Abstract
The less explored, commercially available foxtail millet-milled fractions like whole flour & bran rich fraction were studied for its antioxidant potency. Phytochemicals like alkaloids, phenolics, reducing sugars and flavonoids were found only in methanolic & aqueous extracts, while tannins and terpenoids were present in all the solvent extracts of whole flour & bran rich fraction. Antioxidants were extracted using methanol, ethanol and water. Methanolic extracts of whole flour and bran rich fraction exhibited a significantly higher (P < 0.05) radical scavenging activity (44.62% & 51.80% respectively) using DPPH model system, and reducing power (0.381 & 0.455 respectively) at 2 mg, than the other solvents used for extraction. As bran rich fraction showed the highest antioxidant activity, suggesting the presence of antioxidant components in the bran layer.
Keywords: Antioxidant activity, Commercial foxtail millet, Phytochemicals, Whole flour, Bran rich fraction
Introduction
Natural antioxidants have gained considerable interest in recent years for their role in preventing auto oxidation of fats and oils. Both synthetic and naturally occurring substances may possess health-promoting potential. Since the public call for an ‘additive free’ and ‘natural’ diet, major interest has been devoted to substances naturally occurring in the diet (Verhagen 1993; Caragay 1992). Grains contribute to the significant supply of antioxidant to prevent oxidative stress due to the fact that grains are used as a staple food and are consumed in larger amount in our diets (Choi et al. 2007). Recent epidemiological studies have suggested that increased consumption of whole grains, fruits and vegetables is associated with reduced risks of chronic diseases (Hu 2002). This may be attributed to the presence of natural antioxidants from plant foods such as vitamin C, tocopherol, carotenoids and polyphenols which prevent free radical damage (Diplock et al. 1998). Millets are known to contain phenolic acids, which are located in the pericarp, testa, aleurone layer and endosperm (Hahn et al. 1984). Millets are rich in phenolics acids, tannins and phytate which act as ‘antinutrients’ (Thompson 1993). However, it is now established that these antinutrients are known to reduce the risk for colon and breast cancer in animals (Graf and Eaton 1990). However, millets have been the subject of less interest in any research.
Foxtail millet (Setaria italica) is one of the minor millets, containing high amounts of proteins and minerals (Pawar and Pawar 1997). Simple processing methods like dehulling, soaking and cooking are reported to result in significant decreases in antinutrients and improved bioavailability of minerals like iron and zinc and also protein digestibility (Vithal and Machewad 2006). Extensive information on processing and nutrient composition (Geervani and Eggum 1989) and little information on its antioxidant activity (Asharani et al. 2010) are available. However, these reports are on the foxtail millet varieties developed by Agricultural research institutes. But no study has been reported on the commercially available (market sample) foxtail millet. The foxtail millet grains are processed (dehusking and polishing) before they reach the market, to make them edible and suitable for human consumption. In general dehulling or dehusking and milling process may cause high dehulling losses due to their small grain sizes and are known to affect the taste and keeping quality of millets (Gulia et al. 2007). Our interest was to know the antioxidant potency of commercially available foxtail millet, as this is what the consumers are eventually going to consume. Therefore, in the present study, the less explored commercially available foxtail millet was analyzed for its antioxidant activity and also screened for the presence of phytochemicals using various solvent extracts.
Materials and methods
Chemicals
1, 1-Diphenyl-2-Picryl Hydrazyl (DPPH) was procured from HiMedia Lab Pvt Ltd. (Mumbai, India). BHT was procured form Qualigens fine chemicals (Mumbai, India). All other reagents used were of analytical grade.
Sample preparation
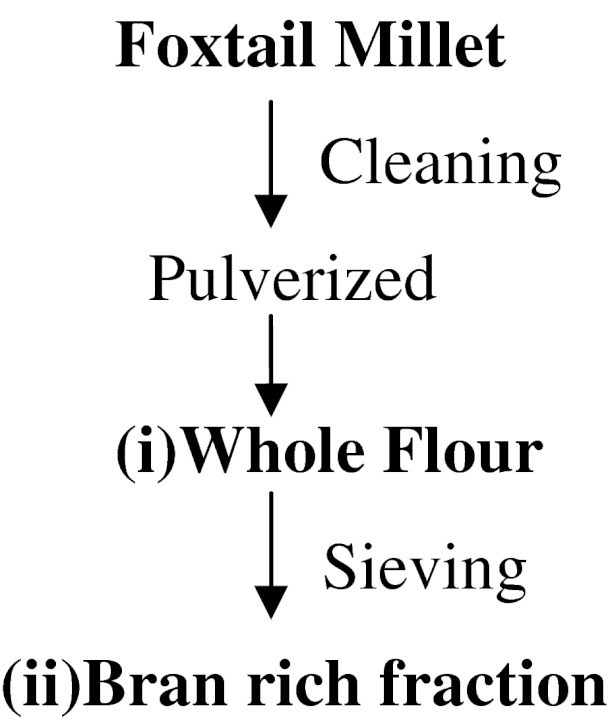
Seeds of foxtail millet were purchased from the local market which was already dehusked and polished (10 degree polishing). It was further cleaned to remove extraneous and foreign material, pulverized in a plate mill into whole flour. A part of the whole flour was sieved through a 44 mesh sieve (BSS) to separate the bran rich fraction (‘+’ fraction) (Fig. 1). Whole flour and the bran rich fraction was stored in air tight containers for further use.
Fig. 1.
Flow chart for production of foxtail millet—milled fractions
Preparation of antioxidant extracts
Whole flour and bran rich fraction (15 g) was extracted with 200 mL of solvent (methanol, ethanol and water respectively) in a mechanical shaker for 24 h, filtered and evaporated to dryness under reduced pressure in a rotary evaporator (Superfit, India). The concentrated methanolic, ethanolic and aqueous extracts were re-dissolved with respective solvents, to a concentration of 4 mg/ml and stored in the refrigerator until analysis.
Screening of phytochemicals
Whole flour & bran rich fraction was subjected to sequential extraction using solvents (petroleum ether  benzene
benzene  chloroform
chloroform  methanol
methanol  water) and screened for the presence of various phytochemicals like alkaloids, tannins, phenols, flavanoids and saponins (Mojah et al. 2003), while triterpenoids, steroids and reducing sugars was screened according to the method of Trease and Evans (1996).
water) and screened for the presence of various phytochemicals like alkaloids, tannins, phenols, flavanoids and saponins (Mojah et al. 2003), while triterpenoids, steroids and reducing sugars was screened according to the method of Trease and Evans (1996).
DPPH radical scavenging activity
The ability of the extracts (methanolic, ethanolic and aqueous extracts) to scavenge free radicals was determined against a very stable free radical DPPH determined spectrometrically (Blois 1958). Aliquot of the sample extract at different concentrations were added to 1 mM methanolic solution of DPPH. The mixture was vortexed vigorously and left for 30 min at room temperature in the dark. The absorbance was measured at 517 nm and activity was expressed as percentage DPPH scavenging relative to control using the following equation:
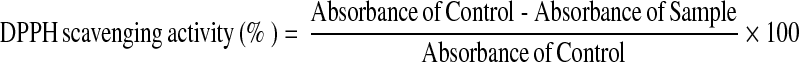 |
Reducing power assay
The ability of extracts (methanolic, ethanolic and aqueous extracts) to reduce iron (III) to iron (II) was assessed by the method of Yildrim et al. 2001. The dried extract (125–1,000 μg) in 1 ml of the corresponding solvent was mixed with 2.5 ml of phosphate buffer (0.2 M, pH 6.6) and 2.5 ml of potassium ferricyanide (K3Fe (CN)6; 10 g l−1), and then the mixture was incubated at 50°C for 30 min. After incubation, 2.5 ml of trichloroacetic acid (100 g l−1) was added and the mixture was centrifuged at 1,650 rpm for 10 min. Finally, 2.5 ml of the supernatant solution were mixed with 2.5 ml of distilled water and 0.5 ml of FeCl3 (1 g l−1) and the absorbance was measured at 700 nm. High absorbance indicates high reducing power.
Statistical analysis
All the experiments were conducted in triplicates and the results were reported as mean values with their respective standard deviations. The data was subjected to analysis of variance (ANOVA) test and the differences between the means were compared for their significance using data analysis tool pack.
Results and discussions
Phytochemical screening
The results of phytochemical screening of foxtail millet whole flour and bran rich fraction are presented in Table 1. Phytochemicals such as terpenoids, and tannins were detected in all the solvent extracts of whole flour and bran rich fractions, while steroids were tested negative in all the solvents used for extraction. Saponin was detected in the aqueous extracts only. Flavonoids, alkaloids, phenolics and reducing sugars were detected in methanol and aqueous extracts. Triterpenoid were detected in the benzene, methanol and aqueous extracts of bran rich fraction; however for petroleum ether and chloroform extracts triterpenoids were detected in whole flour as well as for bran rich fractions. There was a wide variation in detecting the presence of phytochemicals due to differences in the polarity of the extracting solvents. Because methanol is a relatively polar organic solvent compared to other extracting solvents, most of the phytochemicals detected in this study are expected to be polar in nature. The health benefits of whole grains are attributed in part to their unique phytochemical composition (Adom and Liu 2002). Besides the health benefits associated with phytochemicals, these compounds have important functional properties. Phytochemicals in grains contribute to product quality in terms of color, flavor and texture (Holtekjolen et al. 2006). Bound Phytochemicals could survive stomach and intestinal digestion to reach the colon. This may partly explain the mechanism of grain consumption in the prevention of colon cancer, other digestive cancers, breast cancer, and prostate cancer, which is supported by epidemiological studies
Table 1.
Phytochemical screening in the sequential extraction of whole flour & bran rich fraction
| Phytochemical | Pet. ether | Benzene | Chloroform | MtOH | Water | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| WF | BRF | WF | BRF | WF | BRF | WF | BRF | WF | BRF | |
| Flavonoids | − | − | − | − | − | − | ++ | ++ | ++ | ++ |
| Alkaloids | − | − | − | − | − | − | + | ++ | ++ | + |
| Terpenoids | ++ | ++ | + | + | + | + | ++ | ++ | + | + |
| Triterpenoids | ++ | ++ | − | ++ | + | ++ | − | ++ | − | + |
| Steroids | − | − | − | − | − | − | − | − | − | − |
| Tannins | + | + | + | + | + | + | + | + | + | + |
| Phenolics | − | − | − | − | − | − | ++ | ++ | ++ | ++ |
| Saponins | − | − | − | − | − | − | − | − | + | + |
| Reducing sugars | − | − | − | − | − | − | ++ | ++ | ++ | ++ |
MtOH methanol, WF whole flour, BRF bran rich fraction, + traces, ++ average, − absence
Antioxidant activity of the foxtail millet fractions
It is well-known that free radicals cause autoxidation of unsaturated lipids in food. On the other hand, antioxidants are believed to break up the free radical chain of oxidation and donate hydrogen there by forming a stable end product, which does not propagate further oxidation of the lipids (Kaur and Perkins 1991; Sherwin 1978). The antioxidants in foxtail millet whole flour and bran rich fraction was extracted using three solvents (Methanol, ethanol and water) at a concentration of 4 mg/ml. The free radical scavenging potentials of various solvent extracts from foxtail millet- whole flour and bran at different concentrations were tested by the DPPH method and the results are shown in Figs. 2 & 3 respectively. Antioxidant reacts with DPPH, which is a stable free radical, and converts it to α,α-diphenyl-β- picryl hydrazine. The degree of discoloration indicates the scavenging potentials of the antioxidant extracts (Singh et al. 2002). There was a significant difference (P < 0.05) in the DPPH free radical scavenging activity for each solvent at increasing concentrations (0.4–2.0 mg) for both the milled fractions. At lower concentrations (0.4–1.2 mg) aqueous extract showed a significantly higher (P < 0.05) radical scavenging activity when compared to the other solvents in whole flour. While at higher concentrations (1.6–2.0 mg) methanolic extract showed considerably higher radical scavenging activity compared to ethanol and aqueous extracts. Reports have suggested that relatively higher antioxidant activity was observed from methanolic extracts in grains compared to the other solvents including acetone, diethyl ether, ethyl acetate and water (Oki et al. 2000; Ziehnski and Kozlowska 2000). A similar trend was observed in case of bran rich fraction of the foxtail millet where the radical scavenging activity was highest in the methanolic extract (51.8%) followed by alcohol (42.90%) and water (33.60%).This indicates that the methanolic extracts followed by ethanol and aqueous extracts showed a higher radical scavenging activity (2.0 mg), however BHT showed a significantly higher (P < 0.05) radical scavenging activity compared to foxtail millet fraction extracts.
Fig. 2.
Radical scavenging activity of foxtail millet—whole flour extracts (4 mg/Ml) & BHT by DPPH method at different concentrations (n = 4)
Fig. 3.
Radical scavenging activity of foxtail millet—bran rich fraction extracts (4 mg/Ml) & BHT by DPPH method at different concentrations (n = 4)
Reducing power assay
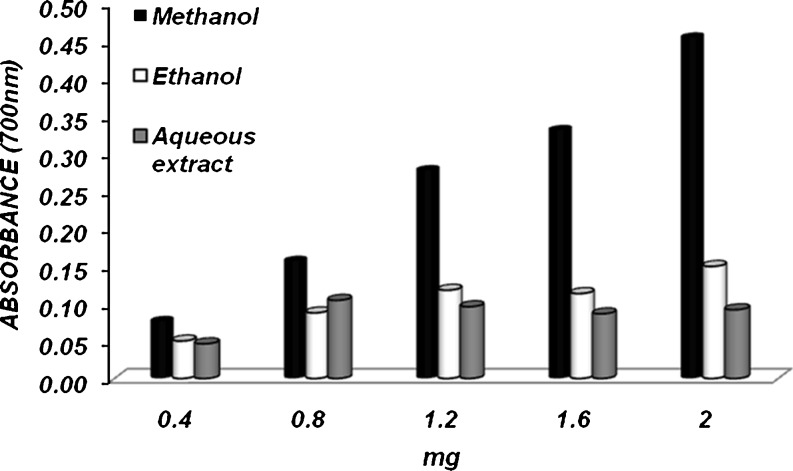
While iron is essential for oxygen transport, respiration, and activity of enzymes it is a reactive metal that catalyzes oxidative damage in the living tissues and cells (Miller 1996). The ferric reducing antioxidant potential of various extracts (4 mg/ml) of whole flour and bran fraction of foxtail millet is shown in Figs. 4 & 5. In this method ferric-ferricyanide complex is reduced to the ferrous form depending in the presence of antioxidants (Amarowicz et al. 2004).The reducing power assay of methanolic and ethanolic extracts in the whole flour showed a significantly (P < 0.05) higher reducing power (A700 = 0.381 & 0.341) respectively followed by aqueous extract (A700 = 0.120) at a concentration of 2.0 mg. The reducing power of methanolic extract was relatively higher than that of the previous findings on foxtail millet (Choi et al. 2007). In the bran fraction, methanolic extract showed the maximum activity (A700 = 0.455) compared to alcoholic and aqueous extracts (A700 = 0.150 & 0.092 respectively) at 2.0 mg. This indicates that methanolic extracts showed a higher ferric reducing antioxidant potential compared to aqueous and ethanolic extracts.
Fig. 4.
Ferric reducing power of foxtail millet—whole flour extracts (4 mg/Ml) at different concentrations (n = 4)
Fig. 5.
Ferric reducing power of foxtail millet—bran rich fraction extracts (4 mg/Ml) at different concentrations (n = 4)
Studies have shown that consumption of whole grains is associated with reduced risk of chronic diseases. Phytochemicals are biologically active organic substance of plant origin having disease preventing and health promoting properties. The screening of these phytochemicals may help in identifying the active compounds responsible for disease prevention. Most of the phytochemicals like polyphenols, Flavonoids are found to have antioxidant activity (Narasinga Rao 2003). In the present investigation, phytochemical screening revealed the presence of flavonoids, phenolics, tannins along with other components which are potent antioxidants. The results indicated that the extraction with methanol showed high antioxidant activity followed by aqueous and ethanolic extracts. High antioxidant activity was observed in the extracts from bran rich fraction compared to whole flour, suggesting the presence of antioxidant components in the bran rich layer. Hence, it can be concluded that the commercially available foxtail millet is a potent source of antioxidants. Further work is in progress to explore its role as an antioxidant in food system.
Acknowledgement
The authors express their sincere thanks to CSIR- New Delhi for the award of Senior Research Fellowship.
References
- Adom KK, Liu RH. Antioxidant activity of grains. J Agric Food Chem. 2002;50(21):6182–6187. doi: 10.1021/jf0205099. [DOI] [PubMed] [Google Scholar]
- Amarowicz R, Pegg RB, Rahimi-Maghaddam P, Barl B. Free radical scavenging capacity and antioxidant selected plant species from the Canadian prairies. Food Chem. 2004;84:551–562. doi: 10.1016/S0308-8146(03)00278-4. [DOI] [Google Scholar]
- Asharani VT, Jayadeep A, Malleshi NG. Natural antioxidants in edible flours of selected small millets. Int J Food Prop. 2010;13:41–50. doi: 10.1080/10942910802163105. [DOI] [Google Scholar]
- Blois MS. Antioxidant determination by the use of a stable free radical. Nature. 1958;26:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Caragay AB. Cancer- Preventive foods and ingredients. Food Technol. 1992;46(4):65. [Google Scholar]
- Choi Y, Jeong H-S, Lee J. Antioxidant activity of methanolic extracts from some grains consumed in Korea. Food Chem. 2007;103:130–138. doi: 10.1016/j.foodchem.2006.08.004. [DOI] [Google Scholar]
- Diplock AT, Charleux LJ, Crozier-Willi G, Kok FT, Rice-Evan C, Roberfroid M. Functional food science and defence against reactive oxidative species. Br J Nutr. 1998;80S:S97–S112. doi: 10.1079/bjn19980106. [DOI] [PubMed] [Google Scholar]
- Geervani P, Eggum BO. Nutrient composition and protein quality of minor millets. Plant Foods Hum Nutr. 1989;39:201–208. doi: 10.1007/BF01091900. [DOI] [PubMed] [Google Scholar]
- Graf E, Eaton JW. Antioxidant functions of phytic acid. Free Radic Biol Med. 1990;8(1):61–69. doi: 10.1016/0891-5849(90)90146-A. [DOI] [PubMed] [Google Scholar]
- Gulia SK, Wilson JP, Carter J, Singh BP. Progress in grain pearl millet research and market development. In: Janik J, Whipkey A, editors. Issues in new crops and new uses. Alexandria: ASHS; 2007. [Google Scholar]
- Hahn DH, Rooney LW, Earp CF. Tannins and phenols of sorghum. Cereal Foods World. 1984;29:776–779. [Google Scholar]
- Holtekjolen AK, Kinitz C, Knutsen SH. Flavanol and bound phenolic acid contents in different barely varieties. J Agric Food Chem. 2006;54(6):2253–2260. doi: 10.1021/jf052394p. [DOI] [PubMed] [Google Scholar]
- Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- Kaur J, Perkins J. The free radical chemistry of food additives. In: Aruoma OI, Halliwell B, editors. In free radicals and food additives. London: Taylor and Francis; 1991. pp. 17–35. [Google Scholar]
- Miller DM. Mineral. In: Fennema OR, editor. Food chemistry. 3. New York: Marcel Dekker; 1996. pp. 618–647. [Google Scholar]
- Mojab F, Kamalinejad M, Ghaderi N, Vahidipour HR (2003) Phytochemical screening of some species of Iranian plants. Iran J Pharm Res 77–82
- Narasinga Rao BS. Bioactive phytochemicals in Indian foods and their potential in health promotion and disease prevention. Asia Pac J Clin Nutr. 2003;12(1):9–22. [PubMed] [Google Scholar]
- Oki T, Masuda M, Kkbayashi M, Nishiba Y, Furuta S, Suda I. Polymeric procyanidins as radical-scavenging components in red-hulled rice. J Agric Food Chem. 2000;50:7524–7529. doi: 10.1021/jf025841z. [DOI] [PubMed] [Google Scholar]
- Pawar VS, Pawar VD. Malting characteristics and biochemical changes of foxtail millet. J Food Sci Technol. 1997;34(5):416–418. [Google Scholar]
- Sherwin ER. Oxidation and antioxidants in fat and oil processing. J Am Oil Chem Soc. 1978;55:809–814. doi: 10.1007/BF02682653. [DOI] [Google Scholar]
- Singh RP, Chimdambara Murthy KN, Jayaprakash GK. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J Agr Food Chem. 2002;50:4791–4795. doi: 10.1021/jf0255735. [DOI] [PubMed] [Google Scholar]
- Thompson LV. Potential health benefits and problems associated with antinutrients in foods. Food Res Int. 1993;26:131–149. doi: 10.1016/0963-9969(93)90069-U. [DOI] [Google Scholar]
- Trease GE, Evans WE. A textbook of pharmacognosy. 14. London: Baillier Tinall Ltd; 1996. [Google Scholar]
- Verhagen H (1993) Cancer prevention by natural food constituents. Int Food Ingred
- Vithal DP, Machewad GM. Processing of foxtail millet for improved nutrient availability. J Food Process Preserv. 2006;30:269–279. doi: 10.1111/j.1745-4549.2006.00064.x. [DOI] [Google Scholar]
- Yildrim A, Mavi A, Kara AA. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem. 2001;49:4083–4089. doi: 10.1021/jf0103572. [DOI] [PubMed] [Google Scholar]
- Ziehnski H, Kozlowska H. Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. J Agric Food Chem. 2000;48:2008–2016. doi: 10.1021/jf990619o. [DOI] [PubMed] [Google Scholar]