Abstract
The present study was conducted to examine the effects of sonication treatments (time intervals of 0, 15, 30, 45 and 60 min.) on phenolics and other antioxidant compounds in starfruits extracted in methanol and water. Overall, methanolic extracts exhibited significantly higher extractability, percentage inhibition of DPPH radicals, ferric reducing antioxidant property (FRAP) value, antioxidant capacity, flavonoids, total phenolics and tannins (p < 0.05) compared to control (0 min) and aqueous extracts. Methanolic extract obtained after 30 min of sonication proved to be the best treatment with regard to various parameters evaluated. Results of the present study clearly indicated sonication treatments to be effective in enhancing the antioxidant compounds in starfruit extracts and could be further explored for commercial purposes to benefit the consumers.
Keywords: Antioxidants, Extraction, Polyphenols, Sonication, Star fruits
Introduction
Incorporation of fresh fruits and vegetables in the normal diet is known to promote health and can overcome some of the degenerative diseases like cardiovascular disease, ageing, brain dysfunction and cancer (Alothman et al. 2009). The positive health effects are linked to the presence of phenolic compounds, which exhibits rich antioxidant activities (Oboh and Ademosun 2011). These facts has led the researchers all over the world to explore the potential of enhancing the bioactive compounds in fresh plant products (fruits and vegetables), especially antioxidants by application of various simple and reliable food processing techniques (Bhat et al. 2011a, b). Ultrasound (sonication) treatment is an emerging, food processing technology, which posses varied applications when applied alone or in combination with other food processing methods. Sonication treatments have been reported to be effective for microbial decontamination along with enhancing certain quality parameters of fruit juices (Rawson et al. 2010; Bhat et al. 2011b). This treatment is considered to be advantageous due to its reduced processing time with lower energy consumption and being environmental friendly (Mason et al. 2005; Tiwari et al. 2008). It has been reported (Wang and Weller 2006) that ultrasound treatments are inexpensive, simple, reliable, and can be an effective alternative to conventional extraction techniques. Furthermore, the same authors have also stated that the major benefits of using ultrasound treatments in solid-liquid extraction include the enhancement of extraction yield and faster kinetics and like Soxhlet extraction, a wide range of solvents can be used for extracting natural bioactive compounds.
Starfruit or carambola (Averrhoa carambola L.) is a popular juicy fruit grown widely in the tropical and subtropical regions of the world. Fresh fruits (matured with greenish-yellow tinge) are widely used as a basic raw material for preparation of clarified juices, jellies, confectionaries and jam (Bhat et al. 2011a). Star fruits is reported to be low in sugar, sodium and acid, and rich in phenolic compounds like (−) epicatechin, vitamin C, proanthocyanidins and carotenoids (Shui and Leong 2004). Nevertheless, no detailed studies have been reported on the antioxidant capacity and the effects of sonication treatments on star fruits. Therefore, the main objective of the present study was (i) to comparatively evaluate the efficacy and/or efficiency of different solvents (methanol and aqueous) on the extraction of polyphenols and antioxidant compounds and (ii) to study the effects of sonication treatment at different time intervals on these compounds in star fruits.
Materials and methods
Fruit samples and chemicals: Fresh star fruit samples were purchased from the local wet market in Penang, Malaysia. All the fruits used in the study were of eating quality with uniform shape, size, color and ripening stage. The selected fruits were devoid of any apparent physical or microbial damage. Further, before use, fruits were washed with water (tap water followed by distilled water, 2–3 times) to remove the surface dirt and adhering particles. Chemicals: 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,4,6-tri (2-pyridyl)-s-triazine (TPTZ), Folin–Ciocalteu reagent, vanillin, sodium carbonate, aluminium chloride hexahydrate, ferric chloride, sodium acetate, ascorbic acid, gallic acid, catechin were purchased from Sigma Chemicals, Germany. All the solvents used in this study were of analytical grade and purchased from Fisher Scientific Sdn. Bhd, Malaysia.
Extraction and ultrasound treatment
Five individual groups (for each time interval including control sample) of fruit slices (25 g) were homogenized in a kitchen blender with methanol and distilled water (1:10, sample to solvent ratio) as extracting solvents. Further, the homogenized samples (juice) along with solvent were kept in a beaker and sonicated at different time intervals of 15, 30, 45 and 60 min. at room temperature (25 ± 1 °C). The ultrasonic treatment was performed using an ultrasonic cleaner (42 kHz, 135 W; Branson ultrasonic corporation, USA). The solvent surface in the beaker was kept at the same level of water in the ultrasonic bath, maintained at a constant room temperature (25 ± 1 °C) and was monitored by an oven thermometer. Whenever a slight change or increase in the temperature in the ultrasonic bath was observed, fresh water was circulated to maintain the stability of water temperature at 25 °C. In order to avoid and minimize the evaporative loss of methanol, the beaker was covered with the aluminium foil. Additionally, all the sonication treatments were performed in the dark to avoid interference of light. After the extraction, the sample residues were dissolved in a known quantity of the respective solvents (100 mL, water and methanol) and were repeatedly extracted until the extracts became clear. The obtained extract was then filtered through Whatman No. 1 filter paper and the filtrate was concentrated in a rotary evaporator (Buchi Rotavapor R-215, Switzerland) under controlled vacuum. The concentrated extract was then dried in a freeze dryer (Labconco, Free Zone 6 Liter, USA) and kept at 4 °C in air tight container until further analysis.
The percentage yield of the samples was calculated as:
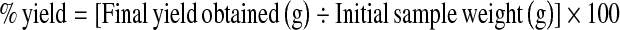 |
Antioxidant activity
Measurement of free radical scavenging activity by DPPH assay
The DPPH radical (1,1-diphenyl-2-picrylhydrazyl) scavenging activity of the methanolic and aqueous extracts of star fruit was measured according to the method described by Blois (1958). The DPPH radical scavenging activity was calculated as:
 |
where, Ao is the absorbance of the control and A1 is the absorbance of the extracts.
Phosphomolybdenum assay
The method described by Prieto et al. (1999) was adapted to measure the antioxidant capacity of the two extracts. A calibration curve was prepared using a standard solution of ascorbic acid (25–300 μg mL−1) and the antioxidant activity was expressed relative to that of ascorbic acid.
Ferric reducing antioxidant property (FRAP assay)
The ability of the sample extracts to reduce ferric ions was measured according to the modified method described by Benzie and Strain (1996). Ferrous sulphate solution with the concentration ranging from 0.1 to 1 μM was used for the preparation of standard calibration curve. The FRAP values were expressed as mM ferrous equivalents per gram of plant material.
Antioxidant compounds
Total phenolics, tannins and flavonoids
The total phenolics content of the extracts was determined using the Folin-Ciocalteu (FC) assay based on the method of Singleton and Rossi (1965) whereas vanillin-HCl method with slight modifications was used to measure the total tannins concentrations in the extracts (Bhat et al. 2007).
The total flavonoids in the extracts were determined based on the method described by Sakanaka et al. (2005). The results were expressed as mg of (+)- catechin equivalent per gram of extract.
Statistical analysis
The results of the present study are presented as mean values of three replicates ± standard deviation. A one way analysis of variance was performed (ANOVA) and the significant differences between mean values were determined by Tukey’s pair wise test at a level of significance of p < 0.05. The statistical analyses were carried out using SPSS 12.01 (SPSS Inc USA).
Results and discussion
In the present study, two different solvent systems: methanol and water were used for extraction of antioxidant compounds from starfruits at 4 different time intervals (15, 30, 45 and 60 min) of sonication with ‘0 min’ serving as control. Earlier, similar experimental setup as that of the present study on the influence of different time intervals of sonication on the extractability of bioactive compounds from different plants and their products have been reported with encouraging results (Li et al. 2007; Paniwnyk et al. 2009). Hence, this encouraged us to take up the present study on starfruits.
The results obtained in the present study are highlighted in Table 1. With regard to aqueous extracts, control samples (extract obtained without sonication or ‘0 min’) showed significantly higher phenolics and antioxidant activities compared to extracts obtained after sonication treatments. A significant (p < 0.05) time dependent decrease in the percentage inhibition of DPPH radicals, FRAP value, antioxidant capacity, total phenolics, total tannins, and total flavonoids were recorded at the prolonged sonication intervals (15, 30, 45 and 60 min). According to the available reports (Mason and Petrier 2004; Paniwnyk et al. 2009) addition of water to a system reduces the antioxidant levels due to poor solubility in the extraction solvent. Also, the generation of free radicals like hydroxyl and peroxyl during sonication process might possibly degrade and react with the antioxidants in the extract.
Table 1.
Effects of sonication treatments on antioxidants in aqueous and methanolic extracts of star fruit*
| Extract | Percentage yield | Percentage inhibition1 | FRAP value2 | Phosphomoly-bdenum assay3 | Total phenolics4 | Total flavonoids5 | Total tannins6 |
|---|---|---|---|---|---|---|---|
| Aqueous extract | |||||||
| 0 min | 4.4 ± 0.10ab | 40.5 ± 0.96c | 1.14 ± 0.03c | 128.9 ± 1.53d | 60.5 ± 0.55c | 28.8 ± 0.35c | 12.4 ± 0.28c |
| 15 min | 4.8 ± 0.12c | 39.6 ± 0.35bc | 1.09 ± 0.01b | 126.2 ± 0.69c | 58.8 ± 0.60bc | 27.6 ± 0.13b | 11.7 ± 0.18b |
| 30 min | 4.7 ± 0.08c | 38.1 ± 0.75ab | 1.06 ± 0.00ab | 122.7 ± 0.31b | 58.5 ± 1.15bc | 26.4 ± 0.35a | 11.2 ± 0.14a |
| 45 min | 4.3 ± 0.10a | 38.0 ± 0.43ab | 1.04 ± 0.01a | 120.2 ± 0.72a | 55.3 ± 0.57a | 26.8 ± 0.61ab | 10.9 ± 0.19a |
| 60 min | 4.6 ± 0.10bc | 37.6 ± 0.82a | 1.08 ± 0.01ab | 123.1 ± 0.81b | 56.9 ± 0.71ab | 27.2 ± 0.48ab | 11.2 ± 0.14ab |
| Methanolic extract | |||||||
| 0 min | 4.8 ± 0.12b | 68.6 ± 1.80a | 2.1 ± 0.01a | 174.1 ± 3.35a | 120.8 ± 0.30a | 63.8 ± 2.24a | 26.9 ± 0.18a |
| 15 min | 4.8 ± 0.08b | 77.2 ± 1.53b | 2.3 ± 0.02b | 179.3 ± 0.83ab | 122.3 ± 1.55b | 65.3 ± 2.32ab | 26.1 ± 0.45a |
| 30 min | 4.7 ± 0.24b | 87.4 ± 0.41d | 2.4 ± 0.00c | 192.7 ± 2.81c | 142.0 ± 0.25e | 79.7 ± 2.09c | 31.8 ± 0.42c |
| 45 min | 4.3 ± 0.16a | 83.3 ± 0.59c | 2.3 ± 0.01b | 182.9 ± 3.44b | 127.0 ± 0.62d | 68.4 ± 1.31ab | 29.2 ± 0.19b |
| 60 min | 4.6 ± 0.10b | 79.1 ± 1.14b | 2.2 ± 0.05a | 185.1 ± 3.78bc | 124.8 ± 0.50c | 69.3 ± 0.61b | 27.0 ± 0.45a |
*Values are the means of three independent replicates ± standard deviation (S.D.). Means in the same column with different superscripts letters denotes significant differences from each other at p < 0.05
1Percentage inhibition of DPPH radicals by samples (100 μg/mL); 2mM ferric reduction to ferrous/g extract (FRAP, Ferric Reducing Antioxidant Power); 3mg ascorbic acid equivalent antioxidant activity/g extract; 4mg gallic acid equivalent/g extract; 5mg catechin equivalent/g extract; 6mg catechin equivalent/g extract
With regard to methanolic extracts, the evaluated parameters like percentage inhibition of DPPH radicals, antioxidant capacity, FRAP value, total phenolics, total tannins, and total flavonoids showed significant increase (p < 0.05) at the treatment time of 30 min, which later on decreased slightly (at 45 and 60 min), but the values were still significantly higher than the control samples (‘0 min’) (p < 0.05). The increase in the antioxidant activities in methanol extracts can be attributed to the differences in the polarity and viscosity, which is much higher compared to solvents with low viscosity, low density and high diffusivity. These features makes it possible for the solvent like methanol to easily diffuse into the pores of the plant materials (plant cell) and leach-out the bioactive compounds (like that of antioxidants) more effectively (Hemwimol et al. 2006; Naczk and Shahidi 2006). Whereas, the decrease in some of the parameters after the extended time duration (at 45 and 60 min) might be attributed to the lower concentration gradient of the solvent, prolonged interval of sonication as well as to the probable degradation that could have occurred due to the generation of free radicals, mainly the highly reactive hydroxyl radicals.
As indicated in Table 1, the methanolic extracts revealed higher antioxidant activities over aqueous extracts. The percentage inhibition of DPPH radicals, FRAP value, total phenolics, total tannins, and total flavonoids of methanolic extracts of starfruit were two fold higher than the aqueous extracts. Solvent extraction of phenolics and antioxidant compounds by using methanol, ethanol, acetone or ethyl acetate have been routinely employed for extraction from fresh fruits (Alothman et al. 2009; Wijekoon et al. 2011). The recovery of polyphenols and antioxidant from plant materials is known to be influenced by the solubility of these compounds in a particular solvent used for extraction process, and is dependent on the solvent polarity (Naczk and Shahidi 2006; Annegowda et al. 2011). In each case, the same plant material might exhibit varied results mainly due to the varied chemical characteristics of antioxidant compounds that necessitate the use of solvents with different polarities to obtain high yield of antioxidants. Sonication treatments using methanol as an extraction solvent has been reported to enhance the bioactive compounds in plants and their products, thus supporting our observations (Wang et al. 2008; Paniwnyk et al. 2009).
Sonication treatment induces microstreaming effect and can enhance the mass transfer produced on the cavitational bubble collapse. This in turn results in the cell wall destruction, thus providing better contact and interactions of solvents in and out of the plant materials. Additionally, sonication treatment has been reported to improve the solvents extractability of materials at low temperatures (Sališová et al. 1997; Vinatoru et al. 2001; Albu et al. 2004).
Generally, when using normal conventional extraction procedures, phenolic compounds including various antioxidants degrade when mild thermal treatments are used. Accordingly, this might not provide optimal yields or accurate results for the evaluated antioxidants. In this regard, ultrasound extraction has been reported to be much better wherein it can provide better optimal yield at lower temperatures (Wu et al. 2001; Xia et al. 2006; Paniwnyk et al. 2009).
Conclusions
In the present study, sonication treatment significantly enhanced various antioxidants in the methanolic extracts (up to 30 min) of the starfruit compared to aqueous extracts. The results also highlight the importance of the solvent system used for ultrasound mediated extractions. Overall, sonication treatments being a physical mode of food processing holds high potential to be explored for industrial applications as an effective environment-friendly method for enhancing the extractability of natural antioxidants, and thus could effectively play a significant role in preventing several physiological and degenerative diseases in consumers.
Acknowledgments
Conflict of interest
No conflict of interest exists in this manuscript.
References
- Albu S, Joyce E, Paniwnyk L, Lorimer JP, Mason TJ. Potential for the use of ultrasound in the extraction of antioxidants from Rosmarinus officinalis for the food and pharmaceutical industry. Ultrason Sonochem. 2004;11:261–265. doi: 10.1016/j.ultsonch.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Alothman M, Bhat R, Karim AA. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009;115:785–788. doi: 10.1016/j.foodchem.2008.12.005. [DOI] [Google Scholar]
- Annegowda HV, Bhat R, Tze LM, Karim AA, Mansor SM (2011) The free radical scavenging and antioxidant activities of pod and seed extract of Clitoria fairchildiana (Howard) - an underutilized legume. J Food Sci Technol (in Press, Corrected Proof, Available online 12 April 2011. doi:10.1007/s13197-011-0370-8) [DOI] [PMC free article] [PubMed]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bhat R, Sridhar KR, Yokotani KT. Effect of ionizing radiation on antinutritional features of velvet bean seeds (Mucuna pruriens) Food Chem. 2007;103:860–866. doi: 10.1016/j.foodchem.2006.09.037. [DOI] [Google Scholar]
- Bhat R, Ameran SB, Karim AA, Liong MT. Quality attributes of starfruit (Averrhoa carambola L.) juice treated with ultraviolet radiation. Food Chem. 2011;127:641–644. doi: 10.1016/j.foodchem.2011.01.042. [DOI] [PubMed] [Google Scholar]
- Bhat R, Nor Shuaidda Bt Che Kamaruddin, Liong M-T, Karim AA (2011b). Sonication improves kasturi lime (Citrus microcarpa) juice quality. Ultrason Sonochem 18:1295–1300 [DOI] [PubMed]
- Blois MS. Antioxidant determination by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Hemwimol S, Pavasant P, Shotipruk A. Ultrasound-assisted extraction of anthraquinones from roots of Morinda citrifolia. Ultrason Sonochem. 2006;13:543–548. doi: 10.1016/j.ultsonch.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Li JW, Ding S-D, Ding XL. Optimization of the ultrasonically assisted extraction of polysaccharides from Zizyphus jujuba cv. Jinsixiaozao J Food Eng. 2007;80:176–183. doi: 10.1016/j.jfoodeng.2006.05.006. [DOI] [Google Scholar]
- Mason TJ, Petrier C. Ultrasound processes. In: Parson S, editor. Advanced oxidation processes for water and wastewater treatment. London: IWA; 2004. pp. 185–208. [Google Scholar]
- Mason TJ, Riera E, Vercet A, Lopez-Bueza P. Application of ultrasound. In: Sun D-W, editor. Emerging technologies for food processing. Amsterdam: Elsevier Academic; 2005. pp. 323–351. [Google Scholar]
- Naczk M, Shahidi F. Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J Pharm Biomed Anal. 2006;41:1523–1542. doi: 10.1016/j.jpba.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Oboh G, Ademosun AO (2011) Characterization of the antioxidant properties of phenolic extracts from some citrus peels. J Food Sci Technol (in Press, Corrected Proof, Available online 22 January 2011. doi:10.1007/s13197-010-0222-y) [DOI] [PMC free article] [PubMed]
- Paniwnyk L, Cai H, Albu S, Mason TJ, Cole R. The enhancement and scale up of the extraction of antioxidant from Rosmarius officinalis using ultrasound. Ultrason Sonochem. 2009;16:287–292. doi: 10.1016/j.ultsonch.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a Phosphomolybdenum Complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Rawson A, Tiwari BK, Patras A, Brunton N, Brennan C, Cullen PJ, O’Donnell CP (2010) Effect of thermosonication on bioactive compounds in watermelon juice. Food Res Int (in Press, Corrected Proof, Available online 15 July 2010. doi:10.1016/j.foodres.2010.07.005)
- Sakanaka S, Tachibana Y, Okada Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha) Food Chem. 2005;89:569–575. doi: 10.1016/j.foodchem.2004.03.013. [DOI] [Google Scholar]
- Sališová M, Toma Š, Mason TJ. Comparison of conventional and ultrasonically assisted extractions of pharmaceutically active compounds from Salvia officinalis. Ultrason Sonochem. 1997;4:131–134. doi: 10.1016/S1350-4177(97)00032-1. [DOI] [PubMed] [Google Scholar]
- Shui G, Leong LP. Analysis of polyphenolic antioxidants in star fruit using liquid chromatography and mass spectrometry. J Chromatogr A. 2004;1022:67–75. doi: 10.1016/j.chroma.2003.09.055. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Tiwari BK, Muthukumarappan K, O’Donnell CP, Cullen PJ. Effects of sonication on the kinetics of orange juice quality parameters. J Agric Food Chem. 2008;56:2423–2428. doi: 10.1021/jf073503y. [DOI] [PubMed] [Google Scholar]
- Vinatoru MT, Paniwnyk L, Mason TJ. Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrason Sonochem. 2001;8:137–142. doi: 10.1016/S1350-4177(01)00071-2. [DOI] [PubMed] [Google Scholar]
- Wang L, Weller CL. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci Technol. 2006;17:300–312. doi: 10.1016/j.tifs.2005.12.004. [DOI] [Google Scholar]
- Wang L, Li D, Bao C, You J, Wang Z, Shi Y, Zhang H. Ultrasonic extraction and separation of anthraquinones from Rheum palmatum L. Ultrason Sonochem. 2008;15:738–746. doi: 10.1016/j.ultsonch.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Wijekoon MMJO, Bhat R, Karim AA (2011) Effects of extraction solvents on the phenolic compounds and antioxidant activities of bunga kantan (Etlingera elatior. Jack) inflorescence. J Food Comp Anal (Available online, doi:10.1016/j.jfca.2010.09.018)
- Wu J, Lin L, Chau F. Ultrasound-assisted extraction of ginseng saponins from ginseng roots and cultured ginseng cells. Ultrason Sonochem. 2001;8:347–352. doi: 10.1016/S1350-4177(01)00066-9. [DOI] [PubMed] [Google Scholar]
- Xia T, Shi S, Wan X. Impact of ultrasonic-assisted extraction on the chemical and sensory quality of tea infusion. J Food Eng. 2006;74:557–560. doi: 10.1016/j.jfoodeng.2005.03.043. [DOI] [Google Scholar]


