Abstract
The process of fruit ripening is normally viewed distinctly in climacteric and non-climacteric fruits. But, many fruits such as guava, melon, Japanese plum, Asian pear and pepper show climacteric as well as non-climacteric behaviour depending on the cultivar or genotype. Investigations on in planta levels of CO2 and ethylene at various stages of fruits during ripening supported the role and involvement of changes in the rate of respiration and ethylene production in non-climacteric fruits such as strawberry, grapes and citrus. Non-climacteric fruits are also reported to respond to the exogenous application of ethylene. Comparative analysis of plant-attached and plant-detached fruits did not show similarity in their ripening behaviour. This disparity is being explained in view of 1. Hypothetical ripening inhibitor, 2. Differences in the production, release and endogenous levels of ethylene, 3. Sensitivity of fruits towards ethylene and 4. Variations in the gaseous microenvironment among fruits and their varieties. Detailed studies on genetic and inheritance patterns along with the application of ‘-omics’ research indicated that ethylene-dependent and ethylene-independent pathways coexist in both climacteric and non-climacteric fruits. Auxin levels also interact with ethylene in regulating ripening. These findings therefore reveal that the classification of fruits based on climacteric rise and/or ethylene production status is not very distinct or perfect. However, presence of a characteristic rise in CO2 levels and a burst in ethylene production in some non-climacteric fruits as well as the presence of system 2 of ethylene production point to a ubiquitous role for ethylene in fruit ripening.
Keywords: Climacteric, Ethylene, Fruit ripening, Non-climacteric, Respiration, System 1, System 2
Postharvest physiology, shelf-life and losses due to decay of fruits are inter-linked processes. They all are primarily governed by last phase of fruit’s maturation called fruit ripening. Fruit ripening involves various physiological, biochemical and developmental changes that occur in a coordinated and genetically regulated manner (Stepanova and Alonso 2005; Etheridge et al. 2006; Barry and Giovannoni 2007). Fruits, in general, show two distinct respiratory patterns during ripening and on this basis they are categorized into climacteric and non-climacteric groups (Biale 1964; Abeles et al. 1992; Lelievre et al. 1997). Importance of respiration can be judged from the fact that the potential shelf-life of plant tissue or plant part after harvest has been shown to be closely related to its rate of respiration (Kader 1987; Kader and Saltveit 2003b; Varoquaux and Ozdemir 2005). Likewise, there is correlation between level of ethylene produced by fruit and its shelf-life and postharvest decay (Gussman et al. 1993; Zheng and Wolff 2000).
In the last few years it has been observed that ripening in many non-climacteric fruits resembles the climacteric pattern of ripening and therefore it has been proposed by Obando et al. (2007) that the classification of fruits into climacteric and non-climacteric categories is an over-simplification. Furthermore there is evidence that climacteric and non-climacteric fruits share some similar pathways of ripening (Barry and Giovannoni 2007). Emerging evidences from the studies carried out on ethylene and ethylene mediated responses have suggested that sensitivity towards ethylene differs in various tissues and/or distinct developmental stages. This is because of signaling interactions of ethylene with other plant hormones, metabolites and environmental signals (Alonso and Stepanova 2004; Stepanova and Alonso 2005; Hall et al. 2007; Kendrick and Chang 2008; Yoo et al. 2009). Identification of key regulators in many plant species have indicated a high level of conservation in ethylene signaling mechanisms that have evolved to function in diverse lifestyles and developmental programs (Klee 2004; Chen et al. 2005; Yoo et al. 2009). This article reviews the ripening behaviour of fruits in the light of recent advances in our knowledge in the area of fruit ripening and ripening related changes especially with respect to respiration and ethylene. It highlights the classical differences between climacteric and non-climacteric fruits, role of ethylene in the process of ripening and system 1 and system 2 of ethylene production. The response and/or ripening behaviour of non-climacteric fruits are discussed in light of recent updates, results and evidences in the area of fruit physiology. The available results and evidence revealing specific responses or behaviour of non-climacteric fruits are discussed. Finally, future perspectives in this area of fruit physiology are presented.
Classical distinctions between climacteric and non-climacteric ripening of fruits
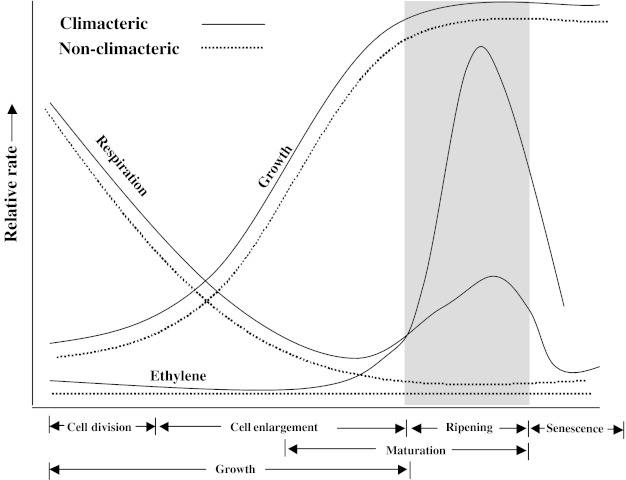
Fruit ripening has received considerable attention because of the dramatic changes in a wide range of metabolic processes that occur before and after this event as it has relevance for basic as well as applied research (Theologies 1992; Zeng et al. 1995; Zeng et al. 1996; Lelievre et al. 1997; Srivastava 1999; Pandey et al. 1998; Reddy and Srivastava 1999a, b; Fallik and Aharoni 2004; Atta-Aly et al. 2000; Brecht et al. 2008). The term climacteric was initially proposed to indicate the dramatic increase in respiration (rise in the production level of CO2) of fruit during ripening. Therefore, fruits have been classified as climacteric or non-climacteric according to their respiratory patterns (Biale 1964). Response of fruit to exogenous ethylene treatment has shown that this can also serve to distinguish between climacteric and non-climacteric fruits (McMurchie et al. 1972; Bufler 1986). Application of propylene (an analogue of ethylene) was reported to initiate an increase in respiration in climacteric fruits as well as in non-climacteric fruits but a propylene mediated induction or rise in endogenous ethylene production occurred only in climacteric fruit (McMurchie et al. 1972; Yamane et al. 2007). It is therefore important to make use of propylene to distinguish fruits for their ripening behaviour however, this practice was not followed in most of the subsequent studies. The process of ripening in climacteric fruits can be triggered and also enhanced by exogenous ethylene treatment (Tucker 1993). Today, the patterns of ethylene and CO2 production are used as criteria to identify climacteric fruits (Abeles et al. 1992). Atta-Aly et al. (2000) suggested that a negative ethylene feedback mechanism (ethylene did not induce its own synthesis) could underlie non-climacteric behaviour, while a positive feedback mechanism (ethylene induces its own synthesis) underlies the climacteric behaviour during ripening. Fruits classified into climacteric and non-climacteric groups are presented in Table 1. Generalized patterns of growth, respiration and ethylene production in these two categories of fruits are shown in Fig. 1.
Table 1.
Classification of fruits into climacteric and non-climacteric groups
| Climacteric | Non-climacteric |
|---|---|
| Apple, Mango, Papaya, Kivifruit, Tomato, Cherimoya, Banana, Pear, Apricot, Peach, Plum, Avocado, Plantain, Fig, Guava, Jackfruit, Muskmelon, Nectarine, Passion fruit, Persimmon, Quince, Blueberry, Cantaloupe, Feijoa, Sapodilla, Breadfruit, Broccoli, Durian, Mangosteen, Sapote, Soursop, Sweetsop | Citrus fruits (orange, grapefruit, lemon, lime, etc.), Berries (cranberry, raspberry, strawberry, cherry, blackberry etc.), Grape, Pineapple, Lychee, Melon, Loquat, Pomegranate, Cucumber, Tamarillo, Carambola, Cashew-apple, Eggplant, Jujube, Longan, Loquat, Okra, Peas, Pepper, Summer squash, Watermelon, Tangerine, Prickly pear, Rambutan, Snap bean, Cacao, Date, Olive, Pumpkin |
Fig. 1.
Generalized patterns of growth, respiration and ethylene during development, maturation and senescence of climacteric and non-climacteric fruits. (Source: Wills et al. 2007)
Climacteric fruits show a dramatic increase in the rate of respiration during ripening and this is referred to as the climacteric rise (Fig. 1). The rise in respiration occurs either simultaneously with the rise in ethylene production or it follows soon afterwards (Burg and Burg 1962; Burg and Burg 1965a; Lelievre et al. 1997; Reddy and Srivastava 2001; Perin et al. 2002; Kays and Paull 2004). The increase in the rate of ethylene production is usually logarithmic (Fig. 1). However, this large change in the magnitude of ethylene production can be misleading. The important point is when the tissue becomes more sensitive to ethylene and internal concentration reaches a threshold concentration required to induce biological responses (Burg 1962). Generally, concentrations of less than 1 μl l−1 saturate the ethylene receptors (Burg and Burg 1962, 1965a). It is important to note that the rate of ethylene production vary widely among species and cultivars of climacteric fruits such as muskmelon, peach and kiwifruit (Kendall and Ng 1988; Miccolis and Saltveit 1991; Klozenbucher et al. 1994 and Xu et al. 1998). A substantial proportion of the rise in rates of respiration is reported to be contributed by cyanide-insensitive respiration in fruits like banana, mango and tomato (Kumar and Sinha 1992; Pandey et al. 1995; Reddy and Srivastava 1998). Differences in the levels of ethylene for climacteric and non-climacteric fruits were reported by Burg and Burg (1962) during the process of ripening (Table 2). The data showed differences in endogenous levels of ethylene not only within the two categories of fruits (higher levels in climacteric fruits) but also for the fruits within a category. The continuous presence of ethylene and its perception is required for the expression of ripening-related genes even at advanced stages of fruit ripening (Theologis 1992; Golding et al. 1998, Hoeberichts and Woltering 2002; Alexander and Grierson 2002). Work on banana (climacteric fruit) where fruits were treated with 1-methylcyclopropene (1-MCP—an action inhibitor of ethylene) after various periods of application of propylene illustrated that ripening related processes once engaged with auto-induced or auto-catalytic ethylene production become partially independent of further ethylene action (Golding et al. 1998). They also suggested that there was a rapid increase in sensitivity towards ethylene as well as enhancement in the number of ethylene receptors after the initiation of ripening (Golding et al. 1999). Thus, ethylene plays a major role in the ripening process of climacteric fruits. Climacteric fruits can ripen fully if they are harvested at completion of their growth period. On the other hand, non-climacteric fruits can ripe fully only if they are allowed to remain attached to the parent plant. Ripening is slow or non-existent in non-climacteric fruits if they are detached from the plant (even if the fruit has completed growth in size). These fruits also do not ripe in response to exogenous ethylene treatment except for the degradation of chlorophyll in citrus fruits and pineapples (Goldschmidt et al. 1993; Noichinda 2000). In contrast to climacteric fruits, cyanide-insensitive respiration is present only to a limited extent in non-climacteric fruits. Further, the upsurge in respiration and ethylene in these fruits is either not observed or it is only transitory even after the application of ethylene (Lurie and Klein 1989; Kays and Paull 2004).
Table 2.
Endogenous concentration of ethylene in selected fruits during ripening
| Climacteric fruit | μl/l | Non-climacteric fruit | μl/l |
|---|---|---|---|
| Apple | 25–2500 | Lemon | 0.1–0.2 |
| Pear | 70–80 | Lime | 0.3–2.0 |
| Peach | 1–21 | Orange | 0.1–0.3 |
| Avocado | 29–74 | Pineapple | 0.2–0.4 |
| Mango | 0.05–3.0 | ||
| Passion fruit | 466–530 | ||
| Plum | 0.2–0.3 |
Burg and Burg (1962)
Role of ethylene in regulation of fruit ripening
Ethylene as the main regulator of ripening in climacteric fruits
Ethylene is a natural plant growth regulator having numerous effects on growth, development and storage life of many fruits. It plays a major role in the ripening process of climacteric fruits (Theologis et al. 1992; Yang 1995; Nagata et al. 1995; Lelievre et al. 1997; Saltveit 1999; Barry et al. 2000; Atta-Aly et al. 2000; Klee 2002; Alexander and Grierson 2002, Hoeberichts and Woltering 2002). Ethylene starts a cascade of events leading to many interactive signaling and metabolic pathways for the progress of ripening in climacteric fruits (Stepanova and Alonso 2005; Etheridge et al. 2006; Barry and Giovannoni 2007). The production of aromas also depends strongly on the levels and action of ethylene (Golding et al. 1998, 1999; Rupasinghe et al. 2000; Alexander and Grierson 2002; Flores et al. 2002). On the basis of analysis of mutants of Arabidopsis, an ethylene signaling pathway has been proposed (Alexander and Grierson 2002; Klee 2004; Kendrick and Chang 2008). Ethylene is perceived by receptors [ETHYLENE RESISTANCE1 (ETR1)] and related proteins (Chang et al. 1993; Hua and Meyerowitz 1998). The ethylene signal is transduced to ETHYLENE INSENSITIVE3 (EIN3) through CONSTITUTIVE TRIPLE RESPONSE1 (CTR1) (Kieber et al. 1993) and ETHYLENE INSENSITIVE2 (EIN2) (Alonso et al. 1999). EIN3 is a transcription factor that plays a crucial role in regulation of expression of ethylene responsive genes (Chao et al. 1997; Solano et al. 1998). Studies have also revealed that in absence of ethylene EIN3 protein is quickly degraded through a ubiquitin–proteasome pathway mediated by two F-box proteins, [EIN3-binding F box protein 1 and 2 (EBF1 and EBF2)] whereas EIN3 protein is stabilized by ethylene itself (Guo and Ecker 2003; Potuschak et al. 2003; Gagne et al. 2004). Recent work on apple by Johnston et al. (2009) has revealed that early and late ripening events differs not only in their dependence on ethylene but also towards their sensitivity to ethylene. The authors therefore suggested that this finding warrants further investigation of the physiology of climacteric and non-climacteric fruits in relation to ripening and ripening associated changes.
Regulation of ethylene production
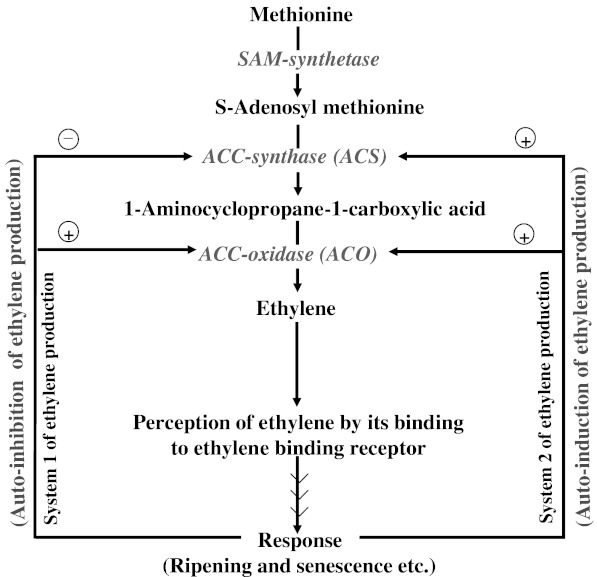
Rates of ethylene production in fruit during the course of ripening are controlled by the ability of tissue to synthesize 1-aminocyclopropane-1-carboxylic acid (ACC) and to convert it in to ethylene (Fig. 2). The two key enzymatic control points are the expression and activity levels of ACC-synthase (ACS) and ACC-oxidase (ACO) (Tucker 1993) (Fig. 2). Both of these enzymes are encoded by multigene families in various plants and regulated by number of factors (Fluhr and Mattoo 1996; Lelievre et al. 1997; Nakatsuka et al. 1998; Barry et al. 2000; Alexander and Grierson 2002). Ethylene (which is the final product of the above reactions) itself also strongly regulates the expression and activity of ACS and ACO (Lelievre et al. 1997) (Fig. 2). The concept of ethylene binding to its receptor in plant systems is widely accepted and proven beyond doubt (Goren et al. 1984; Sisler and Yang 1984; Sisler and Serek 1997; Sisler et al. 2006). Gas diffusion in fruits follows Fick’s law (Burg and Burg 1965b). This law states that the flux of a gas, diffusing normally to a barrier, is dependent on the diffusion coefficient and concentration gradient. Burg and Burg (1965b) and then Solomos (1987) developed relationships between rates of ethylene production by fruit and the internal concentrations and these relationships were surprisingly found similar across many fruit species. However, more has to be learned about the retention and release of ethylene in plant tissues in relation to its resistance towards the diffusion across the boundaries of fruit and physiological activity of ethylene bound to its receptor (Ben-Yehoshua et al. 1985; Goldschmidt et al. 1993). So, to get better understanding of ethylene emission by tomato fruit Genard and Gouble (2005) developed a simulation model called ‘ETHY’.
Fig. 2.
Simplified pathway of ethylene biosynthesis in plants showing auto-inhibition (inhibiting its own production) and auto-induction of ethylene (inducing its own production). These systems are referred as system 1 and system 2 of ethylene production respectively. In system 1, ethylene inhibits its own production by inhibiting (⊝) ACS expression/activity. It may be noted that the ACO activity is enhanced during system 1 but due to the absence of any enhancement in the activity of ACS there is no auto-induction. In system 2, ethylene induces more of its own production by stimulating (⊕) the expression/activity of both of the enzymes (ACS and ACO) simultaneously
Two systems of ethylene production have been defined in plants (McMurchie et al. 1972). The first one is designated as system 1. System 1 operates and functions during normal growth and development and in response to various stresses. System 1 is responsible for the basal level of ethylene production in vegetative tissues and unripe fruit. This system is regulated in an auto-inhibitory manner (Fig. 2). This means that exogenous ethylene inhibits any further synthesis of ethylene. The second system is system 2 and that operates during floral senescence and fruit ripening. This system represents the large increase in ethylene production associated with fruit ripening and gynoecium of some senescing flowers such as carnation. It is regulated in an auto-inductive manner (Fig. 2) (Oetiker and Yang 1995; Lelievre et al. 1997; Nakatsuka et al. 1998; Inaba 2007). This means that exogenous ethylene when applied to climacteric fruits (at mature stage) stimulates ethylene biosynthesis and generally induces rapid fruit ripening. Now, it is only in presence of action inhibitors of ethylene that the response towards the exogenously applied ethylene or auto-inductive rise in the production of ethylene can be suppressed.
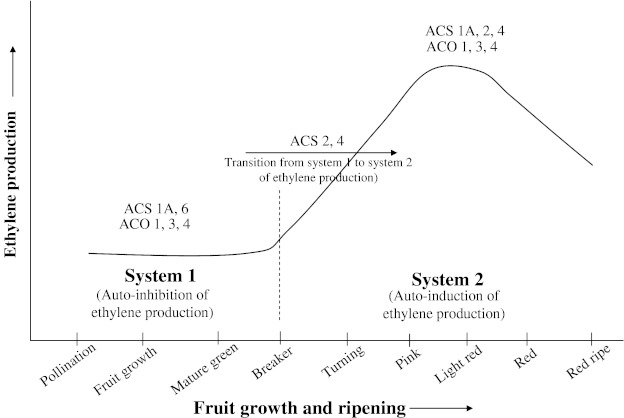
Tomato possesses at least nine ACS (LeACS1A, LeACS1B, and LeACS2-8) and five ACO (LeACO1-5) genes (Zarem-binski and Theologis 1994; Barry et al. 1996; Oetiker et al. 1997; Nakatsuka et al. 1998; Van-der-Hoeven et al. 2002). Expression analysis studies have revealed that at least four ACS (LeACS1A, LeACS2, LeACS4, LeACS6) and three ACO (LeACO1, LeACO3, LeACO4) genes are differentially expressed in tomato fruit (Rottmann et al. 1991; Nakatsuka et al. 1998; Barry et al. 1996, 2000; Barry and Giovannoni 2007) (Fig. 3). LeACO1, LeACO3, and LeACO4 are expressed at low levels in green fruit representing system 1 of ethylene synthesis. Transcripts of each of these genes were found to increase at the onset of ripening when the fruit shows transition from system 1 to system 2 of ethylene production. During ripening, LeACO1 and LeACO4 are sustained in expression, whereas the increase in expression of LeACO3 is transient (Barry et al. 1996; Nakatsuka et al. 1998). Studies on the regulation of ACS gene expression during fruit ripening by Barry et al. (2000) and Barry and Giovannoni (2007) revealed the following information 1. LeACS6 is expressed in wild-type green fruit but the expression declines rapidly at the onset of ripening during the transition from system 1 to system 2 of ethylene synthesis. 2. LeACS6 transcripts persist throughout development and ripening in the rin mutant. 3. LeACS6 is responsible for low-level of ethylene production in preclimacteric fruit. 4. LeACS1A gene may be important in regulating the transition from system 1 to system 2 of ethylene synthesis. 5. LeACS4 is not expressed in green fruit but is induced at the onset of ripening. 6. LeACS2 expression is induced at the onset of ripening, this induction requires ethylene. 7. It seems that LeACS1A and LeACS4 are responsible for initiating system 2 of ethylene synthesis and this is maintained by a combination of LeACS2 and LeACS4. 8. Auto-inhibition of ethylene synthesis during system 1 of ethylene production is mediated by a reduction in expression of LeACS1A and 6 genes of ACS and 9. Auto-induction of ethylene synthesis at the onset of fruit ripening is mediated through ethylene-stimulated expression of LeACS2 and 4 and LeACO1 and 4.
Fig. 3.
Differential expression of ACC-synthase (ACS) and ACC-oxidase (ACO) genes associated with system 1 and system 2 of ethylene synthesis during fruit development and ripening in tomato. See text for details. (Adapted and modified after: Barry and Giovannoni 2007)
One of the most important questions in the physiology of fruit ripening concerns the mechanism that initially induces system 2 of ethylene synthesis. The physiological and molecular pathways that act to initiate the transition from the system 1 to system 2 mode of ethylene synthesis, at the onset of ripening, are largely unknown (Barry and Giovannoni 2007; Cara and Giovannoni 2008). According to Klee (2004), one explanation may be that the cumulative effects of system 1 (even if the level is low) reach a certain limit and induces system 2. The second explanation is that there is a change in the sensitivity of fruit to ethylene (already reported by Biale and Young 1981; McGlasson 1985). Fruit might become more sensitive to system 1 of ethylene as its development progresses. So, the transition from system 1 to system 2 is caused by a change in ethylene sensitivity due to continuous exposure of fruit to ethylene from system 1 (Barry et al. 2000). The involvement of system 1 in the transition process is supported by the observation that the treatment of an immature tomato fruit with ethylene for a short time does not induce system 2 immediately, rather, it shortens the period preceding or required for the onset of system 2 (Yang 1987). In general, stresses such as wounding, water stress, and disease during fruit development induce ethylene production and this shortens the period required for the onset of fruit ripening (Abeles et al. 1992; Nakano et al. 2003). Kevany et al. (2007) demonstrated that the shortened period of ripening by exogenous ethylene is closely related to the level of the ethylene receptor protein (a negative regulator of ethylene signaling). These observations indicate that during development, both exogenous and endogenous ethylene increase the physiological age of fruit and also sensitize the fruit to ethylene. Thus, it has been proposed that the level of the ethylene receptor modulates the timing of the onset of fruit ripening by measuring and memorizing ethylene exposure together with the role of system 1 of ethylene production in initiation of system 2 of ethylene production (Kevany et al. 2007, 2008). In addition, work by Trainotti et al. (2007) with peaches suggested that the transition from growth to ripening also involves changes in synthesis of auxin and its activity. These changes could modulate timing of the onset of ripening.
Recent understanding on ripening behaviour of fruits
Tomato
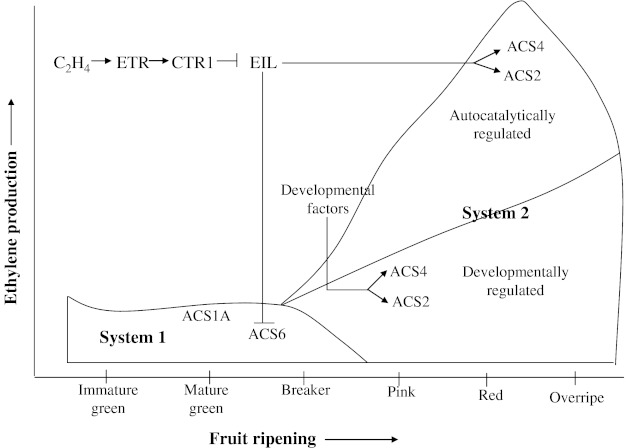
In tomato, four EIN3-like (EIL) genes (LeEIL1–LeEIL4) have been isolated (Tieman et al. 2001; Yokotani et al. 2003). Reduced expression of tomato LeEIL genes by antisense technology modulates ethylene responses, including leaf epinasty, flower senescence and fruit ripening (Tieman et al. 2001). Ito et al. (2008) demonstrated that the ripening inhibitor (RIN protein) exhibits transactivator activity because it binds to the promoter region of LeACS2. Thus, part of the gradual increase in ethylene production as observed in tomato fruit lines where LeEIL genes were suppressed by RNA interference engineering (RiEIL) may be due to the direct up-regulation of the LeACS2 gene by RIN. However, it is unclear whether system 1 alone is enough to have this effect. In an interesting study by Yokotani et al. (2009) an attempt was made to find the answer to the question whether system 2 is completely regulated by an auto-induced system or partly regulated by another mechanism. By using RNA silencing of LeEIL genes (hairpin RNA-induced gene silencing technique to suppress the total expression of LeEIL1–LeEIL4 to trace levels) and application of 1-MCP (1-MCP has been shown to bind to ETR1 protein, a receptor of ethylene, and thereby it inhibits the ethylene signal and thereby its response. In this way, 1-MCP is one of the most potent inhibitors of ethylene action) revealed that ripening-associated ethylene biosynthesis is regulated by both i.e., an auto-induced system of ethylene and ethylene-independent developmental factors. So, fruit can initiate system 2 of ethylene production even without the cumulative effects of system 1 of ethylene production. Suppression of ethylene production would be expected in 1-MCP-treated transgenic fruit if leaky LeEIL mRNA is involved in ethylene production. However, the pattern and level of ethylene production in 1-MCP-treated transgenic fruit were almost identical to those in non-treated transgenic and 1-MCP-treated wild-type fruit. Despite the double block in the ethylene signal, residual ethylene was detected, which indicates that the ethylene production is probably not due to leaky ethylene sensitivity, but rather due to an ethylene-independent developmental factor. Thus, it was concluded that ripening ethylene (system 2) in wild-type tomato fruit consists of two parts: a large part that occurs under auto-induced regulation and a minor part regulated by an ethylene-independent developmental system (Yokotani et al. 2009). Yokotani et al. (2009) (Fig. 4) proposed a model to explain the transition from system 1 to system 2. Ethylene in system 1 is produced via LeACS1A and LeACS6, which are regulated by negative feedback (Nakatsuka et al. 1998; Barry et al. 2000; Alexander and Grierson 2002). Transition of system 1 to system 2, under natural conditions i.e., in absence of exogenous ethylene and stress, occurs mainly via limited expression of LeACS2 and LeACS4. This transition occurs even if the effect of system 1 of ethylene is eliminated. In this way, fruit can initiate system 2 of ethylene production leading to fruit ripening. This transition can be controlled by developmental factor(s) independent of ethylene besides the involvement of ethylene in bringing about this transformation. So, shifting towards system 2 of ethylene production in tomato fruits consists of both, ethylene-dependent (auto-induced) and ethylene-independent (non-auto-induced) mechanisms.
Fig. 4.
Proposed model for the regulation of system 1 and system 2 of ethylene production during growth and ripening of tomato fruit. (Source: Yokotani et al. 2009)
Mango
Two ethylene peaks were recorded during ripening, one at initial stage and the other at final stage of ripening (Reddy and Srivastava 1999a). NADP-malic enzyme activity and respiratory climacteric followed a parallel increase (Reddy and Srivastava 1998). ACC-synthase activity was not limited, but ACC-oxidase was limiting and this therefore resulted in accumulation of ACC with the progress in ripening in both the cultivars (‘Amrapali’ and ‘Dashehari’) of mango (Prasad et al. 1999). The seed embryos also produced considerable amounts of ethylene (Reddy and Srivastava 1999a)
Differences in the surface and internal anatomical features in different cultivars and maturity stages of mango fruit have been observed (Paul et al. 2007). Hypertrophy of lenticels present on the surface of mango fruit was reported during ripening (Larson et al. 1993). The extent of this hypertrophy appears to be inversely related to the density of lenticels present on the surface of the mango fruit (Paul et al. 2007). In addition, lenticel proliferation is also dependent on the cultivars of mango fruits (Paul et al. 2007). Hagenmaier (2005) reported that exchange of gases by fruits through the peel by diffusion through openings (stomates or lenticels) was proportional to the area of such openings. Features on the surface of the mango fruits like epicuticular wax (thickness), cuticle (thickness, surface, structure, cracks and arrangement of cutin platelets), epidermal layer (arrangement/pattern, depositions of lignin and suberin etc.) might play a role in deciding the postharvest behaviour of mango fruit including ripening (Paul et al. 2007). These features, govern the fruit’s microenvironment by determining the permeability of gases (like O2 and CO2), moisture and endogenous levels of ethylene as well. In this way, changes in the resistance to the gaseous diffusion with the development and maturation of mango fruit are expected and this in turn can influence the ripening behaviour of mango fruits. The rate of ripening in mango fruits was found to be associated with anatomical features including cell density, cell size and surface to volume ratio of cells (Paul et al. 2004).
Guava
The classification of guava as a climacteric or non-climacteric fruit is contradictory. Many authors consider guava to be a non-climacteric fruit (Biale and Barcus 1970), while others consider it as climacteric fruit (Akamine and Goo 1979; Brown and Wills 1983 and Mercado-Silva et al. 1998). Reyes and Paull (1995) reported that guavas respond to the exogenous application of ethylene depending upon their maturity. Cultivar ‘Beaumont’ showed enhanced changes in skin colour and softening in response to exogenous application of ethylene only if the fruits were harvested at an immature-green stage but not when harvested at mature-green and quarter-yellow stages. Climacteric or non-climacteric behaviour of guava fruit is a varietal characteristic. A study by Azzolini et al. (2005) showed that ‘Pedro Sato’ guava shows a gradual increase in the rates of respiration and ethylene production after harvest and it completes its ripening with changes in quality attributes. Ethylene was found to be necessary for skin colour changes and firmness loss during ripening. These characteristics classify guava as a climacteric fruit. However, maximum respiratory activity as well as ethylene production was observed when the fruits were already ripe. In addition to this, the exogenous application of ethylene to these fruits at the mature-light green stage had no effect on the ripening process. This evidence contradicts classification of ‘Pedro Sato’ guava as a traditional climacteric fruit. Based on these findings, Azzolini et al. (2005) concluded that classification of guava fruit as climacteric or non-climacteric varies with cultivars. The data obtained with ‘Pedro Sato’ guava show that it does not display typical climacteric behaviour.
Melons
Melon (Cucumis melo) is the most diverse species of the genus Cucumis. As a polymorphous species, it encompasses netted muskmelon, salmon-flesh cantaloupe, smooth-skinned and green-fleshed honey dew, wrinkle-skinned cassaba, long shelf-life hami melon, small and thin-pericarp makuwa and several non-sweet pickling and cooking oriental melons. Harvested mature cantaloupe fruit of variety ‘raticulatis’ exhibited climacteric rise in respiration and ethylene during ripening (Lyons et al. 1962). Some varieties of melons like ‘cantalupensis’ and ‘reticulatus’ produce high levels of ethylene, ripen rapidly and have short shelf-life. They have been categorized as climacteric fruits (Lyons et al. 1962; Hadfield et al. 1995). Other types such as ‘inodorus’ exhibit no auto-induced ethylene production and are slow-ripening. The ‘inodorus’ varieties have been assigned to the non-climacteric category (Pratt et al. 1977; Lester 1988) although honey dew melons, an ‘inodorus’ variety ripen when treated with ethylene. Postharvest treatment with ethylene is a recommended commercial practice (Suslow et al. 2010). Ethylene treatment induces some cell wall softening and development or aroma. Honey dew melon will ripen if left long enough on the vine. The non-climacteric phenotype in melon fruit may be attributed to alteration in either upstream developmental processes or any element of the ethylene signal transduction pathway (Giovannoni 2001). In cantaloupe, as in other climacteric fruit, exogenous ethylene can prematurely induce abscission, ethylene production and ripening. Physiological and genetic analysis in non-climacteric phenotype (showing non-abscising and long shelf-life behaviour) by Perin et al. (2002) involved the study on segregating populations of the cross between a non-climacteric melon, Songwhan Charmi PI 161375 (C. melo var. ‘chinensis’), and a typical climacteric type Charentais melon (C. melo var. ‘cantalupensis’ cv. ‘Vedrantais’). Two loci were found to be responsible for variation in the ripening behaviour. Non-climacteric fruits lack a burst in ethylene production. This therefore reflected inability to synthesize ethylene in an auto-induced manner. This was due to either the blockage in the auto-induced synthesis of ethylene or there was alteration in the early steps of the ripening process itself. Of these two possibilities, the first one was found to be correct because the non-climacteric phenotype ‘PI 161375’ showed alteration in the ethylene perception itself. In this way, the non-climacteric phenotype in fruit tissues was attributable to ethylene insensitivity conferred by the recessive allelic forms derived from ‘PI 161375’ (Perin et al. 2002)
In subsequent studies, when different varieties of melon were investigated, distinct variations were noticed in the levels of ethylene during fruit development, ripening and shelf-life (Liu et al. 2004a; b). Varieties like ‘acidulous’, ‘makuwa’, ‘reticulatus’ and ‘cantalupensis’ showed poor shelf-life while, varieties like ‘saccharinus’ and ‘inodorus’ showed good shelf-life (Liu et al. 2004b). Ethylene plays a major role in the ripening of ‘cantaloupe’ and ‘charentais’ melons as they show climacteric behaviour. Knocking-down the expression of ACC-oxidase in melon fruit did not affect the expression of several of ripening pathways and sensory attributes, suggesting that these ripening-related changes are independent of ethylene (Ayub et al. 2008). Hybrids of the charentais cantalupensis type (showing typical climacteric behaviour) with un-characterized non-ripening ‘charentais’ genotypes have led to the generation of mid or long shelf-life melons (Pech et al. 2008). Genetic analysis of these hybrids showed that the climacteric character is genetically determined and is conferred by two duplicated loci (Pech et al. 2008). However, other studies have generated climacteric fruit by crossing two non-climacteric melons. This therefore indicates the existence of different and complex genetic regulation of the climacteric character (Pech et al. 2008). In view of the enormous amount of information that has been already accumulated, melon have been suggested as an alternative model system for studying fruit ripening, ethylene perception and signaling in addition to the study material to unraveling the basis of the differences between climacteric and non-climacteric fruit (Ezura and Owino 2008).
Plum
Plums are classified as climacteric fruits. However, two distinct patterns of ripening, depending on cultivars of Japanese plum, were observed by Abdi et al. (1997). Cultivars ‘Gulfruby’ and ‘Beauty’ showed a typical climacteric pattern while cultivars ‘Shiro’ and ‘Rubyred’ exhibited a suppressed-climacteric behaviour. Further characterization of these cultivars by Abdi et al. (1998) revealed that cultivars showing suppressed climacteric phenotypes had a reduced capacity to convert ACC to ethylene. Ripening was accelerated when ‘Shire’ and ‘Rubyred’ fruits were treated with propylene or ethylene. Fruits when treated with 1-MCP did not show any rise in either respiration or ethylene unless exogenous propylene was applied. Fruits of an early cultivar ‘Early Golden’ and late cultivar ‘Shiro’ of Japanese plum also showed typical climacteric and suppressed climacteric patterns respectively (El-Sharkawy et al. 2007). The differences between these two cultivars of Japanese plum were explained by differences in the accumulation pattern of four mRNAs of the ethylene signal transduction components including ethylene receptors (El-Sharkawy et al. 2007), ACC-synthase genes (El-Sharkawy et al. 2008), auxin-mediated control of the expression of a gene for ethylene-responsive transcriptional factors (ERFs) (El-Sharkawy et al. 2009, 2010).
Peach
Peach is classified as a climacteric fruit. Miller et al. 1987 reported that concomitant with the ethylene climacteric there was also a significant increase in auxin production in the fruit. Interestingly, it had been reported that auxin can stimulate the synthesis of more climacteric ethylene (Bleecker and Kende 2000) through its inductive action on the expression of the key enzyme i.e., ACC-synthase (Abel and Theologis 1996). Many genes involved in biosynthesis, transport and signaling of auxin were noticed on transcript profiling during ripening of peach fruit (Trainotti et al. 2007). This study also demonstrated the cross-talk between auxin and ethylene because genes in the auxin domain were found to be regulated by ethylene and genes in ethylene domain were also being regulated by auxin. Higher levels of auxin were found to be associated with climacteric nature while lower level of auxin caused suppression in climacteric behaviour. These results, on the role of auxin in regulating the ripening of peaches, are similar to the results for plums (El-Sharkawy et al. 2009, 2010). Similarly, auxin and ethylene interactions during fruit growth and ripening were also demonstrated in kiwifruit (Bregoli et al. 2007).
Pear
European pears (Pyrus communisp) are usually classified as climacteric fruit. During ripening of P. communis var. ‘Bartlett’, the changes in the patterns of free auxin and ABA were found to be remarkably similar (Frenkel 1975). Application of relatively higher concentrations of auxin delayed the maturation but at the same time it temporarily stimulates ethylene production (Frenkel and Dyck 1973; Frenkel 1975). These observations were parallel to the results obtained in grape which is a non-climacteric fruit (Coombe and Hale 1973; Inaba et al. 1976; Weaver and Singh 1978; Davies et al. 1997; Baydar and Harmankaya 2005). In addition to this, there are several cultivars of European pears and species of Chinese and Japanese pears that are usually non-climacteric. The latter two species of pears (also referred as Asian pears) includes a few cultivars that exhibit a climacteric behaviour (Downs et al. 1991; Itai et al. 2002; Yamane et al. 2007). Propylene treatment was used to distinguish climacteric cultivars from non-climacteric cultivars as ethylene production was induced by propylene only in climacteric cultivars (Downs et al. 1991; Yamane et al. 2007). The ethylene levels in different cultivars of Japanese pear fruits were found to vary from 0.1 to 300 μl kg−1 (fr.wt.) h−1 during ripening (Itai et al. 2002). Differences in the ethylene production were thought to be regulated mainly by differential expression of ethylene biosynthetic genes of ACC-synthase (Itai et al. 1999; 2002; 2003)
Ripening behaviour of some attached and detached fruits
Many fruits that belong to climacteric group such as apple and pear enter the climacteric phase soon after harvest but they might not ripe fully for weeks if left on the tree (Gerhardt 1947). The best example for such a differential behaviour is the avocado fruit. Most cultivars of avocado do not ripe or undergo climacteric change if the fruit remain attached to the plant. To explain this, it has been proposed that a substance of unknown nature enters the fruit from the tree and inhibits ripening (Burg and Burg 1965a; Gazit and Blumenfield 1970). It was also hypothesized that ethylene is rendered ineffective by this hypothetical ripening inhibitor (which is supposed to be supplied by the parent plant) and harvesting the fruit removes it from the source of this inhibitor i.e., the parent plant (Burg and Burg 1965a). Further, Reid and Pratt (1970) suggested that endogenous ethylene is not the direct cause of climacteric rise. The hypothetical inhibitor is transported through the phloem from leaf to fruit and prevents the auto-induced production of ethylene (Sfakiotakis and Dilley 1973). However, Mapson and Hulme (1970) had earlier suggested that this inhibitor either blocks ethylene production or raises the threshold concentration at which ethylene becomes physiologically active in promoting the process of ripening. Ripening was observed to be more rapid in fruits such as apple, purple passion fruit and plum (Prunus salicina Lindl.) when they were detached from the tree (Blanpied 1993; Shiomi et al. 1996; Abdi et al. 1997). Study by Lin and Walsh (2008) on apple fruits indicated that the “tree factor” mediated effect on ripening is cultivar dependent and also linked with the growing/ripening season of the fruit. With this historical background, fruit specific examples are described below with the information generated so far.
Avocado
Avocado fruit is a typical climacteric fruit. However, most avocado cultivars lack the ability to ripen as long as the fruit is attached to the tree. This phenomenon is probably related to the fact that avocado fruit attached to the tree produce only trace amounts of ethylene. It has been suggested that some unknown ripening inhibitor moves from the leaves or shoots to the fruit where it inhibits ethylene production and hence ripening (Burg and Burg 1964; Tingwa and Young 1975). Sitrit et al. (1986) reported that inability of preclimacteric avocado fruit, to produce ethylene was due to lack of ACC as there was low activity of ACC-synthase (ACS) enzyme [catalyses the conversion of S-adenosyl methionine (SAM) into 1-aminocyclopropane-1-carboxylic acid (ACC) during biosynthesis of ethylene]. It is also well accepted that malonylation of ACC may play a role in regulation of endogenous levels of ACC and hence ethylene production (Yang and Hoffman 1984). In fact, preclimacteric avocado fruits were reported to contain significant levels of malonylated-ACC (MACC) (Sitrit et al. 1986). This indicates that at least a part of the ACC (which is synthesized in the course of fruit growth) is conjugated to form MACC. Thus, formation of MACC may be a mechanism involved in regulating ethylene production during the preclimacteric stage. However, it does not seem to play a regulatory role in ethylene production at the climacteric stage. At this stage, MACC increases only slightly compared to the marked increase in the level of ACC (Sitrit et al. 1986). These results suggest that the inability of most avocado cultivars to produce ethylene as long as they remain attached to the tree is mainly due to repression of ACS. This repression is removed once the fruit is harvested and this results in normal ripening of harvested avocado fruit however the cause of this repression is not known.
Tomato
Tomato fruits were observed to ripe normally only if they were harvested after completing 40% of their total growth period. If fruits were picked after 20% of the total growth period, then the normal ripening behaviour was not observed (Simons 1970). Exogenous ethylene treatment also did not induce ripening in undeveloped locules of fruits if picked at the completion of about 20% of the total growth period. Keeping in mind that immature tomato fruits are relatively insensitive to exogenously applied ethylene, Simons (1970) suggested that developing seeds produced an inhibitor of ethylene action. But, the other researchers have however explained these results of Simons (1970) by suggesting that the capacity to ripe is dependent on continued seed development. Later, McGlasson and Adato (1977) proposed that developing tomato fruits at about 15 days after anthesis undergo a transition period during which changes required for the expression of ripening are completed.
The induction of ripening of tomato fruit depends on the threshold concentration of ethylene in the fruit tissues and a threshold concentration of 4.9 μl l−1 has been reported by Knegt et al. (1974). Ethylene concentrations were unusually high in attached tomato fruits (ranging from 2 to 13 μl l−1 depending on cultivars and seasonal conditions) while, in detached fruits the values drop to a level of 1.4 μl l−1 on an average (Sawamura et al. 1978). Detachment of tomato fruit advances the ripening and also reduces the threshold value of endogenous ethylene required to promote ripening to a considerable lower level. This supports the concept of a hypothetical labile ripening inhibitor that is translocated from vegetative parts of the plant to the fruits where it antagonizes the action on ethylene (Sawamura et al. 1978). This concept might be true, but the large decrease in the levels of ethylene in detached fruits may be explained by more effective gaseous exchange from the stem scar region of harvested tomato fruit as a result of detachment of the pedicel. This explanation is supported by de Vries et al. (1995) who reported that 85–90% of ethylene is being released by tomato fruit through this stem scar region.
Based on comparative study on attached and detached tomatoes, it was concluded by Saltveit (1993) that a respiratory climacteric per se, which has been considered an intrinsic part of the ripening of climacteric fruits may not be necessary for the ripening of climacteric fruit on the plant but instead it may be an artifact of using harvested fruit. Andrews (1995) challenged these findings for tomato and reported that the respiration climacteric occurred in both attached and detached fruit. As the physiological differences in ripening of attached and detached fruits were ambiguous Saltveit (1999) again reported that once ripening of climacteric fruits has started, the internal ethylene concentration increases quickly to a much higher level (reaching up to 100 μl l−1). The reason for this is the stronger diffusion resistance, especially during later stages of fruit development (Bargel and Neinhuis 2005; Paul and Srivastava 2006). However, as per the classical work of Burg and Burg (1965b) and then by Solomos (1987), more the ethylene production steeper will be the concentration gradient and this results in more release of ethylene from the fruit. This is in accordance to the Fick’s law of diffusion. In an another study, changes in electrolyte efflux pattern in detached and attached tomato fruits by Paul et al. (2005) indicated that the electrolyte efflux (%) was more related to ripening of tomato fruits on plant rather than for the fruits harvested at green mature stage and stored. Detached fruits (at 8 days after harvest) and attached fruits (at pink stage) showed a large increase in electrolyte efflux. Rate of electrolyte efflux was not influenced by slow or fast ripening behaviour of tomato varieties. It was therefore concluded that the electrolyte efflux% could not be a suitable criterion to assess the slow and fast ripening behaviour of tomato varieties either for the fruits detached from the plant or fruits remain attached to the plant.
There are various factors that affect the production and release of ethylene by fruit. In this regard, as already stated, a theory of ethylene emission was developed and used as the base to develop simulation model called “ETHY” by Genard and Gouble (2005). This model was found to be highly sensitive to the factors such as permeability of the fruit skin, internal concentration of O2, CO2 and ACC, changes in fruit growth and temperature, activities of ACC-oxidase and ACC-synthase, concentration of ethylene itself and production status of ATP. Similarly, a model has been proposed for gas exchange in pear fruit (Ho et al. 2008). Hypoxia (low O2 to CO2 ratio) or anaerobic conditions decrease the synthesis as well as the action of ethylene (Kanellis et al. 1989a; Gorny and Kader 1996; Mathooko 1996). Hypoxia in the microenvironment of the tomato fruit was reported to increase the activity of pyruvate decarboxylase and alcohol dehydrogenase (ADH) enzymes (Chen and Chase 1993; Longhurst et al. 1994). As a result, production of acetaldehyde and ethanol was also triggered in tomato (Longhurst et al. 1994). According to Lyons and Pratt (1964), rise in the endogenous concentrations of CO2 and ethylene during ripening of tomato fruit was accompanied by decrease in O2 concentration but this drop in O2 was not sufficient enough to cause anaerobiosis. As per Solomos (1987), skin represents the major barrier to gas exchange for most horticultural produce and diffusivity of the gases for the fruit flesh is 10–20 times higher than the diffusivity from the skin. In view of this, low internal O2 stress was reported to be faced by fruits (including tomato) during the course of ripening due to presence or increase in diffusion barriers in peel as well as in flesh portions of fruits (Longhurst et al. 1994; Solomos and Kanellis 1997). Under such situation, accumulation of ADH enzyme occurs due to induction of ADH2 gene and this leads to appropriate balance of some flavour aldehydes and alcohols in tomato fruits (Longhurst et al. 1994; Speirs et al. 1998). Similarly, mandarins accumulate larger amount of acetaldehyde and ethanol after harvest than grapefruit because of higher activity of ADH in the juice sacs (vesicles) and lesser permeability of peel towards the exit of gases in mandarins (Shi et al. 2007). Anatomical observations revealed that although the total thickness of the peel (comprising the albedo, the white inner layer and the flavedo) was greater in grapefruit but the peel was more densely packed in mandarins. So, it was concluded that mandarins accumulate larger amount of acetaldehyde and ethanol in comparison to grapefruit and this was because of higher activity of the enzyme ADH in the juice sacs in view of the lesser permeability of the peel towards the exit of gases in mandarins (Shi et al. 2007). In this regard it is interesting to note that ethanol and acetaldehyde have already been reported to delay ripening in tomato and other fruits (Beaulieu et al. 1997; Pesis 2005; Pesis 2006).
Surface morphology, anatomical features and mechanical properties of cells undergo a considerable change during growth, development and ripening which can affect resistance to gaseous diffusion (Zagory and Kader 1988; Kader and Saltveit 2003a, b). There are changes in the mechanical properties of the cuticle of tomato fruit with maturity (Bargel and Neinhuis 2005). Gaseous transport in fruit tissue is governed by diffusion as well as permeation. The permeation ability is determined by the overall pressure gradient of a given gas (Ho et al. 2006a, b) and also by cellular arrangement and intercellular spaces within the fruit (Cloetens et al. 2006; Mendoza et al. 2007; Verboven et al. 2008). According to Burg and Burg (1965b), internal concentration of ethylene is inversely related to the pressure if the diffusing medium is gaseous in nature (as represented by intercellular spaces in plant systems). Due to the differences in physical and chemical properties of cellular barriers, concentrations of gases are not uniform throughout the fruit especially in the bulky fruits (Solomos 1987). Presence of such barriers cause restriction in the permeability and therefore internal concentration of ethylene in the fruits may rise to physiologically active levels. The role of surface morphology in determining the ripening behaviour, rate of respiration and water loss by attached and detached tomato fruits was emphasized and there was variety dependent increase in the density of lenticels with the progress of ripening (Paul and Srivastava 2006). Significance of available surface area of stem scar region in determining the rate of ripening and extent of climacteric rise in tomato fruit was reported by Paul et al. (2010a). Variations among cultivars in fruit characteristics that contribute to differences in the endogenous levels of ethylene and thereby ethylene mediated responses were also described by (Paul et al. 2010a, b). The overall role of the gaseous microenvironment in the fruit (including the status of CO2, O2, moisture/water status, ethylene and other non-ethylene volatiles) and various other factors (morphological, anatomical and biochemical etc.) in relation to ripening were reviewed by Paul and Pandey (2011). Further, it is interesting to note that the extent of the 1-MCP mediated delay in ripening depends on the endogenous level of ethylene in the tomato fruits (Zhang et al. 2009). This thereby explains that the cultivar dependent variations in the ripening behaviour of fruits can also be due to the differences in their internal gaseous environment.
Melons
Several cultivated cultivars and wild ecotypes of melons exhibit a climacteric pattern of ripening that is linked with detachment of the fruit from its parent plant (Abeles et al. 1992). There are variations in the magnitude and duration of the respiratory rate depending on the cultivars in harvested melon fruits. However, several melon cultivars that apparently do not abscise show no burst in ethylene production and display a long shelf-life. These melon fruits emit little or no ethylene and therefore behave like non-climacteric fruit (Shiomi et al. 1999). It has been reported that the ripening-associated increase in ethylene production is present but the respiratory climacteric is absent during ripening of melon fruit attached to the plant, leading to the suggestion that climacteric respiration is an artifact of harvest. To address the universality of this phenomenon, ripening behaviour in the melon cultivar ‘charentais’ (Cucumis melo cv. ‘Reticulatus F1 Alpha’), was investigated. The results however, showed that the respiratory climacteric occurs in fruit ripened both on and off the plant (Hadfield et al. 1995). The sensitivity to ethylene in relation to the climacteric respiration is affected by detachment of the fruit (Bower et al. 2002). In an another study, application of ethylene to antisense ACC-oxidase melons stimulated O2 consumption only if they were detached from the vine, showing that attachment to the plant inhibits the effect of ethylene on respiration (Pech et al. 2008). This effect of detachment on sensitivity to ethylene is known as the tree factor by postharvest physiologists as discussed previously. Auxin has been suggested to be a candidate but experimental evidence is still lacking (Pech et al. 2008). In muskmelon also, the detached fruit produced a burst in respiration and ethylene but fruits attached fruits did not exhibit a large increase in respiration in spite of the significant increase in the levels of ethylene (Shellie and Saltveit 1993).
Sweet pepper
Different ripening patterns exist for attached and detached fruits of sweet pepper (Saltveit 1977; Biles et al. 1993; Villavicencio et al. 1999). It was reported by Tadesse et al. (1998) that there is an indication that capsicum fruits of cultivar ‘Domino’ are climacteric with respect to their internal ethylene concentration during ripening. But, the internal CO2 concentration, on the other hand, increased only for the fruits undergoing ripening when attached to the plant and a steady decline in CO2 was recorded in fruits that were detached.
Saskatoon
Comparision of respiration and ethylene production rates at nine different maturity stages of saskatoon (Amelanchier alnifolia, Nutt.) fruits after their harvest with the fruits undergoing maturation and ripening on the plant itself indicated that the respiratory response was very different in these two situations. The increase in respiration associated with ripening was demonstrated only on a whole-fruit basis only in attached fruits (Rogiers and Knowles 1999).
Based on these discussions on ripening behaviour of different fruits, it can be concluded that there are various factors that could contribute differentially in determining the ripening behaviour of attached and detached fruits. Such factors include 1. Supply of a hypothetical ripening inhibitor from plant to fruit, 2. Differences in the production and release of ethylene and thereby endogenous levels of ethylene in the fruit, 3. Differences in the sensitivity of fruit to the endogenous levels of ethylene, 4. Differences in the threshold level of ethylene required to initiate the process of fruit ripening and 5. Overall differences in the endogenous gaseous microenvironment of fruits. In addition to this, variability among varieties and cultivars in these factors could also contribute to the differences in ripening behaviour. To a certain extent, these factors could also explain the fading distinctions in the classical pattern of climacteric and non-climacteric ripening behaviour of fruits.
Role for ethylene in ripening of non-climacteric fruits
Strawberry
Strawberry is considered to be a typical example of non-climacteric fruit and it has served as a model system for this category of fruits (Giovannoni 2001). However, Trainotti et al. (2005) reported an increase in the synthesis of ethylene receptors concomitant with the increased synthesis of ethylene in strawberries. The receptors mostly expressed in ripening strawberries are type-II ones (with a degenerate histidine–kinase domain). According to Cancel and Larsen (2002), the degenerate histidine–kinase domain of type-II receptors would lead to a weaker affinity for the amino-terminal domain of CTR1 than that of type-I receptors. So, even the little ethylene produced by strawberries (Abeles and Takeda 1990; Perkins-Veazie et al. 1996; Iannetta et al. 2000) might be sufficient to trigger ripening-related physiological responses. This means that CTR1 might be released by type-II receptors, like FaEtr2, by lower amounts of ethylene. In addition to this, increase in expression of FaEtr2 (a type II receptor homolog that is closely related to the tomato LeETR4) was also found to be associated with ripening in strawberry (Trainotti et al. 2005). Application of the highly sensitive technique of laser photoacoustic spectroscopy coupled with the specific apparatus to determine in planta ethylene production in fruits and flowers of strawberry during development and ripening revealed an increase in ethylene production and a concomitant rise in respiration rate in red strawberry fruits (Iannetta et al. 2006). Treating strawberry fruit with the ethylene action inhibitor (silver thiosulfate) showed that this increase in ethylene production was under the control of a positive feedback mechanism in ripe fruits and thereby suggesting that a form of auto-induced ethylene production is operational during ripening in strawberry (Iannetta et al. 2006). However, the timing of this ripening related increase in ethylene production in strawberry was distinct from the patterns of ethylene production typically found in ripening of climacteric fruits such as tomato (Iannetta et al. 2006). Manning (1994) has previously demonstrated the negative role of auxin in ripening of this non-climacteric fruit.
Grape berry
Ethylene influences multiple aspects of ripening in grape although grape is regarded as an important example of a non-climacteric fruit. Grape berries have a double sigmoid growth pattern. The last large increase in the size is called veraison stage of grape development. At this stage, berries start accumulating sugars and anthocyanins. Ethylene treatment of grape berries stimulated an increase in the production of anthocyanins along with the increase in the four transcripts encoding enzymes (involved in synthesis of anthocyanins) (El-Kereamy et al. 2003). Similar to strawberry, small but significant increases in ethylene synthesis (at the onset of ripening) has been detected in grape berries also. Chervin et al. (2004) demonstrated the presence of a transient peak of ethylene production in grapes just prior to the onset of ripening. Experiments with 1-MCP (action inhibitor of ethylene) indicated that ethylene is required for the onset of accumulation of anthocyanins, fruit swelling and decrease in acidity associated with ripening of grape berries (Chervin et al. 2004). The results therefore indicate that tissues of grape berry have a fully functional pathway for ethylene synthesis and this pathway is activated and perception of ethylene is critical just before the veraison stage (Chervin et al. 2004). Besides this, there is involvement of ethylene signaling in the regulation of ADH expression in grapevine. Treatment of grape berries with 1-MCP caused partial repression of ripening-induced expression of the VvADH2 gene that encodes an ADH (Tesniere et al. 2004). It was further observed that low doses of ethylene application increase the berry diameter at the inception of veraison stage during ripening (Chervin et al. 2008). These results confirmed the finding of earlier studies also where good correlations between ripening of grape berries and accumulation of various transcripts of polygalacturonase, pectin methylesterase, cellulose synthase and expansins were recorded. In addition to this, auxin has been proposed as a negative regulator (Davies et al. 1997) while ABA is reported to act as a positive regulator of berry ripening (Gagne et al. 2006).
Citrus
Studies on citrus, a non-climacteric fruit, showed that ripening-related colour changes in flavedo portion of the fruit peel are regulated by endogenous as well as exogenous levels of ethylene. Ethylene was reported to play role in regulating the ripening of peel in citrus fruit (Goldschmidt et al. 1993; Goldschmidt 1997). Ethylene stimulates respiration, chlorophyll breakdown and carotenoid accumulation (Stewart and Wheaton 1972; Purvis and Barmore 1981; Goldschmidt et al. 1993; Goldschmidt 1997; Katz et al. 2004). Further studies have shown that some genes (including chlorophyllase) were regulated by ethylene in citrus fruit (Alonso et al. 1995; Jacob-Wilk et al. 1999). Earlier, it was Aharoni (1968) who reported a significant rise in ethylene evolution and respiration in young citrus fruits and latter Eaks (1970) proposed this phenomenon as “psedoclimacteric”. According to McMurchie et al. (1972), citrus (and other non-climacteric fruits) lack a ripening-associated autocatalytic rise in ethylene production. But, at the same time they also suggested that this does not rule out a role for the small but detectable amount of endogenous ethylene in the regulation of ripening process. In a review article Goldschmidt (1997) pointed out the potential significance of even the lowest levels of ethylene present in the non-climacteric fruits in regulation of ripening process. Induction of ripening of citrus fruit peel by ethylene was also found to be opposed by gibberellins and cytokinins and this was possibly due to the reduction in the tissue’s sensitivity to ethylene (as reviewed by Goldschmidt 1997). There is auto-induced ethylene production in citrus fruit (Katz et al. 2004). The harvested immature fruits produce high levels of ethylene and this can be further stimulated by exogenous treatment with ethylene or propylene and inhibited by 1-MCP. This indicates the auto-inductive nature of ethylene production but with altered timing in comparison with similar events in climacteric fruit (Katz et al. 2004). Likewise the developmental regulation of transition from system 1 to system 2 of ethylene production in climacteric fruit (Yokotani et al. 2009), the transition from system 2 behaviour of young fruitlets of citrus to system 1 behaviour by mature fruit was also suggested to be under the developmental regulation (Katz et al. 2004). Changes in the sensitivity to ethylene with development or ripening have already been pointed by many workers in different fruits (Goldschmidt 1997; Golding et al. 1999; Bower et al. 2002, Pech et al. 2008; Yoo et al. 2009; Johnston et al. 2009). These findings thereby necessitate to take up further work not only on the role of the development/developmental signals but also on the mechanism of alteration in the sensitivity of tissue/s with the development of fruit.
Melons
As mentioned earlier, this group of fruits comprises not only climacteric but also non-climacteric genotypes. Crossing climacteric with non-climacteric melons generates climacteric melons. For example, crossing long shelf-life honeydew melon (C. melo var. ‘inodorus’) with cantaloupe charentais type melon (C. melo var ‘cantalupensis’) gives hybrids of the climacteric type (Ezura et al. 2002). It was reported that some lines generated from two non-climacteric melons, Piel de Sapo (var. ‘inodorus’) and Songwhan Charmi PI 161375 (var ‘chinensis’) showed the climacteric character (Obando et al. 2007). Suppression of ethylene production by antisense ACC-oxidase RNA in ‘charentais’ melon (known for its climacteric behaviour) showed that many ripening pathways (synthesis of aroma volatiles, respiratory climacteric and degreening of the exocarp) were regulated by ethylene but some pathways (initiation of climacteric, sugar accumulation, loss of acidity and coloration of pulp) were ethylene-independent (Pech et al. 2008). Similarly, softening of flesh comprised both, ethylene-dependent and independent components that could also be correlated with differential regulation of cell wall degrading genes (Pech et al. 2008). To gain further understanding, near-isogenic lines (NILs) of melon were used to distinguish the aroma profiles associated with climacteric or non-climacteric pattern of ripening. These studies revealed that various volatiles like esters, aldehydes, isobutyl acetate, benzyl acetate and pentanal allow the climacteric NILs to be distinguished from the non-climacteric NILs at harvest using univariate and multivariate analysis (Obando-Ulloa et al. 2008a, b, 2009). Ethylene bioassays in closely related watermelon genotype (having similar phenotypes) at different (green, white, pink and red) stages were performed by Wechter et al. (2008). The study showed that ethylene levels were highest during the green stage of fruit development and this was followed by a decrease in its levels during the white and pink stages of development. The higher level of ethylene production during fruit development in watermelon thereby supports a role for ethylene in development besides its role in ripening of this non-climacteric fruit.
Pepper
Capsicum fruits are classified as non-climacteric based on the patterns of CO2 and ethylene production (Saltveit 1977; Lurie et al. 1986; Lu et al. 1990; Biles et al. 1993). However, some hot pepper cultivars are climacteric (Gross et al. 1986), indicating that classification of capsicum as non-climacteric may be inconclusive. Further, some cultivars seem to be ethylene insensitive, while in others continuous treatment with exogenous ethylene has been shown to accelerate ripening (Armitage 1989) and to up-regulate the expression of ripening specific genes (Ferrarese et al. 1995; Harpster et al. 1997). It was reported by Villavicencio et al. (2001) that all cultivars seem to exhibit intermediate levels of ethylene and CO2 evolution during ripening and therefore peppers appear to fall between the typical characteristics of climacteric and non-climacteric behaviour of fruit ripening. Therefore peppers perhaps constitute an evolutionary intermediate group between climacteric and non-climacteric fruits originated probably as a consequence of selection and breeding practices.
Cucumber
Cucumber fruit are classified as non-climacteric (Biale and Young 1981). However, climacteric-like increase in postharvest ethylene production has also been reported in several cultivars of cucumber. It was noticed that ethylene production patterns were more closely associated with decay than climacteric respiratory patterns or ripening-like physical changes (e.g., colour or softening) (Saltveit and McFeeters 1980). Postharvest changes in surface hue angle (reflecting a loss of green colour) are usually gradual but, postharvest chlorophyll loss of cucumber fruit can also be quite rapid also (Lima et al. 2005; Nilsson 2005). More rapid postharvest quality loss and degreening occurs in cucumber fruit if harvested at a stage of development close to and beyond seed maturation (Frost 1987). Postharvest responses of cucumber fruit to exogenous ethylene have been examined primarily with fruit of commercial maturity and not in fruit of more advanced developmental maturity. Exposure to exogenous ethylene expedites chlorophyll loss (Villalta and Sargent 2004; Lima et al. 2005; Nilsson 2005), and is further associated with increased electrolyte leakage, watersoaking and decay (Villalta and Sargent 2004; Lima et al. 2005; Nilsson 2005). These studies have examined fruit at a single developmental stage corresponding to commercial harvest maturity. However, a recent study by Hurr et al. (2009) investigated the postharvest storage and ethylene responses of cucumber fruit at defined developmental stages based on growth and surface colour parameters. The results showed that ethylene increased respiration in fruit of all stages of development but, ethylene production was detectable only in decaying fruit. The data also showed that the postharvest response of cucumber fruit to ethylene is highly dependent on developmental stage because only older fruits exhibit climacteric-like responses while younger fruits exhibit only tissue watersoaking and general fruit deterioration.
Conclusions and future perspectives
There have been considerable advances in understanding the changes associated with fruit ripening at the physiological, biochemical and molecular levels. These advances include discoveries on the mechanisms and signaling pathways involved and linked with the process of fruit ripening. Detailed and highly sensitive analyses of ripening and ripening related changes especially in the levels of CO2 and of ethylene at different stages of fruit growth and maturation in climacteric as well as non-climacteric fruits challenged the basic classification of many fleshy fruits into these categories. Recent research has revealed that some genes that regulate ripening of fruits have been remained conserved during the course of evolution. The emerging picture points clearly to the coexistence of ethylene-dependent and ethylene-independent pathways in both the categories of fruits. It has been shown clearly that many regulators of fruit development and ripening are common to both climacteric and non-climacteric fruits. Studies have shown that low O2 and high CO2 in the microenvironment of fruit can delay the onset of rise in ethylene and respiration and thereby the ripening of fruit. There exists variability in the surface morphology/anatomical features among the cultivars and this can cause differences in the prevailing gaseous microenvironment of fruits in terms of endogenous concentrations of O2, CO2 and ethylene etc. This aspect thereby provides an additional explanation for the existing variations in the ripening behaviour not only among different types of fruits, among the different cultivars for a given fruit but also for the observed deviations in ripening behaviour by a fruit under plant-attached and plant-detached situations.
In the area of fruit physiology and ripening in particular, there are many aspects that still need the attention of plant scientists. Till we get clear picture, it would be safe to advise that even the non-climacteric fruits need to be kept away from any source of ethylene to avoid any possible effect on ripening and/or quality of these fruits. Some of the important research priorities and questions that need to be answered to understand and solve the confusion regarding the ripening behaviour and grouping of fruits into climacteric and non-climacteric categories are as follows:
Re-evaluate the application of propylene to distinguish between climacteric and non-climacteric fruits and system 1 and system 2.
To carry out further identification and characterization of ethylene-dependent and ethylene-independent gene.
How does exactly the transition occur from system 1 to system 2 of ethylene synthesis at the onset of ripening?
How is system 2 of ethylene synthesis perpetuated?
To probe the changes in ethylene sensitivity rather than ethylene levels that mediate physiological changes during ripening of non-climacteric fruits.
Is it so that non-climacteric fruits carry mutations within the components of ethylene synthesis or signaling pathways?
What is the molecular identity of loci that control climacteric ethylene production within closely related species such as capsicums and melons?
What exactly is the interaction between ethylene and other hormones especially with auxin? This needs to be understood further at mechanism as well as regulatory levels.
Can we harness the available natural variation in the ripening behaviour for generating new varieties with broad consumer appeal and enhanced storage capability?
What are the functions of the individual ethylene signaling components and how is this network of proteins assembled in vivo? Is the expansion of gene families in tomato and other fleshy fruits linked to special ethylene signaling requirements?
What are the downstream targets of the EIN 3 like transcriptional factors (EILs) and ethylene responsive factors (ERFs) in ripening fruits and how do these transcription factors activate or repress specific ripening-related pathways?
What is the exact reason and specific role of the rise in respiration (climacteric rise) during ripening of climacteric fruits?
How ethylene is linked to respiratory metabolism at the biochemical and molecular levels? Alternate respiration also needs attention in terms of its role and significance in climacteric and non-climacteric fruits. Can manipulation of alternate respiration influence the ripening and ripening–related changes?
To integrate genetically identified regulatory components into mechanistic actions at the biochemical, molecular and cellular levels in understanding ethylene signaling and function.
References
- Abdi N, Holford P, McGlasson WB, Mizrahi Y. Ripening behaviour and responses to propylene in four cultivars of Japanese type plums. Postharvest Biol Technol. 1997;12:21–24. doi: 10.1016/S0925-5214(97)00041-0. [DOI] [Google Scholar]
- Abdi N, McGlasson WB, Holford P, Williams M, Mizrahi Y. Responses of climacteric and suppressed-climacteric plums to treatment with propylene and 1-methylcyclopropene. Postharvest Biol Technol. 1998;14:29–39. doi: 10.1016/S0925-5214(98)00031-3. [DOI] [Google Scholar]
- Abel S, Theologis A. Early genes and auxin action. Plant Physiol. 1996;111:9–17. doi: 10.1104/pp.111.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeles FB, Takeda F. Cellulase activity and ethylene in ripening strawberry and apple fruits. Sci Hort. 1990;42:269–275. doi: 10.1016/0304-4238(90)90050-O. [DOI] [Google Scholar]
- Abeles FB, Morgan PW, Saltveit ME. Ethylene in plant biology, Vol 15. 2. San Diego, California, USA: Academic; 1992. [Google Scholar]
- Aharoni Y. Respiration of oranges and grapefruit harvested at different stages of development. Plant Physiol. 1968;43:99–102. doi: 10.1104/pp.43.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akamine EK, Goo T. Respiration and ethylene production in fruits of species and cultivars of Psidium and species of Eugenia. J Am Soc Hort Sci. 1979;104:632–635. [Google Scholar]
- Alexander L, Grierson D. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J Exp Bot. 2002;53:2039–2055. doi: 10.1093/jxb/erf072. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN. The ethylene signaling pathway. Sci. 2004;306:1513–1515. doi: 10.1126/science.1104812. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Chamarro J, Granell A. Evidence for the involvement of ethylene in the expression of specific RNAs during maturation of the orange, a nonclimacteric fruit. Plant Mol Biol. 1995;29:385–390. doi: 10.1007/BF00043661. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Sci. 1999;284:2148–2152. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- Andrews J. The climacteric respiration rise in attached and detached tomato fruit. Postharvest Biol Technol. 1995;6:287–292. doi: 10.1016/0925-5214(95)00013-V. [DOI] [Google Scholar]
- Anonymous (2010) Putting mangosteen quality to the test. In: FSTA reports. Food Info Online FSTA Reports. Food Science and Technology Abstracts. http://www.foodsciencentral.com/fsc/ixid15815
- Armitage AM. Promotion of fruit ripening in ornamental peppers by ethephon. HortSci. 1989;24:962–964. [Google Scholar]
- Atta-Aly M, Brecht JK, Huber DJ. Ethylene feedback mechanisms in tomato and strawberry fruit tissues in relation to fruit ripening and climacteric patterns. Postharvest Biol Technol. 2000;20:151–162. doi: 10.1016/S0925-5214(00)00124-1. [DOI] [Google Scholar]
- Ayub R, Rombaldi C, Lucchetta L, Ginies C, Latche A, Bouzayen M, Pech JC (2008) Mechanisms of melon fruit ripening and development of sensory quality. In: Pitrat M (ed) Cucurbitaceae 2008, Proceedings of the IXth EUCARPIA meeting on Genetics and Breeding of Cucurbitaceae. INRA, Avignon (France), 21–24 May, p 241–248
- Azzolini M, Jacomino AP, Bron HU, Kluge RA, Schiavinato MA. Ripening of “Pedro Sato” guava: study on its climacteric or non-climacteric nature. Braz J Plant Physiol. 2005;17:299–306. doi: 10.1590/S1677-04202005000300004. [DOI] [Google Scholar]
- Bargel H, Neinhuis C. Tomato (Lycopessicon esculentum Mill.) fruit growth and ripening as related to the biomechanical properties of fruit skin and isolated cuticle. J Exp Bot. 2005;56:1049–1060. doi: 10.1093/jxb/eri098. [DOI] [PubMed] [Google Scholar]
- Barry CS, Giovannoni JJ. Ethylene and fruit ripening. J Plant Growth Regul. 2007;26:143–159. doi: 10.1007/s00344-007-9002-y. [DOI] [Google Scholar]
- Barry CS, Blume B, Bouzayen M, Cooper W, Hamilton AJ Grierson D. Differential expression of the 1-aminocyclopropane-1-carboxylate oxidase gene family of tomato. Plant J. 1996;9:525–535. doi: 10.1046/j.1365-313X.1996.09040525.x. [DOI] [PubMed] [Google Scholar]
- Barry CS, Llop-Tous MI, Grierson D. The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato. Plant Physiol. 2000;123:979–986. doi: 10.1104/pp.123.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baydar NG, Harmankaya N. Changes in endogenous hormone levels during the ripening of grape cultivars having different berry set mechanisms. Turk J Agric For. 2005;29:205–210. [Google Scholar]
- Beaulieu JC, Peiser G, Saltveit ME. Acetaldehyde is a causal agent responsible for ethanol induced ripening inhibition in tomato fruits. Plant Physiol. 1997;113:431–439. doi: 10.1104/pp.113.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yehoshua S, Burg SP, Young R. Resistance to citrus fruit to mass transport of water vapours and gases. Plant Physiol. 1985;79:1048–1053. doi: 10.1104/pp.79.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biale JB. Growth, maturation and senescence in fruits. Sci. 1964;146:880–888. doi: 10.1126/science.146.3646.880. [DOI] [PubMed] [Google Scholar]
- Biale JB, Barcus DE. Respiratory patterns in tropical fruits of the Amazon basin. Trop Sci. 1970;12:93–104. [Google Scholar]
- Biale JB, Young RE. Respiration and ripening in fruit-retrospect and prospect. In: Friend J, Rhodes MJC, editors. Recent advances in the biochemistry of fruit and vegetables. New York: Academic; 1981. pp. 1–39. [Google Scholar]
- Biles CL, Wall MM, Blackstone K. Morphological and physiological changes during maturation of New Mexican type peppers. J Soc Hort Sci. 1993;118:476–480. [Google Scholar]
- Blanpied GD (1993) Studies of the “tree factor” that inhibits the ripening of attached apples. Acta Hort 343:6–11
- Bleecker AB, Kende H. Ethylene: a gaseous signal molecule in plants. Ann Rev Cell Dev Biol. 2000;16:1–18. doi: 10.1146/annurev.cellbio.16.1.1. [DOI] [PubMed] [Google Scholar]
- Bower J, Holford P, Latche A, Pech JC. Culture conditions and detachment of the fruit influence the effect of ethylene on the climacteric respiration of the melon. Postharvest Biol Technol. 2002;26:135–146. doi: 10.1016/S0925-5214(02)00007-8. [DOI] [Google Scholar]
- Brecht JK, Ritenour MA, Haard NF, Chism GW (2008) Postharvest physiology of edible plant tissues. In: Damodaran S, Parkin KL, Fennema OR, (eds) Fennema’s food chemistry, 4th edn. CRC Press, p 975–1049
- Bregoli AM, Fabbroni C, Costa F, Raimondi V, Costa G (2007) Auxin and ethylene interaction during fruit growth and ripening of Actinidia deliciosa. In: Ramina A, Chang C, Giovannoni J, Klee H, Perata P, Woltering E, (eds) Advances in plant ethylene research. Proceedings of the 7th International Symposium on the Plant Hormone Ethylene. Springe, p 105–107
- Brown BI, Wills RBH. Post-harvest changes in guava fruit of different maturity. Sci Hort. 1983;19:23–243. doi: 10.1016/0304-4238(83)90069-9. [DOI] [Google Scholar]
- Bufler G. Ethylene-promoted conversion of 1-aminocyclopropane-1-carboxylic acid to ethylene in apple at various stages of fruit development. Plant Physiol. 1986;80:539–543. doi: 10.1104/pp.80.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg SP. The physiology of ethylene formation. Annu Rev Plant Physiol. 1962;13:265–302. doi: 10.1146/annurev.pp.13.060162.001405. [DOI] [Google Scholar]
- Burg SP, Burg EA. The role of ethylene in fruit ripening. Plant Physiol. 1962;37:179–189. doi: 10.1104/pp.37.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg SP, Burg EA (1964) Evidence for a natural occurring inhibitor of fruit ripening. Plant Physiol 39:(Supplement) 10
- Burg SP, Burg EA. Ethylene action and the ripening of fruits. Sci. 1965;148:1190–1196. doi: 10.1126/science.148.3674.1190. [DOI] [PubMed] [Google Scholar]
- Burg SP, Burg EA. Gas exchange in fruits. Physiol Plant. 1965;18:870–886. doi: 10.1111/j.1399-3054.1965.tb06946.x. [DOI] [Google Scholar]
- Cancel JD, Larsen PB. Loss-of-function mutations in the ethylene receptor ETR1 cause enhanced sensitivity and exaggerated response to ethylene in Arabidopsis. Plant Physiol. 2002;129:1557–1567. doi: 10.1104/pp.003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cara B, Giovannoni JJ. Molecular biology of ethylene during tomato fruit development and maturation. Plant Sci. 2008;175:106–113. doi: 10.1016/j.plantsci.2008.03.021. [DOI] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Sci. 1993;262:539–544. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR. Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell. 1997;89:1133–1144. doi: 10.1016/S0092-8674(00)80300-1. [DOI] [PubMed] [Google Scholar]
- Chen ARS, Chase J., Jr Alcohol dehydrogenase and pyruvate decarboxylase induction in ripening and hypoxia tomato fruit. Plant Physiol Biochem. 1993;31:875–885. [Google Scholar]
- Chen YF, Etheridge N, Schaller GE. Ethylene signal transduction. Ann Bot (Lond). 2005;95:901–915. doi: 10.1093/aob/mci100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervin C, El-Kereamy A, Roustan JP, Latche A, Lamon J, Bouzayen M. Ethylene seems required for the berry development and ripening in grape, a non-climacteric fruit. Plant Sci. 2004;167:1301–1305. doi: 10.1016/j.plantsci.2004.06.026. [DOI] [Google Scholar]
- Chervin C, Tira-Umphon A, Terrier N, Zouine M, Severac D, Roustan JP. Stimulation of the grape berry expansion by ethylene and effects on related gene transcripts, over the ripening phase. Physiol Plant. 2008;334:534–546. doi: 10.1111/j.1399-3054.2008.01158.x. [DOI] [PubMed] [Google Scholar]
- Cloetens P, Mache R, Schlenker M, Lerbs-Mache S. Quantitative phase tomography of Arabidopsis seeds reveals intercellular void network. Proc Natl Acad Sci (USA) 2006;103:14626–14630. doi: 10.1073/pnas.0603490103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombe CR, Hale BG. The hormone content of ripening grape berries and the effect of growth substance treatments. Plant Physiol. 1973;51:629–634. doi: 10.1104/pp.51.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C, Boss PK, Robinson SP. Treatment of grape berries, a non-climacteric fruit with a synthetic auxin, retard ripening and alters the expression of developmentally regulated genes. Plant Physiol. 1997;115:1155–1161. doi: 10.1104/pp.115.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries HSM, Harren FJM, Voesenek LACJ, Blom CWPM, Woltering EJ, van der Valk HCPM, Reuss J. Investigation of local ethylene emission from intact cherry tomatoes by means of photothermal deflection and photoacoustic detection. Plant Physiol. 1995;107:1371–1377. doi: 10.1104/pp.107.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs CG, Brady CJ, Campbell J, McGlasson WB. Normal ripening cultivars of Pyrus serotina are either climacteric or non-climacteric. Sci Hort. 1991;48:213–221. doi: 10.1016/0304-4238(91)90129-M. [DOI] [Google Scholar]
- Eaks IL. Respiratory response, ethylene production, and response to ethylene of citrus fruit during ontogeny. Plant Physiol. 1970;45:334–338. doi: 10.1104/pp.45.3.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kereamy A, Chervin C, Roustan JP, Cheynier V, Souquet JM, Moutounet M, Raynal J, Ford C, Latche A, Pech JC, Bouzayen M. Exogenous ethylene stimulates the long-term expression of genes related to anthocyanin biosynthesis in grape berries. Physiol Plant. 2003;119:175–182. doi: 10.1034/j.1399-3054.2003.00165.x. [DOI] [Google Scholar]
- El-Sharkawy I, Kim WS, El-Kereamy A, Jayasankar S, Svircev AM, Brown DCW. Isolation and characterization of four ethylene signal transduction elements in plums (Prunus salicina L.) J Exp Bot. 2007;58:3631–3643. doi: 10.1093/jxb/erm213. [DOI] [PubMed] [Google Scholar]
- El-Sharkawy I, Kim WS, Jayasankar S, Svircev AM, Brown DCW. Diffeential regulation of four members of ACC-synthase gene family in plum. J Exp Bot. 2008;59:2009–3027. doi: 10.1093/jxb/ern056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sharkawy I, Sherif S, Mila I, Bouzayen M, Jayasankar S. Molecular characterization of seven genes encoding ethylene responsive transcriptional factor during plum fruit development and ripening. J Exp Bot. 2009;60:907–922. doi: 10.1093/jxb/ern354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sharkawy I, Mila I, Bouzayen M, Jayasankar S. Regulation of two germin-like protein genes during plum fruit development. J Exp Bot. 2010;61:1761–1770. doi: 10.1093/jxb/erq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge N, Hall BP, Schaller GE. Progress report: ethylene signaling and responses. Planta. 2006;223:387–391. doi: 10.1007/s00425-005-0163-2. [DOI] [PubMed] [Google Scholar]
- Ezura H, Owino WO. Melon, an alternative model plant for elucidating fruit ripening. Plant Sci. 2008;175:121–129. doi: 10.1016/j.plantsci.2008.02.004. [DOI] [Google Scholar]
- Ezura H, Akashi Y, Kato K, Kuzuya M. Genetic characterization of long shelf-life in honeydew (Cucumis melo var inodorus) melon. Acta Hort. 2002;588:369–372. [Google Scholar]
- Fallik E, Aharoni Y. Postharvest physiology, pathology and handling of fresh produce. Israel: Lecture Notes. International Research and Development Course on Postharvest Biology and Technology. The Volcani Center; 2004. p. 30. [Google Scholar]
- Ferrarese L, Trainotti L, Moretto P, Polverino de Laureto P, Rascio N, Casadoro G. Differential ethylene-inducible expression of cellulase in pepper plants. Plant Mol Biol. 1995;29:735–747. doi: 10.1007/BF00041164. [DOI] [PubMed] [Google Scholar]
- Flores F, El-Yahyaoui F, de Billerbeck G, Romojaro F, Latche A, Bouzayen M, Pech JC, Ambid C. Role of ethylene in the biosynthesis pathway of aliphatic ester aroma volatiles in Charentais Cantaloupe melons. J Exp Bot. 2002;53:201–206. doi: 10.1093/jexbot/53.367.201. [DOI] [PubMed] [Google Scholar]
- Fluhr R, Mattoo AK. Ethylene biosynthesis and perception. CRC Crit Rev Plant Sci. 1996;15:479–523. [Google Scholar]
- Frenkel C. Oxidative turnover of auxin in relation to the onset of ripening in Bartlett pear. Plant Physiol. 1975;55:480–484. doi: 10.1104/pp.55.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel C, Dyck R. Auxin inhibition of ripening in Bartlerr pears. Plant Physiol. 1973;51:6–9. doi: 10.1104/pp.51.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost DJ (1987) The influence of calcium deficiency on embryo and fruit development of Cucumis sativus L. Ph.D. Dissertation. College of Food, Agricultural, and Environmental Sciences. Columbus, OH: The Ohio State University
- Gagne JM, Smalle J, Gingerich DJ, Walker JM, Yoo SD, Yanagisawa S, Vierstra RD. Arabidopsis EIN3-binding F-box 1 and 2 form ubiquitin-protein ligases that repress ethylene action and promote growth by directing EIN3 degradation. Proc Natl Acad Sci (USA) 2004;101:6803–6808. doi: 10.1073/pnas.0401698101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne S, Esteve K, Deytieux C, Saucier C, Geny L. Influence of abscisic acid in triggering version in grape berry skins of Vitis vinifera L. cv. Cabernet Sauvignon. J Int Sci Vigne Vin. 2006;40:7–14. [Google Scholar]
- Gazit S, Blumenfield A. Response of mature avocado fruits to ethylene treatments before and after harvest. J Am Soc Hort Sci. 1970;95:229–231. [Google Scholar]
- Genard M, Gouble B. ETHY. A theory of fruit climacteric ethylene emission. Plant Physiol. 2005;139:531–545. doi: 10.1104/pp.105.063339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt F. A comparison of the ripening of attached apple and pear fruits. Wash State Hort Assoc Proc. 1947;43:57–59. [Google Scholar]
- Giovannoni J. Molecular biology of fruit maturation and ripening. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:725–749. doi: 10.1146/annurev.arplant.52.1.725. [DOI] [PubMed] [Google Scholar]
- Golding JB, Shearer D, Wyllie SG, McGlasson WB. Application of 1-MCP and propylene to identify ethylene-dependent ripening processes in mature banana fruit. Postharvest Biol Technol. 1998;14:87–98. doi: 10.1016/S0925-5214(98)00032-5. [DOI] [Google Scholar]
- Golding JB, Shearer D, McGlasson WB, Wyllie SG. Relationship between respiration, ethylene and aroma production in ripening banana. J Agric Food Chem. 1999;47:1646–1651. doi: 10.1021/jf980906c. [DOI] [PubMed] [Google Scholar]
- Goldschmidt EE. Ripening of citrus and other non-climacteric fruits: a role for ethylene. Acta Hort. 1997;463:335–340. [Google Scholar]
- Goldschmidt EE, Huberman M, Goren R. Probing the role of endogenous ethylene in degreening of citrus-fruit with ethylene antagonists. J Plant Growth Regul. 1993;12:325–329. doi: 10.1007/BF00027214. [DOI] [Google Scholar]
- Goren R, Mattoo AK, Anderson JD. Ethylene binding during leaf development and senescence and its inhibition by silver nitrate. J Plant Physiol. 1984;117:243–248. doi: 10.1016/S0176-1617(84)80006-1. [DOI] [PubMed] [Google Scholar]
- Gorny JR, Kader AK. Controlled atmosphere suppression of ACC synthase and ACC-oxidase in ‘Golden Delicious’ apple during long-term cold storage. J Am Soc Hort Sci. 1996;121:751–755. [Google Scholar]
- Gross KC, Watada AE, Kang MS, Kim SD, Lee SW. Biochemical changes associated with the ripening of hot pepper fruit. Physiol Plant. 1986;66:31–36. doi: 10.1111/j.1399-3054.1986.tb01227.x. [DOI] [Google Scholar]
- Guo H, Ecker JR. Plant responses to ethylene gas are mediated by SCFEBF1/EBF2-dependent proteolysis of EIN3 transcription factor. Cell. 2003;115:667–677. doi: 10.1016/S0092-8674(03)00969-3. [DOI] [PubMed] [Google Scholar]
- Gussman CD, Goffredz JC, Gianfagna TJ. Ethylene production and fruit-softening rates in several apple fruit ripening variants. HortSci. 1993;28:135–137. [Google Scholar]
- Hadfield KA, Rose JKC, Bennett AB. The respiratory climacteric is present in Charentais (Cucumis melo cv. Reticulatus F1 Alpha) melons ripened on or off the plant. J Exp Bot. 1995;46:1923–1925. doi: 10.1093/jxb/46.12.1923. [DOI] [Google Scholar]
- Hagenmaier RD. A comparison of ethane, ethylene and CO2 peel permeance for fruits with different coatings. Postharvest Biol Technol. 2005;37:56–64. doi: 10.1016/j.postharvbio.2005.02.012. [DOI] [Google Scholar]
- Hall BP, Shakeel SN, Schaller GE. Ethylene receptors: ethylene perception and signal transduction. J Plant Growth Regul. 2007;26:118–130. doi: 10.1007/s00344-007-9000-0. [DOI] [Google Scholar]
- Harpster MH, Lee KY, Dunsmuir P. Isolation and characterization of gene encoding endo-β-1, 4-glucanase from pepper (Capsicum annuum L.) Plant Mol Biol. 1997;33:47–59. doi: 10.1023/A:1005795028489. [DOI] [PubMed] [Google Scholar]
- Ho QT, Verlinden BE, Verboven P, Nicolai BM. Gas diffusion properties at different positions in the pear. Postharvest Biol Technol. 2006a;41:113–120. doi: 10.1016/j.postharvbio.2006.04.002. [DOI] [Google Scholar]
- Ho QT, Verlinden BE, Verboven P, Vandewalle S, Nicolai BM. A permeation-diffusion-reaction model of gas transport in cellular tissue of plant material. J Exp Bot. 2006b;57:4215–4224. doi: 10.1093/jxb/erl198. [DOI] [PubMed] [Google Scholar]
- Ho QT, Verboven P, Verlinden BE, Lammertyn J, Vandewalle S, Nicolai BM. A continuum model for metabolic gas exchange in pear fruit. PLoS Computational Biol. 2008;4:e1000023. doi: 10.1371/journal.pcbi.1000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeberichts FA, Woltering EJ. Multiple mediators of plant programme cell death: interplay of conserved cell death mechanisms and plant-specific regulators. Bioassays. 2002;25:47–57. doi: 10.1002/bies.10175. [DOI] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell. 1998;94:261–271. doi: 10.1016/S0092-8674(00)81425-7. [DOI] [PubMed] [Google Scholar]
- Hurr BM, Huber DJ, Vallejos CE, Talcott ST. Developmentally dependent responses of detached cucumber (Cucumis sativus L.) fruit to exogenous ethylene. Postharvest Biol Technol. 2009;52:207–215. doi: 10.1016/j.postharvbio.2008.12.006. [DOI] [Google Scholar]
- Iannetta PP, Loorhoven LJ, Davies HV, Harren F (2000) Ethylene production by strawberry flowers and the ripening fruit. In: Communication to the 9th International Workshop on LASER based Photoacoustic Trace Gas Detection in Life Science. Nijmegen: The Netherlands
- Iannetta PPM, Laarhoven LJ, Medina-Escobar N, James EK, McManus MT, Davies HV, Harren FJM. Ethylene and carbon dioxide production by developing strawberries show a correlative pattern that is indicative of ripening climacteric fruit. Physiol Plant. 2006;127:247–259. doi: 10.1111/j.1399-3054.2006.00656.x. [DOI] [Google Scholar]
- Inaba A. Studies on the internal feedback regulation of ethylene biosynthesis and signal transduction during fruit ripening, and the improvement of fruit quality. J Jan Soc Hort Sci. 2007;76:1–12. doi: 10.2503/jjshs.76.1. [DOI] [Google Scholar]
- Inaba A, Jshida M, Sobojima Y. Changes in endogenous hormone concentrations during berry development in relation to the ripening “Delaware” grapes. J Jpn Soc Hort Sci. 1976;15:245–252. doi: 10.2503/jjshs.45.245. [DOI] [Google Scholar]
- Itai A, Kawata T, Tanabe K, Tamura F, Uchiyama M, Tomomitsu M, Shiraiwa N. Identification of 1-aminocyclopropane-1-carboxylic acid synthase genes controlling the ethylene level of ripening fruit in Japanese pear (Pyrus pyrifolia Nakai) Mol Gen Genet. 1999;261:42–49. doi: 10.1007/s004380050939. [DOI] [PubMed] [Google Scholar]
- Itai A, Tanabe K, Tamura F. Mechanism of ethylene production during fruit ripening in Asian pears. Acta Hort. 2002;587:497–504. [Google Scholar]
- Itai A, Kotaki T, Tanabe K, Tamura F, Kawaguchi D, Fukuda M. Rapid identification of 1-aminocyclopropane-1-carboxylate (ACC) synthase genotypes in cultivars of Japanese pear (Pyrus pyrifolia Nakai) using CAPS markers. Theor Appl Genet. 2003;106:1266–1272. doi: 10.1007/s00122-002-1186-8. [DOI] [PubMed] [Google Scholar]
- Ito Y, Kitagawa M, Ihashi N, Yabe K, Kimbara J, Yasuda J, Ito H, Inakuma T, Hiroi S, Kasumi T. DNA-binding specificity, transcriptional activation potential, and the rin mutation effect for the tomato fruit-ripening regulator RIN. Plant J. 2008;55:212–223. doi: 10.1111/j.1365-313X.2008.03491.x. [DOI] [PubMed] [Google Scholar]
- Jacob-Wilk D, Holland D, Goldschmidt EE, Riov J, Eyal Y. Chlorophyll breakdown by chlorophyllase: isolation and functional expression of the Chlase1 gene from ethylene treated citrus fruit and its regulation during development. Plant J. 1999;20:653–661. doi: 10.1046/j.1365-313X.1999.00637.x. [DOI] [PubMed] [Google Scholar]
- Johnston JW, Gunaseelan K, Pidakala P, Wang M, Schaffer RJ. Co-ordination of early and late ripening events in apples is regulated through differential sensitivities to ethylene. J Exp Bot. 2009;60:2689–2699. doi: 10.1093/jxb/erp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kader AA. Respiration and gas exchange of vegetable. In: Weichmann J, editor. Postharvest physiology of vegetables. New York: Marcel Dekker; 1987. pp. 25–43. [Google Scholar]
- Kader AA, Barrett DM. Classification, composition of fruits and postharvest maintenance of quality. In: Somogyi LP, Ramaswamy HS, Hui YH, editors. Processing fruits: Science and technology, Vol 1, Biology, principles and applications. Lancaster: Technomic Publishing Company; 1996. pp. 1–24. [Google Scholar]
- Kader AA, Saltveit ME. Respiration and gas exchange. In: Bartz JA, Brecht JK, editors. Postharvest physiology and pathology of vegetables. New York: Marcel Dekker; 2003a. pp. 7–29. [Google Scholar]
- Kader AA, Saltveit ME. Atmosphere modification. In: Bartz JA, Brecht JK, editors. Postharvest physiology and pathology of vegetables. New York: Marcel Dekker; 2003b. pp. 229–246. [Google Scholar]
- Kanellis AK, Solomos T, Mattoo AK. Hydrolytic enzyme activities and protein pattern of avocado fruit ripened in air and in low oxygen with and without ethylene. Plant Physiol. 1989a;90:259–266. doi: 10.1104/pp.90.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E, Lagunes PM, Riov J, Weiss D, Goldschmidt EE. Molecular and physiological evidence suggests the existence of a system II-like pathway of ethylene production in non-climacteric citrus fruit. Planta. 2004;219:243–252. doi: 10.1007/s00425-004-1228-3. [DOI] [PubMed] [Google Scholar]
- Kays SJ, Paull RE. Metabolic process in harvested products. In: Post harvest biology. Exon Press, Athens, GA; 2004. pp. 79–136. [Google Scholar]
- Kendall SA, Ng TJ. Genetic variation of ethylene production in harvested muskmelon fruit. HortSci. 1988;23:759–761. [Google Scholar]
- Kendrick MD, Chang C. Ethylene signaling: new levels of complexity and regulation. Curr Opin Plant Biol. 2008;11:479–485. doi: 10.1016/j.pbi.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevany BM, Tieman DM, Taylor MG, Cin VD, Klee H. Ethylene receptor degradation controls the timing of ripening in tomato fruit. The Plant J. 2007;51:458–467. doi: 10.1111/j.1365-313X.2007.03170.x. [DOI] [PubMed] [Google Scholar]
- Kevany BM, Taylor MG, Klee H. Fruit-specific suppression of the ethylene receptor LeETR4 results in early-ripening tomato fruit. Plant Biotechnol J. 2008;6:295–300. doi: 10.1111/j.1467-7652.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-B. [DOI] [PubMed] [Google Scholar]
- Klee HJ. Control of ethylene mediated processes in tomato at the level of receptors. J Exp Bot. 2002;53:2057–2063. doi: 10.1093/jxb/erf062. [DOI] [PubMed] [Google Scholar]
- Klee HJ. Ethylene signal transduction. Moving beyond Arabidopsis. Plant Physiol. 2004;135:660–667. doi: 10.1104/pp.104.040998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klozenbucher KA, Altman SA, McIntosh MS, Walsh CS. Effect of cultivar on endogenous ethylene evolution and its relationship to increase of soluble protein in peach mesocarp tissue. Fruit Var J. 1994;48:20–26. [Google Scholar]
- Knegt E, Kramer SJ, Bruinsma J. Pectin changes and internal ethylene concentrations in ripening tomato fruit. Colloques Internationaux CNRS. 1974;238:355–358. [Google Scholar]
- Kumar S, Sinha SK. Alternative respiration and heat production in ripening banana fruits (Musa paradisiaca var. Mysore Kadali) J Exp Bot. 1992;43:1639–1642. doi: 10.1093/jxb/43.12.1639. [DOI] [Google Scholar]
- Larson KD, Schaffer B, Davies FS. Flood water oxygen content, ethylene production and lenticel hypertrophy in flooded mango (Mangifera indica L.) trees. J Exp Bot. 1993;44:665–671. doi: 10.1093/jxb/44.3.665. [DOI] [Google Scholar]
- Lelievre JM, Latche A, Jones B, Bouzayen M, Pech JC. Ethylene and fruit ripening. Physiol Plant. 1997;101:727–739. doi: 10.1111/j.1399-3054.1997.tb01057.x. [DOI] [Google Scholar]
- Lester G. Comparison of ‘Honey Dew’ and netted muskmelon fruit tissues in relation to storage life. HortSci. 1988;23:180–182. [Google Scholar]
- Lima LCO, Hurr BM, Huber DJ. Deterioration of beit alpha and slicing cucumbers (Cucumis sativus L.) during storage in ethylene or air: responses to suppression of ethylene perception and parallels to natural senescence. Postharvest Biol Technol. 2005;37:265–276. doi: 10.1016/j.postharvbio.2005.04.010. [DOI] [Google Scholar]
- Lin S-f, Walsh CS (2008) Studies of the “tree factor” and its role in maturation and ripening of ‘Gala’ and ‘Fuji’ apples. Postharvest Biol Technol 48:99–106
- Liu L, Kakihara F, Kato M. Ethylene changes during development and ripening of fruit with reference to variety of Cucumis melo L. Breeding Sci. 2004;54:297–300. doi: 10.1270/jsbbs.54.297. [DOI] [Google Scholar]
- Liu L, Kakihara F, Kato M. Characterization of six varieties of Cucumis melo L. based on morphological and physiological characters, including shelf-life of fruit. Euphytica. 2004;135:305–313. doi: 10.1023/B:EUPH.0000013330.66819.6f. [DOI] [Google Scholar]
- Longhurst TJ, Lee E, Brady CJ, Speirs J. Structure of the tomato Adh2 gene and Adh2 pseudo-gene and the study of Adh2 gene expression in fruit. Plant Mol Biol. 1994;26:1073–1084. doi: 10.1007/BF00040690. [DOI] [PubMed] [Google Scholar]
- Lu G, Yang C, Liag H, Lu Z. ‘Changjiao’ hot peppers are non-climacteric. HortSci. 1990;25:807. [Google Scholar]
- Lurie S, Klein JD. Cyanide metabolism in relation to ethylene production and cyanide insensitive respiration in climacteric and non-climacteric fruits. J Plant Physiol. 1989;135:518–521. [Google Scholar]
- Lurie S, Shapiro B, Ben-Yehoshua S. Effects of water stress and degree of ripeness on rate of senescence of harvested bell pepper fruit. J Am Soc Hort Sci. 1986;111:880–885. [Google Scholar]
- Lyons JM, McGlasson WB, Pratt HK. Ethylene production, respiration and internal gas concentration in cantaloupe fruits at various stages of maturity. Plant Physiol. 1962;37:31–36. doi: 10.1104/pp.37.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons JM, Pratt HK. Effect of stage of maturity and ethylene treatment on respiration and ripening of tomato fruits. J Am Soc Hort Sci. 1964;84:491–500. [Google Scholar]
- Manning K. Changes in gene expression during strawberry fruit ripening and their regulation by auxin. Planta. 1994;194:62–68. doi: 10.1007/BF00201035. [DOI] [Google Scholar]
- Mapson W, Hulme AC. The biosynthesis, physiological effects and mode of action of ethylene. In: Reinhold L, Liwschitz Y, editors. Progress in phytochemistry. 2. London: Interscience; 1970. pp. 343–384. [Google Scholar]
- Mathooko FM. Regulation of ethylene biosynthesis in higher plants by carbon dioxide. Postharvest Biol Technol. 1996;7:1–26. doi: 10.1016/0925-5214(95)00026-7. [DOI] [Google Scholar]
- McGlasson WB. Ethylene and fruit ripening. HortSci. 1985;20:51–54. [Google Scholar]
- McGlasson WB, Adato I. Relationship between the capacity to ripen and ontogeny in tomato fruits. Aust J Plant Physiol. 1977;4:451–458. doi: 10.1071/PP9770451. [DOI] [Google Scholar]
- McMurchie EJ, McGlasson WB, Eaks IL. Treatment of fruit with propylene gives information about biogenesis of ethylene. Nature. 1972;237:235–236. doi: 10.1038/237235a0. [DOI] [PubMed] [Google Scholar]
- Mendoza F, Verboven P, Mebatsion HK, Kerckhofs G, Wevers M, Nicolai B. Three-dimensional pore space quantification of apple tissue using X-ray computed microtomography. Planta. 2007;226:559–570. doi: 10.1007/s00425-007-0504-4. [DOI] [PubMed] [Google Scholar]
- Mercado-Silva E, Bautista PB, Garcia-Velasco MA. Fruit development, harvest index and ripening changes of guava produced in central maxico. Postharvest Biol Technol. 1998;13:143–150. doi: 10.1016/S0925-5214(98)00003-9. [DOI] [Google Scholar]
- Miccolis V, Saltveit ME., Jr Morphological and physiological changes during fruit growth and maturation of seven melon cultivars. J Am Soc Hort Sci. 1991;116:1025–1029. [Google Scholar]
- Miller AN, Walsh CS, Cohen JD. Measurement of indole-3-acetic acid in peach fruits (Prunus persica L. Batsch cv. Redhaven) during development. Plant Physiol. 1987;84:491–494. doi: 10.1104/pp.84.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata M, Mori H, Tabei Y, Sato T, Hirai M, Imaseki H. Modification of tomato fruit ripening by transformation with sense or antisense chimeric 1-aminocyclopropane-1-carboxylate synthase gene. Acta Hort. 1995;394:213–218. [Google Scholar]
- Nakano R, Ogura E, Kubo Y, Inaba A. Ethylene biosynthesis in detached young persimmon fruit is initiated in calyx and modulated by water loss from the fruit. Plant Physiol. 2003;131:276–286. doi: 10.1104/pp.010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuka A, Murachi S, Okunishi H, Shiomi S, Nakano R, Kubo Y, Inaba A. Differential expression and internal feedback regulation of l-aminocyclopropane-l-carboxylate synthase, 1-aminocyclopropane-l-carboxylate oxidase and ethylene receptor genes in tomato fruit during development and ripening. Plant Physiol. 1998;118:1295–1305. doi: 10.1104/pp.118.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T. Effects of ethylene and 1-MCP on ripening and senescence of European seedless cucumbers. Postharvest Biol Technol. 2005;36:113–125. doi: 10.1016/j.postharvbio.2004.11.008. [DOI] [Google Scholar]
- Noichinda S. Effect of modified atmosphere on quality and storage-life of mangosteen (Garcinia mangostana L.) fruit M.Sc. Thesis (In Thai) Bangkok: Kasetsart Univ; 1992. p. 74. [Google Scholar]
- Noichinda S. Respiration rate and ethylene production of lime fruit. National J King Mongkut’s Inst Tech (North Bangkok) 2000;7:27–31. [Google Scholar]
- O’Hare TJ. Postharvest physiology and storage of rambutan. Postharvest Biol Technol. 1995;6:189–199. doi: 10.1016/0925-5214(95)00022-X. [DOI] [Google Scholar]
- Obando J, Miranda C, Jowkar MM, Moreno E, Souri MK, Martinez IA, Arus P, Garcia-Mas J, Monforte AJ, Fernandez-Trujillo JP. Creating climacteric melon fruit from non-climacteric parentals: postharvest quality implications. In: Ramina A, Chang J, Giovannoni J, Klee H, Perata P, Woltering E, editors. Advances in plant ethylene research. Proceedings of the 7th International Symposium on Plant Hormone Ethylene. Dordrecht: Kluwer; 2007. pp. 197–205. [Google Scholar]
- Obando-Ulloa JM, Jowkar MM, Moreno E, Souri MK, Dos-Santos N, Sanmartin P, Bueso MC, Kessler M, Martinez JA, Alarcon A, Nicolai B, Lammertyn J, Garcia-Mas J, Monforte AJ, Fernandez-Trujillo JP. Near-isogenic lines of melon with different climacteric behavior as a tool to characterize fruit senescence trait. In: Pitrat M, editor. Proceeding of the IXth EUCARPIA meeting on Genetics and Breeding of Cucurbitaceae. Avignon (France): INRA; 2008a. pp. 109–113. [Google Scholar]
- Obando-Ulloa JM, Moreno E, Garcia-Mas J, Nicolai B, Lammertyn J, Monforte AJ, Fernandez-Trujillo JP. Climacteric or non-climacteric behavior in melon fruit 1. Aroma volatiles. Postharvest Biol. Technol. 2008b;49:27–37. doi: 10.1016/j.postharvbio.2007.11.004. [DOI] [Google Scholar]
- Obando-Ulloa JM, Nicolai B, Lammertyn J, Bueso MC, Monforte AJ, Fernandez-Trujillo JP. Aroma volatiles associated with the senescence of climacteric or non-climacteric melon fruit. Postharvest Biol Technol. 2009;52:146–155. doi: 10.1016/j.postharvbio.2008.11.007. [DOI] [Google Scholar]
- Oetiker JH, Yang SF. The role of ethylene in fruit ripening. Acta Hort. 1995;398:167–178. [Google Scholar]
- Oetiker JH, Olson DC, Shiu OY, Yang SF. Differential induction of seven 1-aminocyclopropane-1-carboxylate synthase genes by elicitor in suspension cultures of tomato (Lycopersicon esculentum) Plant Mol Biol. 1997;34:275–286. doi: 10.1023/A:1005800511372. [DOI] [PubMed] [Google Scholar]
- Oslund CR, Davenport TL. Ethylene and carbon dioxide in ripening fruit of Averrhoa carambola. HortSci. 1983;18:229–230. [Google Scholar]
- Palapol Y, Ketsa S, Stevenson D, Cooney JM, Allan AC, Ferguson IB. Colour development and quality of mangosteen (Garcinia mangostana L.) fruit during ripening and after harvest. Postharvest Biol Technol. 2009;51:349–353. doi: 10.1016/j.postharvbio.2008.08.003. [DOI] [Google Scholar]
- Pandey M, Zeng Y, Prasad NK, Srivastava GC. Alternate respiration during ripening of tomato fruits. Indian J Plant Physiol. 1995;38:182–183. [Google Scholar]
- Pandey M, Srivastava GC, Prasad NK. Physiological changes associated with ripening of two mango varieties. Indian J Plant Physiol. 1998;3:94–96. [Google Scholar]
- Paul V, Srivastava GC. Role of surface morphology in determining the ripening behaviour of tomato (Lycopersicon esculentum Mill.) fruits. Sci Hort. 2006;110:84–92. doi: 10.1016/j.scienta.2006.06.023. [DOI] [Google Scholar]
- Paul V, Pandey R (2011) Fruit ripening and storability: role of fruit’s internal gaseous microenvironment – a review. J Food Sci Technol (Communicated) [DOI] [PMC free article] [PubMed]
- Paul V, Malik SK, Srivastava GC (2004) Ripening behaviour in relation to anatomy of mango (Mangifera indica L.) fruits. In: Book of abstracts, National Seminar on Physiological Basis of Improving Agricultural, Horticultural and Medicinal Plant Productivity. 27–29 Dec., 2004 at Department of Botany, University of Pune, Maharashtra (India) p 74
- Paul V, Srivastava GC, Singh VP. Changes in electrolyte efflux pattern in detached and attached tomato fruits in slow and fast ripening varieties. Indian J Plant Physiol. 2005;10:25–31. [Google Scholar]
- Paul V, Malik SK, Srivastava GC. Intervarietal differences in the surface morphology and anatomy of mango (Mangifera indica L.) fruits. Phytomorphol. 2007;57:211–220. [Google Scholar]
- Paul V, Pandey R, Srivastava GC (2010a) Ripening of tomato (Solanum lycopersicum L.). Part II: Regulation by its stem scar region. J Food Sci Technol 47:527–533 [DOI] [PMC free article] [PubMed]
- Paul V, Pandey R, Srivastava GC (2010b) Ripening of tomato (Solanum lycopersicum L.). Part I: 1- Methylcyclopropene mediated delay at higher storage temperature. J Food Sci Technol 47:519–526 [DOI] [PMC free article] [PubMed]
- Pech JC, Bouzayen M, Latche A. Climacteric fruit ripening: Ethylene-dependent and independent regulation of ripening pathways in melon fruit. Plant Sci. 2008;175:114–120. doi: 10.1016/j.plantsci.2008.01.003. [DOI] [Google Scholar]
- Perin C, Gomez-Jimenez M, Hagen L, Dogiment C, Pech JC, Latche A, Pitral M, Lelievre JM. Molecular and genetic characterization of a non-climacteric phenotype in melon reveals two loci conferring altered ethylene response in fruit. Plant Physiol. 2002;129:300–309. doi: 10.1104/pp.010613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins-Veazie PM, Huber DJ, Brecht JK. In vitro growth and ripening of strawberry fruit in the presence of ACC, STS or propylene. Ann App Biol. 1996;128:105–116. doi: 10.1111/j.1744-7348.1996.tb07094.x. [DOI] [Google Scholar]
- Pesis E. The role of the anaerobic metabolites, acetaldehyde and ethanol in fruit ripening, enhancement of fruit quality and fruit deterioration. Postharvest Biol Technol. 2005;37:1–19. doi: 10.1016/j.postharvbio.2005.03.001. [DOI] [Google Scholar]
- Pesis E. Postharvest treatments prior to storage with anaerobiosis or anaerobic metabolites to improve fruit quality. In: Advances in postharvest technology for horticultural crops, Trivandrum, India, Research Signpost; 2006. pp. 251–274. [Google Scholar]
- Potuschak T, Lechner E, Parmentier Y, Yanagisawa S, Grava S, Koncz C, Genschik P. EIN3-dependent regulation of plant ethylene hormone signaling by two Arabidopsis F box proteins: EBF1 and EBF2. Cell. 2003;115:679–689. doi: 10.1016/S0092-8674(03)00968-1. [DOI] [PubMed] [Google Scholar]
- Prasad NK, Srivastava GC, Pandey M. Studies on mango fruit ripening with reference to superoxide dismutase and polygactouronase enzyme activity under different storage conditions. J Plant Biol. 1999;26:161–164. [Google Scholar]
- Pratt HK, Goeschl JD, Martin FW. Fruit growth and development, ripening and role of ethylene in the ‘Honey Dew’ muskmelon. J Am Soc Hort Sci. 1977;102:203–210. [Google Scholar]
- Purvis AC, Barmore CR. Involvement of ethylene in chlorophyll degradation in peel of citrus fruits. Plant Physiol. 1981;68:854–856. doi: 10.1104/pp.68.4.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy YV, Srivastava GC. Malic enzyme and cyanide resistant respiration in mango during ripening. Indian J Plant Physiol. 1998;3:185–187. [Google Scholar]
- Reddy YV, Srivastava GC. Ethylene biosynthesis and respiration in mango fruits during ripening. Indian J Plant Physiol. 1999;4:32–35. [Google Scholar]
- Reddy YV, Srivastava GC. Regulation of cell wall softening enzymes during ripening of mango fruits. Indian J Plant Physiol. 1999;4:194–196. [Google Scholar]
- Reddy YV, Srivastava GC. Ethylene biosynhesis and respiration during ripening of mango cultivars. Indian J Plant Physiol. 2001;6:361–364. [Google Scholar]
- Reid MS, Pratt HK. Ethylene and respiratory climacteric. Nature. 1970;226:976–977. doi: 10.1038/226976b0. [DOI] [PubMed] [Google Scholar]
- Reyes MU, Paull RE. Effect of storage temperature and ethylene treatment on guava (Psidium guajava L.) fruit ripening. Postharvest Biol Technol. 1995;6:357–365. doi: 10.1016/0925-5214(95)00007-S. [DOI] [Google Scholar]
- Rogiers SY, Knowles NR. A comparison of preharvest and postharvest ethylene production and respiration rates of saskatoon (Amelanchier alnifolia Nutt.) fruit during development. Can J Bot. 1999;77:323–332. [Google Scholar]
- Rottmann WH, Peter GF, Oeller PW, Keller JA, Shen NF, Nagy BP, Taylor LP, Campbell AD, Theologies A. 1 Aminocyclopropane-1-carboxylate synthase in tomato is encoded by a multigene family whose transcription is induced during fruit and floral senescence. J Mol Biol. 1991;222:937–962. doi: 10.1016/0022-2836(91)90587-V. [DOI] [PubMed] [Google Scholar]
- Rupasinghe HPV, Murr DP, Paliyath G, Skog L. Inhibitory effect of 1-MCP on ripening and superficial scald development in ‘McIntosh’ and ‘Delicious’ apples. J Hort Sci Biotechnol. 2000;75:271–276. [Google Scholar]
- Saltveit ME. Carbon dioxide, ethylene and color development in ripening mature green bell peppers. J Am Soc Hort Sci. 1977;102:523–525. [Google Scholar]
- Saltveit ME., Jr Internal carbon dioxide and ethylene levels in ripening tomato fruit attached to or detached from plant. Physiol Plant. 1993;89:204–210. doi: 10.1111/j.1399-3054.1993.tb01807.x. [DOI] [Google Scholar]
- Saltveit ME. Effect of ethylene on quality of fresh fruit and vegetables. Postharvest Biol Technol. 1999;15:279–292. doi: 10.1016/S0925-5214(98)00091-X. [DOI] [Google Scholar]
- Saltveit ME, Jr, McFeeters RF. Polygalacturonase activity and ethylene synthesis during cucumber fruit development and maturation. Plant Physiol. 1980;66:1019–1023. doi: 10.1104/pp.66.6.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salunkhe DK, Bolin HR, Reddy HR. Storage, processing and nutritional quality of fruits and vegetables. Fresh fruits and vegetables: CRC Press, Boca Raton; 1991. [Google Scholar]
- Sawamura M, Knegt E, Bruinsma J. Levels of endogenous ethylene, carbon dioxide, and soluble pectin and activities of pectin methylesterase and polygalacturonase in ripening tomato fruits. Plant Cell Physiol. 1978;19:1061–1069. [Google Scholar]
- Sfakiotakis EM, Dilley DP. Internal ethylene concentrations in apple fruits attached to or detached from the tree. J Am Soc Hort Sci. 1973;98:501–503. [Google Scholar]
- Shellie KC, Saltveit ME., Jr The lack of a respiratory rise in muskmelon fruit ripening on the plant challenges the definition of climacteric behaviour. J Exp Bot. 1993;44:1403–1406. doi: 10.1093/jxb/44.8.1403. [DOI] [Google Scholar]
- Shi JX, Goldschmidt EE, Goren R, Porat R. Molecular, biochemical and anatomical factors governing ethanol fermentation metabolism and accumulation of off-flavours in mandarins and grapefruit. Postharvest Biol Technol. 2007;46:242–251. doi: 10.1016/j.postharvbio.2007.05.009. [DOI] [Google Scholar]
- Shiomi S, Wamocho LS, Agong SG (1996) Ripening characteristics of purple passion fruit on and off the vine. Postharvest Biol Technol 7:161–170
- Shiomi S, Yamamoto M, Nakamura R, Inaba A. Expression of ACC synthase and ACC oxidase genes in melons harvested at different stage of maturity. J Jpn Soc Hort Sci. 1999;68:10–17. doi: 10.2503/jjshs.68.10. [DOI] [Google Scholar]
- Simons DH. Possible relationships of ethylene to tomato fruit development and ripening. Ph.D: Dissertation, University of California, Davis; 1970. [Google Scholar]
- Sisler EC, Yang SF. Antiethylene effect of cis-2-butene and cyclic olefine. Phytochemistry. 1984;23:2765–2768. doi: 10.1016/0031-9422(84)83011-3. [DOI] [Google Scholar]
- Sisler EC, Serek M. Inhibitors of ethylene responses in plants at receptor level: Recent developments. Physiol Plant. 1997;100:577–582. doi: 10.1111/j.1399-3054.1997.tb03063.x. [DOI] [Google Scholar]
- Sisler EC, Grichko VP, Serek M. Interaction of ethylene and other compounds with the ethylene receptor: Agonists and antagonists. In: Khan NA, editor. Ethylene action in plants. Berlin, Heidelberg: Springer Verlag; 2006. pp. 1–34. [Google Scholar]
- Sitrit Y, Riov J, Blumenfeld A. Regulation of ethylene biosynthesis in avocado fruit during ripening. Plant Physiol. 1986;81:130–135. doi: 10.1104/pp.81.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR. Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev. 1998;12:3703–3714. doi: 10.1101/gad.12.23.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomos T. Principles of gas exchange in bulky plant tissue. HortSci. 1987;22:766–771. [Google Scholar]
- Solomos T, Kanellis AK. Hypoxia and fruit ripening. In: Kanellis AK, Chang C, Kende H, Grierson D, editors. Biology and biotechnology of plant hormone ethylene. Dordrecht, The Netherlands: Kluwer; 1997. pp. 239–252. [Google Scholar]
- Speirs J, Lee E, Holt K, Kim YD, Scott NS, Loveys B, Schuch W. Genetic manipulation of alcohol dehydrogenase levels in ripening tomato fruit affects the balance of some flavour aldehydes and alcohols. Plant Physiol. 1998;117:1047–1058. doi: 10.1104/pp.117.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava GC. Physiological and biochemical basis of ripening and senescence. In: Manual, Post harvest management of fruits, vegetables and flowers. ICAR Sponsored Programme; 1999. pp. 30–33. [Google Scholar]
- Stepanova AN, Alonso JM. Ethylene signaling and response pathway a unique signaling cascade with a multitude of inputs and outputs. Physiol Plant. 2005;123:195–206. doi: 10.1111/j.1399-3054.2005.00447.x. [DOI] [Google Scholar]
- Stewart I, Wheaton TA. Carotenoids in citrus—their accumulation induced by ethylene. J Agric Food Chem. 1972;20:448–449. doi: 10.1021/jf60180a024. [DOI] [Google Scholar]
- Suslow TV, Cantwell M, Mitchell J (2010) Honeydew melon—Recommendations for maintaining postharvest quality. Available at http://postharvest.ucdavis.edu/Produce/ProduceFacts/Fruit/honeydew.shtml
- Tadesse T, Nichols MA, Hewett EW. Ripening of attached and detached sweet pepper fruit cv. ‘Domino’. Acta Hort. 1998;464:503. [Google Scholar]
- Tesniere C, Pradal M, El-Kereamy A, Torregrosa L, Chatelet P, Roustan JP, Chervin C. Involvement of ethylene signaling in a non-climacteric fruit: new elements regarding the regulation of ADH expression in grapevine. J Exp Bot. 2004;55:2235–2240. doi: 10.1093/jxb/erh244. [DOI] [PubMed] [Google Scholar]
- Theologis A. One rotten apple spoils the whole bushel: The role of ethylene in fruit ripening. Cell. 1992;70:181–184. doi: 10.1016/0092-8674(92)90093-R. [DOI] [PubMed] [Google Scholar]
- Theologis A, Zarembinski TI, Oeller PW, Liang X, Abel S. Modification of fruit ripening by suppressing gene expression. Plant Physiol. 1992;100:549–551. doi: 10.1104/pp.100.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieman DM, Ciardi JA, Taylor MG, Klee HJ. Members of the tomato LeEIL (EIN3-like) gene family are functionally redundant and regulate ethylene responses throughout plant development. Plant J. 2001;26:47–58. doi: 10.1046/j.1365-313x.2001.01006.x. [DOI] [PubMed] [Google Scholar]
- Tingwa PO, Young RE. Studies on the inhibition of ripening in attached avocado (Persea Americana Mill.) fruits. J Am Soc Hort Sci. 1975;100:447–449. [Google Scholar]
- Trainotti L, Pavanello A, Casadoro G. Different ethylene receptors show an increased expression during the ripening of strawberries: does such an increment imply a role for ethylene in the ripening of these non-climacteric fruits? J Exp Bot. 2005;56:2037–2046. doi: 10.1093/jxb/eri202. [DOI] [PubMed] [Google Scholar]
- Trainotti L, Tadiello A, Casadoro G (2007) Variations of the peach fruit transcriptome during ripening and in response to hormone treatments. Caryologia 60:156–159
- Tucker GA. Introduction. In: Seymour G, Talor J, Tucker G, editors. Biochemistry of fruit ripening. London: Chapman & Hall; 1993. pp. 1–51. [Google Scholar]
- Van-der-Hoeven R, Ronning C, Giovannoni J, Martin G, Tanksley S. Deductions about the number, organization, and evolution of genes in the tomato genome based on analysis of a large expressed sequence tag collection and selective genomic sequencing. Plant Cell. 2002;14:1441–1456. doi: 10.1105/tpc.010478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoquaux P, Ozdemir IS. Packaging and produce degradation. In: Lamikanra O, Imam S, Ukuku D, editors. Produce degradation pathways and prevention. Boca Raton: CRC Press; 2005. pp. 117–153. [Google Scholar]
- Verboven P, Kerckhofs G, Mebatsion HK, Ho QT, Temst K, Wevers M, Cloetens P, Nicolai BM. Three-dimensional gas exchange pathways in pome fruit characterized by synchrotron X-ray computed tomography. Plant Physiol. 2008;147:518–527. doi: 10.1104/pp.108.118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalta AM, Sargent SA. Response of beit alpha-type cucumbers (Cucumis sativus L., ‘Manar’) to continuous ethylene exposure. Proc Fla State Hort Soc. 2004;117:368–372. [Google Scholar]
- Villavicencio LE, Blankenship SM, Sanders DC, Swallow WH. Ethylene and carbon dioxide production in detached fruit of selected pepper cultivars. J Am Soc Hort Sci. 1999;124:402–406. [Google Scholar]
- Villavicencio LE, Blankenship SM, Sanders DC, Swallow WH. Ethylene and carbon dioxide concentrations in attached fruits of pepper cultivars during ripening. Sci Hort. 2001;91:17–24. doi: 10.1016/S0304-4238(01)00249-7. [DOI] [Google Scholar]
- Weaver RJ, Singh IS. Occurrence of endogenous ethylene and effect of plant regulators on ethylene production in grapevine. Am J Enol Vitic. 1978;29:282–285. [Google Scholar]
- Wechter WP, Levi A, Harris KR, Davis AR, Fei Z, Katzir N, Giovannoni JJ, Salman-Minkov A, Hernandez A, Thimmapuram J, Tadmor Y, Portnoy V, Trebitsh T. Gene expression in developing watermelon fruit. BMC Genomics. 2008;9:275. doi: 10.1186/1471-2164-9-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills RBH, McGlasson WB, Graham D, Joyce DC (2007) Physiology and biochemistry. In: Postharvest: An introduction to physiology and handling of fruits, vegetables and ornamentals, 5th edn. UK: UNSW Press, CAB International p 29
- Xu ZC, Ikoma Y, Yano M, Ogawa K, Hyodo H. Varietal differences in the potential to produce ethylene and gene expression of ACC-synthase and ACC-oxidase between ‘Kuimi’ and ‘Hong Xin’ of Chinese kiwifruit. J Jpn Soc Hort Sci. 1998;67:204–209. doi: 10.2503/jjshs.67.204. [DOI] [Google Scholar]
- Yamane M, Abe D, Yasui S, Yokotani N, Kimata W, Ushijima K, Nakano R, Kubo Y, Inaba A. Differential expression of ethylene biosynthetic genes in climacteric and non-climacteric Chinese pear fruit. Postharvest Biol Technol. 2007;44:220–227. doi: 10.1016/j.postharvbio.2006.12.010. [DOI] [Google Scholar]
- Yang SF. The role of ethylene and ethylene synthesis in fruit ripening. In: Thompson W, Nothnagel E, Huffaker R, editors. Plant senescence: its biochemistry and physiology. Rockville: The American Society of Plant Physiologists; 1987. pp. 156–165. [Google Scholar]
- Yang SF. The role of ethylene in fruit ripening. Acta Hort. 1995;398:167–178. [Google Scholar]
- Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol. 1984;35:155–189. doi: 10.1146/annurev.pp.35.060184.001103. [DOI] [Google Scholar]
- Yokotani N, Tamura S, Nakano R, Inaba A, Kubo Y. Characterization of a novel EIN3-like gene (LeEIL4) J Exp Bot. 2003;54:2775–2776. doi: 10.1093/jxb/erg308. [DOI] [PubMed] [Google Scholar]
- Yokotani N, Nakano R, Imanishi S, Nagata M, Inaba A, Kubo Y. Ripening-associated ethylene biosynthesis in tomato fruit is autocatalytically and developmentally regulated. J Exp Bot. 2009;60:3433–3442. doi: 10.1093/jxb/erp185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S-D, Cho Y, Sheen J. Emerging connections in the ethylene signaling network. Trends Plant Sci. 2009;14:270–279. doi: 10.1016/j.tplants.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagory D, Kader AA. Modified atmosphere packaging of fresh produce. Food Technol. 1988;42:70–77. [Google Scholar]
- Zarem-binski TI, Theologis A. Ethylene biosynthesis and action: A case of conservation. Plant Mol Biol. 1994;26:1579–15970. doi: 10.1007/BF00016491. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Pandey M, Prasad NK Srivastava GC. Ripening associated changes in enzymes and respiratory activities in three varieties of mango (Mangifera indica L.) Indian J Plant Physiol. 1995;38:73–76. [Google Scholar]
- Zeng Y, Pandey M, Prasad NK, Srivastava GC. Hydrolysing enzymes and respiration during ripening of tomato (Lycopersicon esculentum) fruits. Curr Sci. 1996;70:1017–1018. [Google Scholar]
- Zhang Z, Huber DJ, Hurr BM, Rao J. Delay of tomato fruit ripening in response to 1-methylcyclopropene is influenced by internal ethylene levels. Postharvest Biol Technol. 2009;54:1–8. doi: 10.1016/j.postharvbio.2009.06.003. [DOI] [Google Scholar]
- Zheng XY, Wolff DW. Ethylene production, shelf-life and evidence of RFLP polymorphisms linked to ethylene genes in melon (Cucumis melo L.) Theor Appl Genet. 2000;101:613–624. doi: 10.1007/s001220051523. [DOI] [Google Scholar]