Abstract
Multigrain composite mixes were prepared from different cereals, legumes, millets, nuts along with condiments by different processes. Multigrain composite mixes had 10 to 12% moisture, 56 to 61% carbohydrate, 15 to 20% protein, 9 to 13% crude lipid and 2 to 3% ash. Energy value ranged from ~1600 to 1700 kJ/100 g. Among the vitamins studied, thiamine and riboflavin content varied from 0. 23 to 0.45 mg% and from 8.7 to 21.6 microgram% respectively. Dietary fibre was in the range of 12.4–16.5%. Swelling power of these mixes was about 10; however solubility varied from 17 to 22%. In-vitro Starch digestibility varied from 60 to 76%. Phytic acid content in these multigrain composite mixes varied from 0.6 to 0.8%. Poly-phenols ranged from 1.2 to 1.5%, DPPH free radical scavenging activity ranged from 75.2–86.2% and metal chelating activity ranged from 1.9 to 3.9%. Pasting profile by a Brabender Viscograph of these mixes indicated that they have cross linked starch type behaviour. These multigrain composite mixes can be used for the preparation of food formulations, savory products, pan cake, snacks preparation like muruku and chakli.
Keywords: Cereals, Legumes, Millets, Functional properties, Dietary fibre, Anti-nutritional factors
Introduction
The consumption of cereals and legumes all over the world gives them an important position in international nutrition. Besides the high starch and protein content as energy source, these grains provide dietary fibre, nutritious protein and lipids rich in essential fatty acids. Important micronutrients present in cereals are vitamins, especially many B vitamins, minerals, antioxidants and phyto-chemicals. However, processing may change the levels of the bioactive components in grains. In addition, interactions between cereals and legumes with nuts, oil seeds and condiments have effects on the nutritional quality of food products.
Arya (1990) reviewed variety of different type of grain based and convenience food prepared either at home or at small scale industry. Alternative cereals like barley, sorghum, millet, amaranth and along with other grains like flax seed, quinoa were used for extrusion (Eastman and Lee 2005). Whole grain flour from barley, millet, rye and sorghum were evaluated individually and along with blends of wheat flour and their pasting behaviour were studied by Rapid Visco-analyzer, these blends were used for making bread, cake and cookie or snack products. A multigrain snack helps in high intake of fibre and health enhancing components (Ragaee and Abddel Aal 2006). Multigrain snacks were also prepared by making dough, sheeting, cutting into pieces and baking (Faa and Lai 2008). In another study, a multigrain shelf stable savory and sweet snacks were prepared which was high in fibre and low in fat (Karwowski et al. 2008). Kraft foods holdings prepared new breakfast foods which aim at reducing childhood obesity and increasing health benefits (Holay 2007). Reduced calorie, palatable snack products were prepared by using whole grain by NIR partial cooking followed by extrusion and deep freezing (Huber et al. 1992).
In this laboratory-the nutrient composition and physicochemical properties of Indian medicinal rice Njavara was studied by Deepa et al. (2008) and reported that the dehusked rice of Njavara had higher content of protein, thiamine, riboflavin, niacin and dietary fiber compared to two non-medicinal rice I R - 64 and Jyothi. Odunayo and Singh (2007) reported the effect of cooking on the profile of phenolics, tannins, phytate, amino acid, fatty acid and mineral contents of whole grain and decorticated cowpea. They also studied the functional properties of flours and starches of the same cowpea varieties and concluded that starches posses higher viscographic profile compared to the flour of the same variety (Odunayo and Singh 2008). Bong and Singh (2009) studied the cooking behavior of rice and black gram in the preparation of idli, a traditional fermented product of Indian origin and reported that pasting profile of fine rice was different compared to the coarse rice and their behavior along with black gram on cooking differs compared to the individual commodities. Anti-oxidant activity and chemical composition of mung bean seeds were studied by Anwar et al. (2007). Similar properties were also studied for hot air dried and freeze-dried pumpkin flours by Fei Que et al. (2008). In the present work, an attempt has been made to prepare common multigrain composite mixes, by using whole grains of cereals, pulses and millets which can be made use of in the preparation of many products like savory, pan cake, snacks like muruku etc. Nutrients, anti-oxidant properties and some functional properties of these multigrain composite mixes were studied and they are reported in this paper.
Materials and methods
Cereals and millets-rice (Oryza sativa L.), wheat (Triticum aestivum), ragi (Eleusine coracana L.), pulses-whole chick pea (Cicer arietinum), whole green gram (Phaseolus aureus Roxb), puffed bengal gram (Cicer arietinum), defatted soya powder (Glycine max Merr), nuts and oil seeds: almond (Prunus amygdalus), cashew nut (Anacardium occidentale), sesame (Sesamum indicum), condiments-poppy seeds (Papaner somniferum) were purchased from local market in Mysore, Karnataka, India, from a single batch. The grains were cleaned, kept in air tight polyethylene bags, in a cool and dry place prior to use. Gallic acid, Folin-Ciocalteu’s reagent, phytic acid, riboflavin, thiamine standards and enzymes for digestion of starch and dietary fibre estimation, pepsin, pancreatin, termamyl (A 3403) and amyloglucosidase were from M/s Sigma Chemical Co., (St. Louis, MO, USA). All other chemicals were of analytical grade.
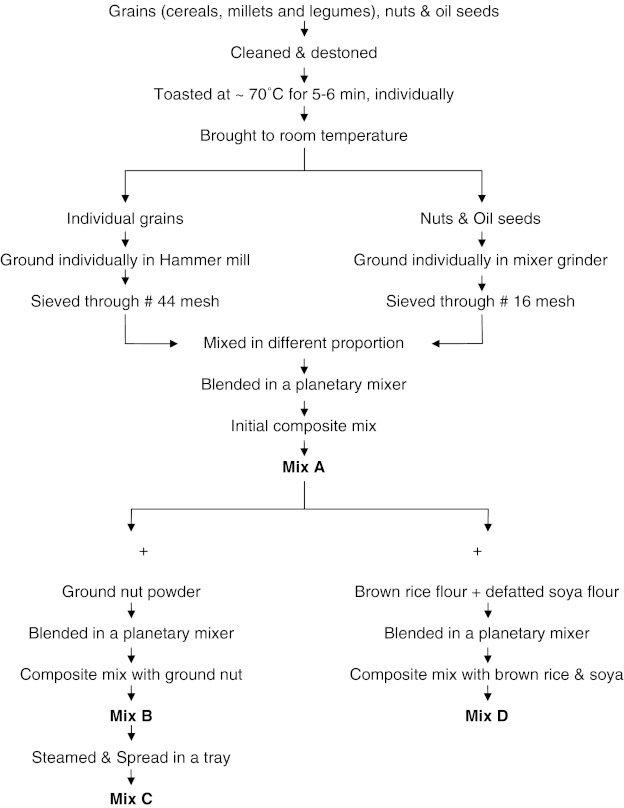
Flow chart for the preparation of four different multigrain composite mixes Fig. 1.
Fig. 1.
Flow chart for the preparation of multigrain composite mixes
In Mix A, 1 kg of finger millet, 0.25 kg of each of wheat, rice, Bengal gram, green gram, puffed Bengal gram, 0.1 kg of cashew, almond, sesame, poppy seeds. Other treatments have been informed in the following steps for the preparation of Mix B (Mix A with 0.1 kg ground nut), Mix C (steamed mix B) and Mix D (Mix A with 0.1 kg defatted soya flour). These were processed as informed in the Fig. 1. These mixes were analysed for the following parameters.
Proximate analyses
The moisture content of the multigrain composite mixes was determined by drying at 105 °C until a constant weight was attained as per AOAC 2000. The micro Kjeldhal method was employed to determine the total nitrogen and the crude protein content (Nx6.25) (AOAC 2000). Crude lipid was estimated by extraction with petroleum ether (60–80 °C), with a soxhlet apparatus and ash contents were determined as per AOAC 2000. Dietary fibre was estimated using the rapid enzymatic assay of Asp Nils et al. (1983). Vitamins viz., riboflavin and thiamine content were estimated as per AOAC methods (2000). Gross energy (kJ/100 g dry matter) was calculated based on the formula: (crude protein × 16.7) + (crude lipid × 37.7) + (crude carbohydrates × 16.7) (Ekanayakke et al. 1999).
Colour
Color values of samples are determined by using hunter lab scan XE model (M/S Hunter associate laboratory Inc., Reston-V.A., USA) with a view angle of 2°. Colour values of the samples were determined by the hunter system L, a, b values. In the hunter system ‘L’ indicates brightness or whiteness, positive ‘a’ value indicates redness and negative value indicates greenness. Positive ‘b’ value indicates yellowness and negative ‘b’ indicates blueness, ‘∆E’ indicates the overall average colour.
Functional properties
Water absorption capacity (WAC) of multigrain composite mixes was determined according to the method of Anderson et al. (1969). Water solubility index (WSI) of these mixes was determined from the amount of dried solids recovered by evaporating the supernatant from the multigrain composite mixes according to the method of Anderson et al. (1969). Swelling and Solubility were measured at boiling water temperature according to the method of Singh et al. (2000). Sedimentation volume was determined as per the method of Bhattacharya and Ali (1976). Bulk density was determined according to the method of Wang and Kinsella (1976).
Antinutritional factors
Phytic acid was determined according to the method described by Odunayo and Singh (2007). The concentration of total phenolic compounds in multigrain composite mixes was measured according to the spectrophotometric method of Singleton et al. (1995). Gallic acid was used as standard and phenolic compounds were expressed as gallic acid equivalents (GAE; g/100 g of dry matter).
Antioxidant activities
Free radical scavenging activity of the mixes was measured using 1, 1-diphenyl-2-picryl-hydrazil (DPPH) by the method of Shimada et al. (1992). Metal chelating activity of multigrain composite mixes on Fe+2 was measured according to the method of Dinis et al. (1994).
In-vitro starch digestibility
This was estimated according to the method described by Ngo Som et al. (1992).
Pasting characteristic
This was studied with a Brabender Viscograph, Type VSK 4 (Duisburg, FRG) fitted with a 700 cmg sensitivity cartridge. Brief procedure followed was as described in Bong and Singh (2009).
Statistical analyses
The results are expressed as mean ± standard deviation. All the data were analyzed by one way analysis of variance (ANOVA) followed by multiple comparison test (Tukey’s test) at 5% level of significance. A value of p < 0.05 was considered statistically significant.
Results and discussion
Proximate composition
Moisture content of the multigrain composite mixes varied from 10 to 12% (Table 1). Carbohydrate, determined by difference varied from 56 to 61%, among which Mix A had highest carbohydrate, as this mix contained grains which are rich sources of carbohydrate compared to other mixes. Mix B, Mix C and Mix D had lower content of carbohydrate as they contained groundnut powder and defatted soya flour as one of the ingredients, which had least carbohydrate content. Protein content was in the range of 15 to 20%. Mix A had lower protein compared to Mix D, Mix B and Mix C had almost same protein contents (Table 1). The difference is due soya and ground nut contents. Lipid content varied from ~ 9–13%. Mix B had the highest and Mix D had the least lipid content. This could be due to presence of ground nut in the former and defatted soya flour in the latter. Other two composite mixes had similar lipid content. Ash content was highest in Mix D and least in Mix A, which may due to the presence of seed coat in the grains, as majority of the grains were used along with the seed coat. Mix B showed the highest energy content, which was around 1700 kJ/100 g while the least energy was in the Mix D (1600 kJ/100 g). Mix A and Mix C had intermediate energy value, thus indicating no substantial change in nutritive value and energy content due to processing.
Table 1.
Mean proximate composition and selected vitamins of multigrain composite mixes
| Components | Mix A | Mix B | Mix C | Mix D |
|---|---|---|---|---|
| Moisture (%) | 10.0a ± 0.12 | 10.9b ± 0.06 | 11.6d,c ± 0.06 | 11.5c ± 0.00 |
| Total carbohydrateA (%) | 61.1a ± 0.16 | 55.9b ± 0.43 | 56.6d ± 0.10 | 56.3c ± 0.27 |
| Protein (%) (Nx6.25) | 14.8a ± 0.15 | 18.1b ± 0.25 | 18.2db ± 0.05 | 20.4c ± 0.00 |
| Crude lipid (%) | 11.8a ± 0.02 | 12.7b ± 0.04 | 11.2d ± 0.01 | 8.9c ± 0.02 |
| Ash (%) | 2.2a ± 0.01 | 2.5b ± 0.01 | 2.4d ± 0.01 | 2.9c ± 0.01 |
| Energy value (kJ/100 g) | 1711.5a ± 3.32 | 1713.8a ± 2.68 | 1673.9b,c ± 0.76 | 1616.8c ± 3.76 |
| Thiamine mg (%) | 0.39a ± 0.05 | 0.23b ± 0.00 | 0.45a ± 0.03 | 0.27a,b ± 0.02 |
| Riboflavin μg (%) | 8.9a ± 0.54 | 8.7a ± 0.12 | 21.6b ± 0.27 | 10.0a ± 0.96 |
| Insoluble Dietary fibre (%) | 11.1a ± 0.00 | 14.0b ± 0.30 | 13.5db ± 0.01 | 9.7c ± 0.00 |
| Soluble Dietary fibre (%) | 2.7a ± 0.00 | 2.5b ± 0.04 | 2.4d,b ± 0.01 | 2.6c ± 0.01 |
| Total Dietary fibre (%) | 13.9a ± 0.01 | 16.5b ± 0.34 | 15.9d,b ± 0.02 | 12.4c ± 0.01 |
Values are mean ± standard deviation of three independent determinations. Means with the same superscript (a, b) within the same row do not differ (p < 0.05)
Mix A-initial composite mix, Mix B-composite mix with ground nut, Mix C-steamed composite Mix B, Mix D-composite mix with Brown rice and soya. ABy difference as 100-(moisture + protein + ash + fat)
Vitamins
Thiamine content of Mix A and Mix C was high, which was around 0.39 and 0.45 mg% respectively (Table 1). The thiamine content in Mix C was high, probably while steaming, the vitamins will move inside the kernel from the seed coat and aleurone layers of the grain and hence while processing of the grains, loss will be less compared to other grains; and hence the values of thiamine content may be high (Singh et al. 2004). Mix B and Mix D had around 0.23–0.27 mg%, which were lower compared to Mix A and Mix C. In the case of riboflavin, Mix C has 21.6 μg%, again proving the fact as explained above. In the case of other multigrain composite mixes almost same values were noticed.
Dietary fibre
Total dietary fibre content in multigrain composite mixes varied from ~12.4–16.5%. The soluble dietary fibre was high in Mix A and Mix D (Table 1). Each of the grains used for preparation of composite mixes were rich sources of soluble fibre. Soluble dietary fibre was almost same in Mix B and Mix C. Total as well as insoluble dietary fiber was high in Mix B and Mix C, indicating that the groundnut addition has contributed substantially to these fractions.
Colour
Mix A was comparatively bright, as the L value was high, followed by Mix D, Mix C and least being Mix B with ground nut powder (Table 2). Similarly it was noticed that delta E was lowest in Mix A indicating highest brightness in this specific mix. Lower L values indicated that browning occurred during various processing steps of preparing the Mix, where sugar and amino acid may be playing a role. Higher a-value indicates more redness as seen in the Mix B with ground nut powder and higher b value indicated less yellowness, as the values varied from ~12.6 to 13.2.
Table 2.
Colour measurement of multigrain composite mixes
| L | a | b | Delta E | |
|---|---|---|---|---|
| Mix A | 65.3a ± 0.45 | 1.8a ± 0.04 | 13.2a ± 0.07 | 28.5a ± 0.41 |
| Mix B | 59.7b ± 0.03 | 1.9a ± 0.09 | 12.6b ± 0.12 | 33.3b ± 0.05 |
| Mix C | 61.8c ± 0.16 | 2.3c ± 0.05 | 12.9a ± 0.09 | 31.5c ± 0.18 |
| Mix D | 64.8a ± 0.07 | 1.4b ± 0.03 | 13.1a ± 0.09 | 28.8a ± 0.10 |
Values are mean ± standard deviation of three independent determinations
Means with the same superscript (a, b) within the same column do not differ (p < 0.05)
Mix A, Mix B, Mix C, Mix D-terms are as per Table 1
Water absorption capacity (WAC) and water solubility index (WSI)
In the present study, grains were toasted and ground for size reduction. As these were multigrain composite mixes, only insignificant differences were noticed between the values of WAC, as all values were between 2.3 and 2.4(g/g) (Table 3). The polar amino acid residue in proteins as well as polysaccharides have been reported to lead to varying water absorption capacity (Kinsella 1976). In the case of WSI, the values varied from 4.8–6%. Highest was shown by Mix D (~ 6%), indicating degradation of starch and denaturation of proteins to some extent. In other composite mixes values were almost same.
Table 3.
Water absorption capacity and Water solubility index, Swelling and Solubility, Sedimentation volume and Bulk density of multigrain composite mixes
| Functional property | Mix A | Mix B | Mix C | Mix D |
|---|---|---|---|---|
| Water absorption capacity (g/g) | 2.3a ± 0.11 | 2.3a ± 0.05 | 2.3a ± 0.06 | 2.4a ± 0.06 |
| Water solubility index (%) | 4.8a ± 0.51 | 5.0a ± 0.49 | 5.0a ± 0.45 | 6.0a ± 0.84 |
| Swelling power | 9.9a ± 0.78 | 9.9a ± 0.37 | 10.1a ± 0.38 | 9.5a ± 0.18 |
| Solubility (%) | 18.9a ± 0.30 | 21.9a ± 0.91 | 17.3a ± 2.38 | 19.1a ± 2.11 |
| Sedimentation volume (ml) | 5.2a ± 0.00 | 4.8b ± 1.90 | 4.8d,b ± 1.91 | 5.4c ± 0.10 |
| Bulk density (g/ml) | 0.6a ± 0.01 | 0.7b ± 0.01 | 0.7dc ± 0.01 | 0.7c ± 0.01 |
Values are mean ± standard deviation of three independent determinations
Means with the same superscript (a, b) within the same row do not differ (p < 0.05)
Mix A, Mix B, Mix C, Mix D-terms are as per Table 1
Sedimentation volume
Sedimentation volume indicates the extent of gelatinization in the material. In the present study, the composite mixes viz., Mixes A, B and D were made from toasted grains, while Mix C was also steamed in addition to toasting. The values indicate that the extent of gelatinization was similar in Mix B and Mix C (Table 3). However Mix A and Mix D had higher values compared to Mix B and Mix C.
Swelling and solubility
Swelling power at boiling condition of water was almost same in all the composite mixes (9.5 to 10) (Table 3), but some differences were observed in the solubility. Solubility values were high indicating greater extent of the starch degradation and vice versa. Least solubility indicates that the starch degradation was quite less, where leaching of the linear components were less. Mix C showed low solubility, indicating it has undergone less degradation under the condition of the steaming experiments carried out. Mix B in which ground nut powder was one of the ingredients and starch from other grains had undergone high degradation; hence the value noticed was high.
Bulk density
The values of the bulk density of composite mixes indicated that the values were almost similar in the case of Mix B, Mix C and Mix D (Table 3). However, the values were high for these mixes compared to Mix A, resulting probably from the groundnut and soya flour components in these mixes. In the Mix A the value was quite less (0.6 g/ml), indicating the differences in composition of the composite mixes.
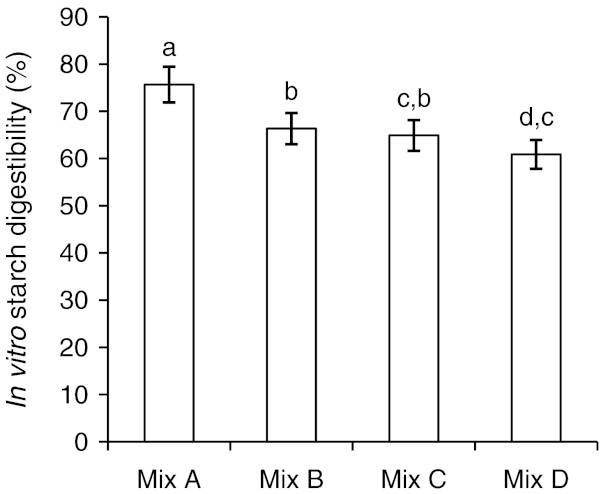
In-vitro Starch digestibility
Starch digestibility was high in Mix A (76%) indicating that the starch in this mix is easily accessible to alpha - amylase. Least starch digestibility was seen in the case of Mix D with defatted soya flour and brown rice flour (60%) (Fig. 2) and it was not significantly different from Mix C. Both the mixes contain protein-rich flour like groundnut and defatted soya and their interaction with starch is likely to lead to a protein-starch network, where starch may not be easily accessible to < alpha > - amylase.
Fig. 2.
In-vitro starch digestibility of multigrain composite mixes. Mix A-initial composite mix, Mix B-composite mix with ground nut, Mix C-steamed composite. Mix B, Mix D-composite mix with Brown rice and soya
Antinutritional factors
Phytic acid being an anti-nutrient, it lowers the bioavailability of minerals and inhibits the digestibility of proteins. Mix B and Mix C had highest phytic acid content (0.8%) and Mix D had least (0.6%) (Table 4). In between value was shown by composite Mix A. Steaming and roasting were more effective in reducing phytic acid in chickpea and pigeon pea than black gram, mungbean and soybean (Chitra et al. 1996).
Table 4.
Antinutritional factors of multigrain composite mixes
| Antinutritional factor | Mix A | Mix B | Mix C | Mix D |
|---|---|---|---|---|
| Phytic acid (%) | 0.7a ± 0.03 | 0.8a ± 0.13 | 0.8a ± 0.07 | 0.6a,b ± 0.02 |
| Polyphenols GAE (%) | 1.2a ± 0.11 | 1.4b ± 0.05 | 1.5c,b ± 0.03 | 1.2a,d ± 0.07 |
Values are mean ± standard deviation of three independent determinations
Means with the same superscript (a, b) within the same row do not differ (p < 0.05)
Mix A, Mix B, Mix C, Mix D-terms are as per Table 1
Total phenolic compounds in all the four different composite mixes varied from 1.2 to 1.5% of the methanolic extract (Table 4). Phenolic compounds usually form insoluble complexes with protein, there by interfering with their bioavailability (Liener 1994). The phenolic compounds were similar in Mix A and Mix D (~ 1.2%) except in the case of Mix B and Mix C, where it was high by about 1.4–1.5%. This may be due to the effect of added groundnut powder in these mixes. Generally it is well known that while steaming nutrients move towards endosperm as it occurs while parboiling of rice (Sashikala et al. 2005). Antonio et al. (2003), observed an increase in hydroxyl methyl furfural, an intermediate in Maillard reaction, in plums dried between 60 and 85 °C. Steam exposure might also have contributed to high total phenol in Mix C, as the free phenolic groups may be exposed during denaturation of proteins and consequent destabilization of the protein-polyphenol complex.
Viscographic parameters
On cooking these composite mixes individually, various parameters measured in the Brabender Viscograph are shown in Table 5. At lower concentration like 8% and 10%, peak viscosity could not be measured. Hence 12% concentration was considered, as measurable parameters could be seen with this concentration. Gelatinization temperature of the Mix A was high (85 °C); indicating that the semi-crystalline nature of starch granules in this mix will begin to lose at high temperature. Mix C had least gelatinization temperature, indicating the granules in this mix loses their birefringence at an early temperature compared to other composite mixes, due to the pre—gelatinization occurring at the steaming stage itself. Other two composite mixes showed almost same gelatinization temperature.
Table 5.
Viscographic parameters of multigrain composite mixes
| MCM | GT (0C) | PV (BU) | HPV (BU) | CPV (BU) | BD | SB | SBt |
|---|---|---|---|---|---|---|---|
| Mix A | 85a ± 0.70 | 520a ± 30 | 410a ± 10 | 1260a ± 20 | 110a ± 10 | 740a ± 20 | 850a ± 10 |
| Mix B | 82b ± 0.40 | 280b ± 10 | 280b ± 10 | 560b ± 20 | 0.0b ± 00 | 280b ± 10 | 280b ± 10 |
| Mix C | 80bd ± 0.40 | 340de ± 20 | 340de ± 20 | 770d ± 20 | 0.0db ± 00 | 430d ± 10 | 430d ± 10 |
| Mix D | 82cb ± 1.90 | 280cb ± 10 | 280cb ± 10 | 650c ± 10 | 0.0cb ± 00 | 370c ± 20 | 370c ± 20 |
Values are mean ± standard deviation of three independent determinations
Means with the same superscript (a, b) within the same column do not differ (p < 0.05)
Mix A, Mix B, Mix C, Mix D-terms are as per Table 1. MCM-Multigrain composite mixes GT Gelatinization temperature, PV Peak viscosity, HPV Hot paste viscosity, CPV cold paste viscosity, BD Break down, SB Set Back, SBt total set back
Peak viscosity is the viscosity where the starch granules reach highest swelling while cooking. We observed that the Mix A showed highest peak viscosity of about 520 B U, indicating the combined starch granules of each grain in this mix will try to swell maximum compared to other composite mixes along with leacheates of protein molecules of the mix, which may be enhancing the viscosity. Next highest was shown by Mix C, where the value was around 340 B U. Probably as the Mix C had undergone steaming, its viscosity might have reduced compared to the Mix A. The extent of swelling was almost same in the case of Mix B as well as Mix D, indicating these grains has achieved cooked nature, while processing the grains, and hence the peak viscosity was less compared to other two composite mixes.
Hot paste viscosity (HPV) is the viscosity registered at the end of cooking. It was observed that in the Mix A, the HPV was 410 B U, where the swollen granules had broken down and hence the viscosity had come down compared to the peak viscosity. In the case of Mix B, Mix C and Mix D, the values did not change compared to peak viscosity. It was an indication that the swollen granules did not change or did not break, indicating that these three composite mixes were behaving like cross linked starch, as cross linked starch will not break down while cooking. Break down value was noted only in the case of Mix A, where as in others the values were zero, further indicating the behaviour of cross linked type starch, in these composite mixes.
The cold paste viscosity (CPV) values were high in the case of Mix A, however in other composite mixes the values were less. Correspondingly the Set Back were high in Mix A, but in others these values were less indicating the fact that in the Mix A higher retrogradation had taken place and in others lesser retrogradation had taken place compared to the Mix A.
Total set back was also high in the case of Mix A, indicating that retrogradation was high in this mix. In others the total set back was less and hence the quantity of linear molecules was less in these composite mixes, hence precipitation of these molecules was also less in these composite mixes.
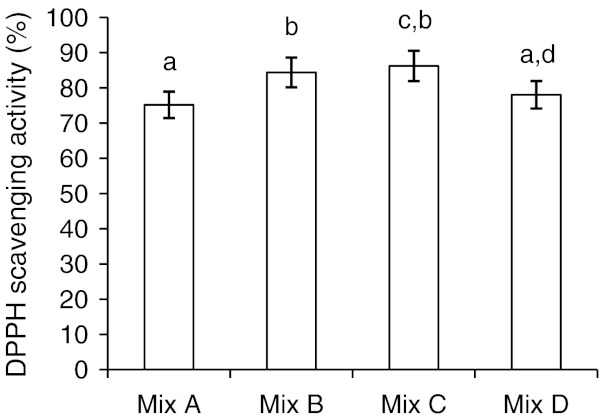
Free radical scavenging activity
The effect of antioxidants on DPPH radical scavenging was thought to be because of their hydrogen donating ability. DPPH is a stable free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule (Soares et al. 1997). The reduction capability of DPPH radicals was determined by the decrease in its absorbance at 517 nm induced by the antioxidants. Lower absorbance of the reaction mixture indicates higher free radical scavenging activity. Scavenging activity varied from ~75 to 86% (Fig. 3). Mix C showed highest value followed by Mix B, Mix D and Mix A. The presence of non-antioxidant compounds like amino acids in the methanolic extracts may produce more antioxidant capacity and may interfere with the polyphenols present in the food matrix, producing a higher antioxidant capacity which is observed in steamed composite mix (Perez-Jimenez and Saura-Calixto 2006).
Fig. 3.
DPPH free radical scavenging activity of methanol extracts of multigrain composite mixes. Mix A, Mix B, Mix C, Mix D-terms are as per Fig. 2
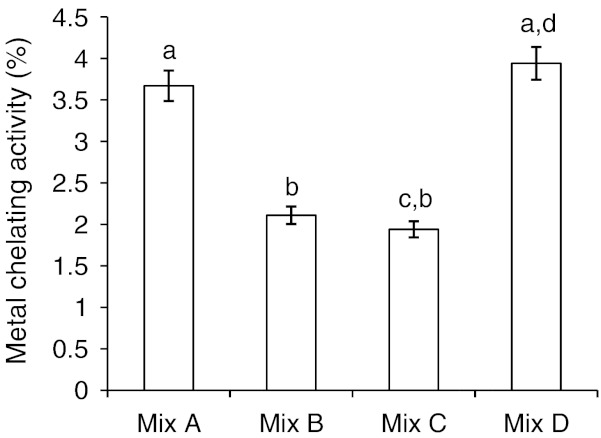
Metal chelating capacity
It is an important property as it reduces the concentration of the catalyzing transition metal in lipid peroxidation (Yildirim et al. 2001). An extract with higher binding ability would prevent or inhibit reaction such as Fenton’s type reaction, which generates reactive hydroxyl radicals. Ferrozine can quantitatively form complexes with Fe ++. In the presence of chelating agents, the complex formation is disrupted with the result that the red colour of the complex is decreased. Measurement of colour reduction therefore allows the estimation of chelating activity of the co-existing chelator. The range and the mean Fe2+ chelating capacities varied among the four mixes. From Fig. 4, it is clear that Mix A and Mix D showed almost same chelating activity towards higher values, however Mix B and Mix C showed lower chelating activities. It was reported that the compounds with structures containing functional groups: -OH, -SH, -COOH, -PO3H2, C = O, -NR2, -S- and -O- is favourable to metal chelation activity (Lindsay 1996; Yuan et al. 2005). Thus molecules including organic acids such as citric, malic, tartaric, oxalic, succinic, lipoic, phytic acid and polyphenols can increase the chelating ability and chelate pro-oxidant metal ions, like iron and copper thus preventing free radical formation from these pro-oxidants (Bhadari and Kawabata. 2004). Mix A and Mix D showed higher chelating activity as they had substantial amount of above mentioned functional groups compared to that of Mix B and Mix C.
Fig. 4.
Metal chelating activities of methanol extracts of multigrain composite mixes. Mix A, Mix B, Mix C, Mix D-terms are as per Fig. 2
Conclusion
Generally traditional food items are prepared with various combinations of food grains-cereals, millets, legumes etc. Taking into consideration their contribution to either nutrition or functional properties these multigrain composite mixes can be used for the preparation of various or specific food items. An attempt has been made to prepare the multigrain composite mixes. Its various functional, nutritional, anti-oxidant properties have been studied. These multigrain composite mixes have been used for the preparation of food formulations which finds use in the preparation of savory products, snacks, pan cake, snacks like muruku etc.
Acknowledgements
The authors are thankful to Director, Dr. V. Prakash, Central Food Technological Research Institute, for showing keen interest and encouraging us in all respects in carrying out this work. One of the authors (Singh) gratefully acknowledges financial support in the form of a 11th Five year plan Project awarded by Govt. of India.
References
- Official methods of analysis. 17. Washington, DC: Association of Official Analytical Chemists; 2000. [Google Scholar]
- Anderson RA, Conway HF, Pfeifer VF, Griffin EL. Roll and extrusion-cooking of grain sorghum grits. Cereal Sci Today. 1969;14:372–380. [Google Scholar]
- Antonio P, Alssandra DC, Giampaola C. From plums to prunes: Influence of drying parameters on polyphenols and antioxidant activity. J Agric food chem. 2003;51:3675–3681. doi: 10.1021/jf021207+. [DOI] [PubMed] [Google Scholar]
- Anwar F, Latif S, Przbylski R, Sultana B, Ashraf M. Chemical composition and antioxidant activity of seeds of different cultivars of mungbean. J Food Sci. 2007;72:503–509. doi: 10.1111/j.1750-3841.2007.00462.x. [DOI] [PubMed] [Google Scholar]
- Arya SS. Grain based snack and convenience foods. Ind food pack. 1990;44(5):17–38. [Google Scholar]
- Asp Nils G, Johansson Claes G, Hallmer H, Siljestrom M. Rapid enzymatic assay of insoluble and soluble dietary fiber. J Agric Food Chem. 1983;31:476–482. doi: 10.1021/jf00117a003. [DOI] [PubMed] [Google Scholar]
- Bhadari MR, Kawabata J. Organic acid, phenolic content and antioxidant activity of wild yam (Dioscorea spp.) tubers of Nepal. Food Chem. 2004;88:163–168. doi: 10.1016/j.foodchem.2003.12.027. [DOI] [Google Scholar]
- Bhattacharya KR, Zakiuddin Ali S. A sedimentation test for pregelatinized rice products. Lebensm Wiss Technol. 1976;9:36–37. [Google Scholar]
- Bong KK, Singh V. Cooking behavior of rice and black gram in the preparation of Idli, A traditional fermented product of Indian origin, by viscography. J text studies. 2009;40:36–50. doi: 10.1111/j.1745-4603.2008.00168.x. [DOI] [Google Scholar]
- Chitra U, Singh U, Rao PV. Phytic acid, in vitro protein digestibility, dietary fibre and minerals of pulses influenced by processing methods. Plant Foods Human Nutri. 1996;49:307–316. doi: 10.1007/BF01091980. [DOI] [PubMed] [Google Scholar]
- Deepa G, Singh V, Akhilender Naidu K. Nutrient composition and Physicochemical properties of Indian medicinal rice-Njavara. Food chem. 2008;106(1):165–171. doi: 10.1016/j.foodchem.2007.05.062. [DOI] [Google Scholar]
- Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivates (acetoaminophen, salycilate, and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- Ekanayakke S, Jansz ER, Nair BM. Proximate composition, mineral and amino acid content of mature Canavalia gladiata seeds. Food chem. 1999;66:115–119. doi: 10.1016/S0308-8146(99)00041-2. [DOI] [Google Scholar]
- Eastman J, Lee G (2005) Whole grains in extruded products. Cer Foods World 50(4): 168, 170–172
- Faa P, Lai R (2008) Production of multi-grain, whole grain, soft and cruncy sheeted snacks. PN: US 2008/0050493 A1, (US2008/0050493A1)
- Que F, Mao L, Fang X, Tao Wu. Comparison of hot air- drying and freeze-drying on the physicochemical properties and antioxidant activities of pumpkin (Cucurbita moschata Duch.) flours. Inter J Food Sci Technol. 2008;43:499–507. doi: 10.1111/j.1365-2621.2007.01590.x. [DOI] [Google Scholar]
- Holay A (2007) The cereal thing. Prepared foods, 176(3): 33–34, 37–38, 41
- Huber Gr, Wenger Ml, Sevatson Es (1992) Reduced calorie, palatable snack product and method of producing same. PN: US 5132 133; (US5132133)
- Karwowski J, Vemulapalli V, Wang CY (2008) Production of whole grain- containing composite food products. PN: US 2008/0003340 A1; (US2008/0003340A1)
- Kinsella JE. Functional properties of proteins in foods: A survey. CRC Reviews Food Sci Nutri. 1976;7:219–240. doi: 10.1080/10408397609527208. [DOI] [Google Scholar]
- Liener IE. Implications of antinutritional components in soybean foods. CRC Reviews Food Sci Nutri. 1994;34:31–67. doi: 10.1080/10408399409527649. [DOI] [PubMed] [Google Scholar]
- Lindsay RC (1996) Food additives. In: Fennema, O.R. (Ed.) Marcel Dekker Inc., New York, Food Chem p. 778 (Chapter 12)
- Ngo Som J, Prajwala M, Daniel VA, Malleshi NG, Venkat Rao S. Digestibility of protein and starch in malted weaning foods. J Food Sci Technol. 1992;29:262–263. [Google Scholar]
- Odunayo CA, Singh V. Effect of cooking on the profile of phenolics, tannins, phytate, amino acid, fatty acid, mineral nutrients of whole-grain and decorticated vegetable cowpea Vigna unguiculata (L.) Walp. J Food Quali. 2007;30:1101–1120. doi: 10.1111/j.1745-4557.2007.00155.x. [DOI] [Google Scholar]
- Odunayo CA, Singh V. Physico-chemical properties of the flours and starches of two cowpea varieties (Vigna unguiculata (L.)Walp) Innovative Food Sci Emer Technol. 2008;9:92–100. doi: 10.1016/j.ifset.2007.06.003. [DOI] [Google Scholar]
- Perez-Jimenz, Saura-Calixato F. Effect of solvent and certain food constituents on different antioxidant capacity assays. Food Res Inter. 2006;39:791–800. doi: 10.1016/j.foodres.2006.02.003. [DOI] [Google Scholar]
- Ragaee S, Abddel Aal ESM. Pasting properties of starch and protein in selected cereals and quality of their food products. Food chem. 2006;95(1):9–18. doi: 10.1016/j.foodchem.2004.12.012. [DOI] [Google Scholar]
- Sashikala IS, Singh V, Ali SZ. Changes in physicochemical properties of Basmati paddy upon parboiling. Trends Carbo Chem. 2005;9:53–59. [Google Scholar]
- Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthine on autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40:945–948. doi: 10.1021/jf00018a005. [DOI] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raentos RM. Analyses of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth Enzymol. 1995;299:152–171. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Singh V, Okadome H, Toyoshima S, Ohtsubo K. Thermal and physico- chemical properties of rice grain, flour and starch. J Agric food chem. 2000;48:2638–2647. doi: 10.1021/jf990374f. [DOI] [PubMed] [Google Scholar]
- Soares JR, Dins TCP, Cunha AP, Ameida LM. Antioxidant activity of some extracts of Thymus zyges. Free Radical Research. 1997;26:469–478. doi: 10.3109/10715769709084484. [DOI] [PubMed] [Google Scholar]
- Singh V, Jayadeep A, Lakshmishree HR, Ashwathanarayana KN. Status of micronutrients in different degree milled rice before and after parboiling (ICFOST) Mysore, Karnataka, India: Central Food Technological Research Institute and Defence Food Research Laboratory; 2004. [Google Scholar]
- Yildirim A, Mavi A, Kara AA. Determination of antioxidant and antimicrobial activities of Rumex crispus L.extracts. J Agric Food chem. 2001;49:4083–4089. doi: 10.1021/jf0103572. [DOI] [PubMed] [Google Scholar]
- Yuan YV, Bone DE, Carrington MF. Antioxidant activity of dulse (Palmaria palmate) extract evaluated in-vitro. Food Chem. 2005;91:485–494. doi: 10.1016/j.foodchem.2004.04.039. [DOI] [Google Scholar]
- Wang JC, Kinsella JE. Functional properties of novel proteins: Alfalfa leaf protein. J Food Sci. 1976;41:286–292. doi: 10.1111/j.1365-2621.1976.tb00602.x. [DOI] [Google Scholar]