it negatively, while TA and CI contribute negatively to PC2, which means that grouping of the samples was performed according to the hue value in correlation with the maceration time and time of wine aging.
Conclusion
The results from this study provide valuable information about the Vranec wines, the most important wine grape variety of the Balkan region, as well as of R. Macedonia. Furthermore, different winemaking treatments were applied for wine preparation in order to study the changes in phenolic content of wines. The results suggest that maceration time and SO2 affect the content of total phenolics, anthocyanins, flavonoids, flavan-3-ols, as well as color intensity and hue in the Vranec wines, increasing their content. During wine aging, when polymerization of phenolics occurs, slight decrease of total phenolics with intensive decrease of anthocyanins and colour was observed in the wines macerated for 3 days, while no changes of the other phenolic content were noticed in the wines produced with 6 and 10 days of maceration. Temperature of storage significantly influences the content of anthocyanins, causing their decrease when the wines were stored at room temperature, while the effect of yeast was not significant on polyphenolics extraction from grape during maceration.
Acknowledgement
Authors gratefully acknowledge to Dr. Veronique Cheynier from INRA-Sciences Pour l’Oenologie, Montpellier, France, for the valuable discussions during the preparation of the manuscript.
References
- Alcalde-Eon C, Escribano-Bailón MT, Santos-Buelga C, Rivas-Gonzalo JC. Changes in the detailed pigment composition of red wine during maturity and ageing. A comprehensive study. Anal Chim Acta. 2006;563:238–254. doi: 10.1016/j.aca.2005.11.028. [DOI] [Google Scholar]
- Arnold RA, Noble AC, Singleton VL. Bitterness and astringency of phenolic fractions in wine. J Agric Food Chem. 1980;28:675–678. doi: 10.1021/jf60229a026. [DOI] [Google Scholar]
- Bakker J, Timberlake CF. The mechanism of color changes in aging port wine. Am J Enol Vitic. 1986;37:288–292. [Google Scholar]
- Bakker J, Bridle P, Honda T, Kuwano H, Saito N, Terahara N, Timberlake CF. Identification of an anthocyanin occurring in some red wines. Phytochemistry. 1997;44:1375–1382. doi: 10.1016/S0031-9422(96)00707-8. [DOI] [Google Scholar]
- Bautista-Ortín AB, Fernádez-Fernádez JI, López-Roca JM, Gómez-Plaza E. Wine-making of high coloured wines: extended pomace contact and run-off of juice prior to fermentation. Food Sci Technol Int. 2004;10:287–295. doi: 10.1177/1082013204047565. [DOI] [Google Scholar]
- Berg HW, Akiyoshi MA. On the nature of reactions responsible for color behavior in red wine: a hypothesis. Am J Enol Vitic. 1975;26:134–143. [Google Scholar]
- Burns J, Gardner PT, O’Neil J, Crawford S, Morecroft I, Mc Phail DB, Lister C, Matthews D, MacLean MR, Lean MEJ, Crozier A. Relationship among antioxidant activity, vasodilation capacity, and phenolic content of red wines. J Agric Food Chem. 2000;48:220–230. doi: 10.1021/jf9909757. [DOI] [PubMed] [Google Scholar]
- Canals R, Llaudy MC, Valls J, Canals JM. Influence of ethanol concentration on the extraction of color and phenolic compounds from the skin and seeds of Tempranillo grapes at different stages of ripening. J Agric Food Chem. 2005;53:4019–4025. doi: 10.1021/jf047872v. [DOI] [PubMed] [Google Scholar]
- Cuinier C. Influence des levures sur les composés phénoliques du vin. Bull OIV. 1988;689–690:596–601. [Google Scholar]
- Di Stefano R, Cravero MC, Gentilini N 1989. Metodi per lo studio dei polifenoli dei vini. L’Enotecnico I. Maggio, 83–89
- Fulcrand H, Doco T, Es-Safi NE, Cheynier V, Moutounet M. Study of the acetaldehyde induced polymerisation of flavan-3-ols by liquid chromatography-ion spray mass spectrometry. J Chromatogr A. 1996;752:85–91. doi: 10.1016/S0021-9673(96)00485-2. [DOI] [Google Scholar]
- Fulcrand H, Benabdeljalil C, Rigaud J, Cheynier V, Mountounet M. A new class of wine pigments generated by reaction between pyruvic acid and grape anthocyanins. Phytochemistry. 1998;47:1401–1407. doi: 10.1016/S0031-9422(97)00772-3. [DOI] [PubMed] [Google Scholar]
- Gil-Muñoz R, Gómez-Plaza E, Martínez A, López-Roca JM. Evolution of the CIELAB and other spectrophotometric parametars during wine fermentation. Influence of some pre and postfermentative factors. Food Res Int. 1997;30:699–705. doi: 10.1016/S0963-9969(98)00029-5. [DOI] [Google Scholar]
- Gil-Muñoz R, Gómez-Plaza E, Martínez A, López-Roca JM. Evolution of phenolic compounds during wine fermentation and post-fermentation: influence of grape temperature. J Food Compos Anal. 1999;12:259–272. doi: 10.1006/jfca.1999.0834. [DOI] [Google Scholar]
- Girard B, Yuksel D, Cliff MA, Delaquis P, Reynolds AG. Vinification effects on the sensory, colour, and GC profiles of Pinot noir wines from British Colombia. Food Res Int. 2001;34:483–499. doi: 10.1016/S0963-9969(00)00177-0. [DOI] [Google Scholar]
- Glories Y. La couleur des vins rouges. Mesure, origine et interprétation. Partie I. Connaiss. Vigne Vin. 1984;18:195–217. [Google Scholar]
- Glories Y. La couleur des vins rouges II, Connaissance de la vigne et du vin. Vigne vin. 1984;18:253–271. [Google Scholar]
- Gómez-Plaza E, Gil-Nuñoz R, López-Roca JM, Martínez A. Color and phenolic compounds of a young red wine. Influence of wine-making techniques, storage temperature, and length of storage time. J Agric Food Chem. 2000;48:736–741. doi: 10.1021/jf9902548. [DOI] [PubMed] [Google Scholar]
- Ho P, Da Conceição M, Silva M, Hogg TA. Changes in the colour of the phenolic composition during the early stages of maturation of prot in wood, stainless steel and glass. J Sci Food Agric. 2003;81:1269–1280. doi: 10.1002/jsfa.938. [DOI] [Google Scholar]
- Ivanova V, Stefova M, Vojnoski B. Assay of the phenolic profile of Merlot wines from Macedonia: effect of maceration time, storage, SO2 and temperature of storage. Maced J Chem Chem Eng. 2009;28:141–149. [Google Scholar]
- Ivanova V, Stefova M, Chinnici F. Determination of polyphenol contents in Macedonian grapes and wines assessed by standardized spectrophotometric methods. J Serb Chem Soc. 2010;75:45–59. doi: 10.2298/JSC1001045I. [DOI] [Google Scholar]
- Marinova D, Ribarova F, Atanassova M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J Univ Chem Tech Metall. 2005;40:255–260. [Google Scholar]
- Mayen M, Merida J, Medina M. Flavonoid and non-flavonoid compounds during fermentation and post-fermentation standing of musts from cabernet sauvignon and Tempranillo grapes. Am J Enol Vitic. 1995;46:255–261. [Google Scholar]
- Mazauric JP, Salmon JM. Interactions between yeast lees and wine polyphenols during simulation of wine aging: I. Analysis of remnant polyphenolic compounds in the resulting wines. J Agric Food Chem. 2005;53:5647–5653. doi: 10.1021/jf050308f. [DOI] [PubMed] [Google Scholar]
- Mazza G, Fukumoto L, Delaquis P, Girard B, Ewert B. Anthocyanins, phenolics, and color of Cabernet Franc, Merlot, and Pinot Noir wines from British Columbia. J Agric Food Chem. 1999;47:4009–4017. doi: 10.1021/jf990449f. [DOI] [PubMed] [Google Scholar]
- Morata A, Gómez-Corovés MC, Suberviola J, Bartolomé B. Adsorption of anthocyanins by yeast cell walls during the fermentation of red wines. J Agric Food Chem. 2003;51:4084–4088. doi: 10.1021/jf021134u. [DOI] [PubMed] [Google Scholar]
- Remy S, Fulcrand H, Labarbe B, Cheynier V, Moutounet M. First confirmation in red wine of products resulting from direct anthocyanin-tannin reactions. J Sci Food Agric. 2000;80:745–751. doi: 10.1002/(SICI)1097-0010(20000501)80:6<745::AID-JSFA611>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Ribereau-Gayon P. La couleur des vins. Aliment Vie. 1965;53:232–248. [PubMed] [Google Scholar]
- Robichaud JL, Noble AC. Astringency and bitterness of selected phenolics in wine. J Sci Food Agric. 1990;53:343–353. doi: 10.1002/jsfa.2740530307. [DOI] [Google Scholar]
- Sacchi KL, Bisson LF, Adams DO. A review of the effect of winemaking techniques on phenolic extraction in red wines. Am J Enol Vitic. 2005;56:197–206. [Google Scholar]
- Slinkard K, Singleton VL. Total phenol analysis: automation and comparison with manual methods. Am J Enol Vitic. 1977;28:49–55. [Google Scholar]
- Somers TC. The polymeric nature of wine pigments. Phytochemistry. 1971;10:2175–2186. doi: 10.1016/S0031-9422(00)97215-7. [DOI] [Google Scholar]
- Thies M, Fischer R. New reaction for microchemical detection and the quantitative determination of catechins. Mikrochim Acta. 1971;1:9–13. doi: 10.1007/BF01216876. [DOI] [Google Scholar]
- Zhishen J, Mengeheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]