Abstract
Perilla Frutescens is a traditional Chinese medicinal herb. Microwave-assisted extraction (MAE) technique was developed for the fast extraction of flavonoids from Perilla Frutescens leaves. Several influential parameters of the MAE procedure (microwave power, extraction cycles, solvent to material ratio, irradiation time and pH value) were investigated for the optimization of the extraction using single factor and Box-Behnken experimental design. Response surface methodology analysis showed good correspondence between experimental and predicted values. It was found that the most effective parameter was solvent to material ratio, which was in good agreement with the experimental value. The adjusted coefficient of determination (R2adj) for the model was 93.45%. Probability value (P < 0.001) demonstrated a very high significance for the regression model. The maximum yield of flavonoids with MAE was obtained by dual extraction with solvent to material ratio of 1: 16.5, irradiation time of 23 min, and pH value of 8.4 at microwave power of 600 W. The optimal yield with MAE 6.07 mg/g were slightly lower than that of Soxhlet extracted and higher than that of heat reflux extraction with water. The extracts exhibited high scavenging effects on DPPH radical. With increasing concentration between 0.10 and 0.80 mg/mL, the scavenging rate achieved 62.3%.
Keyword: Perilla Frutescens, Flavonoid, Microwave-assisted extraction, Box-Behnken design, Scavenging effect, DPPH radical
Introduction
Perilla frutescens is an annual herb of Lamiaceae with multiple important usefulness and is mainly distributed in Japan, Korea, and China (Longvah and Deosthale. 1998). The leaves of P. frutescens are a traditional Chinese medicinal herb, have been used for centuries to treat various diseases including depression, tumor, bacterial and fungal infections, allergy and some intestinal disorders. The main active constituents of P frutescens leaves include flavonoids, saponins, polysaccharides, aminoacids, and trace elements (Chen et al. 2003; Zhang 2007). Recent studies reveal that the flavonoids of P frutescens show strong antioxidant activity and pharmacological properties.
Several techniques are available for the extraction of flavonoids including Soxhlet, ultrasound assisted extraction and heat reflux extraction (Kamaljit, et al. 2008; Charalampos and Michael 2008). Methanol was often used as extraction solvent. The use of large amounts of organic hazardous solvents in sample preparation and the increasing cost of solvent waste disposal have created a growing demand for better and more environment friendly extraction methods (Ho et al. 2008). Water is an interesting alternative to the usual solvents, particularly in view of its low cost, polarity and non-toxic character. The use of water as an extraction solvent for phenolic compounds has been proposed.
Microwave energy is a non-ionizing radiation that causes molecular motion by migration of ions and rotation of dipoles (Chen et al. 2008; Gu et al. 2008). Many reports have been published on the application of microwave-assited extraction( MAE ) of secondary metabolites from plants (Chattip et al. 2008; Guo et al. 2001; Chen et al. 2007). Nevertheless, few publications on flavonoid extraction from P frutescens by MAE are available till now.The main advantages of MAE are the considerable reduction in time and solvent as compared to conventional techniques.
Response surface methodology is a statistical method, which uses quantitative data from an appropriate experimental design to determine or simultaneously solve multivariate equations (Jiang et al. 2006). In this study, MAE was employed to extract flavonoids from P frutescens. The effects of microwave power, extraction cycles, irradiation time, solvent to material ratio and pH value on flavonoid yield were investigated. Response surface methodology was used to build a model between the flavonoid yield and these independent factors, and to optimise the extraction conditions.
Materials and methods
Materials and reagents
Perilla frutescens were bought from Hangzhou Tong Ren Tang Group Co., Ltd., in May 2008. The cut pieces were ground with a blade-mill to obtain a relatively homogenous drug powder and then sieved through 10-mesh screen. The powder was dried at 50 °C until constant weight and was well blended before use.
Rutin was purchased from Sigma Chemical Company (St. Louis, MO). All other chemicals were of analytical grade.
MAE extraction method
The process of MAE was performed in a experimental microwave equipment (Model NJL07-3, Jiequan microwave equipment Co., Ltd., China). 40 g of drug powder of Perilla frutescens leaves varied according to the water to material ratio were put into extraction vessel under different MAE conditions. After extraction, the vessels were allowed to cool at room temperature before opening. Then, extarcts were obtained by vaccum drying.
Several influential parameters of the MAE procedure (microwave power, extraction cycles, water to material ratio, irradiation time and pH value) were investigated for the optimization of the extraction using single factor and Box-Behnken experimental disign.
Experimental design
Firstly, the single factor experiment for MAE was performed with the analysis of the effects of two factors (microwave power and extraction cycles) on flavonoids yield from Perilla frutescens leaves. Secondly, the optimization of extraction process parameters of water to solid ratio, irradiation time and pH value was performed using Box-Behnken design and a model was developed. The pH selection range is from 7 to 9 in the study in view of the alcaline flavonoids in Perilla Frutescens Leaves. The higher pH value of the extraction solution can promote the oxidation of phenolics. Finally, the model validation and comparement with Soxhlet and heat flux extraction on flavonoids yield were carried out.
Box-behnken design
A Box-Behnken design was employed to study the response Y, namely flavonoids yield (Wu et al. 2007). The independent variables were X1, X2, and X3 representing water-solid ratio, duration and pH value, respectively. Three repeated experiments at the center (0, 0, 0) of the design were performed to allow the estimation of the pure error. The experimental design was shown in Table 1. All experiments were carried out in a randomized order to minimize the effect of unexpected variability in the observed response due to extraneous factors.
Table 1.
Independent variables and their levels for Box-Behnken design
| Independent variables | Codes | Variable levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Solvent to material ratio(ml/g) | X1 | 1:10 | 1:14 | 1:18 |
| Irradiation time(min) | X2 | 10 | 20 | 30 |
| pH value | X3 | 7 | 8 | 9 |
As for the optimization for flavonoids yield , the responses were analyzed using Matlab software (Matlab 7.0, Beijing, China) (Olivieri et al. 2004). A quadratic polynomial regression model was assumed for predicting response. The model proposed for each response of Y was
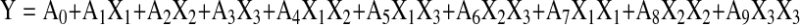 |
1 |
Where A0 is constant; A1, A2, and A3 are linear coefficients; A4, A5, and A6 are cross-product coefficients; A7, A8, and A9 are quadratic coefficients.
Determination of concentrations of flavonoids
An aliquot of 6 mL flavonoids solution was put into a 25 mL flask, and then 1 mL 5 wt% NaNO2 was added in. After 6 min, 1 mL 10 wt% Al(NO3)3 was added and after another 6 min, 10 mL 5 wt% KOH was added. After mixing, water was added to the flask to make to the volume. The solution was allowed to stand for 15 min at room temperature, and the absorbance at 500 nm was determined with a UV1600 spectrophotometer (Beijing Ruili Instrument Co., Beijing). Flavonoids concentration was calculated using rutin as the calibration standard. A good linear relationship was obtained over the range of 0.013–0.104 mg/mL, and the regression equation is y = 0.9518x + 0.0311(correlation coefficient R2 = 0.9976 ) where y is the absorbance at 500 nm and x is the concentration of flavonoids (mg/mL).
Free radical scavenging activity on DPPH
The DPPH(purchased from Sigma Corp.) radical scavenging test was carried out following the method used in reference (Armata et al. 2008). In brief, 2 mL of 0.2 mmol/L ethanolic solution of DPPH ( freshly prepared daily) was mixed with 2 mL of various dilutions of the crude flavoinds extract. The mixture was deposited in the dark at room temperature for 30 min. And the decrease of absorbance at 515 nm was measured. The scavenging rate was calculated as follows: [1-(AS-A0)/A], where AS is the OD value of flavoinds extract ;A0 is the OD value of the blank sample(without DPPH); A is the OD value of the control(without flavoinds extract).
Soxhlet extraction was performed in a Soxhlet apparatuswith methanol. 20.0 g drug powder was placed in an extraction bag filter and performed at 85°C for about 4 h with 360 ml methanol.
Statistical analysis
All trials were carried out in triplicate and the averages of flavonoids yields were taken as responsive value. Analysis of variance (ANOVA) were used to determine the significant difference in flavonoids yield extracted from P frutescens under different conditions. A second-order polynomial regressed equation was established on the basis of analysis of Box-Behnken experimental data, and the optimal conditions for extraction were found using the software of Matlab 7.0 version.
Results and discussion
Effect of microwave power
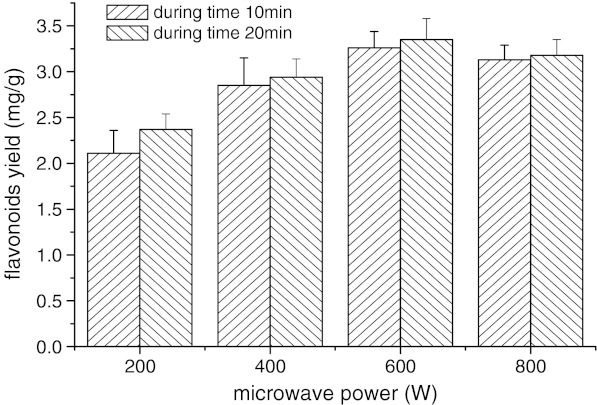
An amount of 40.0 g was extracted under different microwave powers (200, 400, 600, 800 W) by dual extraction (see Fig. 1). Generally, the higher exaction temperature is profitable for the extraction and reduces the reaction time. Temperature of the extraction medium increased with increasing microwave forward power (Xiao et al. 2008).
Fig. 1.
Effects of microwave power on flavonoids yield during different duration time
In general, the extraction efficiency was improved by raising microwave power from 200 to 800 W. During irradiation time (10 and 20 min), flavonoids yield was enhanced with microwave power increasing. The difference of the flavonoids yield among 200 to 800 W appears more significant with short irradiation times compared to long irradiation times. The accelerated extraction of flavonoids by increasing microwave power is related to the direct effects of microwave energy on biomolecules by ionic conduction and dipole rotation which result in power dissipated inside the solvent and plant material and then generate molecular movement and heating (Vadivambal and Jayas 2007; Gentry and Roberts 2005). More electromagnetic energy was transferred to the extraction system quickly and improved extraction efficiency when the microwave power increased from 200 to 800 W. Therefore, microwave power of 600 W was used in the following experiments.
Effect of extraction cycles
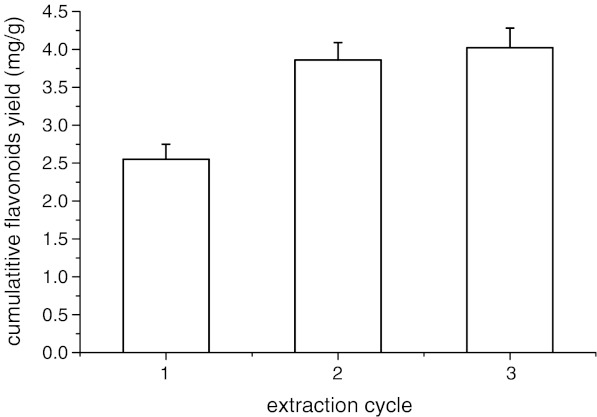
The effect of extraction cycle was investigated in this experiment (see Fig. 2). An amount of 40.0 g drug powder was extracted for 20 min under solvent to material ratio 20 ml/g . The residue was taken back and re-extracted three times under the above-mentioned conditions. The yield of flavonoids in three cycles was 4.02 mg/g. The yields of two cycles accounted for 96.0% of the yield of three cycles and the third extraction accounted for only 4.0% of the yield of three cycles.
Fig. 2.
Effects of extraction cycle on cumulative flavonoids yield
RSM model fitting
The statistical combination of the independent variables in coded and natural values along with the predicted and experimental response was presented in Table. 2.
Table 2.
Box-Benhen design and experiment data
| Run | X1 | X2 | X3 | Experimental data (mg/g) | Predicted data (mg/g) | Error (%) |
|---|---|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 2.02 | 1.98 | 1.98 |
| 2 | −1 | 1 | 0 | 3.84 | 4.02 | 4.69 |
| 3 | 1 | −1 | 0 | 4.87 | 4.59 | 5.75 |
| 4 | 1 | 1 | 0 | 5.23 | 5.46 | 4.40 |
| 5 | 0 | −1 | −1 | 2.45 | 2.57 | 4.89 |
| 6 | 0 | −1 | 1 | 3.68 | 3.90 | 5.97 |
| 7 | 0 | 1 | −1 | 4.47 | 4.22 | 5.60 |
| 8 | 0 | 1 | 1 | 5.53 | 5.28 | 4.52 |
| 9 | −1 | 0 | −1 | 2.57 | 2.54 | 1.17 |
| 10 | 1 | 0 | −1 | 4.36 | 4.39 | 0.68 |
| 11 | −1 | 0 | 1 | 3.57 | 3.54 | 0.84 |
| 12 | 1 | 0 | 1 | 5.79 | 5.82 | 0.52 |
| 13 | 0 | 0 | 0 | 5.71 | 5.62 | 1.57 |
| 14 | 0 | 0 | 0 | 5.64 | 5.62 | 0.35 |
| 15 | 0 | 0 | 0 | 5.50 | 5.62 | 2.18 |
In order to determine whether or not the quadratic model is significant, it is necessary to conduct ANOVA analysis, in which the regression sum of square was subdivided into two parts that attributed to linear regression and that attributed to the quadratic model. The P-values were used as a tool to check the significance of each coefficient, which also indicated the interaction strength of each parameter. The smaller the p-values are, the bigger the significance of the corresponding coefficient. Here, the p-value of the model was smaller than 0.001, which indicated that the model was suitable for use in this experiment. The P-value of “lack of fit” was 0.063 (P > 0.01), which indicated that “lack of fit” was insignificant relative to the pure error. The coefficient of determination (R2) and adjust coefficient of determination (Adj.R2) were also shown in Table 3. The values indicated that, the accuracy and general availability of the polynomial model were adequate.
Table 3.
Analysis of variance (ANOVA) for the fitted quadratic polynomial model
| Source | Sum of squares | d.f. | Mean square | F-value | Probability(P) |
|---|---|---|---|---|---|
| Model | 22.51 | 9 | 2.50 | 23.18 | <0.001a |
| Linear | 15.92 | 3 | 5.31 | 49.17 | <0.001a |
| Quadratic | 6.13 | 3 | 2.04 | 18.93 | <0.01a |
| Cross product | 0.47 | 3 | 0.16 | 1.44 | 0.34 |
| Lack of fit | 0.52 | 3 | 0.17 | 15.06 | 0.06 |
| Pure error | 0.02 | 2 | 0.01 | ||
| Total | 23.05 | 14 | |||
| R2 = 97.66%, Adj R2 = 93.45% | |||||
aSignificant at 1% level
The regression coefficients and the corresponding p-values were presented in Table 4. From the p-values of each model term, it could be concluded that the regression coefficients of the linear term X1, X2, X3, and all the quadratic terms had significant effect on flavonoids yield. They were all significant at 1% level.
Table 4.
Results of regression analysis of a full second order polynomial model
| Term | Coefficients estimated | p-Value |
|---|---|---|
| Intercept | 5.6167 | |
| X1 | 1.0313 | 0.0003a |
| X2 | 0.7475 | 0.0013a |
| X3 | 0.6063 | 0.0036a |
| X1X2 | −0.3150 | 0.1132 |
| X1X3 | 0.1075 | 0.5417 |
| X2X3 | −0.0450 | 0.6770 |
| X21 | −0.7771 | 0.0060a |
| X22 | −0.8496 | 0.0040a |
| X23 | −0.7671 | 0.0060a |
aSignificant at 1% level
Using the designed experimental data (Table 2), the polynomial model for the flavonoids yield was regressed and shown as below (in term of coded factors):
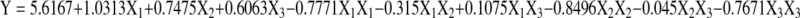 |
2 |
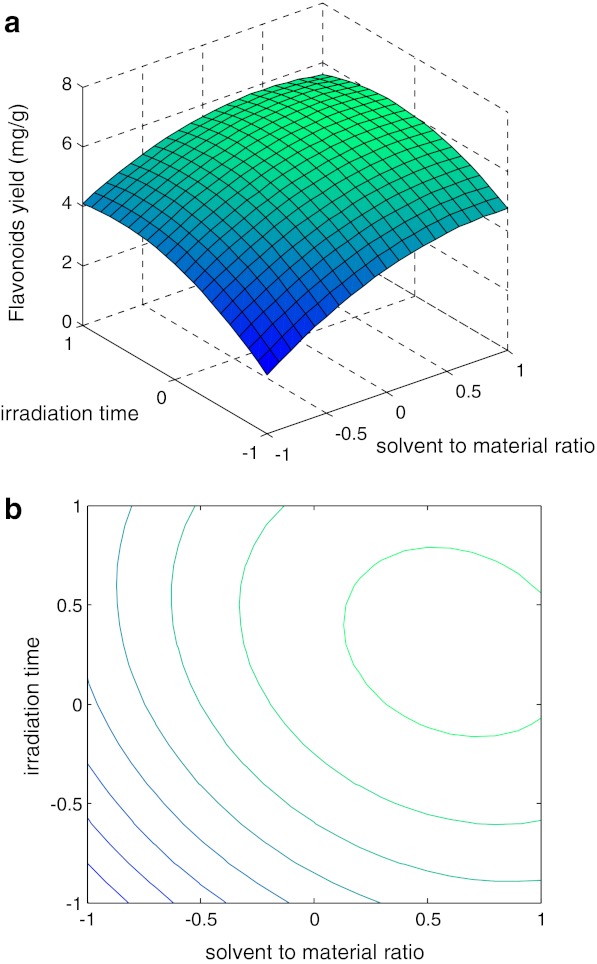
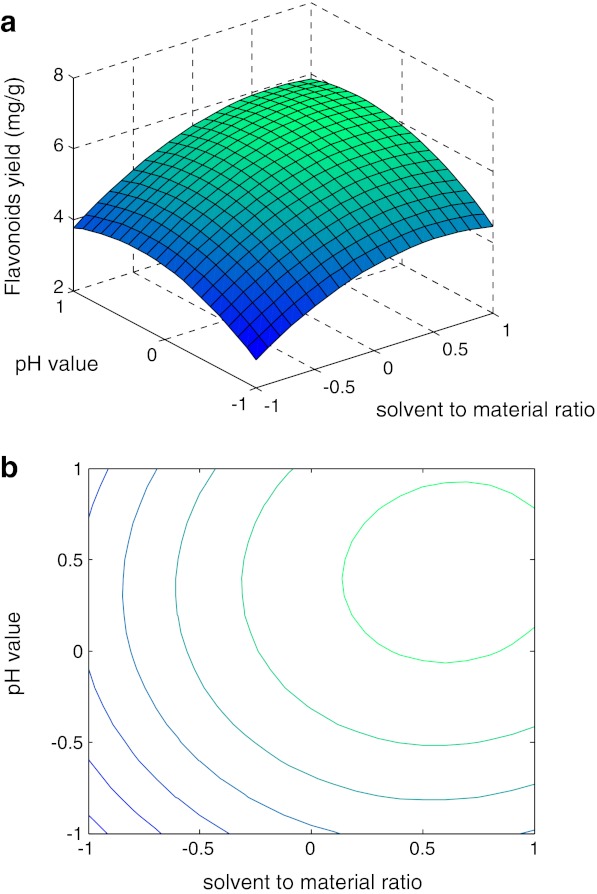
Many parameters can influence the performance of flavonoids yield from Perilla frutescens. Eq. 2 showed that flavonoids yield has a complex relationship with independent variables that encompass both first and second-order polynomials and may have more than one maximum point. The best way of expressing the effect of any parameter on the yield within the experimental space under investigated was to generate response surface plots of the equation. The effects of irradiation time, solvent to material ratio and pH value on the flavonoid yield of Perilla frutescens , as well as their interactions, are shown in Figs. 3–4. From the shape of contour plots, the significance of the mutual interactions between the independent variables could be estimated. An elliptical profile of the contour plots indicates remarkable interaction between the independent variables.
Fig. 3.
Response surface curve (a) and contour plot (b) showing predicted response surface of flavonoids yield as a function of solvent to material ratio and irradition time (pH value=8)
Fig. 4.
Response surface curve (a) and contour plot (b) showing predicted response surface of flavonoids yield as a function of solvent to material ratio and pH value (irradiation time=25 min)
The plots in Figs. 3–4 showed similar relationships with respect to the effects of each variable. The response obtained were convex nature suggesting that there were well-defined optimum operating conditions. However, the convexity was not high enough, as the surfaces were rather symmetrical and a little flat near the optimum which meant that the response optimized value based on combined effects may not vary widely from the single variable optimized conditions.
Contour plot and response surface curve showing predicted response surface of flavonoids yield as a function of solvent to material ratio and irradiation time was shown in Fig. 3. An increasing solvent to material ratio resulted in a higher extraction yield, while the flavonoid yield reached a maximum when solvent to material ratio amount was up to a certain value, with no significantly further improvement thereafter (Fig. 3). It was indicated that yield was sensitive to irradiation time (Gao et al. 2006; Sheetal and Laxmi 2007). An increase in flavonoids yield was observed with the increasing of irradiation time at first. But the trend was reversed when the ratio reached a certain value. Overexposure in the microwave may cause the loss of flavonoids. Therefore, around 23 min and 1:17 were chosen as the optimal solvent to material ratio and irradiation time for MAE, respectively.
Interaction of solvent to material ratio and pH value on flavonoids yield was presented in Fig. 4. Influence of pH value on yield showed significant variation both above and below the optimum values. With the increase of pH value, we observed an increase at first and decrease subsequently in the amount of yield. The extraction yield of flavonoids increased as the pH values increased from 7 to 8, but it decreased as the pH values higher than 8 were used, which indicated that the extraction pH value significantly affected the extraction yield. The increased extraction yield under the low pH conditions could be due to the inhibition of the enzymatic oxidation of phenolics and/or the maintenance of the extracted flavonoids stability. It is also likely that alkaline soluable flavonoids were extracted. So, pH value of about 8 was therefore suggested. The interaction of irradiation time and pH value on flavonoids yield was studied. With the increase of irradiation time and pH value, we observed an increase at first and decrease subsequently.
Validation of the model
The model was processed by partial differential coefficient operation to this model , and equal to zero. The point of curved surface was determined (X1 = 0.61,X2 = 0.31, X3 = 0.43). According to RSM result, an experiment with solvent to material ratio of 1: 16.5, irradiation time of 23 min, and pH value of 8.4 was conducted in order to investigate the effect of RSM. The experiment was carried out at the optimized conditions. flavonoids yield of 6.07 mg/g was obtained and was in good agreement with the predicted one. The accuracy of the model was validated with triplicate experiments under the aforementioned optimal reaction conditions. As a result, the model was considered to be accurate and reliable for predicting the flavonoids yield .
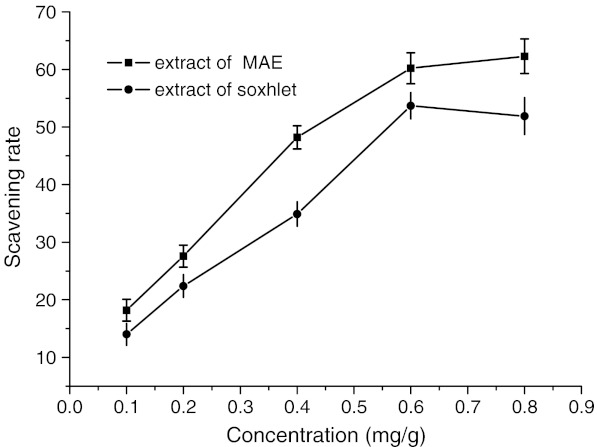
Free radical scavenging activity on DPPH test provides an easy and rapid way to evaluate the antiradical activities of flavonoids. It is a very stable free radical. The DPPH radical exhibits an absorption at 515 nm, but upon reduction by the extract with an antioxidant function, the absorption at 515 nm disappear. The flavonoids could scavenge it effectively as shown in Fig. 5. The extract obtained with MAE had higher antioxidant activity than those of Soxhlet extraction. The lower activity of Soxhlet extract could be resulted from extended extraction time, hence exposure to unfavorable conditions such as light and oxygen. With increasing concentration between 0.10 and 0.80 mg/mL, the scavenging effects of flavonoids extract of MAE increased significantly. When the scavenging rate achieved 62.3%, it lay in a steady state, even if concentration increased.
Fig. 5.
DPPH radical scavenging activity of flavonoids extarct of MAE and Soxhlet method
Comparison of MAE with Soxhlet extraction and heat reflux extraction
Soxhlet extraction was performed in a Soxhlet apparatus. Exhaustive extraction with methanol was performed on 20.0 g drug powder, placed in an extraction bag filter, and impregnated with methanol. Extraction was performed at 85 °C for about 4 h with 360 ml methanol. Heat reflux extraction was conducted in a water bath at 80 °C. An amount of 20.0 g rug powder were placed into a 1000 ml glass flask with 360 ml 85% (v/v) aqueous ethanol and extracted for two 2 h cycles.
It can be seen in Table 5 that the flavonoids yield of Soxhlet method is maximum among the three methods and MAE is the second highest yield method. Though the flavonoids yield was slightly lower than that of Soxhlet, MAE took only one by eighth time of Soxhlet and the extraction solvent (pure water )was much safer than methanol used in Soxhlet extraction. The flavonoid yield of MAE is much higher than that of heat reflux extraction. Therefore, MAE can save a lot of time as compared to Soxhlet and heat reflux method and bring higher yield of flavonoids. It is worth noting that MAE is a good alternative to heat reflux extraction in the practical production of Perilla frutescens falvonoids. This shows that MAE extraction of flavonoids from P has a relatively higher commercial value.
Table 5.
Comparison of MAE with other extraction methods
| Extraction methods | Extraction time | Extraction solvent | Flavonoids yield (mg/g) |
|---|---|---|---|
| Soxhlet | 4 h | methanol | 6.37 |
| Soxhlet | 4 h | water | 2.69 |
| Heat reflux extraction | 2 h*2 | 85% ethanol | 3.15 |
| MAE | 23 min | water | 6.07 |
Stability of flavonoid during MAE
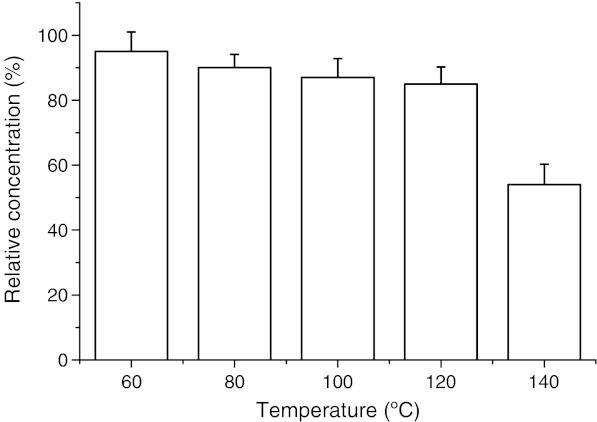
In order to evaluate the performance of different extraction conditions, stability of Perilla frutescens flavonoid was determined. The stability study was performed using the extract obtained by the optimized condition as listed previously.The extract was centrifuged for 15 min, filtered through filter paper and stored at −10 °C. The selected temperature range is between 60 and 140 °C.
The flavonoid stability under extraction conditions for 23 min using different temperatures was investigated. The individual relative concentration of flavonoid in the extract submitted to different temperatures for 23 min was shown in Fig. 6. The values are relative to the initial flavonoid concentration in the MAE extract (100%). The stability of extracted flavonoids was decreased with the increased temperature from 60 to 140 °C. when the temperature was 140 °C, the relative concentration of flavonoids decreasd by 50%.
Fig. 6.
Stability of extracted flavonoids at different temperatures for 28 min
Conclusion
Microwave-assisted extraction (MAE) technique was developed for the fast extraction of flavonoids from Perilla Frutescens leaves. Response surface methodology analysis showed good correspondence between experimental and predicted values.
It was found that the most effective parameter was solvent to material ratio, which was in good agreement with the experimental value. The adjusted coefficient of determination (R2adj) for the model was 93.45%. Probability value (P < 0.001) demonstrated a very high significance for the regression model. The maximum yield of flavonoids with MAE was obtained by dual extraction with solvent to material ratio of 1: 16.5, irradiation time of 23 min, and pH value of 8.4 at microwave power of 600 W. The optimal yield with MAE 6.07 mg/g were slightly lower than that of Soxhlet extracted and higher than that of heat reflux extraction with water. The extracts exhibited high scavenging effects on DPPH radical. With increasing concentration between 0.10 and 0.80 mg/mL, the scavenging rate achieved 62.3%.
Acknowledgment
This work was financially supported by Shizi Co.,LTD of Zhejiang Province. And the authors also gratefully acknowledge the contribution of the following to this work: Shuo Lin and Xin Ma for their valuable advices and helpful comments on this manuscript.
Contributor Information
Ping Shao, Phone: +86-571-88320137, FAX: 86-571-88320345, Email: pingshao325@yahoo.com.cn.
Peicheng Zhao, Phone: +86-571-88320137, FAX: 86-571-88320345, Email: pingshao325@tom.com.
References
- Armata M, Gabrieli C, Termentzi A, Zervou M, Kokkalou E. Constituents of Sideritis syriaca. ssp. syriaca (Lamiaceae) and their antioxidant activity. Food Chem. 2008;111:179–186. doi: 10.1016/j.foodchem.2008.03.061. [DOI] [Google Scholar]
- Charalampos P, Michael K. Application of microwave-assisted extraction to the fast extraction of plant phenolic compounds. LWT. 2008;41(4):652–659. doi: 10.1016/j.lwt.2007.04.013. [DOI] [Google Scholar]
- Chattip P, Wanchai DE, Artiwan S. Extraction of flavonoids and carotenoids from Thai silk waste and antioxidant activity of extracts. Sep Sci Technol. 2008;62:444–448. [Google Scholar]
- Chen JH, Xia ZH, Tan RX. High performance liquid chromatographic analysis of bioactive triterpenes in perilla frutescens. J Pharm Biomed Anal. 2003;32:1175–1179. doi: 10.1016/S0731-7085(03)00160-2. [DOI] [PubMed] [Google Scholar]
- Chen Y, Xie MY, Gong XF. Microwave-assistedextraction usedfor the isolation of total triterpenoid saponins from Ganoderma atrum. J Food Eng. 2007;81:162–170. doi: 10.1016/j.jfoodeng.2006.10.018. [DOI] [Google Scholar]
- Chen LG, Jin HY, Ding L, Zhang HR, et al. Dynamic microwave-assisted extraction of flavonoids from Herba Epimedii. Sep Sci Technol. 2008;59:50–57. [Google Scholar]
- Gao M, Song BZ, Liu CZ. Dynamic microwave-assisted extraction of flavonoids from Saussurea medusa maxim cultured cells. Biochem Eng J. 2006;32:79–83. doi: 10.1016/j.bej.2006.09.004. [DOI] [Google Scholar]
- Gentry TS, Roberts JS. Design and evaluation of a continuous flow microwave pasteurization system for apple cider. LWT. 2005;38(3):227–238. doi: 10.1016/j.lwt.2004.05.016. [DOI] [Google Scholar]
- Gu ZY, Chen DM, Han Y, et al. Optimization of carotenoids extraction from Rhodobacter sphaeroides. LWT. 2008;41:1082–1088. doi: 10.1016/j.lwt.2007.07.005. [DOI] [Google Scholar]
- Guo Z, Jin Q, Fan G, et al. Microwave-assisted extraction of effective constituents from a Chinese herbal medicine Radix puerariae. Anal Chim Acta. 2001;436:41–47. doi: 10.1016/S0003-2670(01)00900-X. [DOI] [Google Scholar]
- Ho CHL, Cacace JE, Mazza G. Mass transfer during pressurized low polarity water extraction of ligans from flaxseed meal. J Food Eng. 2008;89(1):64–71. doi: 10.1016/j.jfoodeng.2008.04.003. [DOI] [Google Scholar]
- Jiang S-T, Shao P, Pan L-J, Zhao Y-Y. Molecular distillation for recovering tocopherol and fatty acid methyl esters from rapeseed oil deodoriser distillate. Biosyst Eng. 2006;93(4):383–391. doi: 10.1016/j.biosystemseng.2006.01.008. [DOI] [Google Scholar]
- Kamaljit V, Raymond M, Lloyd S, Darren B. Application and opportunities for ultrasound assisted extraction in the food industry- A review. Innov Food Sci Emerg Technol. 2008;9(2):161–169. doi: 10.1016/j.ifset.2007.04.014. [DOI] [Google Scholar]
- Longvah T, Deosthale YG. Effect of dehulling, cooking and roasting on the protein quality of Perilla frutescens seed. Food Chem. 1998;63(4):519–523. doi: 10.1016/S0308-8146(98)00030-2. [DOI] [Google Scholar]
- Olivieri AC, Goicoechea HC, Iñón FA. An integrated matlab toolbox for first order multivariate calibration. Chemom Intell Lab Syst. 2004;73:189–197. doi: 10.1016/j.chemolab.2004.03.004. [DOI] [Google Scholar]
- Sheetal MC, Laxmi Enzyme aided extraction of lycopene from tomato tissues. Food Chem. 2007;102(1):77–81. doi: 10.1016/j.foodchem.2006.04.031. [DOI] [Google Scholar]
- Vadivambal R, Jayas DS. Changes in quality of microwave-treated agricultural products-a review. Biosyst Eng. 2007;98(1):1–16. doi: 10.1016/j.biosystemseng.2007.06.006. [DOI] [Google Scholar]
- Wu Y, Cui SW, Tang J, Gu XH. Optimization of extraction process of crude polysaccharides from boat-fruited sterculia seeds by response surface methodology. Food Chem. 2007;105(4):1599–1605. doi: 10.1016/j.foodchem.2007.03.066. [DOI] [Google Scholar]
- Xiao W-H, Han L-J, Shi B. Microwave-assisted extraction of flavonoids from Radix Astragali. Sep Sci Technol. 2008;62:616–620. [Google Scholar]
- Zhang T. Studies on in vitro flowering and fruiting of Perilla frutescens. Agricultural Sciences in China. 2007;6(1):33–37. doi: 10.1016/S1671-2927(07)60014-5. [DOI] [Google Scholar]