Abstract
Onion solid wastes were analysed using HPLC and LC / MS and the major polyphenols detected were quercetin, quercetin 4′-O-glucoside, accompanied by protocatechuic acid and a benzofuranone derivative. The latter two compounds have been demonstrated as some of the degradation products that might arise as a result of peroxidase-mediated decomposition of quercetin. These four substances, along with two principal enzymes implicated in flavonols breakdown, β-glycosidase and peroxidise, were monitored throughout an examination period of 48 h, in ground onion solid waste. The determinations showed that quercetin 4′-O-glucoside content increased by 13.3%, while quercetin, benzofuranone derivative and protocatechuic acid contents increased by 68.6, 37.5 and 58.4%, respectively. β-Glycosidase activity exhibited fluctuations and increased by 38.2%, whereas the POD showed a constant increasing trend, leading in 21.7% higher activity. On such a ground, a hypothesis was set up to explain transformations of flavonols.
Keywords: Food wastes, Phenolics, Onion, Oxidation, Quercetin
Introduction
The increasing consumer interest in healthier foods has led industries in the search of the most convenient and cost-effective technologies to incorporate in traditional foods functional ingredients, which are claimed to contribute to an improved health status. In this concept, there has been a considerable interest in natural antioxidants, which might prevent deleterious reactions in foods, but also provide the organism a “shield” against detrimental biochemical reactions implicated in cell damage (Kapiszewska et al. 2005; Valenzuela et al. 2003).
The trend for fortification of foods with natural polyphenols, which are considered as functional ingredients but also protect labile compounds from oxidation, has led in the search of rich residual sources, such as food by-products and wastes. Although fruit processing residues have gained prominent attention (Djilas et al. 2009), vegetable wastes have been a subject of current research, with regard to their potential as residual sources of bioactive substances (Domínguez-Perles et al. 2010; Stojceska et al. 2008).
Onions are a unique source of dietary flavonoids and other bioactive substances (Corzo-Martínez et al. 2007; Griffiths et al. 2002; Lanzotti 2006; Slimestad et al. 2007; Rodriguez et al. 2009, 2010) and there has been in vivo evidence for the hypolipidemic and antioxidant effects of onion consumption (Park et al. 2007; Vidyavati et al. 2010). It is estimated that more than 450,000 tonnes of onion solid waste is produced each year in Europe (Moure et al. 2001). The non-edible portions of the onion bulb, which are considered as waste of onion consumption and processing, contain phenolics with very peculiar structures, not encountered in the edible parts (Furusawa et al. 2003; Ly et al. 2005; Ramos et al. 2006). However, recent studies on the recovery of flavonols from onion solid wastes clearly showed that mainly quercetin and to a lesser extent, quercetin glucosides are the major phenolics in onion trimmings and the outer dry layers, and account for strong antioxidant effects (Beesk et al. 2010; Khiari et al. 2008; Kiassos et al. 2009; Suh et al. 1999).
Plant material that is discarded as waste is usually not processed immediately after generation, and because of inherent instability factors, such as high moisture levels and degrading enzymes, the value-added compounds to be recovered might suffer extensive decomposition. On this conceptual basis, this study was dealt with the transformations that might take place in major flavonols occurring in onion wastes, as a result of the activity of two principal enzymes implicated, β-glycosidase (β-GL) and peroxidise (POD). The investigations included the detection of compounds that might represent key-decomposition products of quercetin, in an effort to identifying possible routes and mechanisms of flavonol decomposition in onion wastes.
Materials and methods
Chemicals
All solvents used for chromatographic analyses were of HPLC grade. Protocatechuic acid, p-nitrophenyl-β-D-glucoside (p-NGP), and quercetin were from Sigma (St. Louis, MO, U.S.A.). Hydrogen peroxide was from Merck (Darmstadt, Germany).
Onion waste and treatments
Onions were purchased from a local grocery (Chania, Crete). The outer dry layers and the apical trimmings (Khiari et al. 2008) were manually separated from the bulb with a sharp cutter and ground in a domestic blender, to facilitate contact of phenolic substrates with degrading enzymes. The ground material was left to macerate for 48 h under regular ambient conditions. Samples were taken after predetermined time intervals for chromatographic and enzyme analyses.
β-Glycosidase activity
A lot of 0.5 g of onion waste was ground with a pestle and a mortar, with 5 mL acetate buffer (0.1 M, pH 4.6) and 200 mg PVPP (polyvinyl polypyrrolidone). The paste formed was centrifuged at 3000 g for 15 min, and the clear supernatant was used as the crude enzyme source. An aliquot of 0.1 mL of p-NPG (9 mM) was mixed with 0.8 mL acetate buffer (0.1 M, pH 4.6) and 0.1 mL crude enzyme extract and incubated for 15 min at 50 °C, in a water bath. Following this, 0.5 mL of sodium carbonate solution (0.2 M) was added and the absorbance was obtained at 400 nm. One enzyme unit (U) was defined as A400 min−1 and the specific activity (υs) as U mg−1 of protein. Control samples were prepared by replacing enzyme extract with buffer solution.
Peroxidase activity
Extraction of the enzyme was performed as for β-glycosidase assay, but sodium phosphate buffer (50 mM, pH 7) was used instead of acetate buffer. To assay peroxidase activity, 0.1 mL of quercetin solution (0.5 mM in dimethylformamide–DMF), and 0.1 mL of extract were mixed in a 1.5-mL microcentrifuge tube. The reaction was initiated by adding 0.8 mL H2O2 (1 mM in phosphate buffer, pH 7). The decrease in absorbance at 370 nm (λmax of quercetin) was recorded using a HP8452A diode array spectrophotometer (Hirota et al. 1998). One peroxidase unit was defined as ΔA370 min−1 and the specific activity (υs) as U mg−1 of protein. Control samples were prepared by replacing enzyme extract with buffer solution.
Protein determination
Protein content was determined according to Bradford 1976, using bovine serum albumin as standard.
Sample preparation for HPLC and LC / MS
An amount of 1 g of waste sample was placed in a 30-mL glass vial with 20 mL MeOH and stirred on a magnetic stirrer at 400 rpm for 20 min. Following this, the extract was filtered through paper filter to remove debris. This procedure was repeated twice more. The combined filtrates were taken to dryness in vacuo (T ≤ 40 °C) and reconstituted in 5 mL MeOH. All samples were filtered through 0.45 μm syringe filters prior to chromatographic analyses.
HPLC analysis
The equipment utilized was an HP 1090, series II liquid chromatograph, coupled with an HP 1090 diode array detector and controlled by Agilent ChemStation software. The column was a LiChrosphere RP18, 5 μm, 250 × 4 mm (Merck), protected by a guard volume packed with the same material. Both columns were maintained at 40 °C. Eluent (A) and eluent (B) were 0.1% aqueous trifluoroacetic acid (TFA) and MeCN/water (6/4, v/v), containing 0.1% TFA, respectively. The flow rate was 1 mL min−1, and the elution programme used was as follows; 5 min., 0% B; 55 min., 100% B; 65 min., 100% B. Monitoring of the eluate was performed at 290 nm. Results were reported as peak area at 290 nm per g of dry waste.
LC / MS
A Finnigan MAT Spectra System P4000 pump was used coupled with a UV6000LP diode array detector and a Finnigan AQA mass spectrometer. Analyses were carried out on a Superspher RP-18, 125 × 2 mm, 4 μm, column (Macherey-Nagel, Germany), protected by a guard column packed with the same material, and maintained at 40 °C. Analyses were carried out employing electrospray ionization (ESI) at the positive ion mode, with acquisition set at 12 and 80 eV, capillary voltage 3.5 kV, source voltage 4.9 kV, detector voltage 650 V and probe temperature 450 °C. Eluent (A) and eluent (B) were 2.5% aqueous acetic acid and MeOH, respectively. The flow rate was 0.33 mL min−1 and the elution programme used was as follows; 0–5 min., 80% A; 5–25 min., 20% A; 25–30 min., 80% B. The eluate was probed at 290 nm.
Statistical analyses
All determinations were carried out at least in triplicate and values were averaged and given along the standard deviation (±S. D.). For all statistics, Microsoft Excel™ 2000 and SigmaPlot 11™ were used.
Results and discussion
An important issue that arises in the exploitation of plant food residual sources is undisputedly the stability of the material to be used for the recovery of value-added substances. With respect to onions, although there has been substantial evidence for the usefulness of extracts, deriving mainly from the outer inedible layers, as antioxidants (Kefalas and Makris 2006; Škerget et al. 2009), antibrowning (Lee 2007; Roldán et al. 2008) and colouring agents (Guinot et al. 2007), no study up to date provided any information on the stability of the major components accounting for the aforementioned properties.
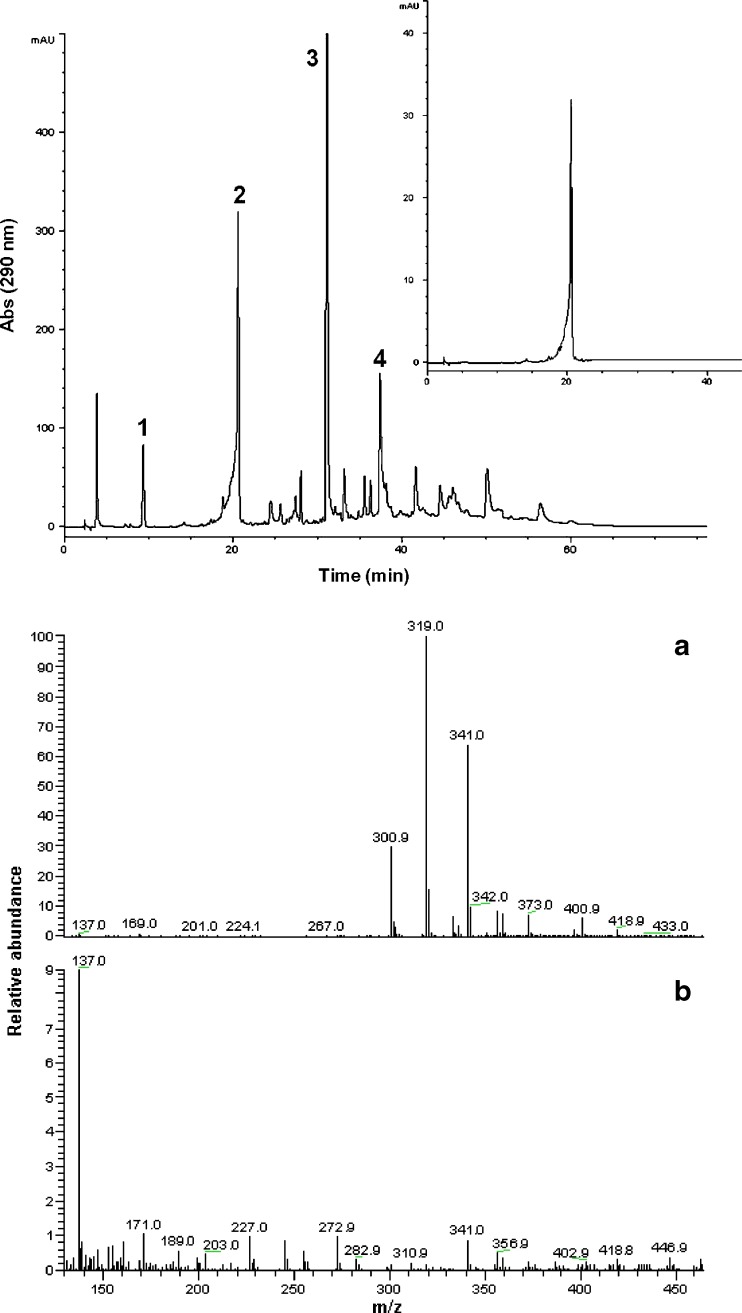
A typical chromatogram of a methanolic onion waste extract is given in Fig. 1. Monitoring of the eluate at 290 nm was proven convenient, as it revealed all major polyphenols occurring in the extract. PA and Qt, which correspond to peaks 1 and 4, could easily be identified by comparison of retention times and UV-vis spectra with those of original standards. For peaks 2 and 3, LC / MS analyses were performed, in an effort to obtain some information on their structure. Peak 2 showed a molecular ion at m/z 319 [M + H]+, an adduct with Na+ at m/z 341 [M + 23]+, and a dehydroxylation species at m/z 301 [M–17]+. Further fragmentation induced by increased source voltage resulted in one daughter ion of m/z 137, which represents a B-ring fragment. These data were consistent with those previously reported (Gülşen et al. 2007). On the basis of these observations the structure was tentatively assigned to 2-(3,4-dihydroxybenzoyl)-2,4,6-trihydroxybenzofuran-3(2H)-one (BD). Peak 3 yielded ions with m/z = 465 and 303, and was ascribed to quercetin 4′-O-glucoside (Qgl). These data were in accordance with previous ones (Khiari et al. 2008; Turner et al. 2006).
Fig. 1.
Chromatogram obtained at 290 nm, showing major phenolics detected in onion solid wastes. 1: PA; 2, BD; 3, Qgl; 4, Qt. The insert picture shows the BD standard. The mass spectrum (lower figure) corresponds to BD. Spectra A and B were obtained with 12 and 80 eV collision energy, respectively
The monitoring of these compounds over a period of 48 h (Fig. 2), during which the ground waste was left under regular atmospheric conditions, showed that Qgl increased up to almost 20% within the first 6 h, but it declined thereafter to a level 13.3% higher than the initial one. To the contrary, all the other three compounds exhibited a continuous increasing trend, and by the end of the treatment PA, BD and Qt levels were higher by 58.4, 37.5 and 68.6%, respectively.
Fig. 2.
Evolution of the content of the major phenolics detected in onion solid wastes, during the examination period (48 h). Every point corresponds to a mean value of three replicates (n = 3)
A continuous increasing trend was seen for POD activity, which was up to 21.7% higher after 48 h. On the other hand, β-GL showed an increase by 46.5% within the first 6 h, but the overall increase after 48 h was limited to 38.2% (Fig. 3). Onions are known to contain primarily quercetin 3,4′-O-diglucoside (Qdgl) and quercetin 4′-O-glucoside (Qmgl), and upon autolysis the aglycon quercetin is formed (Price and Rhodes 1997). The relevant reactions, which explained the transformations observed quantitatively, were thought to involve hydrolysis of Qdgl and generation of Qmgl and subsequent hydrolysis of Qmgl to quercetin. Therefore, it would be reasonable to hypothesise that β-glycosidases are involved.
Fig. 3.
Evolution of the activity of β-glycosidase (β-GL) and peroxidise (POD), the two enzymes implicated in transformations of quercetin 4′-O-glucoside and quercetin in onion solid wastes. Every point corresponds to a mean value of three replicates (n = 3)
The examination of the solid wastes tested indicated that there is activity of β-GL, which might increase even after 48 h of exposure to ambient conditions (Fig. 3). Thus the declining tendency observed for Qmgl (Fig. 2) could be attributed to β-GL activity. The fact that the content of this particular metabolite increased during the first 6 h of maceration, might be an indication of its release from other glycosides with higher degree of conjugation. Although Qdgl was not one of the major glucosides detected in the waste extract, the occurrence of other, minor quercetin glycosides cannot be ruled out (Price and Rhodes 1997).
Likewise, the constant increase in quercetin throughout the examination period might as well represent its release from Qmgl hydrolysis. On the other hand, the detection of products arising from quercetin oxidation evidenced the activity of POD. Indeed, the detection of PA and BD, which are known to derive from POD-mediated quercetin oxidation (Osman et al. 2008; Takahama and Hirota 2000), is a sound argument for POD activity upon quercetin. The fact that POD was found active during 48 h corroborates this assumption. The evolution of the content of both PA and BD indicated that there might be a concomitant formation of these products as the liberation of quercetin from its conjugates progressed.
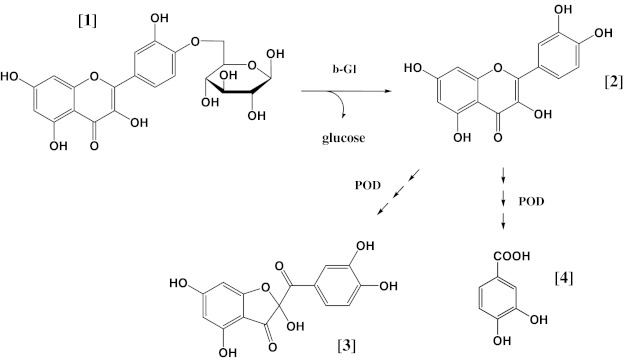
On the hypothesis established above, a putative degradation pathway can be proposed (Fig. 4). First, Qmgl is hydrolysed upon the action of a β-glycosidase, yielding the aglycone quercetin. Following this, quercetin is in turn acted upon by POD to produce PA and BD. Because other oxidation products that have been previously identified (Osman et al. 2008) were not detected, it can be presumed that the pathway proposed might be favourable under the conditions employed, which represent conditions that could be encountered in both domestic and industrial processing of onions.
Fig. 4.
Putative pathway(s) accounting for decomposition of quercetin 4′-O-glucoside and quercetin, and generation of protocatechuic acid and benzofuranone derivative
Conclusions
The investigation of the stability of the major flavonols detected in onion solid wastes showed that:
Glycosides such as quercetin 4′-O-glucoside probably undergo hydrolysis as a first step of breakdown, since β-GL was found active for at least 48 h under usual ambient conditions. The same holds true for POD, which is known to act only on the aglycone quercetin but not on its glycosides.
The detection of phenolics that represent fragments of the quercetin skeleton upon action of POD suggests that oxidative cleavage is responsible for quercetin degradation in onion wastes. On the other hand, the fact that within 48 h quercetin exhibited an increasing trend might indicate that release of quercetin from their glucosides through hydrolysis proceeds faster than quercetin decomposition.
To the extent these reactions can be controlled to increase quercetin content in onion wastes, the exploitation of this residual source for quercetin recovery might be more efficient. This could probably be achieved by inactivating or inhibiting peroxidase, thus hindering excessive quercetin losses.
Abbreviations
- BD
Benzofuranone derivative
- β-GL
β-glycosidase
- DMF
Dimethylformamide
- LC / MS
Liquid chromatography / mass spectrometry
- p-NGP
p-nitrophenyl-β-D-glucoside
- PA
Protocatechuic acid
- POD
Peroxidase
- PVPP
Polyvinyl polypyrrolidone
- TFA
Trifluoroacetic acid
- Qgl
Quercetin 4′-O-glucoside
- Qt
Quercetin
References
- Beesk N, Perner H, Schwarz D, George E, Kroh LW, Rohn S. Distribution of quercetin-3, 4′-O-diglucoside, quercetin-4′-O-monoglucoside, and quercetin in different parts of the onion bulb (Allium cepa L.) influenced by genotype. Food Chem. 2010;122:566–571. doi: 10.1016/j.foodchem.2010.03.011. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Corzo-Martínez M, Corzo N, Villamiel M. Biological properties of onions and garlic. Trends Food Sci Technol. 2007;18:609–625. doi: 10.1016/j.tifs.2007.07.011. [DOI] [Google Scholar]
- Djilas S, Čanadanović-Brunet J, Ćetković G. By-products of fruits processing as a source of phytochemicals. Chem Ind Chem Eng Q. 2009;15:191–202. doi: 10.2298/CICEQ0904191D. [DOI] [Google Scholar]
- Domínguez-Perles R, Martínez-Ballesta MC, Carvajal M, García-Viguera C, Moreno DA. Broccoli-derived by-products—A promising source of bioactive ingredients. J Food Sci. 2010;75:383–392. doi: 10.1111/j.1750-3841.2010.01606.x. [DOI] [PubMed] [Google Scholar]
- Furusawa M, Tsuchiya H, Nagayama M, Tanaka T, Nakaya K, Iinuma M. Anti-platelet and membrane-ridifying flavonoids in brownish scale of onion. J Health Sci. 2003;49:475–480. doi: 10.1248/jhs.49.475. [DOI] [Google Scholar]
- Griffiths G, Trueman L, Crowther T, Thomas B, Smith B. Onions-A global benefit to health. Phytother Res. 2002;16:603–615. doi: 10.1002/ptr.1222. [DOI] [PubMed] [Google Scholar]
- Guinot P, Benonge I, Nicolet G, Gargadennec A, Andary C, Rapiot S. Combined dyeing and antioxidative properties of some plant by-products. Acta Bot Gal. 2007;154:43–52. [Google Scholar]
- Gülşen A, Turan B, Makris DP, Kefalas P. Copper(II)-mediated biomimetic oxidation of quercetin: generation of a naturally occurring oxidation product and evaluation of its in vitro antioxidant properties. Eur Food Res Technol. 2007;225:435–441. doi: 10.1007/s00217-006-0437-3. [DOI] [Google Scholar]
- Hirota S, Shimoda T, Takahama U. Tissue and special distribution of flavonol and peroxidase in onion bulbs and stability of flavonol glucosides during boiling of the scales. J Agric Food Chem. 1998;46:3497–3502. doi: 10.1021/jf980294w. [DOI] [Google Scholar]
- Kapiszewska M, Sołtys E, Visioli F, Cierniak A, Zając G. The protective ability of the Mediterranean plant extracts against the oxidative DNA damage. The role of the radical oxygen species and the polyphenol content. J Physiol Pharmacol. 2005;56:183–197. [PubMed] [Google Scholar]
- Kefalas P, Makris DP. Exploitation of agri-food solid wastes for recovery of high added-value compounds: the case of grape pomace and onion peels. Bulletin USAMV-CN. 2006;62:276–281. [Google Scholar]
- Khiari Z, Makris DP, Kefalas P. Recovery of bioactive flavonols from onion solid wastes employing water/ethanol-based solvent systems. Food Sci Tech Inter. 2008;14:497–502. doi: 10.1177/1082013208100707. [DOI] [Google Scholar]
- Kiassos E, Mylonaki S, Makris DP, Kefalas P. Implementation of response surface methodology to optimise extraction of onion (Allium cepa) solid waste phenolics. Inn Food Sci Emer Tech. 2009;10:246–252. doi: 10.1016/j.ifset.2008.10.004. [DOI] [Google Scholar]
- Lanzotti V. The analysis of onion and garlic. J Chrom A. 2006;1112:3–22. doi: 10.1016/j.chroma.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Lee MK. Inhibitory effect of banana polyphenol oxidase during ripening of banana by onion extract and Maillard reaction products. Food Chem. 2007;102:146–149. doi: 10.1016/j.foodchem.2006.05.012. [DOI] [Google Scholar]
- Ly TN, Hazama C, Shimoyamada M, Ando H, Kato K, Yamauchi R. Antioxidative compounds from the outer scales of onion. J Agric Food Chem. 2005;53:8183–8189. doi: 10.1021/jf051264d. [DOI] [PubMed] [Google Scholar]
- Moure A, Cruz JM, Franco D, Domínguez JM, Sineiro J, Domínguez H, Núñez MJ, Parajó JC. Natural antioxidants from residual sources. Food Chem. 2001;72:145–171. doi: 10.1016/S0308-8146(00)00223-5. [DOI] [Google Scholar]
- Osman A, Makris DP, Kefalas P. Investigation on biocatalytic properties of a peroxidase-active homogenate from onion solid wastes: an insight into quercetin oxidation mechanism. Process Biochem. 2008;43:861–867. doi: 10.1016/j.procbio.2008.04.003. [DOI] [Google Scholar]
- Park J, Kim J, Kim MK. Onion flesh and onion peel enhance antioxidant status in aged rats. J Nutr Sci Vitaminol. 2007;53:21–29. doi: 10.3177/jnsv.53.21. [DOI] [PubMed] [Google Scholar]
- Price KR, Rhodes MJC. Analysis of the major flavonol glycosides present in four varieties of onion (Allium cepa) and changes in composition resulting from autolysis. J Sci Food Agric. 1997;74:331–339. doi: 10.1002/(SICI)1097-0010(199707)74:3<331::AID-JSFA806>3.0.CO;2-C. [DOI] [Google Scholar]
- Ramos FA, Takaishi Y, Shirotori M, Kawaguchi Y, Tsuchiya K, Shibata H, Higuti T, Tadokoro T, Takeuchi M. Antibacterial and antioxidant activities of quercetin oxidation products from yellow onion (Allium cepa) skin. J Agric Food Chem. 2006;54:3551–3557. doi: 10.1021/jf060251c. [DOI] [PubMed] [Google Scholar]
- Rodriguez AS, Pérez-Gregorio MR, García-Falcón MS, Simal-Gándara J. Effect of curing and cooking on flavonols and anthocyanins in traditional varieties of onion bulbs. Food Res Inter. 2009;42:1331–1336. doi: 10.1016/j.foodres.2009.04.005. [DOI] [Google Scholar]
- Rodriguez AS, Pérez-Gregorio MR, García-Falcón MS, Simal-Gándara J. Effect of post-harvest practices on flavonoid content of red and white onion cultivars. Food Control. 2010;21:878–884. doi: 10.1016/j.foodcont.2009.12.003. [DOI] [Google Scholar]
- Roldán E, Sánchez-Moreno C, de Ancos B, Cano MP. Characterisation of onion (Allium cepa L.) by-products as food ingredients with antioxidant and antibrowning properties. Food Chem. 2008;108:907–916. doi: 10.1016/j.foodchem.2007.11.058. [DOI] [PubMed] [Google Scholar]
- Škerget M, Majhenič L, Bezjak M, Knez Ž. Antioxidant, radical scavenging and antimicrobial activities of red onion (Allium cepa L.) skin and edible part extracts. Chem Biochem Eng Q. 2009;23:435–444. [Google Scholar]
- Slimestad R, Fossen T, Vågen IM. Onions: a source of unique dietary flavonoids. J Agric Food Chem. 2007;55:10067–10080. doi: 10.1021/jf0712503. [DOI] [PubMed] [Google Scholar]
- Stojceska V, Ainsworth P, Plunkett A, İbanoğlou E, İbanoğlou S. Cauliflower by-products as a new source of dietary fibre, antioxidants and proteins in cereal based ready-to-eat expanded snacks. J Food Eng. 2008;87:554–563. doi: 10.1016/j.jfoodeng.2008.01.009. [DOI] [Google Scholar]
- Suh HJ, Lee JM, Cho JS, Kim YS, Chung SH. Radical scavenging compounds in onion skin. Food Res Inter. 1999;32:659–664. doi: 10.1016/S0963-9969(99)00141-6. [DOI] [Google Scholar]
- Takahama U, Hirota S. Deglucosidation of quercetin glucosides to the aglycone and formation of antifungal agents by peroxidase-dependent oxidation of quercetin on browning of onion scales. Plant Cell Physiol. 2000;41:1021–1029. doi: 10.1093/pcp/pcd025. [DOI] [PubMed] [Google Scholar]
- Turner C, Turner P, Jacobson G, Almgren K, Waldebäck M, Sjöberg P, Karlsson EN, Markides KE. Subcritical water extraction and β-glucosidase-catalysed hydrolysis of quercetin glycosides in onion waste. Green Chem. 2006;8:949–959. doi: 10.1039/b608011a. [DOI] [Google Scholar]
- Valenzuela A, Sanhueza J, Nieto S. Natural antioxidants in functional foods: from food safety to health benefits. Gracas Aceites. 2003;54:295–303. [Google Scholar]
- Vidyavati HG, Manjunatha H, Hemavathy J, Srinivasan K. Hypolipidemic and antioxidant efficacy of dehydrated onion in experimental rats. J Food Sci Technol. 2010;47:55–60. doi: 10.1007/s13197-010-0015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]