Abstract
Present investigation was undertaken with the overall objective of optimizing the enzymatic parameters i.e. moisture content during hydrolysis, enzyme concentration, enzyme ratio and incubation period on wild apricot kernel processing for better oil extractability and increased oil recovery. Response surface methodology was adopted in the experimental design. A central composite rotatable design of four variables at five levels was chosen. The parameters and their range for the experiments were moisture content during hydrolysis (20–32%, w.b.), enzyme concentration (12–16% v/w of sample), combination of pectolytic and cellulolytic enzyme i.e. enzyme ratio (30:70–70:30) and incubation period (12–16 h). Aspergillus foetidus and Trichoderma viride was used for production of crude enzyme i.e. pectolytic and cellulolytic enzyme respectively. A complete second order model for increased oil recovery as the function of enzymatic parameters fitted the data well. The best fit model for oil recovery was also developed. The effect of various parameters on increased oil recovery was determined at linear, quadric and interaction level. The increased oil recovery ranged from 0.14 to 2.53%. The corresponding conditions for maximum oil recovery were 23% (w.b.), 15 v/w of the sample, 60:40 (pectolytic:cellulolytic), 13 h. Results of the study indicated that incubation period during enzymatic hydrolysis is the most important factor affecting oil yield followed by enzyme ratio, moisture content and enzyme concentration in the decreasing order. Enzyme ratio, incubation period and moisture content had insignificant effect on oil recovery. Second order model for increased oil recovery as a function of enzymatic hydrolysis parameters predicted the data adequately.
Keywords: Wild apricot kernel, Enzymatic pretreatment, Oil recovery, Incubation period
Introduction
Shortage of traditional edible oils in India has stimulated studies on tree-borne oilseeds. The wild apricot, locally called Chullu is found in abundance in the hilly regions of the states of Himachal Pradesh, Kashmir and Uttarakhand in India. Wild apricot is utilized by the tribal people for the preparation of distilled alcoholic liquor, and the oil is used for lighting lamps, cooking and medicinal purposes by hill people. Wild apricot kernel oil contains a high percentage of unsaturated fatty acids, so the skin readily absorbs it. For this reason, it is one of the major ingredients in the formulation of products such as suntan protective lotion and other protective creams and soaps.
Like other oilseeds, oil extraction of wild apricot kernel can be done by mechanical expellers or by solvent extraction. The oilseeds require to undergo preparatory treatments prior to oil extraction. These pretreatments may include such operations as dehulling, size reduction operations like splitting, cracking/breaking, grinding or flaking and thermal treatments like cooking or steaming (Singh and Agrawal 1988). The purpose of these operations is to break the cell walls and make oil available for easy extraction.
Enzymatic hydrolysis, a bioconversion process has recently been shown to be another option for pretreatment of oilseeds. In such process, enzyme acts on cellulosic material converting cellulose to glucose (Chusky et al. 1982). The cell walls, made up of cellulosic material, degenerate and open-up, leading to easy extraction of oil. Further, enzyme acts on complex lipo-protein biomolecules and breaks it down to simple molecules of lipid and protein making extra oil available for extraction. Application of microbial enzymes in oilseed processing makes available extra oil for extraction as it breaks-up lipo-protein and lipo-polysaccharide molecules and release the lipid fraction which is otherwise not extracted.
Oil content in wild apricot kernel is in the range 40–56 g/100 g. However, because of the economical value of the oil and due to the high value of oil content, it is a valuable raw material for oil extraction. Microbial enzymes facilitate mechanical expression and solvent extraction of oilseed and reduce refining operation.
In the last decade, statistical experimental methods such as Plackett-Burman and Response surface methodology (RSM) have been applied to optimize media for industrial purposes. RSM is a collection of statistical techniques for designing experiments, building models, evaluating the effects of various factors and searching for the optimum conditions. RSM has been successfully used in the optimization of bioprocesses (Majumdar and Goyal 2008).
The present study was aimed to study the effect of enzyme concentration, enzyme quantity, enzyme ratio and incubation period on oil recovery and to use RSM for further optimization to enhance the yield using the influential process variables.
Materials and methods
The present study was conducted on wild apricot (Prunus armeniaca L.) obtained from the market of Chamba, located in Garhwal region of Uttarakhand. Seeds were cleaned and decorticated using apricot decorticator. Clean and unbroken kernels were collected. The moisture content was determined by single stage hot air oven method (ISI 1967). Moisture content of the sample was adjusted to desired experimental level of moisture during hydrolysis by uniformly distributing the desired enzyme solution and appropriate amount of distilled water over the sample and manually shaking it. Enzyme concentration varied from 12–16% v/w basis. The flasks were plugged with cotton and were kept in refrigerator for 6 h at 10 °C temperature for diffusion of the enzyme solution. The flasks were then incubated at 45 °C for desired experimental period of incubation. After incubation, the samples were dried on the petridishes at 70 °C to inactivate the enzyme (Sosulski et al. 1988 and Sarkar et al. 1998) and also to reduce moisture content to 3–6% (w.b.) desired for further extraction for oil. The wild apricot kernels were ground in the food processor (Jaipan) for 30 s for obtaining the average size of particles less than 0.5 mm. Ground wild apricot kernel sample weighing about 20 g was treated with crude enzyme in clean, sterilized 100 ml conical flask. The foundation culture of the Aspergillus foetidus and Trichoderma viride was obtained from National Chemical Laboratories, Pune and stored in refrigerator. The foundation culture was multiplied using potato dextrose agar (PDA) media.
Pectolytic enzyme
For the preparation of enzyme from Aspergilus foietidus, synthetic media was used as it produced good amount of pectate and pectinase enzymes. Two different media were used for the production of pectinesterase (Pectate) and polygalacturonase (Pectinase) enzyme. The final products of two fermentations were mixed to formulate the ultimate pectolytic enzyme preparation.
Pectinesterase (Pectyl hydrolase-EC 3.1.1.11): A strain of Aspergillus foietidus was cultured for 7 days in agar slant at 45 °C. Inoculums of 10 ml sterile water washing of 3 to 5 slants was added to a glucose free medium in a conical flask after adjusting its pH to 4.5. The composition of medium per litre was: 10 g pectin, 10 g NaNO3, 5 g KH2PO4, 2.5 g MgSO4. 7H2O, and 1 g CaCl2 and traces of FeCl3 and vitamin B2. The culture was aerated and agitated with 120 rpm at 45 °C for 7 to 9 days, within this period a sufficient enzyme activity was observed. The liquid cultured fluid was centrifuged at 6,000 rpm for 20 min. The precipitated mycelium was discarded and the enzyme was recovered.
Polygalacturonase [poly (1,4,a–D galacturonoide) – EC 3.2.1.15]: Aspergillus foietidus was cultured in a sterile medium composed of 12.5 g of pectin, 10 g of peptone, 0.5 g of K2HPO4, 0.5 g of NH4NO3, 0.2 g of MgSo4.7H2O and 0.3 ml of FeCl in 1 l of distilled water. The initial pH was adjusted to 5.4 by adding 1 N HCl. Cultivation was carried out at 45 °C for 4–6 days under aerobic condition in an incubator shaker at 120 rpm. Fermentation time was adjusted on the basis of activity of enzyme assayed in the sample drawn from the conical flask. The sample was filtered through filter cloth and solution was centrifuged at 6,000 rpm for 20 min. The crude enzyme broth thus obtained was analyzed for protein content and specific polygalacturonase activity.
Cellulolytic enzyme
For the preparation of enzyme from Trichoderma viride, conventional wheat bran medium was used. Forty gram of wheat bran was thoroughly mixed in one litre of distilled water by stirring with glass rod in a beaker. The pH of the medium was adjusted at 7.0. The medium thus prepared was transferred into the conical flask in incubator shaker. The cooled medium was inoculated with two loops of fungal inoculum cultured YPSS medium using laminar flow bench setup for the proper inoculation and to avoid contamination. The culture broth was fermented at 45 °C for 7 days under aerobic conditions. The medium was filtered through a muslin cloth and whatman no. 1 filter paper to separate the fungal mycelium. The filtrate was centrifuged at 6,000 rpm 20 min. The supernatant thus obtained was used as crude enzyme for cellulose/crude fibre breakdown.
The parameters considered for the experiment were moisture content during hydrolysis, enzyme concentration, combination of pectolytic and celulolytic enzyme and incubation period. All these parameters were taken at five levels each. The treated samples were dried at 70 °C to approximately 3–7% moisture content and kept in cotton plugged test tube for deoiling by solvent extraction. Preliminary experiments were conducted to find out influential parameters, standardizing procedures and techniques.
Experimental plan
The experiments were conducted in two phases. In the first phase, the effect of conventional pretreatment unit operation i.e. size reduction in conjunction with enzymatic hydrolysis was studied and the optimum combination of this treatment parameters was determined with respect to maximum oil recovery and oil quality parameters. Size reduction was done by grinding the wild apricot kernel in food processor. The range of variables selected for the experiment was moisture content during hydrolysis from 20–32% (w.b.), enzyme concentration from 12–16% (volume per unit weight of sample), enzyme ratio from 3:7–7:3 (pectolytic: cellulolytic) and incubation period from 12–16 h. The prepared cellulolytic enzyme was characterized by cellulase activity and protein concentration. The prepared cellulolytic enzyme had the cellulase activity of 0.74 IU/ml and the protein concentration of 4.0338 mg/ml of solution. In the pectolytic enzyme the pectinesterase activity and polygalacturonase activity were 1.0749 PEU/g and 0.7319 PGU/ml, respectively. The protein content of the pectinesterase and polygalacturonase was 2.01 and 3.76 mg/ml of solution, respectively. The wild apricot kernel so treated by different process combinations were analyzed for oil recovery using soxhlet extractor in 8 h duration. By using the optimal combination of pretreatments, the enzymatic parameters were optimized for oil recovery. The experimental design and analysis in this phase was based on Response Surface Methodology.
In the second phase, the maximized oil recovery for wild apricot kernel processed through optimal pretreatment as determined in phase first was experimentally confirmed, and further its mechanical expellability was investigated at optimum condition obtained in at the first phase. Laboratory carver press was used for determination of expellability. Wild apricot kernel having approximately 4–7% moisture content was used for the treatment. Enzyme concentration varied from 12–16% v/w basis. Soxhlet butt tube extraction apparatus was used for determination of extractability of oil from wild apricot kernels processed through optimum pretreatments.
Response surface methodology was adopted for experimental design considering the parameters i.e. moisture content during hydrolysis, enzyme concentration, enzyme ratio and incubation period as it provided sufficient information for statistically acceptable results and optimization of process (Box and Hunter 1978). The whole experiment comprised of four independent variables at five levels each following central composite rotatable design. Design expert 8 and Surfer 8 were used for the optimization and for plotting the contour maps.
Results and discussion
Increased oil recovery
Data on increased oil recovery from enzyme pretreated wild apricot kernel are presented in Tables 1 and 2. The actual oil content of untreated kernel was 53.08%. It is seen that incremental oil recovery could be as high as 2.53% compared to the control i.e. enzyme untreated kernel. Such increased oil recovery has also been reported by Sosulski et al. (1988) and Sarkar et al. (1998). The increased oil recovery ranged from 0.14 to 2.53%. The minimum oil recovery was at experimental run no. 2 with experimental conditions as 29% (w.b.), 13% v/w of the sample, 40:60 (pectolytic:cellulolytic enzyme), 13 h of moisture content during hydrolysis, enzyme concentration, enzyme ratio, incubation period, respectively. The corresponding conditions for maximum oil recovery (exp. no. 30) were 23% (w.b.), 15 v/w of the sample, 60:40 (pectolytic:cellulolytic), 13 h. It was observed that the optimum value of moisture content during hydrolysis, enzyme concentration, incubation period were close to the reported values for soybrokens, rapeseed (Sarkar et al. 1998), soyflakes (Kashyap et al. 1997) and sesame seed by (Singh et al. 1999), respectively. Increase in extractable oil may be attributed to the action of crude enzymes on ground wild apricot kernel. Enzymes enhance hydrolysis of the linkages in lipo-polysaccharides, lipo-proteins and some other bound compounds, rendering easier extraction and therefore improvement in oil recovery (Fullbrook 1983; Sosulski et al. 1988).
Table 1.
Coded and uncoded values of variables and their levels
| Independent variable | Coded levels | −2 | −1 | 0 | 1 | 2 |
|---|---|---|---|---|---|---|
| Moisture content during hydrolysis (%,wb) | X1 | 20 | 23 | 26 | 29 | 32 |
| Enzyme concentration (%, v/w) | X2 | 12 | 13 | 14 | 15 | 16 |
| Enzyme ratio (Pectolytic:cellulolytic) | X3 | 30:70 | 40:60 | 50:50 | 60:40 | 70:30 |
| Incubation period (h) | X4 | 12 | 13 | 14 | 15 | 16 |
Table 2.
Increased oil recovery in enzymatic hydrolysis of wild apricot kernel
| Exp. No. | Uncoded value | Oil recovery, g/100 g | Oil increased, g/100 g | |||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | |||
| 1 | −1 | −1 | −1 | −1 | 54.35 ± 0.04 | 1.28 |
| 2 | 1 | −1 | −1 | −1 | 53.22 ± 0.89 | 0.14 |
| 3 | −1 | 1 | −1 | −1 | 54.45 ± 0.38 | 1.38 |
| 4 | 1 | 1 | −1 | −1 | 53.27 ± 1.24 | 0.19 |
| 5 | −1 | −1 | 1 | −1 | 53.73 ± 0.99 | 0.65 |
| 6 | 1 | −1 | 1 | −1 | 53.63 ± 1.83 | 0.55 |
| 7 | −1 | 1 | 1 | −1 | 55.61 ± 1.63 | 2.53 |
| 8 | 1 | 1 | 1 | −1 | 54.85 ± 0.47 | 1.77 |
| 9 | −1 | −1 | −1 | 1 | 54.69 ± 1.34 | 1.61 |
| 10 | 1 | −1 | −1 | 1 | 54.63 ± 0.20 | 1.56 |
| 11 | −1 | 1 | −1 | 1 | 54.19 ± 0.07 | 1.11 |
| 12 | 1 | 1 | −1 | 1 | 54.66 ± 1.03 | 1.58 |
| 13 | −1 | −1 | 1 | 1 | 54.08 ± 0.98 | 1.00 |
| 14 | 1 | −1 | 1 | 1 | 54.21 ± 0.70 | 1.14 |
| 15 | −1 | 1 | 1 | 1 | 53.47 ± 1.01 | 0.39 |
| 16 | 1 | 1 | 1 | 1 | 54.38 ± 0.05 | 1.31 |
| 17 | −2 | 0 | 0 | 0 | 54.99 ± 0.63 | 1.91 |
| 18 | 2 | 0 | 0 | 0 | 53.83 ± 0.48 | 0.75 |
| 19 | 0 | −2 | 0 | 0 | 55.51 ± 1.09 | 2.43 |
| 20 | 0 | 2 | 0 | 0 | 53.84 ± 0.91 | 0.76 |
| 21 | 0 | 0 | −2 | 0 | 54.71 ± 1.16 | 1.63 |
| 22 | 0 | 0 | 2 | 0 | 54.03 ± 0.08 | 0.95 |
| 23 | 0 | 0 | 0 | −2 | 54.39 ± 0.16 | 1.31 |
| 24 | 0 | 0 | 0 | 2 | 54.78 ± 1.28 | 1.70 |
| 25 | 0 | 0 | 0 | 0 | 54.98 ± 0.81 | 1.90 |
| 26 | 0 | 0 | 0 | 0 | 54.83 ± 0.24 | 1.75 |
| 27 | 0 | 0 | 0 | 0 | 54.62 ± 1.04 | 1.54 |
| 28 | 0 | 0 | 0 | 0 | 54.69 ± 0.57 | 1.61 |
| 29 | 0 | 0 | 0 | 0 | 55.01 ± 0.19 | 1.94 |
| 30 | 0 | 0 | 0 | 0 | 55.43 ± 0.07 | 2.35 |
The oil recovery data were analyzed employing multiple regression technique. The results of complete second order model are given in Table 3. It shows that the model predicting increased oil yield is significant at 5% level of significance. P values more than 0.05 indicate that the model terms are not significant. Moisture content during hydrolysis, enzyme concentration, enzyme ratio (pectolytic:cellulolytic enzyme) and incubation period did not affect the oil yield significantly at linear level.
Table 3.
ANOVA for increased oil recovery
| Source | Coeff. | Sum of Squares | DF | Mean Square | F-Value | P-value |
|---|---|---|---|---|---|---|
| Model | 1.8486 | 7.4188 | 14 | 0.5299 | 2.0508 | 0.0900 |
| X1 | −0.1683 | 0.6794 | 1 | 0.6794 | 2.6293 | 0.1257 |
| X2 | −0.0412 | 0.0408 | 1 | 0.0408 | 0.1577 | 0.6969 |
| X3 | −0.0361 | 0.0314 | 1 | 0.0314 | 0.1213 | 0.7324 |
| X4 | 0.0829 | 0.1647 | 1 | 0.1647 | 0.6376 | 0.4371 |
| X1X2 | 0.0369 | 0.0219 | 1 | 0.0219 | 0.0846 | 0.7751 |
| X1X3 | 0.1306 | 0.2729 | 1 | 0.2729 | 1.0560 | 0.3204 |
| X1X4 | 0.2899 | 1.3445 | 1 | 1.3445 | 5.2036 | 0.0376a |
| X2X3 | 0.1869 | 0.5591 | 1 | 0.5591 | 2.1638 | 0.1620 |
| X2X4 | −0.2606 | 1.0869 | 1 | 1.0869 | 4.2065 | 0.0582 |
| X3X4 | −0.2839 | 1.2901 | 1 | 1.2901 | 4.9931 | 0.0411a |
| X21 | −0.1779 | 0.8684 | 1 | 0.8684 | 3.3609 | 0.0867 |
| X22 | −0.1127 | 0.3482 | 1 | 0.3482 | 1.3476 | 0.2638 |
| X23 | −0.1882 | 0.9712 | 1 | 0.9712 | 3.7587 | 0.0716 |
| X24 | −0.1343 | 0.4949 | 1 | 0.4949 | 1.9157 | 0.1866 |
| Residual | 3.8758 | 15 | 0.2583 | |||
| Lack of Fit | 3.4575 | 10 | 0.3457 | 4.1323 | 0.0654 | |
| Pure Error | 0.4183 | 5 | 0.0837 | |||
| Total | 11.2946 | 29 |
X1-Moisture content, X2-Enzyme concentration, X3-Enzyme ratio, X4-Incubation period
aSignificant at 5% level of significance
The effect of interaction of incubation period with moisture content during hydrolysis and enzyme ratio was significant on oil yield at 5% level of significance. Table 3 shows that the interaction between incubation period and moisture content was more significant and positive suggesting oil recovery would be minimum at centre point and would increase with increase in the levels of both parameters above or below centre point. Oil yield, however, would decrease when one of the parameters is above centre point and other is below centre point.
Table 3 also shows that the interaction between incubation period with enzyme ratio is negative suggesting that the level of one parameter can be increased above centre point while that of other decreased below centre point for the same response. That means with increase in enzyme ratio, the incubation period decreases which is logical.
The predictive best fit regression equation for increased oil yield is
 |
1 |
where,
- Y1
Increased oil yield, g/100 g extractable oil present in moisture free sample
- X1
Moisture content during hydrolysis,% w.b.
- X2
Enzyme concentration,% v/w of sample
- X3
Enzyme ratio, (pectolytic:cellulolytic)
- X4
Incubation period, h
Effect of process parameters on oil yield
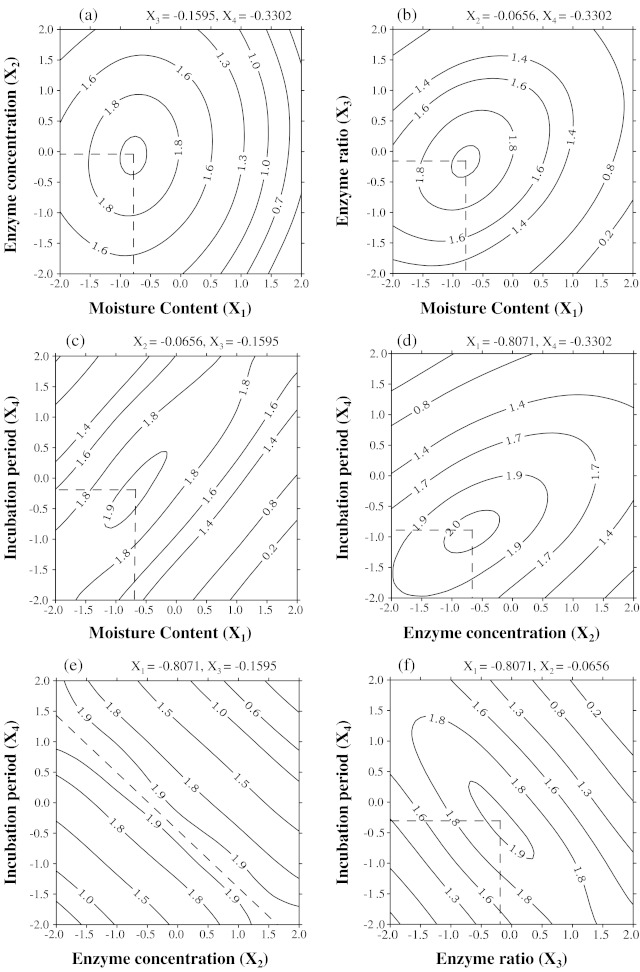
The effect of moisture content during hydrolysis and enzyme concentration, enzyme ratio and incubation period on increased oil yield is shown in Fig. 1a–f, respectively. The optimization was done using response surface methodology and shown in the figures that the increased oil recovery is maximum near the centre point (uncoded form) of enzyme concentration (14%), enzyme ratio (5:5) and incubation period (14 h) and moisture content at level (23%). The increased oil recovery decreased from this point with increase or decrease of parameters.
Fig. 1.
Contour plots showing the effect of two parameters on increased oil recovery (at optimum values of other parameters)
Fig. 1d shows the effect of enzyme concentration and enzyme ratio on increased oil yield. It is clear from the figure that the maximum increased oil yield can be obtained for the combination of enzyme concentration of about 13.75% v/w of sample and enzyme ratio of 4:6 (uncoded form). Fig. 1e shows that the maximum increased oil yield can be obtained using the combination of enzyme concentration and incubation period levels on the diagonal representing both maximum levels. Fig. 1f shows the maximum increased oil yield nearly at the centre point of both incubation period and enzyme ratio.
Mechanical expellability of wild apricot kernel processed through optimal enzymatic pretreatment
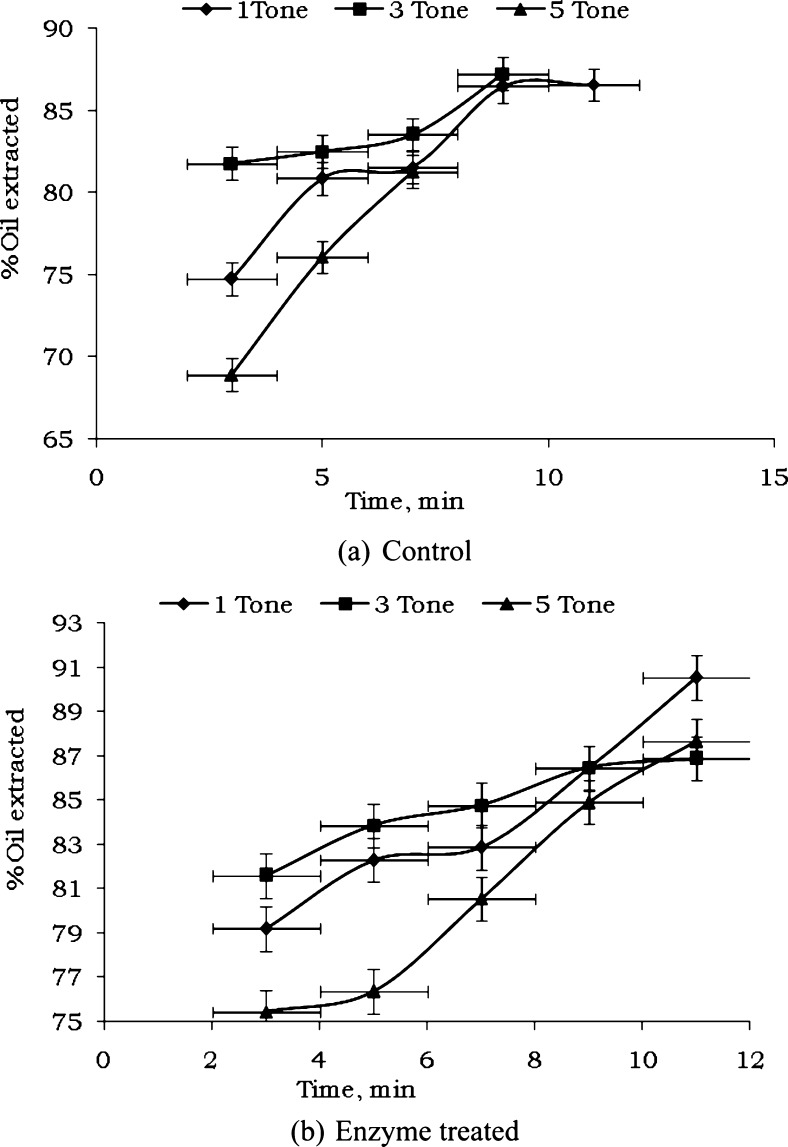
Oil recovery values for different time (3–11 min with 2 min interval) of compression in case of treated and untreated sample employing constant (1–5 tonne with 2 tonne interval) load are given in Table 4. The data were plotted between time of compression and% oil extractability for control and enzyme treated sample and are shown in Fig. 2. It is clear that per cent oil extracted increases with increase in holding time at various pressures. It was observed that enzyme treated sample resulted higher rate of recovery as compared to untreated sample.
Table 4.
Percent oil extracted
| Load, tone (pressure, MPa) | Time, min | Oil extracted (g/100 g) | Increased oil recovery,% | |
|---|---|---|---|---|
| Control sample | Treated sample | |||
| 1 (15.14) | 3 | 74.76 | 79.21 | 5.9 |
| 5 | 80.88 | 82.31 | 1.8 | |
| 7 | 81.56 | 82.89 | 1.6 | |
| 9 | 86.49 | 86.44 | −0.1 | |
| 11 | 86.58 | 90.55 | 4.6 | |
| 3 (45.43) | 3 | 81.77 | 81.61 | −0.2 |
| 5 | 82.51 | 83.86 | 1.6 | |
| 7 | 83.54 | 84.78 | 1.5 | |
| 9 | 87.23 | 86.48 | −0.9 | |
| 11 | 85.89 | 86.89 | 1.2 | |
| 5 (75.71) | 3 | 68.92 | 75.46 | 9.5 |
| 5 | 76.09 | 76.38 | 0.4 | |
| 7 | 81.27 | 80.56 | −0.8 | |
| 9 | 79.46 | 84.91 | 6.7 | |
| 11 | 82.02 | 87.67 | 6.9 | |
Fig. 2.
Per cent oil extracted vs holding time of wild apricot kernel a control and b enzyme treated
It was observed that, at pressure 15.14 MPa and 11 min holding time, 90.55% oil was extracted. At 45.43 MPa, the oil extractability was found to be 86.89%. In case of 75.71 MPa, the oil extractability rate was decreased. The data showed an exponential correlation between holding time (X) and% oil extracted (Y) as expected. Therefore, equation of the following form was fitted in the data
 |
2 |
The values of ‘a’ and ‘b’ varied from 61.23 to 80.19 and 0.0078 to 0.020 for all the experimental parameters.
Summary and Conclusions
On the basis of experimental data and analysis it could be concluded that the percent oil extracted from treated sample increases with increase in holding time. It is found that enzyme treated sample resulted higher rate of oil recovery as compare to untreated sample. Recommended enzymatic optimum hydrolysis parameters for increased oil recovery are stated as 28.04% (w.b.) moisture content, 12.17% (v/w of sample) enzyme concentration and enzyme ratio, 42.5:57.5 (pectolytic:cellulolytic) for incubation period of 15.58 h. Under optimum enzymatic hydrolysis conditions, the oil increase is reported to be 2.76% which was experimentally verified as 2.57%.
References
- Box GEP, Hunter JS. Statistics for experiments: an introduction to data analysis and model building. New York: Wiley; 1978. [Google Scholar]
- Chusky SM, Frein EM, Mountanecourt BS, Evelleigh DE (1982) In: over production of microbial products, Academic press, London, 405–416
- Fullbrook PD. The use of enzyme in processing of oilseed. J Am Oil Chem Soc. 1983;60(2):476–478. doi: 10.1007/BF02543552. [DOI] [Google Scholar]
- ISI (1967) IS: 3579–1966. Method for test for oilseeds, New Delhi
- Kashyap MC, Agrawal YC, Sarkar BC, Singh BPN. Response surface analysis of enzyme a.ided extraction of soybean. J Food Sci Technol. 1997;34(5):386–390. [Google Scholar]
- Majumdar A, Goyal A. Enhanced production of exocellular glucansucrase from Leuconostoc dextranicum NRRL B-1145 using response surface method. Bioresour Technol. 2008;99:3685–3691. doi: 10.1016/j.biortech.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Sarkar BC, Singh BPN, Agrawal YC, Gupta DK. Optimization of enzyme pre-treatment of rapeseed for enhanced oil recovery. J Food Sci Technol. 1998;35(2):183–186. [Google Scholar]
- Singh BPN, Agrawal YC (1988) Soybean processing and utilization in India, edited by N Ali, AP Gandhi & TP Ojha, Central Institute of Agricultural Engineering, Bhopal, India, pp. 414–423
- Singh RK, Sarkar BC, Kumbhar BK, Agrawal YC, Kulshreshtha MK. Response surface analysis of enzyme assisted oil extraction factors for sesame, groundnut and sunflower seeds. J Food Sci Technol. 1999;36(6):511–514. [Google Scholar]
- Sosulski K, Sosulski FW, Coxworth E. Carbohydrase hydrolysis of canola to enhance oil extraction with hexane. J Am Oil Chem Soc. 1988;65(3):357–361. doi: 10.1007/BF02663076. [DOI] [Google Scholar]