Abstract
Freshly harvested Red delicious apples were dipped in calcium chloride solution of varying concentrations (0.5–2.0% w/v) for 1 h prior to irradiation at dose level of 0.4 kGy. Fruits after radiation treatment were stored at 2 ± 1°C, RH 90% and evaluated at intervals of 30 days for various quality parameters. Results revealed significant (p ≤ 0.05) retention in firmness, juice yield and ascorbic acid content in samples treated with combination of calcium chloride at 2.0% w/v and gamma irradiation (0.4 kGy) during storage. Water soluble pectin was inversely correlated with firmness (r = 0.88) and was significantly (p ≤ 0.05) lower in samples subjected to combination treatment of 2.0% w/v CaCl2 and 0.4 kGy irradiation throughout the storage. The combination treatment of 2.0% CaCl2 and 0.4 kGy irradiation gave about 4.3 log reduction in yeast and mold count of apple samples. Results of the post refrigeration weight loss, firmness and overall acceptability revealed that combination treatment was helpful in extending the shelf-life of Red Delicious apples by around 20–25 days at 17 ± 2°C, RH 75% following 90 days of refrigeration.
Keywords: Red delicious apples, Calcium treatment, Gamma irradiation, Physico-chemical parameters, Storage quality, Shelf-life extension
The state of Jammu and Kashmir, owing to its topography and climate offers a huge potential for growing of pome and stone fruits as well as berries of temperate nature. Among the pome fruits presently grown in Kashmir valley, apple has a leading position both in terms of cultivation and production. Apple occupies around 50% of the total area under its cultivation and constitutes about 90% of total fruit produced in the state (Anon 2007-08). Among the commercially grown varieties, Red Delicious has gained much popularity and commercial importance due to its overall sensory qualities, acceptability and market returns. Factors affecting the overall quality of apple during storage include loss of water, respiration, metabolism and microbial spoilage (Alak and Goswami 2006). Microbial contamination of fresh produce poses potential health risks besides the losses. Of the various fungal toxins which are generally associated with fresh apples, patulin is of prime concern. Patulin is a mycotoxin produced by fungal genera of Penicillium, Aspergillus and Byssochlamys (Shephard and Leggott 2000; Varga et al. 2003; Boonzaaijer et al. 2005). The representative symptom of patulin is acute digestive disease, especially in infants (Lai et al. 2000; Drusch et al. 2007). Patulin also acts as a mutagen and influences the nervous and immune systems (Yun et al. 2008). Therefore, patulin should be controlled as an important quality factor in apple and processed apple products. Further, due to inappropriate post-harvest management practices, lack of proper storage and prompt transportation facilities, huge losses of the order of 20–40% occur in the fresh produce during handling, packaging, transportation, marketing and storage (Roy 1993). Therefore, post-harvest treatment of fresh apple has become necessary to maintain the quality and provide longer life to fruit. Also, in order to facilitate international trade and overcome quarantine barriers, it is needed to inactivate both food-borne pathogens as well as spoilage microorganisms in fresh produce and simultaneously extend the storage life.
Gamma irradiation is a non-thermal process in nature and has emerged as a potential alternate method of preservation of fruits and vegetables, obviating the use of chemical preservatives. Gamma irradiation has proved to be effective in reducing bacterial and mold contamination, inactivating food-borne pathogens in fresh produce as well as delaying the ripening of climacteric fruits (Baskaran et al. 2007; Niemira et al. 2003; Prakash et al. 2000; Bidawid et al. 2000; King and Josephson 1982; Kader 1986). The use of gamma irradiation to enhance the shelf-life of fruits and vegetables and to ensure microbiological safety is increasing, as it appeared to be effective both on cells and spores (DeRuiter and Dwyer 2002). Zegota et al. (2001) studied the influence of ionizing radiations on the patulin content of apple juice concentrate and reported that patulin, at an initial concentration of about 2 mg/kg disappeared after irradiation of the concentrate with doses as low as 2.5 kGy. Irradiation of the concentrate with doses sufficient for patulin disappearance did not affect the quality of concentrate. Yun et al. (2008) studied the radio degradation of patulin in apple model system and reported that dose of 1.0 kGy is effective for patulin degradation. The viable cell counts of the inoculated conidia in the apple showed 2 log reduction at a dose of 1.0 kGy. Our earlier study (Hussain et al. 2008) revealed that gamma irradiation dose of 0.4 kGy was effective in extending the shelf-life of apple fruit by 30 days under ambient storage. Combinatory treatments have also been widely investigated as they often result in synergistic effects. Gamma irradiation in combination with other treatments (e.g. heat, washing, waxing, refrigeration) decreased the microbial contamination level of fresh produce, leading thus to an improvement of quality and shelf-life (Spalding and Reeder 1986; Lacroix et al. 1991).
Calcium chloride is used as an economical processing aid in the fresh-cut produce industry to minimize tissue damage during processing. Applying calcium treatment may contribute to cell wall integrity by increasing the amount of endogenous calcium available to bind with deesterified pectic residues. In addition to preserving firmness, post-harvest calcium dips reduce respiration, decrease ethylene production and delay senescence in fresh produce such as carrots (Izumi and Watada 1994), Kiwi fruit (Agar et al. 1999), strawberries (Rosen and Kader 1989) and tomatoes (Artes et al. 1999). The concentration of the calcium dip depends on the fruit or vegetable being treated and many studies report that 1–2% calcium salts are most effective for diced tomatoes (Castaldo et al. 1996), cantaloupes (Luna-Guzman and Barrett 2000) and pears (Dong et al. 2000). Literature regarding the combination treatments of irradiation and calcium dip with respect to quality maintenance during storage and shelf-life extension of fresh produce are limited, although few investigations have been carried out in minimally processed apple products (Kovacs et al. 1998; Gunes et al. 2001; Fan et al. 2005). Therefore, this work aims to investigate the effects of calcium chloride dip treatment and gamma irradiation on storage quality and shelf-life extension of fresh whole “Red Delicious” apples. The assessment of the treatments is based on the evaluation of physico-chemical parameters, overall acceptability and microbial load as yeast and mold count.
Materials and methods
Red Delicious apples of proper maturity (180 days after full bloom) and firm texture were procured from the local apple orchards of Zakura, Srinagar. Selection of apples was done from single orchard. After harvesting, the fruit was pre-cooled at 2°C for 24 h in a cold storage chamber in order to remove the field heat. The pre-cooled fruit was graded manually for achieving uniformity in size and any blemished or diseased fruits present were discarded. The whole graded fruit was dipped for 1 h in various concentrations of calcium chloride solution (0.5, 1.0, 1.5 and 2% w/v). The temperature of the dip solution was 10 ± 2°C. During treatment, the fruits were turned manually to facilitate uniform dipping. After dipping, the samples were drained and the adhering water was whipped out using blotting paper. Following the calcium chloride treatment, the fruit was packed in cardboard boxes of size (0.5 m × 0.3 m × 0.3 m) each containing ninety fruits.
Gamma irradiation treatment
The packaged fruit was subjected to γ-irradiation at dose level of 0.4 kGy using PANBIT irradiator having Co-60 as γ-ray source. The fruit was irradiated at a mean dose rate of 195 Gy/h. The dose rate was determined by Fricke dosimetry. To ensure uniformity of dose, boxes were rotated by 180° halfway through the procedure, resulting in an over dose ratio (Dmax/Dmin) of 1.6. After irradiation, the fruit was stored under refrigerated conditions (temp.2 ± 1°C, RH 90%) for evaluation of physico-chemical and microbiological parameters at intervals of 30 days of storage. For each treatment three boxes of fruits were used. Fruits neither dipped in calcium chloride nor gamma irradiated served as control.
Fruit quality analysis
Prior to the measurement of quality attributes, fruits were allowed to attain the room temperature. Firmness of apples was determined by a hand penetrometer model “FT-327” (EFFEGI, Italy) provided with a round plunger (11.2 mm diameter). To avoid the interference of skin, fruits were peeled at the points where firmness was to be measured. Triplicate samples of ten fruits were selected randomly and evaluated for firmness measurement on three sides of each whole fruit and average value was reported in kg. The fruits initially used for firmness measurement were subjecting to juice extraction using an Omini mixer (Philips make). Total soluble solids (TSS) were determined by hand refractometer model “ SP-05A” (SIPCON, Japan) having range of 0–32%. Titratable acidity (% malic acid) and total sugars were determined as per the methods of Ranganna (1986). Ascorbic acid was determined by AOAC (1984) method using 2, 6-dichlorophenol-indophenol dye solution. Water soluble pectin (WSP) was evaluated by modifying the method of Ranganna (1986). A sample of 50 g was first extracted with 100 ml of 95% ethanol. The residue left after alcoholic extraction was dried at 30°C. To obtain water soluble pectin (WSP), 30 g of the leftover alcohol insoluble solids (AIS) were extracted in 250 ml boiling water for 30 min. The extract was filtered, cooled and volume made up to 250 ml with double distilled water. 100 ml aliquot of the filtrate was precipitated for 1 h with 25 ml of calcium chloride (1 N). The precipitate obtained was dried and weighed in order to calculate the amount of WSP as
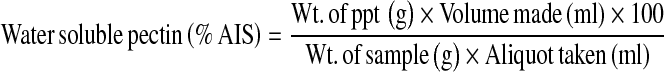 |
Juice yield was determined by extracting the juice from known weight of sample. Weight loss was determined by periodical weighing of samples. Overall acceptability based on sensory attributes viz. firmness, appearance and taste was done by a trained panel of five judges using 4-point scale where, 4 = excellent, 3 = good, 2 = fair and 1 = poor. For each treatment eight fruits were selected randomly, coded and served to judges. Microbial load as yeast and mold count was determined by the serial dilution method using potato dextrose agar media (Aneja 1996). All the analyses were done in triplicates for each treatment.
Statistical analysis
The data was analyzed statistically using completely randomized design experiment (Cochran and Cox 1975). For each measurement, three replicates of samples were tested per treatment and mean±standard deviation values were reported. Analysis of variance (ANOVA) of the data was performed using MINITAB statistical analysis software package (Minitab, version 11.12, 32 bit, Minitab). Difference between means of data was compared by least significant difference (LSD) and Student’s t test was applied to determine if the difference was statistically significant. Differences at p ≤ 0.05 were considered to be statistically significant. Duncan’s multiple range test was used to compare the mean values at each storage period. Coefficient of correlation was determined by Karl Pearson method.
Results and discussion
Red delicious apples at harvest had 9.4 ± 0.35 kg firmness, 10.6 ± 0.20 0Bx total soluble solids, 0.38 ± 0.02% acidity, 8.2 ± 0.25% total sugars, 4.8 ± 0.14 mg/100 g ascorbic acid, 0.08 ± 0.01 water soluble pectin (%AIS) and 67.2 ± 1.4% juice yield. Results of the change in quality parameters of Red delicious apple due to calcium chloride dip and gamma irradiation during storage are discussed as under.
Firmness
Effect of calcium chloride and gamma irradiation treatments on firmness of apples is depicted in Table 1. Statistical analysis of the data revealed that after 30 days of storage, firmness of control apples and those treated with CaCl2 at levels 0.5 and 1.0% w/v was marginally (p ≥ 0.05) different with respect to each other, but significantly (p ≤ 0.05) lower than rest of the treatments. Among other treatments, firmness of 0.4 kGy irradiated apples and those treated with CaCl2 alone at 1.5 and 2.0% w/v or combination of CaCl2 (0.5 and 1.0% w/v) and gamma irradiation (0.4 kGy) was also statistically non-significant (p ≥ 0.05) with respect to each other over the same storage period. Treatment of CaCl2 (1.5 and 2.0% w/v) in combination with irradiation (0.4 kGy) proved effective in maintaining the significantly (p ≤ 0.05) higher firmness of apples over the same storage period. As the storage period advanced, firmness recorded a further decrease; the decrease was significantly (p ≤ 0.05) higher in control, 0.5 and 1.0% w/v CaCl2 treated apples. After 90 days of storage, control fruits recorded a decrease of 32.9% in firmness as against the 22.3 and 10.6% in 0.4 kGy irradiated fruits and those subjected to combination of CaCl2 and irradiation (2.0% w/v, 0.4 kGy). The retention of firmness in samples either calcified only or calcified and irradiated is due to fact that calcium plays an important role in maintaining cell wall structure by interaction with pectic acids in the cell walls to form calcium pectate (Poovaiah 1986; Conway and Sams 1987). Cell wall integrity is preserved when deesterified pectic acid residues form cross-bridges between negatively charged carboxylic groups and divalent cations such as calcium, thus minimizing pectic substance solubilization (Grant et al. 1973; Krall and Mc Feeters 1998).Also calcium appears to serve as an intermolecular binding agent that stabilizes protein-pectin complexes of the middle lamella (Dey and Brinson 1984), thus plays a role in maintaining cell wall structure by interacting with pectic acids in the cell wall to form calcium pectate. Further, both irradiation as well as calcium is known to delay the natural physiological processes like respiration, ripening and senescence responsible for the solubilization and depolymerization of pectic substances and other cell wall polymers (Izumi and Watada 1994; Agar et al. 1999; Rosen and Kader 1989; Artes et al. 1999; Floros et al. 1992; Kader 1986). Thus, slower decrease of firmness in samples subjected either to calcification or combination of calcification and irradiation is related to delayed physiological processes, thereby resulting in reduction in the rate of increase of soluble pectic fractions. Hence the normal conversion of insoluble to soluble pectins during storage appears to have been markedly retarded both by calcification and irradiation ( El Assi et al. 1997; d’Amour et al. 1993; Howard et al. 1995; Kovacs et al. 1997; Prakash et al. 2002).
Table 1.
Effect of calcium chloride dip and gamma irradiation treatments on firmness, water soluble pectin (WSP) and juice yield of Red Delicious apples during storage under refrigerated conditions
| Storage period (days) | Treatments | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | LSD@0.05 | |
| Firmness (kg) | |||||||||||
| 30 | 8.1 ± 0.30a,C | 8.6 ± 0.11b,C | 8.1 ± 0.25a,C | 8.3 ± 0.23a,C | 8.6 ± 0.30b,C | 8.6 ± 0.25b,C | 8.6 ± 0.30b,C | 8.6 ± 0.11b,C | 9.1 ± 0.14c,C | 9.1 ± 0.23c,C | 0.20 |
| 60 | 7.4 ± 0.13a,B | 8.1 ± 0.25b,B | 7.5 ± 0.23a,B | 7.5 ± 0.22a,B | 7.9 ± 0.21b,B | 8.2 ± 0.25b,B | 8.2 ± 0.23b,B | 8.1 ± 0.26b,B | 8.8 ± 0.15c,B | 8.8 ± 0.11c,B | 0.30 |
| 90 | 6.3 ± 0.25a,A | 7.3 ± 0.23b,A | 6.5 ± 0.33a,A | 6.5 ± 0.31a,A | 7.3 ± 0.30b,A | 7.5 ± 0.30b,A | 7.4 ± 0.25b,A | 7.5 ± 0.26b,A | 7.9 ± 0.18c,A | 8.4 ± 0.12d,A | 0.20 |
| 32.9*f | 22.3* d | 30.8* e | 30.8* e | 22.3* d | 20.2* c | 21.2* c | 20.2* c | 13.8* b | 10.6* a | 1.4 | |
| LSD@0.05 | 0.45 | 0.33 | 0.42 | 0.44 | 0.35 | 0.30 | 0.25 | 0.22 | 0.20 | 0.22 | |
| Water soluble pectin (%AIS) | |||||||||||
| 30 | 0.18 ± 0.02c,A | 0.11 ± 0.03a,A | 0.18 ± 0.02c,A | 0.16 ± 0.01c,A | 0.13 ± 0.02b,A | 0.11 ± 0.01a,A | 0.11 ± 0.02a,A | 0.12 ± 0.03a,A | 0.10 ± 0.02a,A | 0.10 ± 0.02a,A | 0.02 |
| 60 | 0.35 ± 0.03c,B | 0.20 ± 0.03a,B | 0.33 ± 0.04c,B | 0.32 ± 0.02b,B | 0.30 ± 0.01b,B | 0.21 ± 0.03a,B | 0.20 ± 0.03a,B | 0.21 ± 0.04a,B | 0.20 ± 0.03a,B | 0.19 ± 0.02a,B | 0.02 |
| 90 | 0.68 ± 0.04f,C | 0.47 ± 0.03c,C | 0.66 ± 0.04e,C | 0.66 ± 0.03e,C | 0.49 ± 0.02d,C | 0.48 ± 0.04c,C | 0.48 ± 0.03c,C | 0.47 ± 0.02c,C | 0.45 ± 0.01b,C | 0.43 ± 0.02a,C | 0.01 |
| 88.2*,f | 82.9*,c | 87.9*,e | 87.9*,e | 83.7*,d | 83.4*,c | 83.4*,c | 82.9*,c | 82.3*,b | 81.4*,a | 0.50 | |
| LSD@0.05 | 0.06 | 0.05 | 0.07 | 0.06 | 0.05 | 0.06 | 0.06 | 0.05 | 0.04 | 0.04 | |
| Juice yield (%) | |||||||||||
| 30 | 60.2 ± 1.3a,C | 62.6 ± 1.2b,C | 60.2 ± 1.1a,C | 62.2 ± 1.4b,C | 62.4 ± 1.6b,C | 62.6 ± 1.4b,C | 62.6 ± 1.3b,C | 63.5 ± 1.1b,C | 63.7 ± 1.4b,C | 64.2 ± 1.3c,C | 1.5 |
| 60 | 54.5 ± 1.6a,B | 59.8 ± 1.5c,B | 55.1 ± 1.4a,B | 58.3 ± 1.4b,B | 58.6 ± 1.1b,B | 59.2 ± 1.5b,B | 59.5 ± 1.4b,B | 60.2 ± 1.3c,B | 60.7 ± 1.2c,B | 62.4 ± 1.2d,B | 1.3 |
| 90 | 47.8 ± 1.2a,A | 54.5 ± 1.1b,A | 48.2 ± 1.4a,A | 53.2 ± 1.1b,A | 53.7 ± 1.4b,A | 54.2 ± 1.1b,A | 54.4 ± 1.2b,A | 55.4 ± 1.5c,A | 55.8 ± 1.3c,A | 58.8 ± 1.4d,A | 1.6 |
| 28.9*e | 18.7*c | 28.2*e | 20.7*d | 20.0*c | 19.2*c | 18.9*c | 17.4*b | 16.8*b | 12.4*a | 1.5 | |
| LSD@0.05 | 1.1 | 1.4 | 1.2 | 1.6 | 1.2 | 1.3 | 1.5 | 1.6 | 2.0 | 1.6 | |
Values are means ± SD, n = 3; LSD = Least significant difference (p ≤ 0.05); AIS = Alcohol insoluble solids
* = Percentage decrease in firmness/Juice yield and percentage increase in water soluble pectin after 90 days of storage
Values with different superscript uppercase letters (A–C) within a column and superscript lowercase letters (a–f) within a row differ significantly (p ≤ 0.05)
T1 = Control; T2 = 0.4 kGy; T3 = 0.5% CaCl2; T4 = 1.0% CaCl2; T5 = 1.5% CaCl2; T6 = 2.0% CaCl2;
T7 = 0.5% CaCl2, 0.4 kGy; T8 = 1.0% CaCl2, 0.4 kGy; T9 = 1.5% CaCl2, 0.4 kGy; T10 = 2.0% CaCl2, 0.4 kGy
Water soluble Pectin (WSP)
The presence of endogenous and exogenous calcium has stabilizing effect on the integrity of the cell wall, thus minimizing pectic substance solubilization (Buescher and Hobson 1982; Krall and Mc Feeters 1998). As calcium chloride dip concentration increased from 0.5 to 2.0% w/v, increase in WSP levels decreased (p ≤ 0.05) (Table 1). After 30 days of storage, WSP levels of control samples and those treated with CaCl2 at levels 0.5 and 1.0% w/v differed non-significantly with respect to each other. During further storage WSP levels exhibited increasing trend and after 90 days of storage increase was significantly (p ≤ 0.05) higher in control samples than all other treatments. Among the treatments, combination of CaCl2 dip (1.5 and 2.0%) and irradiation (0.4 kGy) maintained (p ≤ 0.05) lowest WSP levels even after 90 days of storage. After 90 days of storage, WSP levels were increase by 88.2% for control as against the 81.4% for the combinatory treatment of CaCl2 dip (2.0%w/v) and irradiation (0.4 kGy). The data also revealed that, there was no significant increase in WSP levels of apples due to irradiation at 0.4 kGy when compared with other treatments, thereby inferring that low dose irradiation does not result in significant solubilization of pectic substance. The lower levels of WSP in samples treated with combination of CaCl2 dip and gamma irradiation is partly attributed to calcium absorption into the cell wall during dipping and to delaying of ripening and enzymatic activity by the synergistic effect of CaCl2 dip and irradiation. This inhibitory effect of CaCl2 and irradiation resulted in reduction in the rate of conversion of insoluble pectin to soluble pectin, thus maintaining the lowest levels of WSP (Hussain et al. 2008; Poovaiah 1986). Our results also revealed that WSP fraction was inversely correlated with firmness (r = 0.88). Similar significant negative correlations were also found by Gunes et al. (2001).
Juice yield
The juice yield of apple samples showed a declining trend during storage irrespective of treatments (Table 1). After 30 days of storage, juice yield of control and calcified (0.5% CaCl2) samples was marginally (p ≥ 0.05) different with respect to each other. Juice yields of 0.4 kGy irradiated samples and those treated with CaCl2 alone at levels 1.0–2.0% w/v or combination of CaCl2 (0.5–1.5% w/v) and irradiation (0.4 kGy) also differed non-significantly after 30 days of storage. Combinatory treatment of 2.0% CaCl2 and 0.4 kGy irradiation proved effective in maintaining significantly (p ≤ 0.05) higher juice yield of apples than all other treatments throughout the whole storage. With progress of storage, juice yield decreased significantly (p ≤ 0.05) when compared with the initial value. After the end of 90 days of storage, juice yield decreased by 28.9% for control, 18.7% for irradiated (0.4 kGy) and 12.4% for samples treated with combination of CaCl2 dip (2.0% w/v) and irradiation (0.4 kGy) respectively. The significantly (p ≤ 0.05) lower decrease in juice yield in samples treated with calcium chloride prior to irradiation can be explained due to the inhibitory effect of combinatory treatments on physiological processes responsible for loss of turgor pressure and membrane integrity (Hussain et al. 2008). Positive correlations (r = 0.78) were obtained between firmness and juice yield, thus explaining the higher firmness of apples treated with combination of CaCl2 dip (2.0% w/v) and gamma irradiation.
Total soluble solids (TSS) and total sugars
The total soluble solids and total sugars of apple samples showed an increasing trend irrespective of treatments (Table 2). After 30 days of storage, increase in TSS and total sugars was significantly (p ≤ 0.05) lower in samples either irradiated, CaCl2 treated at levels above 0.5% w/v or treated with combination of CaCl2 and irradiation. Among treatments, significantly (p ≤ 0.05) lower TSS (10.8) and total sugars (8.1%) were recorded in samples treated with combination of CaCl2 at levels 1.5 and 2.0% w/v and 0.4 kGy irradiation over the same storage period. The data also revealed that CaCl2 treatment up to 1.0% w/v had no significant effect on preventing the TSS as well as total sugar increase when compared with control. Positive correlations (r = 0.78) were obtained between TSS and total sugars, indicating that total sugars increased with increase in TSS. With further advancement of storage, TSS recorded a significant increasing trend. Similar pattern was observed in total sugars during further storage. Among the treatments, increase was significantly (p ≤ 0.05) lower in samples treated with combination of 2.0% w/v CaCl2 and 0.4 kGy irradiation even after 90 days of storage. After 90 days of storage, control samples recorded an increase of 22.1 and 29.9% in TSS and total sugars as against the 10.2 and 17.2% in samples subjected to combination of CaCl2 dip (2.0% w/v) and irradiation (0.4 kGy).The increase in TSS and total sugars is attributed to the enzymatic conversion of higher polysaccharides such as starches and pectins in to simple sugars during ripening (Hussain et al. 2008). Since, both CaCl2 and gamma irradiation delay the natural physiological processes like ripening, senescence and respiration, responsible for increase as well as decrease of TSS and total sugars; due to inhibitory effect on the activities of enzymes involved in hydrolysis (Izumi and Watada 1994; Agar et al. 1999; Rosen and Kader 1989; Kader 1986; Artes et al. 1999). Therefore, the synergistic effect of CaCl2 dip and gamma irradiation resulted in delaying the increase in TSS as well as total sugars in samples subjected to combination treatments of CaCl2 and irradiation even after 90 days of storage.
Table 2.
Effect of calcium chloride dip and gamma irradiation treatments on total soluble solids (TSS), total sugars and titratable acidity of Red Delicious apples during storage under refrigerated conditions
| Storage period (days) | Treatments | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | LSD@0.05 | |
| Total soluble solids (0BX) | |||||||||||
| 30 | 11.6 ± 0.14c,A | 11.1 ± 0.11b,A | 11.7 ± 0.22d,A | 11.4 ± 0.20c,A | 11.2 ± 0.14b,A | 11.1 ± 0.21b,A | 11.2 ± 0.15b,A | 11.2 ± 0.30b,A | 10.8 ± 0.22a,A | 10.8 ± 0.24a,A | 0.20 |
| 60 | 12.2 ± 0.13c,B | 11.8 ± 0.25b,B | 12.4 ± 0.23d,B | 12.1 ± 0.22c,B | 12.1 ± 0.21c,B | 11.6 ± 0.25b,B | 11.7 ± 0.23b,B | 11.8 ± 0.26b,B | 11.4 ± 0.15a,B | 11.2 ± 0.11a,B | 0.20 |
| 90 | 13.6 ± 0.25g,C | 12.8 ± 0.23d,C | 13.6 ± 0.33g,C | 13.4 ± 0.31f,C | 13.1 ± 0.30e,C | 12.6 ± 0.30c,C | 12.6 ± 0.25c,C | 12.5 ± 0.26c,C | 12.2 ± 0.18b,C | 11.8 ± 0.12a,C | 0.15 |
| 22.1*f | 17.2*d | 22.1*f | 21.0*f | 19.1*e | 15.9*c | 15.9*c | 15.2*c | 13.1*b | 10.2*a | 1.5 | |
| LSD@0.05 | 0.20 | 0.17 | 0.22 | 0.26 | 0.23 | 0.20 | 0.25 | 0.28 | 0.26 | 0.24 | |
| Total sugars (%) | |||||||||||
| 30 | 9.5 ± 0.15c,A | 8.8 ± 0.21b,A | 9.6 ± 0.22c,A | 8.9 ± 0.23b,A | 8.8 ± 0.24b,A | 8.8 ± 0.21b,A | 8.9 ± 0.25b,A | 8.8 ± 0.22b,A | 8.1 ± 0.22a,A | 8.1 ± 0.20a,A | 0.20 |
| 60 | 10.6 ± 0.23d,B | 9.8 ± 0.22b,B | 10.7 ± 0.23d,B | 10.6 ± 0.24d,B | 10.4 ± 0.24c,B | 9.9 ± 0.25b,B | 9.9 ± 0.20b,B | 9.9 ± 0.26b,B | 9.3 ± 0.21a,B | 9.3 ± 0.16a,B | 0.10 |
| 90 | 11.7 ± 0.25e,C | 10.8 ± 0.23c,C | 11.6 ± 0.33e,C | 11.5 ± 0.31e,C | 11.2 ± 0.30d,C | 10.5 ± 0.30b,C | 10.5 ± 0.25b,C | 10.3 ± 0.26b,C | 10.3 ± 0.18b,C | 9.9 ± 0.1a,C | 0.20 |
| 29.9*f | 24.1*d | 29.3*f | 28.7*f | 26.8*e | 21.9*c | 21.9*c | 20.4*b | 20.4*b | 17.2*a | 1.4 | |
| LSD@0.05 | 0.35 | 0.45 | 0.36 | 0.33 | 0.41 | 0.45 | 0.42 | 0.36 | 0.35 | 0.28 | |
| Titratable acidity (% malic acid) | |||||||||||
| 30 | 0.31 ± 0.30a,C | 0.32 ± 0.11a,C | 0.31 ± 0.25a,C | 0.32 ± 0.23a,C | 0.33 ± 0.30b,C | 0.33 ± 0.25b,C | 0.33 ± 0.30b,C | 0.34 ± 0.11b,C | 0.34 ± 0.14b,C | 0.34 ± 0.23b,B | 0.01 |
| 60 | 0.24 ± 0.13a,B | 0.28 ± 0.25b,B | 0.24 ± 0.23a,B | 0.28 ± 0.22b,B | 0.28 ± 0.21b,B | 0.28 ± 0.25b,B | 0.28 ± 0.23b,B | 0.30 ± 0.26c,B | 0.31 ± 0.15c,B | 0.31 ± 0.11c,A | 0.01 |
| 90 | 0.18 ± 0.25a,A | 0.22 ± 0.23b,A | 0.19 ± 0.33a,A | 0.22 ± 0.31b,A | 0.23 ± 0.30b,A | 0.23 ± 0.30b,A | 0.24 ± 0.25b,A | 0.24 ± 0.26b,A | 0.26 ± 0.18c,A | 0.29 ± 0.12d,A | 0.02 |
| 52.6*g | 42.1*e | 50.0*f | 42.1*e | 39.5*d | 39.5*d | 36.8*c | 36.8*c | 31.6*b | 23.7*a | 1.4 | |
| LSD@0.05 | 0.01 | 0.02 | 0.02 | 0.03 | 0.02 | 0.01 | 0.02 | 0.03 | 0.02 | 0.02 | |
Values are means ± SD, n = 3; LSD = Least significant difference (p ≤ 0.05);
* = Percentage increase in TSS and total sugars after 90 days of storage/Percentage decrease in acidity after 90 days of storage
Values with different superscript uppercase letters (A–C) within a column and superscript lowercase letters (a–g) within a row differ significantly (p ≤ 0.05)
T1 = Control; T2 = 0.4 kGy; T3 = 0.5% CaCl2; T4 = 1.0% CaCl2; T5 = 1.5% CaCl2; T6 = 2.0% CaCl2;
T7 = 0.5% CaCl2, 0.4 kGy; T8 = 1.0% CaCl2, 0.4 kGy; T9 = 1.5% CaCl2, 0.4 kGy; T10 = 2.0% CaCl2, 0.4 kGy
Acidity
The acidity values of apples showed a declining trend during storage irrespective of treatments (Table 2). The decrease in acidity was significantly (p ≤ 0.05) lower in samples treated with CaCl2 alone at levels 1.5 and 2.0% w/v and combination of CaCl2 (0.5–2.0% w/v) and irradiation after 30 days of storage. After 60 day of storage, the decrease in acidity values of samples treated with combination of CaCl2 (at levels above 0.5% w/v) and gamma irradiation was significantly (p ≤ 0.05) lower than rest of the treatments. Towards the end of the storage, combinatory treatment of 2.0% w/v CaCl2 and 0.4 kGy irradiation proved beneficial in maintaining significantly (p ≤ 0.05) higher acidity values of apple samples. After 90 days of storage, control samples recorded a loss of 52.6% in acidity compared to 23.7% in samples treated with combination of CaCl2 (2.0% w/v) and irradiation. The loss of acid values is largely due to the utilization of organic acids as respiratory substrates and as carbon skeleton for the synthesis of new compounds during ripening. Also accumulation of sugars during ripening contributes to decrease of acidity as a result of increase in TSS acid ratio (Wani et al. 2008). The retention of higher values of acidity in samples subjected to combinatory treatments of CaCl2 (2.0% w/v) dip and irradiation (0.4 kGy) is due to the delay in ripening process because of synergistic effect of the treatment.
Ascorbic acid
Ascorbic acid also recorded decreasing trend in all the treatments (Table 3). After 30 days of storage, ascorbic acid of control, 0.5 and 1.0% w/v CaCl2 treated samples differed marginally (p ≥ 0.05) with respect to each other. Among all other treatments, ascorbic acid was significantly (p ≤ 0.05) higher in samples subjected to combination of calcium chloride dip at levels 1.5 and 2.0% w/v and irradiation at 0.4 kGy. Ascorbic acid content of 0.4 kGy irradiated samples and those treated with CaCl2 alone at levels 1.5 and 2.0%w/v also differed non-significantly after 30 days of storage. With advancement of storage, ascorbic acid decreased significantly (p ≤ 0.05). After 90 days of storage, control and 0.5% w/v CaCl2 treated samples recorded a decrease of 45.8% in ascorbic acid as compared to 25% in samples treated with combination of CaCl2 (1.5–2.0% w/v) and irradiation (0.4 kGy). The irradiation alone at 0.4 kGy caused a slight decrease in ascorbic acid, but the decrease was statically non-significant when compared with samples treated with Calcium chloride alone at levels 1.5 and 2.0% w/v. Thus, it can be inferred that main loss of ascorbic acid is due to storage rather than irradiation. The ascorbic acid loss during storage is known to be due to its antioxidant activity especially under postharvest storage conditions (Davey et al. 2000). The beneficial effect of calcium in preventing decline of ascorbic acid during storage is due to the regulation of oxidative processes is the cytosol (Faust and Shear 1972).
Table 3.
Effect of calcium chloride dip and gamma irradiation treatments on ascorbic acid, weight loss and yeast and mold count of Red Delicious apples during storage under refrigerated conditions
| Storage period (days) | Treatments | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | LSD@0.05 | |
| Ascorbic acid (mg/100 g) | |||||||||||
| 30 | 3.8 ± 0.14a,C | 4.1 ± 0.11b,C | 3.8 ± 0.22a,C | 3.8 ± 0.20a,C | 4.1 ± 0.14b,C | 4.1 ± 0.21b,C | 4.1 ± 0.15b,C | 4.2 ± 0.30b,C | 4.5 ± 0.22c,C | 4.5 ± 0.24c,C | 0.20 |
| 60 | 3.2 ± 0.11a,B | 3.7 ± 0.15b,B | 3.4 ± 0.13a,B | 3.4 ± 0.12a,B | 3.6 ± 0.11b,B | 3.7 ± 0.15b,B | 3.6 ± 0.13b,B | 3.7 ± 0.16b,B | 4.1 ± 0.15c,B | 4.1 ± 0.11c,B | 0.20 |
| 90 | 2.6 ± 0.11a,A | 3.1 ± 0.13c,A | 2.6 ± 0.13a,A | 2.8 ± 0.11b,A | 3.1 ± 0.12c,A | 3.1 ± 0.14c,A | 3.0 ± 0.15c,A | 3.2 ± 0.14d,A | 3.6 ± 0.11e,A | 3.6 ± 0.12e,A | 0.10 |
| 45.8*f | 35.4*c | 45.8*f | 41.7*e | 35.4*c | 35.4*c | 37.5*d | 33.4*b | 25.0*a | 25.0*a | 1.5 | |
| LSD@0.05 | 0.20 | 0.20 | 0.30 | 0.20 | 0.30 | 0.30 | 0.30 | 0.40 | 0.20 | 0.20 | |
| Weight loss (%) | |||||||||||
| 30 | 1.6 ± 0.20c,A | 1.2 ± 0.13a,A | 1.6 ± 0.22c,A | 1.4 ± 0.14b,A | 1.4 ± 0.21b,A | 1.3 ± 0.11b,A | 1.2 ± 0.15a,A | 1.2 ± 0.14a,A | 1.1 ± 0.12a,A | 1.1 ± 0.12a,A | 0.10 |
| 60 | 4.1 ± 0.30e,B | 3.0 ± 0.25b,B | 4.1 ± 0.23e,B | 3.5 ± 0.22d,B | 3.2 ± 0.15c,B | 3.2 ± 0.25c,B | 3.1 ± 0.13b,B | 2.9 ± 0.12b,B | 2.6 ± 0.15a,B | 2.4 ± 0.11a,B | 0.20 |
| 90 | 5.8 ± 0.20g,C | 4.1 ± 0.23d,C | 5.6 ± 0.33g,C | 4.9 ± 0.21f,C | 4.6 ± 0.15e,C | 4.4 ± 0.24e,C | 3.9 ± 0.15c,C | 3.8 ± 0.12c,C | 3.4 ± 0.11b,C | 3.0 ± 0.12a,C | 0.20 |
| LSD@0.05 | 0.50 | 0.60 | 0.60 | 0.70 | 0.70 | 0.50 | 0.50 | 0.60 | 0.40 | 0.40 | |
| Yeast and mold count (log cfu/g of sample) | |||||||||||
| 30 | 3.6 ± 0.32c,A | ND | 3.5 ± 0.33b,A | 3.3 ± 0.35a,A | 3.3 ± 0.37a,A | 3.3 ± 0.23a,A | ND | ND | ND | ND | 0.03 |
| 60 | 3.8 ± 0.42c,B | ND | 3.7 ± 0.43b,B | 3.7 ± 0.33b,B | 3.7 ± 0.32b,B | 3.5 ± 0.25a,B | ND | ND | ND | ND | 0.03 |
| 90 | 4.1 ± 0.44e,C | 3.3 ± 0.31b | 4.1 ± 0.41e,C | 3.9 ± 0.42d,C | 3.9 ± 0.31d,C | 3.7 ± 0.20c,C | 3.3 ± 0.32b | 3.1 ± 0.42a | 3.1 ± 0.33a | ND | 0.05 |
| LSD@0.05 | 0.03 | – | 0.03 | 0.05 | 0.05 | 0.04 | – | – | – | – | |
Values are means ± SD, n = 3; LSD = Least significant difference (p ≤ 0.05); ND = Not detected
* = Percentage decrease in ascorbic acid after 90 days of storage
Values with different superscript uppercase letters (A–C) within a column and superscript lowercase letters (a–g) within a row differ significantly (p ≤ 0.05)
T1 = Control; T2 = 0.4 kGy; T3 = 0.5% CaCl2; T4 = 1.0% CaCl2; T5 = 1.5% CaCl2; T6 = 2.0% CaCl2;
T7 = 0.5% CaCl2, 0.4 kGy; T8 = 1.0% CaCl2, 0.4 kGy; T9 = 1.5% CaCl2, 0.4 kGy; T10 = 2.0% CaCl2, 0.4 kGy
Loss in weight
The statistically analysis of data revealed that weight loss of control and 0.5% w/v calcium chloride treated samples differed marginally (p ≥ 0.05) with respect to each other, but differed significantly (p ≤ 0.05) when compared with other treatments at the end of 30 days of storage (Table 3). The weight loss of samples irradiated to 0.4 kGy and those treated with combination of Calcium chloride (0.5–2.0% w/v) and irradiation (0.4 kGy) also differed marginally (p ≥ 0.05) with respect to each other over the same storage period. As the storage period progressed, weight loss also increased (p ≤ 0.05) and was significantly higher in control samples (5.8 ± 0.20) followed by calcium chloride treated apples at levels of 0.5% w/v (5.6 ± 0.33%) at the end of 90 days of storage. The samples treated with combination of CaCl2 dip (2.0% w/v) and irradiation (0.4 kGy) recorded significantly (p ≤ 0.05) lower weight loss than all other treatments over the same storage period. The lower weight loss in samples treated with combination of CaCl2 dip (2.0% w/v) and irradiation (0.4 kGy) is due to the synergistic effect of CaCl2 and gamma irradiation on the delaying of natural physiological processes like respiration, onset of the climacteric, ripening process and senescence (Dong et al. 1994; Wani et al. 2008)
Yeast and mold count
Yeast and mold count of apples was markedly reduced by irradiation alone (0.4 kGy) and in combination with CaCl2 dip treatment (0.5–2.0% w/v). No yeast and mold count was recorded in samples treated with irradiation alone (0.4 kGy) and combination of CaCl2 dip (0.5–2.0% w/v) and irradiation (0.4 kGy) after 30 days of storage (Table 3). Among CaCl2 treated fruits, yeast and mold count was marginally (p ≥ 0.05) different in samples treated at levels above 0.5% w/v after 30 days of storage. After 60 days of storage, yeast and mold count of samples treated with CaCl2 at levels 0.5–1.5% w/v differed marginally (p ≥ 0.05) with respect to each other, but differed significantly (p ≤ 0.05) when compared with samples treated with 2.0% w/v CaCl2. After 90 days of storage yeast and mold count of 4.1 log cfu/g sample was recorded in control and 0.5% w/v CaCl2 treated samples as against the 3.3 log cfu/g sample in 0.4 kGy irradiated samples. In fruits treated with CaCl2 alone at levels above 0.5% w/v or combination of CaCl2 (0.5–1.5% w/v) and irradiation (0.4 kGy), yeast and mold count was in the range of 3.1–3.9 log cfu/ g sample after 90 days of storage. No yeast and mold count was observed in samples treated with combination of 2.0% w/v CaCl2 and 0.4 kGy gamma irradiation even after 90 days of storage. The combination treatment of 2.0% w/v CaCl2 and 0.4 kGy irradiation gave about 4.3 log reduction in yeast and mold count of apple samples. The yeast and molds identified were Candida Sp. and Penicillium Sp.
Post refrigeration studies under ambient conditions (temp. 17 ± 2°C, RH 75%)
Firmness and weight loss
Effect of calcium chloride and gamma irradiation treatments on post refrigeration firmness and weight loss at 17 ± 2°C, RH 75% is depicted in Table 4. Statistical analysis of data revealed that firmness of control, 0.5 and 1.0% w/v CaCl2 treated apples differed marginally (p ≥ 0.05) with respect to each other over the entire storage period and was below detection level after 20 days of storage. Firmness of 0.4 kGy irradiated and those of 1.5% w/v CaCl2 treated samples was below detection level after 25 days of storage. Firmness of samples treated with 2.0% w/v CaCl2 and combination of CaCl2 (0.5–1.0% w/v) and irradiation (0.4 kGy) was below detection level after 30 days of storage. Among the treatments, firmness was detectable up to 30 days only in samples subjected to combination of CaCl2 dip at levels 1.5–2.0% w/v and irradiation (0.4 kGy), however the marketable and acceptable firmness was observed between 20–25 days of storage. Inverse correlations (r = 0.76) were obtained between firmness and weight loss, indicating that firmness decreased with increase in weight loss. Weight loss through transpiration is a major cause of quality deterioration in fresh horticultural crops after harvest. Weight loss not only results in direct quantitative losses, but also causes losses in appearance, textural quality (softening, loss of crispness and juiciness) and nutritional quality. If weight loss is more than 10%, surface of the fruit becomes prone to quality defects like wilting and shriveling and the commodity becomes unmarketable. Data of weight loss revealed that maximum shelf-life of control, 0.5 and 1.0% w/v CaCl2 treated apples is about 10 days at 17 ± 2°C, RH 75% following 90 days of refrigeration. Data further indicated that shelf-life of fruits irradiated at 0.4 kGy and those treated with CaCl2 alone (2.0% w/v) or combination of CaCl2 (0.5 and 1.0% w/v) and irradiation (0.4 kGy) is 15 days under similar storage conditions. On the other hand, shelf-life of fruits subjected to combination treatments of 2.0% w/v CaCl2 and 0.4 kGy irradiation is about 20–25 days at 17 ± 2°C, RH 75% following 90 days of refrigeration.
Table 4.
Effect of calcium chloride dip and gamma irradiation treatments on firmness, weight loss and overall acceptability of Red Delicious apples during additional ambient storage at 17 ± 2°C, RH following refrigeration
| Storage period (days) | Treatments | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | LSD@0.05 | |
| Firmness (kg) | |||||||||||
| 5 | 4.4 ± 0.30a,C | 5.7 ± 0.11b,D | 4.8 ± 0.25a,C | 4.8 ± 0.23a,C | 5.4 ± 0.30b,D | 5.7 ± 0.25b,E | 5.5 ± 0.30b,E | 5.7 ± 0.11b,E | 6.2 ± 0.14c,F | 6.8 ± 0.23d,F | 0.40 |
| 10 | 3.6 ± 0.13a,B | 4.2 ± 0.25b,C | 3.6 ± 0.23a,B | 3.8 ± 0.22a,B | 3.9 ± 0.21a,C | 4.6 ± 0.25c,D | 4.3 ± 0.23b,D | 4.8 ± 0.26c,D | 5.4 ± 0.15d,E | 5.9 ± 0.11e,E | 0.30 |
| 15 | 2.2 ± 0.25a,A | 3.6 ± 0.23c,B | 2.4 ± 0.33a,A | 2.5 ± 0.31a,A | 3.1 ± 0.30b,B | 3.9 ± 0.30c,C | 3.7 ± 0.25c,C | 3.9 ± 0.26c,C | 4.3 ± 0.18d,D | 4.9 ± 0.12e,D | 0.30 |
| 20 | BDL | 2.7 ± 0.23b,A | BDL | BDL | 2.2 ± 0.30a,A | 3.1 ± 0.30c,B | 2.9 ± 0.25b,B | 3.3 ± 0.26 c,B | 3.7 ± 0.18d,C | 4.1 ± 0.12e,C | 0.20 |
| 25 | – | – | – | – | BDL | 2.4 ± 0.30b,A | 2.1 ± 0.25a,A | 2.6 ± 0.26b,A | 3.0 ± 0.18c,B | 3.6 ± 0.12d,B | 0.20 |
| 30 | – | – | – | – | – | BDL | BDL | BDL | 2.4 ± 0.18a,A | 3.0 ± 0.12b,A | 0.20 |
| LSD@0.05 | 0.45 | 0.33 | 0.42 | 0.44 | 0.35 | 0.30 | 0.25 | 0.22 | 0.20 | 0.22 | |
| Weight loss (%) | |||||||||||
| 5 | 7.5 ± 0.20g,A | 5.4 ± 0.13c,A | 7.6 ± 0.22g,A | 6.8 ± 0.14f,A | 6.2 ± 0.21e,A | 5.9 ± 0.11d,A | 5.5 ± 0.15c,A | 5.5 ± 0.14c,A | 4.2 ± 0.12b,A | 3.7 ± 0.12a,A | 0.20 |
| 10 | 10.4 ± 0.30g,B | 6.7 ± 0.25c,B | 10.2 ± 0.23g,B | 9.5 ± 0.22f,B | 7.8 ± 0.15e,B | 7.2 ± 0.25d,B | 6.6 ± 0.13c,B | 6.4 ± 0.12c,B | 5.5 ± 0.15b,B | 5.1 ± 0.11a,B | 0.30 |
| 15 | UM | 8.5 ± 0.23c,C | UM | UM | 9.6 ± 0.15d,C | 9.2 ± 0.24d,C | 8.7 ± 0.15c,C | 8.2 ± 0.12b,C | 7.9 ± 0.11b,C | 7.2 ± 0.12a,C | 0.40 |
| 20 | – | 10.2 ± 0.33c,D | – | – | UM | 10.8 ± 0.15d,D | 10.3 ± 0.12c,D | 10.2 ± 0.11c,D | 9.3 ± 0.12b,D | 8.6 ± 0.13a,D | 0.30 |
| 25 | – | UM | – | – | – | UM | UM | UM | 10.5 ± 0.12b,E | 9.4 ± 0.11a,E | 0.20 |
| 30 | – | – | – | – | – | – | – | – | UM | 10.6 ± 0.12F | – |
| LSD@0.05 | 0.50 | 0.60 | 0.60 | 0.70 | 0.70 | 0.50 | 0.50 | 0.60 | 0.40 | 0.40 | |
| Overall acceptability (OAA) | |||||||||||
| 5 | 3.4 ± 0.30a,C | 3.8 ± 0.11b,D | 3.5 ± 0.25a,C | 3.6 ± 0.23a,C | 3.8 ± 0.30b,D | 3.8 ± 0.25b,D | 3.8 ± 0.30b,D | 3.8 ± 0.11b,D | 4.0 ± 0.14b,D | 4.0 ± 0.23b,D | 0.20 |
| 10 | 2.8 ± 0.13a,B | 3.2 ± 0.25b,C | 3.0 ± 0.23a,B | 3.0 ± 0.22a,B | 3.3 ± 0.21b,C | 3.4 ± 0.25b,C | 3.4 ± 0.23b,C | 3.4 ± 0.26b,C | 3.8 ± 0.15c,D | 3.8 ± 0.11c,D | 0.30 |
| 15 | 2.0 ± 0.25a,A | 2.7 ± 0.23c,B | 2.3 ± 0.33b,A | 2.3 ± 0.31b,A | 2.7 ± 0.30c,B | 2.7 ± 0.30c,B | 2.9 ± 0.25c,B | 2.9 ± 0.26c,B | 3.3 ± 0.18d,C | 3.5 ± 0.12d,C | 0.20 |
| 20 | P | 2.1 ± 0.23a,A | P | P | 2.0 ± 0.30a,A | 2.1 ± 0.30a,A | 2.4 ± 0.25b,A | 2.3 ± 0.26b,A | 2.9 ± 0.18c,B | 3.0 ± 0.12c,B | 0.20 |
| 25 | – | P | – | – | P | P | P | P | 2.5 ± 0.18a,A | 2.9 ± 0.12b,B | 0.20 |
| 30 | – | – | – | – | – | – | – | – | P | 2.5 ± 0.12A | – |
| LSD@0.05 | 0.45 | 0.33 | 0.42 | 0.44 | 0.35 | 0.30 | 0.25 | 0.22 | 0.20 | 0.22 | |
Values are means ± SD, n = 3; LSD = Least significant difference (p ≤ 0.05); BDL = Below detection level; UM = Unmarketable; Values with different superscript uppercase letters (A–F) within a column and superscript lowercase letters (a–g) within a row differ significantly (p ≤ 0.05); P = Poor; T1 = Control; T2 = 0.4 kGy; T3 = 0.5% CaCl2; T4 = 1.0% CaCl2; T5 = 1.5% CaCl2; T6 = 2.0% CaCl2;
T7 = 0.5% CaCl2, 0.4 kGy; T8 = 1.0% CaCl2, 0.4 kGy; T9 = 1.5% CaCl2, 0.4 kGy; T10 = 2.0% CaCl2, 0.4 kGy
Overall acceptability
Overall acceptability based on texture, taste and visual appearance was significantly (p ≤ 0.05) lower in control, 0.5 and 1.0% w/v CaCl2 treated samples after first 5 days of ambient storage following refrigeration (Table 4). The overall acceptability of all other treatments was marginally (p ≥ 0.05) different with respect to each other over the same storage period. After 15 days of ambient storage, overall acceptability was significantly (p ≤ 0.05) higher in samples treated with combination of CaCl2 at levels 1.5 and 2.0% w/v and 0.4 kGy irradiation. Beyond 20 days of storage, overall acceptability of all the samples was rated poor except for those subjected to combination treatment of CaCl2 at levels 1.5 and 2.0% w/v and 0.4 kGy gamma irradiation. Overall acceptability of samples treated with combination of 2.0% w/v CaCl2 and 0.4 kGy irradiation was rated almost good (2.9) on a 4-point scale even after 25 days of ambient storage following 90 days of refrigeration. The higher overall acceptability of samples subjected to the above mentioned combinatory treatment is due to the synergistic effect of the treatment on delaying the processes responsible for decaying, solubilization of pectic substance and loss of volatiles. Hence the higher retention of quality attributes like firmness and appearance in sample treated with combination of CaCl2 (2.0% w/v) dip and irradiation (0.4 kGy) was responsible for their higher overall acceptability values.
Conclusion
The study reveals that combination treatment of CaCl2 dip (2.0% w/v) and gamma irradiation (0.4 kGy) proved significantly (p ≤ 0.05) effective in maintaining the quality of Red Delicious apples during storage. Results of the post refrigeration weight loss and other quality parameters like firmness and overall acceptability revealed that combination treatment was helpful in extending the shelf-life of Red Delicious apples by around 20–25 days at 17 ± 2°C, RH 75% following 90 days of refrigeration.
References
- Agar IT, Massantini R, Hess-Pierce B, Kader AA. Post harvest CO2 and ethylene production and quality maintenance of fresh cut kiwi fruit slices. J Food Sci. 1999;64:433–440. doi: 10.1111/j.1365-2621.1999.tb15058.x. [DOI] [Google Scholar]
- Alak KS, Goswami TK. Controlled atmosphere storage of fruits and vegetables: a review. J Food Sci Technol. 2006;43(1):1–7. [Google Scholar]
- Aneja KR (1996) Experiments in microbiology, plant pathology, tissue culture and Mushroom cultivation. 2nd edn, New Age International (P) Ltd., New Delhi
- Anon (2007–2008) Annual Report, Department of Horticulture, Directorate of Horticulture, Jammu and Kashmir, Rajbagh, Srinagar, p 101–102
- Official methods of analysis. 15. Washington DC: Association of Official Analytical Chemists; 1984. [Google Scholar]
- Artes F, Conesa MA, Hermandez S, Gill MI. Keeping quality of fresh-cut tomato. Postharvest Biol Technol. 1999;17:153–162. doi: 10.1016/S0925-5214(99)00044-7. [DOI] [Google Scholar]
- Baskaran R, Devi AU, Nayak CA. Effect of low dose gamma irradiation on the shelf life and quality characteristics of minimally processed potato cubes under modified atmosphere packaging. Rad Phy Chem. 2007;76:1042–1049. doi: 10.1016/j.radphyschem.2006.10.004. [DOI] [Google Scholar]
- Bidawid S, Farber JM, Sattar SA. Inactivation of hepatitis A virus (HAV) in fruits and vegetables by gamma irradiation. Int J Food Microbiol. 2000;57:91–97. doi: 10.1016/S0168-1605(00)00235-X. [DOI] [Google Scholar]
- Boonzaaijer G, Bobeldijik I, Osenbruggen WA. Analysis of patulin in dutch food, an evaluation of a SPE based method. Food Con. 2005;16:587–591. doi: 10.1016/j.foodcont.2004.06.020. [DOI] [Google Scholar]
- Buescher RW, Hobson GE. Role of calcium and chelating agents in regulating the degradation of tomato fruit tissue by polygalacturonase. J Food Biochem. 1982;61:147–160. doi: 10.1111/j.1745-4514.1982.tb00682.x. [DOI] [Google Scholar]
- Castaldo D, Villari G, Laratta B, Impembo M, Giovane A, Fasanaro G, Servillo L. Preservation of high-consistency diced tomatoes by immersion in calcifying solutions: a pilot study. J Agric Food Chem. 1996;44:2600–2607. doi: 10.1021/jf9602030. [DOI] [Google Scholar]
- Cochran WG, Cox GM. Experimental designs. New York: John Willey and Sons. Inc; 1975. pp. 95–100. [Google Scholar]
- Conway WS, Sams CE. The effect of post harvest infiltration of calcium, magnesium or strontium on decay, firmness, respiration and ethylene production in apples. J Am Soc Hort Sci. 1987;112:300–303. [Google Scholar]
- d’Amour J, Gosselin C, Arul J, Castaigne F, Willemot C. Gamma irradiation affects cell wall composition of strawberry. J Food Sci. 1993;58:182–185. doi: 10.1111/j.1365-2621.1993.tb03239.x. [DOI] [Google Scholar]
- Davey MW, Montagu MV, Inze D, Sanmartin M, Kanellis A, Smirnoff N, Benzie IJJ, Strain JJ, Favell D, Fletcher J. Plant L-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. J Sci Food Agric. 2000;80:825–860. doi: 10.1002/(SICI)1097-0010(20000515)80:7<825::AID-JSFA598>3.0.CO;2-6. [DOI] [Google Scholar]
- DeRuiter FE, Dwyer J. Consumer acceptance of irradiated foods: dawn of a new era? Food Ser Technol. 2002;2:47–58. doi: 10.1046/j.1471-5740.2002.00031.x. [DOI] [Google Scholar]
- Dey PM, Brinson K. Plant cell walls. Adv Carbohy Chem Biochem. 1984;42:265–382. doi: 10.1016/S0065-2318(08)60127-4. [DOI] [Google Scholar]
- Dong CZ, Montillet JL, Triantaphylides C. Effect of gamma-irradiation on the plasma membrane of suspension- cultivated apples cells. Rapid irreversible inhibition of H + -AT Pase activity. Physiol Plant. 1994;90:307–312. doi: 10.1111/j.1399-3054.1994.tb00392.x. [DOI] [Google Scholar]
- Dong X, Wrolstad RE, Sugar D. Extending shelf life of fresh-cut pears. J Food Sci. 2000;65:181–186. doi: 10.1111/j.1365-2621.2000.tb15976.x. [DOI] [Google Scholar]
- Drusch S, Kopka S, Kaeding J. Stability of patulin in a juice like aqueous model system in the presence of ascorbic acid. Food Chem. 2007;100:192–197. doi: 10.1016/j.foodchem.2005.09.043. [DOI] [Google Scholar]
- El Assi N, Huber DJ, Brecht JK. Irradiation induced changes in tomato fruit and pericarp firmness, electrolyte efflux, and cell wax enzyme activity as influenced by ripening stage. J Ame Soc Hort Sci. 1997;122:100–106. [Google Scholar]
- Fan X, Niemera BA, Mattheis JP, Zhuang H, Olson DW. Quality of fresh-cut apple slices as affected by low dose ionizing radiation and calcium ascorbate treatment. J Food Sci. 2005;70(2):143–148. doi: 10.1111/j.1365-2621.2005.tb07119.x. [DOI] [Google Scholar]
- Faust M, Shear CB. The Effect of calcium on respiration of apples. J Ame Soc Hort Sci. 1972;97:437–439. [Google Scholar]
- Floros JD, Ekanayake A, Abide GP, Nelson PE. Optimization of a diced tomato to calcification process. J Food Sci. 1992;57:1144–1148. doi: 10.1111/j.1365-2621.1992.tb11284.x. [DOI] [Google Scholar]
- Grant GT, Morris ER, Rees DA, Smith PJC, Thom D. Biological interactions between polysaccharides and divalent cations: the egg-box model; Fed. Eu Biochem Soc /Let. 1973;32:195–198. doi: 10.1016/0014-5793(73)80770-7. [DOI] [Google Scholar]
- Gunes G, Hotchkiss JH, Watkins CB. Effect of gamma irradiation on the texture of minimal processed apple slices. J Food Sci. 2001;66:63–67. doi: 10.1111/j.1365-2621.2001.tb15582.x. [DOI] [Google Scholar]
- Howard LR, Miller GH, Wagner AB. Microbiological, chemical and sensory changes in irradiated Pico de gallo. J Food Sci. 1995;60:461–464. doi: 10.1111/j.1365-2621.1995.tb09803.x. [DOI] [Google Scholar]
- Hussain PR, Dar MA, Meena RS, Mir MA, Shafi F, Wani AM. Changes in quality of apple (Malus domestica) cultivars due to gamma irradiation and storage conditions. J Food Sci Technol. 2008;45(1):44–449. [Google Scholar]
- Izumi H, Watada AE. Calcium treatments affect storage quality of shredded carrots. J Food Sci. 1994;59:106–109. doi: 10.1111/j.1365-2621.1994.tb06908.x. [DOI] [Google Scholar]
- Kader AA. Potential applications of ionizing radiation in post harvest handling of fresh fruits and vegetables. Food Technol. 1986;6:117–121. [Google Scholar]
- King BL, Josephson ES. Action of radiation on protozoa and helminths. In: Josephson ES, Peterson MS, editors. Preservation of foods by ionizing radiation. Boca Raton: CRC; 1982. pp. 245–267. [Google Scholar]
- Kovacs E, Van Burn JP, Pitifer LA, Hoch HC, Terhume BT. Effect of irradiation and storage on cell wall structure of Golden Delicious and Empire apples. Acta Aliment. 1997;26:171–190. [Google Scholar]
- Kovacs E, Keresztes A, Kovacs J. The effect of gamma irradiation and calcium treatment on the ultra structure of apples and pears. Food Microstru. 1998;7:1–14. [Google Scholar]
- Krall SM, Mc Feeters RF. Pectin hydrolysis; effect of temperature, degree of methylation. pH, and calcium on hydrolysis rates. J Agric Food Chem. 1998;46:1311–1315. doi: 10.1021/jf970473y. [DOI] [Google Scholar]
- Lacroix M, Jobin M, Laterille B, Laponite M, Gagnon M. Hot water immersion and irradiation effect on mangoes keeping quality after air shipment from Thailand and Canada. Microbial Alim Nutr. 1991;9:155–160. [Google Scholar]
- Lai CL, Fuh YM, Shih DYC. Detection of mycotoxin patulin in apple juice. J Food Drug Anal. 2000;8:85–96. [Google Scholar]
- Luna-Guzman I, Barrett DM. Comparison of calcium chloride and calcium lactate effectiveness in maintaining shelf stability and quality of fresh cut Cantaloupes. Postharvest Biol Technol. 2000;19:61–72. doi: 10.1016/S0925-5214(00)00079-X. [DOI] [Google Scholar]
- Niemira BA, Fan X, Sokoria KJB, Sommers CH. Ionizing radiation sensitivityof Listeria monocytogenes and L. innocua inoculated on endive (Cichorium endive) J Food Prot. 2003;66(6):993–998. doi: 10.4315/0362-028x-66.6.993. [DOI] [PubMed] [Google Scholar]
- Poovaiah BW. Role of calcium in prolonging storage life of fruits and vegetables. Food Technol. 1986;40:86–89. [Google Scholar]
- Prakash A, Inthajak P, Huibregste H, Caporaso F, Foley DM. Effect of low dose gamma irradiation and conventional treatments on shelf life and quality characteristics of diced celery. J Food Sci. 2000;65(6):1070–1075. doi: 10.1111/j.1365-2621.2000.tb09420.x. [DOI] [Google Scholar]
- Prakash A, Manley J, DeCosta S, Caporaso F, Foley DM. The effect of gamma irradiation on the microbiological, physical and sensory qualities of diced tomatoes. Radiat Phys Chem. 2002;63:387–390. doi: 10.1016/S0969-806X(01)00529-1. [DOI] [Google Scholar]
- Ranganna S (1986) Handbook of analysis and quality control for fruit and vegetable Products. 2nd ed. Tata MacGraw Hill Pub Co New Delhi
- Rosen JC, Kader AA. Postharvest Physiology and quality maintenance of sliced pear and strawberry fruits. J Food Sci. 1989;54:656–659. doi: 10.1111/j.1365-2621.1989.tb04675.x. [DOI] [Google Scholar]
- Roy SK (1993) Research achievement in the field of post-harvest technology of fruits and vegetables. Proc workshop Appropriate Technology of Agro-Processing. CIPHET Ludhina p 26
- Shephard GS, Leggott NL. Chromatographic determination of the mycotoxin patulin in fruit and fruit juices. J Chromatogr A. 2000;882:17–22. doi: 10.1016/S0021-9673(99)01341-2. [DOI] [PubMed] [Google Scholar]
- Spalding DH, Reeder WF. Decay and acceptability of mangoes treated with combination of hot water, immazalel and gamma irradiation. Plant Dise. 1986;11:49–51. [Google Scholar]
- Varga J, Rigo K, Toth B, Teren J, Kozakiewicz Z. Evolutionary relationships among Asprgillus species producing economically important mycotixins. Food Technol Biotechnol. 2003;41:29–36. [Google Scholar]
- Wani AM, Hussain PR, Meena RS, Dar MA. Effect of gamma irradiation and refrigerated storage on the improvement of quality and shelf life extension of pear (Pyrus communis L., Cv. Bartlett/William) Rad Phy Chem. 2008;77:983–989. doi: 10.1016/j.radphyschem.2008.04.005. [DOI] [Google Scholar]
- Yun HJ, Lim SY, Yang SH, Lee WY, Kwon JH, Lim BL, Kim DH. Effect of gamma irradiation on the growth and patulin production of Penicillium griseofulvum in an apple model system. Food Sci Biotechnol. 2008;17(4):723–727. [Google Scholar]
- Zegota H, Zegota A, Bachmann S. Effect of irradiation and storage on patulin disappearance and some chemical constituents of apple juice concentrate. Z Lebensm Unters Forsch. 2001;187:321–324. doi: 10.1007/BF01454421. [DOI] [PubMed] [Google Scholar]


