Abstract
Effect of water activity (aw, 0.99), pH (4.5) and their interaction on the growth inhibition of Aspergillus parasiticus was studied on potato dextrose agar (PDA) using various antimicrobial agents (citral, carvacrol, eugenol, cineole, thymol guaiacol, vanillin, anethol, potassium sorbate and sorbic acid). The results demonstrate that colony diameter (mm) exhibited a constant increase with time (zero order kinetics) for all antimicrobials evaluated. Eugenol and sorbic acid inhibited the test fungi at 300 and 600 ppm, respectively. Radial growth rate (RGR) of A. parasiticus was significantly (p < 0.05) different among different antimicrobials as well as the concentrations tested. However, this difference was not observed with higher concentration of citral, eugenol, vanillin and sorbic acid. Among the antimicrobials evaluated potassium sorbate, cineole, anethol and guaiacol were least effective. Thymol, eugenol and carvacrol were more effective in inhibiting A. parasiticus even with low concentration (150 ppm) as their mean RGR was zero even after 20 days of incubation (pH 4.5).
Keywords: Water activity, Radial growth rate, Essential oils, Antifungal agents, Growth inhibition
Introduction
Chemical compounds are used to combat the fungus, but the consumer perception of chemical preservatives drives attention towards natural alternatives. Interest has been focused on the potential application of plant derived essential oils (EOs). Wilkins and Board (1989) reported that more than 1,340 plants are known to be potential source of antimicrobials; about 60 are mentioned by Nychas (1995) and Beuchat (1994).The use of natural antimicrobial compounds is important not only in the preservation of food but also in the control of human and plant disease of microbial origin (Baratta et al. 1998). Among the complex interactions which occur during fungal growth, intrinsic and extrinsic factors play an important role. pH, temperature, time, moisture, gaseous composition, and antimicrobial substances are some of the factors that are being evaluated in combination against fungistatic/fungicidal activity. Although fungal growth is usually controlled using synthetic chemicals, natural antimicrobials have also demonstrated important antifungal properties (Lopez-Malo et al. 1997).
It has been reported that a variety of plant extracts and phenolic compounds have a wide antimicrobial spectrum (Paster et al. 1990; Elgayyar et al. 2001; Dorman and Deans 2000; Mahmoud 1994). Antimicrobial activity of several EOs such as thymol, carvacrol, vanillin, citral etc., could be a result of impairment of variety of cell systems including those involved in energy production and cell synthesis (Conner and Beuchat 1984a, b). Nychas (1995) indicated that phenolic compounds could denature enzymes responsible for spore germination or interfere with amino acids involved in germination. Studies made by Hope et al. (2003) has shown that specific EOs and phenolic compounds can retard/control the growth rate and spore germination time of deteriorating fungi. Lopez-Malo et al. (2005) found that the germination time and radial growth rate (RGR) were significantly affected by the concentration of vanillin, pH and incubation temperature. They also reported that inhibition condition depends on the type of mold (the most resistant sp. A. niger was inhibited at 1000 ppm of vanillin, while A. ochraceous at 500 ppm at pH 3.0). Further, Paster et al. (1994) observed that 400 ppm of thyme and oregano EOs inhibited the growth of three Aspergillus sp. viz., A. ochraceous, A. flavus and A. niger. Complete inhibition was recorded at 600 ppm of thyme and 400 ppm of oregano. However, the different environmental factors such as pH, water activity (aw) might affect the effectiveness of these synthetic antimicrobials as well as phenolic compounds in relation to fungal growth and proliferation. The purpose of this work was to study the effect of few antimicrobial agents such as natural antimicrobials and few chemical preservatives on the growth of A. parasiticus on potato dextrose agar (PDA) designed with selected aw (0.99) and pH (4.5).
Material and methods
Fungal culture and preparation of inoculum
A. parasiticus strain used in this study was kindly supplied by Dr. Vincente Sanchis, Food Technology Department, Lleida University, Lleida, Spain. The pure culture of the organism was cultivated on PDA slants (Hi-media pvt Ltd., Mumbai) and incubated for 10 days at 25 ± 1 °C. At the end of incubation period, the spores were harvested with 10 ml of sterile 0.1% Tween-80 (Merk India Ltd.,) solution. This suspension was passed through sterilized membrane filter (0.45 μ) to remove debris and other agar particles, if any. The final concentration of spore suspension (≈1 × 106 spores/ml) was made using haemocytometer just before use.
Chemicals
Citral, carvacrol, eugenol, cineole and thymol were purchased from Sigma-Aldrich, Germany; while guaiacol and vanillin were from Sisco Research Laboratories, Mumbai. Anethol was purchased from Merk India and potassium sorbate and sorbic acid were from Hi-Media Ltd., Mumbai.
Protocol of the experiment
The effect of water activity (0.99), pH (4.5) and antimicrobial compounds such as citral, anethol, guaiacol, vanillin, thymol, carvacrol, eugenol, cineole, potassium sorbate and sorbic acid, and their concentrations (from 150 to 1500 ppm) were assessed on radial growth rate (RGR) of A. parasiticus by factorial designs according to Montgomery (1984). The culture plates were prepared using sterile PDA medium, with varied concentrations of above antimicrobials. All sets of PDA plates formulated accordingly were replicated thrice.
Media preparation, their combination and incubation
Initially we have tested the solutes to be used in this study, such as KCl, NaCl, glucose and glycerol to know the growth of test organism (A. parasiticus) in PDA. It was observed that the organism grew well in PDA plates formulated with KCl media. The PDA systems were prepared with 1.2% of KCl as prescribed by Chen (1989) to achieve 0.99 aw, and the media was autoclaved, cooled and acidified with 0.1 N HCl to adjust the pH to the desired level. The sterilized agar solutions were aseptically divided and required quantity of every antimicrobial agent was added and thoroughly mixed before being poured into sterile glass Petri plates. Triplicate sets of each system was inoculated in the middle of PDA plates by adding 5μL of the spore suspension (≈1.0x106 spores/ml). Control sets with no antimicrobial agents were also run along with test plates as above. The plates were kept in clean plastic square boxes (25x25cm), and incubated for 20 days at 25 ± 0.5 °C. To avoid dehydration in culture plates, saturated sodium chloride solution was used. Each set of incubated plates were removed for 5–10 min to measure the size of colony (right angles to each other) and re-incubated, soon after.
Growth and colony measurement
The plates were observed periodically for 3 weeks (once after first week and 4 times in 2 weeks thereafter) using stereoscopic microscope (Magnus, Olympus India Pvt Ltd.,). Increase in size of colony (diameter) in each plate was plotted as incubation time and radial growth rate (RGR). This was obtained from the slope by linear regression (Horner and Anagnostopoulos 1973). For each set of experiment, mean RGR and standard deviations were calculated and presented. The germination time or lag was calculated using linear equation which was extrapolated to zero increase in diameter and the intercept on the time axis was defined as lag (Lopez-Malo et al. 1995).
Statistical analysis
To establish the significant difference between treatments and level of treatments of different variables (aw and pH), analysis of variance (ANOVA) was performed using Duncan’s new multiple range test (DMRT) at p < 0.05 (Duncan 1955).
Results and discussion
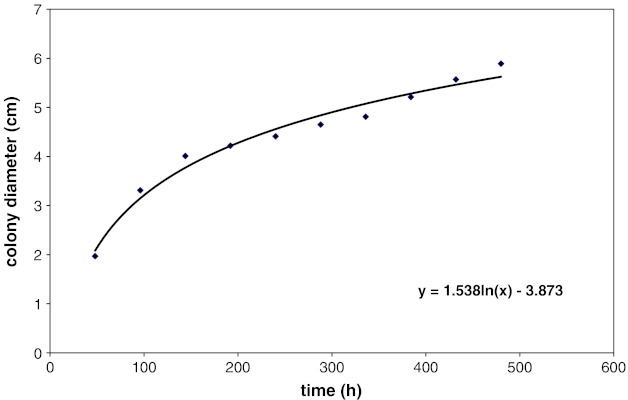
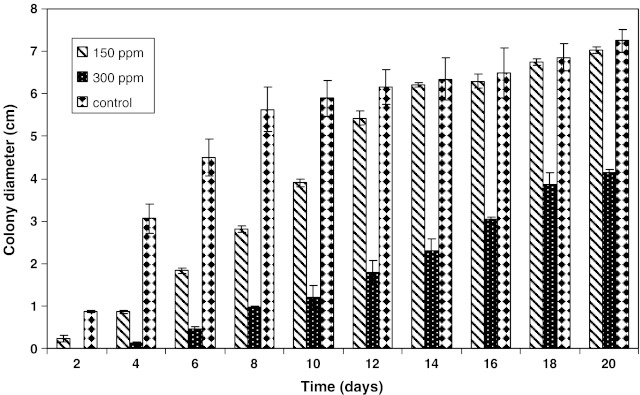
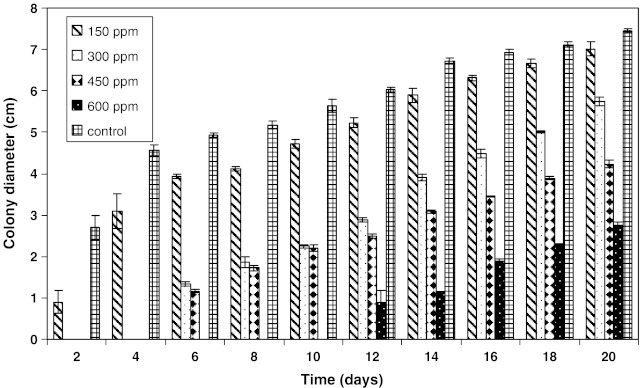
The combined effect of aw (0.99), pH (4.5) and antimicrobial agents (citral, anethol, guiacol, cineole, eugenol, thymol, carvacrol, vanillin, potassium sorbate and sorbic acid) on the growth of A. parasiticus were evaluated in PDA. Based on our preliminary experiments, we have choosen KCl as a suitable agent to adjust aw in PDA systems, although there was some growth with glycerol. It appeared that the use of glycerol to adjust the medium is not economical. Therefore, we have used KCl as an suitable agent to adjust aw throughout the experiment. The increase in colony diameter of A. parasiticus inoculated in PDA and incubated for 20 days at 25 ± 1 °C with 0.99 aw, pH 4.5 and cineole concentration at 150 ppm is shown in Fig. 1. It indicated that the colony diameter exhibited a constant increase with time. Almost same results were obtained for other antimicrobial agents investigated. Regression coefficients were obtained for the sigmoid growth phase, with standard deviation/mean values. It has been established that a linear increase in size of colony (diameter) as a function of incubation time for molds in solid medium and found significant differences among radial growth rate (Gonzalez et al. 1987). Figures 2 and 3 presents the growth of A. parasiticus in presence of different concentrations eugenol and sorbic acid, respectively at pH 4.5 and 0.99 aw. In general, the growth was directly proportional to the concentration of antimicrobials used. Although there was very little growth with eugenol upto 300 ppm concentration, above 450 ppm total inhibition was observed. Almost similar results were obtained with 600 ppm of sorbic acid (Figs. 2 and 3). However, the other antimicrobials such as citral and vanillin have shown inhibition at >750 ppm (data not shown). The least effective antimicrobials being anethol, guaiacol, cineole and potassium sorbate.
Fig. 1.
Growth and colony diameter of A. parasiticus in PDA formulated with 0.99 aw at 4.5 pH and 1500 ppm of cineole
Fig. 2.
Growth of A. parasiticus in potato dextrose agar formulated with 0.99 aw at 4.5 pH in presence of eugenol
Fig. 3.
Growth of A. parasiticus in potato dextrose agar formulated with 0.99 aw at 4.5 pH in presence of sorbic acid
The antimicrobial activity of sorbate is influenced by number of factors such as species, strains, substrate composition, pH, aw, additives present, temperature and inoculum size (Sofos 1989; Steels et al. 2000). It also depends on the dissociation constant (pKa). The dissociation constant for sorbate is 4.75. At this pH value, 50% of the acid is in the effective undissociated form. The more favourable antimicrobial effect at higher pH levels obtained with sorbate is due to their more pKa values compared to propionates/benzoates. Therefore, sorbate has maximum pH for activity around 6.0–6.5 pH, while those for propionates and benzoates are 5.0–5.5 and 4.0–4.5, respectively.
With regard to pH, the antimicrobial activity of sorbate increases as the pH decreases (Sofos and Busta 1981; Cerruti et al. 1990), this is attributed to the increased amount of undissociated acid present (Lund et al. 1987; Skirdal and Eklund 1993). Although activity is greater at lower pH values, sorbates have the advantage of being effective at pH values as high as 6.5 (Sofos and Busta 1981). Therefore, in the present study, we preferred to use 4.5 pH for assessing the mold growth (A. parasiticus). However, few studies have shown that it’s activity by sorbate at pH values as high as 7.0 (Chung and Lee 1982; Statham and McMeekin 1988). Contrary to this, the maximum pH for antimicrobial activity by most other common chemical food preservatives is lower; for example, 5.0 to 5.5 and 4.0 to 4.5 for propionate and benzoate, respectively (Sofos and Busta 1981). Also, Lopez-Malo et al. (2005) found an important antimicrobial differences being, the natural antimicrobials are less pH dependant than chemical preservatives.
A Number of factors are related to fungal physiology, morphology, cell enlargement and branch formation with respect to RGR (Brancato and Golding 1953). Table 1 represents mean RGR of A. paraciticus inoculated in PDA with different antimicrobials formulated with 0.99 aw at 4.5 pH value. At this condition, RGR of A. parasiticus was significantly (p < 0.05) different among the different antimicrobials as well as the concentrations tested. RGR was shown as zero, in the plates where there is no growth even after 20 days of incubation. This was observed with thymol and carvacrol which were more efficient in inhibiting the growth of A. parasiticus even at low concentration (150 ppm). However, there was very little growth at 150 and 300 ppm of eugenol. Further, eugenol with higher concentrations (450 ppm and above) no growth was observed (Table 1). It is known that many fungal sp. are capable of growing in wide range of pH. It also affects growth rate and other extrinsic and intrinsic growth limits (Holmquist et al. 1983). Thompson (1990) suggested that pH might affect in vitro growth of Aspergillus strains; for six different strains of A. flavus studied, decrease in pH from 6.0 to 4.0 reduced the mycelial production upto 13%, while for two strains of A. parasiticus, the reduction was 9%. Lopez-Malo et al. (1995) demonstrated that vanillin concentration and the type of agar significantly (p < 0.05) affected the RGR of Aspergilli. Also, differences among mold responses were evaluated; A. niger was most resistant, followed by A. flavus and A. parasiticus. Nevertheless, vanillin at 1000 ppm level inhibited A. ochraceous growth for more than 2 months at 25 °C in PDA, where as growth of A. niger, A. flavus and A. parasiticus was inhibited by 1500 ppm.
Table 1.
Mean radial growth rate (mm/h) of A. parasiticus in PDA formulated with 0.99 aw at 4.5 pH with different concentrations (ppm) of antimicrobials
| Chemicals | Concentration of antimicrobials (ppm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 150 | 300 | 450 | 600 | 750 | 900 | 1050 | 1200 | 1350 | 1500 | |
| Citral | 1.03ab | 0.92c | 0.83c | 0.72c | 0.62c | 0.00a | 0.00a | 0.00a | 0.00a | 0.00a |
| Eugenol | 0.00a | 0.00a | 0.00a | 0.00a | 0.00a | 0.00a | 0.00a | 0.00a | 0.00a | 0.00a |
| Thymol | 0.00a | 0.00a | 0.00a | 0.00a | 0.00a | 0.00a | 0.00a | 0.00a | 0.00a | 0.00a |
| Carvacrol | 0.00a | 0.00a | 0.00a | 0.00a | 0.00a | 0.00a | 0.00a | 0.00a | 0.00a | 0.00a |
| Anethol | 1.29b | 1.13d | 0.91c | 0.82c | 0.73d | 0.57d | 0.42c | 0.36c | 0.28b | 0.09a |
| Cineole | 1.28b | 1.26f | 1.24e | 1.24e | 1.16f | 1.14f | 1.09e | 1.03e | 0.96d | 0.92a |
| Guaicol | 1.23ab | 1.18e | 1.09d | 1.03d | 0.95e | 0.85e | 0.74d | 0.71d | 0.65c | 0.57b |
| Vanillin | 1.07ab | 0.94c | 0.87d | 0.76c | 0.66c | 0.49c | 0.00a | 0.00a | 0.00a | 0.00a |
| Potassium | ||||||||||
| sorbate | 1.03ab | 0.76b | 0.57b | 0.53b | 0.41b | 0.40b | 0.30b | 0.27b | 0.24b | 0.22d |
| Sorbic acid | 9.83c | 5.70g | 4.60f | 1.75f | 0.00a | 0.00a | 0.00a | 0.00a | 0.00a | 0.00a |
Mean values in the same column with different letters are significantly different at p < 0.05 by Duncan’s New Multiple Range Test (DMRT).
In the present study, for most of the antimicrobials tested, an analysis of variance (ANOVA) demonstrated that antimicrobial concentrations used (150 to 1500 ppm) significantly (p < 0.005) affected A. parasiticus RGR (Table 1). However, this difference was not observed at higher concentration of few antimicrobials such as citral, eugenol, vanillin and sorbic acid. In general, as antimicrobial concentration increased, longer lag periods were observed, this is because the sensitivity of the fungus directly depends on concentration of the antimicrobial used. As such in our study, A. parasiticus exhibited higher sensitivity to thymol, carvacrol and eugenol than to other antimicrobials tested at same aw (0.99) and pH (4.5).
A number of authors have been reviewed the possible modes of action of phenolic compounds (Wilkins and Board 1989; Beuchat 1994; Davidson and Naidu 2000). At lower pH levels, thymol molecule is largely undissociated and therefore more easily dissolved in the lipidic phase. Kabara (1991) mentioned that undissociated phenolic groups were more active as antimicrobials than dissociated forms, suggesting that phenols can act on a wide range of pH (3.5–8.0). At low concentration, phenols could affect enzyme activity, especially of those enzymes associated with energy production, where as at higher concentrations, they cause protein denaturation. Lis-Balchin and Deans (1997) reported that the strong antimicrobial activity could be correlated with essential oils containing a high%ge of monoterpenes, eugenol, cinnamic aldehyde and thymol. Although exact cause for mode of action of phenolics (thymol, eugenol, carvacrol and vanillin) could not be determined, they may however interfere with essential enzymes and react with cell membrane or disturb the genetic functionality of the organism (Davidson 2001).
Phyto-chemicals of antimicrobial in nature are yet to be completely exploited; and also the exact mechanism(s) for food antimicrobials are often not known. Spices, herbs and their essential oils have varying degree of biological activity. Of the 70 herbs and spices officially recognized as useful food ingredients (Lindsay 1996), only a handful have demonstrated significant antimicrobial activity. Often, the antimicrobial concentration of compounds in spices and herbs are very low to be used effectively without untoward effects on the sensory qualities of a food. The use of plant essential oils, herbs and spices are very limited as antimicrobials in foods, as they require higher minimum inhibitory concentrations (MICs) (Castanon et al. 1999). Added to this they may also impart off flavours. These objectionable off flavours could be minimized to certain extent, if natural compounds are used combining with other stress factors such as reduced aw and pH (in the frame of “hurdle technology”).
Acknowledgement
The authors wish to thank the Director, CFTRI, Mysore for providing necessary facilities during the course of this investigation. We also thank Dr. R. Ravi, for analysing the statistical data.
References
- Baratta MT, Dorman HJD, Deans SG, Biondi DM, Ruberto G. Chemical composition, antibacterial and antioxidative activity of laurel, sage, rosemary, oregano and coriander essential oils. J Essent Oil Res. 1998;10:618–627. [Google Scholar]
- Beuchat LR. Antimicrobial properties of spices and their essential oils. In: Dillon VM, Board RG, editors. Natural Antimicrobial Systems and Food Preservation. Wallingford: CAB Intl; 1994. pp. 167–179. [Google Scholar]
- Brancato FP, Golding NS. The diameter of the mold colony as a reliable measure of growth. Mycologia. 1953;45:848–864. [Google Scholar]
- Castanon X, Argaiz A, Lopez-Malo A. Effect of storage temperature on the microbial and color stability of banana purees prepared with the addition of vanillin or potassium sorbate. Food Sci Technol Int. 1999;5:56–60. doi: 10.1177/108201329900500105. [DOI] [Google Scholar]
- Cerruti P, Alzamora SM, Chirife J. A multi-parameter approach to control the growth of Saccaromyces cerevisiae in laboratory media. J Food Sci. 1990;55:837–840. doi: 10.1111/j.1365-2621.1990.tb05243.x. [DOI] [Google Scholar]
- Chen CS. Water activity— concentration molds for solutions of sugars, salts and acids. J Food Sci. 1989;54(5):1318–1321. doi: 10.1111/j.1365-2621.1989.tb05982.x. [DOI] [Google Scholar]
- Chung YM, Lee JS. Potassium sorbate inhibition of microorganisms isolated from seafood. J Food Prot. 1982;45:1310. doi: 10.4315/0362-028X-45.14.1310. [DOI] [PubMed] [Google Scholar]
- Conner DE, Beuchat LR. Effects of essential oils from plants on growth of food spoilage yeasts. J Food Sci. 1984;49:429–434. doi: 10.1111/j.1365-2621.1984.tb12437.x. [DOI] [Google Scholar]
- Conner DE, Beuchat LR. Sensitivity of heat-stressed yeasts to essential oils of plants. Appl Environ Microbiol. 1984;47:229–233. doi: 10.1128/aem.47.2.229-233.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PM. Chemical preservatives and naturally antimicrobial compounds. In: Doyle MP, Beuchat LR, Montville TJ, editors. Food Microbiology. Fundamentals and Frontiers. 2. Washington: ASM Press; 2001. pp. 593–628. [Google Scholar]
- Davidson PM, Naidu AS. Phyto-phenols. In: Naidu AS, editor. Natural Food Antimicrobial Systems. Boca Raton: CRC Press; 2000. pp. 265–294. [Google Scholar]
- Dorman HJD, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- Duncan DB. Multiple range and multiple ‘F’ test. Biometrics. 1955;11:1–42. doi: 10.2307/3001478. [DOI] [Google Scholar]
- Elgayyar M, Draughon FA, Golden DA, Mount JR. Antimicrobial activity of essential oils from plants against selected pathogenic and saprophytic microorganisms. J Food Prot. 2001;64:1019–1024. doi: 10.4315/0362-028x-64.7.1019. [DOI] [PubMed] [Google Scholar]
- Gonzalez HHL, Resnik SL, Vaamonde G. Influence of inoculum size on growth rate and lag phase of fungi isolated from Argentine corn. Int J Food Microbiol. 1987;4:111–117. doi: 10.1016/0168-1605(87)90017-1. [DOI] [PubMed] [Google Scholar]
- Holmquist GU, Walker HW, Stahr HM. Influence of temperature, pH, water activity and antifungal agents on growth of Aspergillus flavus and A. parasiticus. J Food Sci. 1983;48:778–782. doi: 10.1111/j.1365-2621.1983.tb14897.x. [DOI] [Google Scholar]
- Hope R, Jestoi M, Magan N. Multitarget environmental approach for control of growth and toxin production by Fusarium culmorum using essential oils and antioxidants. In: Credland PE, Armitage DM, Bell CH, Cogan PM, Highley E, editors. Advances in Stored Product Protection. Cambridge: CABI Publishing; 2003. pp. 486–492. [Google Scholar]
- Horner KJ, Anagnostopoulos GD. Combined effect of water activity, pH and temperature on the growth and spoilage potential of fungi. J Appl Bacteriol. 1973;36:427–436. doi: 10.1111/j.1365-2672.1973.tb04124.x. [DOI] [PubMed] [Google Scholar]
- Kabara JJ. Phenols and chelators. In: Russel NJ, Gould GW, editors. Food Preservatives. Glasgow: Blackie & Son; 1991. pp. 44–71. [Google Scholar]
- Lindsay RC. Food additives. In: Fennema OR, editor. Food chemistry. 3. New York: Marcel Dekker; 1996. pp. 767–823. [Google Scholar]
- Lis-Balchin M, Deans SG. Bioactivity of selected plant essential oils against Listeria monocytogenes. J Appl Microbiol. 1997;82:759–762. doi: 10.1046/j.1365-2672.1997.00153.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Malo A, Alzamora SM, Argaiz A. Effect of natural vanillin on germination time and radial growth of moulds in fruit-based agar systems. Food Microbiol. 1995;12:213–219. doi: 10.1016/S0740-0020(95)80100-6. [DOI] [Google Scholar]
- Lopez-Malo A, Alzamora SM, Argaiz A. Effect of vanillin concentration, pH and incubation temperature on Aspergilllus flavus, A. niger, A. ochraceous, and A. parasiticus growth. Food Microbiol. 1997;14:117–124. doi: 10.1006/fmic.1996.0078. [DOI] [Google Scholar]
- Lopez-Malo A, Alzamora SM, Palou E. Aspergillus flavus growth in the presence of chemical preservatives and naturally occurring antimicrobial compounds. Int J Food Microbiol. 2005;99:119–128. doi: 10.1016/j.ijfoodmicro.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Lund BM, George SM, Franklin JG. Inhibition of type A and type B (proteolytic) Clostridium botulinum by sorbic acid. Appl Environ Microbiol. 1987;59:935–941. doi: 10.1128/aem.53.5.935-941.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud ALE. Antifungal action and anti aflatoxigenic properties of some essential oil constituents. Let Appl Microbiol. 1994;19:110–113. doi: 10.1111/j.1472-765X.1994.tb00918.x. [DOI] [PubMed] [Google Scholar]
- Montgomery DC. Design and Analysis of Experiments. New York: John Wiley; 1984. [Google Scholar]
- Nychas GJE. Natural antimicrobials from plants. In: Gould GW, editor. New Methods of Food Preservation. Glasgow: Blackie Academics and Professional; 1995. pp. 58–89. [Google Scholar]
- Paster N, Juven BJ, Shaaya E, Menassherov M, Nitzan R, Weisslowicz H, Ravid U. Inhibitory effect of oregano and thyme essential oils on moulds and food borne bacteria. Let Appl Microbiol. 1990;11:33–37. doi: 10.1111/j.1472-765X.1990.tb00130.x. [DOI] [Google Scholar]
- Paster N, Menasherov M, Ravid U, Juven B. Antifungal activity of oregano and thyme essential oils applied as fumigants against fungi attacking stored grain. J Food Prot. 1994;58:81–85. doi: 10.4315/0362-028X-58.1.81. [DOI] [PubMed] [Google Scholar]
- Skirdal IM, Eklund T. Microculture model studies on the effect of sorbic acid on Penicillium chrysogenum, Cladosporium cladosporioides and Ulocladium atrum at different pH levels. J Appl Bacteriol. 1993;74:191–195. doi: 10.1111/j.1365-2672.1993.tb03014.x. [DOI] [PubMed] [Google Scholar]
- Sofos JN. Sorbate Food Preservatives. Boca Raton: CRC Press; 1989. [Google Scholar]
- Sofos JN, Busta FF. Alternatives to the use of nitrite as an antibotulinal agent. Food Technol. 1980;34:244–251. [Google Scholar]
- Sofos JN, Busta FF. Antimicrobial activity of sorbate. J Food Prot. 1981;44:614–622. doi: 10.4315/0362-028X-44.8.614. [DOI] [PubMed] [Google Scholar]
- Statham JA, McMeekin TA. The effect of potassium sorbate on the structural integrity of Alteromonas putrefaciens. J Appl Bacteriol. 1988;65:469–476. doi: 10.1111/j.1365-2672.1988.tb01919.x. [DOI] [Google Scholar]
- Steels H, James SA, Roberts IN, Stratford M. Sorbic acid resistance: the inoculum effect. Yeast. 2000;16:1173–1183. doi: 10.1002/1097-0061(20000930)16:13<1173::AID-YEA617>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Thompson DP. Influence of pH on the fungitoxic activity of naturally occurring compounds. J Food Prot. 1990;53:482–429. doi: 10.4315/0362-028X-53.5.428. [DOI] [PubMed] [Google Scholar]
- Wilkins KM, Board RG. Natural antimicrobial system. In: Gould GW, editor. Mechanism of Action of Food Preservation Procedures. New York: Elsevier; 1989. pp. 285–362. [Google Scholar]