Abstract
Functional properties of the Lactobacillus fermentum CFR 2195, isolated from healthy infant feces have been evaluated. The adherence of L. fermentum to HT-29 and Caco-2 cell-lines were found to be 197.66 ± 15.62 and 100.33 ± 15.69 per 100 cells, respectively. The effect of different concentrations of FOS (0.5, 1.0, 1.5, 2.0 and 2.5%) on the growth rate of L. fermentum was checked and 2.0% FOS was selected for further studies. The synbiotic preparation containing L. fermentum and FOS exhibited significant antimicrobial activity against a few tested common food borne pathogens. The proteolytic activity of the L. fermentum was significant and the total amino acid content in milk fermented with L. fermentum was 555 mg/l. In addition, it was found to produce 29.45 ng vitamin B12/g dry biomass in submerged fermentation (96 h) with successive anaerobic and aerobic phases of 48 h.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-011-0345-9) contains supplementary material, which is available to authorized users.
Keywords: L. fermentum, HT-29, Caco-2, Fermented milk, Fructooligosaccharides, Vitamin B12
Introduction
There is growing interest in the design of functional foods beneficial to human health. LAB and food products incorporated with LAB are believed to confer a variety of important nutritional and therapeutic benefits such as inhibition of pathogenic organism, antimutagenic and reduction of blood cholesterol (Salminen et al. 1996) etc. The production of acetic acid, ethanol, aroma compounds, bacteriocins, exopolysaccharides and several enzymes by LAB is of importance (Sridevi et al. 2010). The tremendous increase in consumer’s demand for products containing natural ingredients, changing food patterns and convenience have led to the development of minimally processed products using LAB cultures (Joshi and Sharma 2010).
The desirable attributes of LAB are that, they must be of human origin, and should be able to survive through the GI tract, resistance to gastric acidity, withstand physiological concentration of bile and adhere to the intestinal epithelial cells. Bacterial adhesion to the intestinal epithelium is considered a requisite for probiotic selection, since it may influence the residence time of the bacteria in the intestinal tract (Servin and Coconnier 2003). Bacterial adhesion is evaluated in vivo using cultures of human intestinal epithelial cell lines, like Caco-2 and HT-29 (Lenaerts et al. 2007). Fermented milk and milk products are resultant of intense bacterial activity of the starter cultures, leading to production of lactic acid and biologically active compounds. LAB are fastidious microorganisms towards nutritional substances (Madhukumar and Muralikrishna 2011). Furthermore, some of them have limited biosynthetic ability, requiring an exogenous source of amino acids or peptides for optimum growth (Chopin 1993). Since milk is deficient in such low-molecular components, the growth of the starter bacteria depends on their proteolytic systems to hydrolyze caseins. The amino acids released due to bacterial fermentation affect the nutritional and biological value of the fermented product. Amino acids contribute indirectly to the flavor and aroma of fermented milks, since they act as precursors of a number of reactions that produce carbonyl compounds. The spectrum and level of free amino acids in fermented milk depend on several variables such as type of milk, composition of the starter, method of preparation and storage conditions etc. A synbiotic formulation, a combination of probiotic and prebiotic (Gibson and Roberfroid 1995), which would ultimately be used for fermentation experiments and be an effective antimicrobial agent against common enteropathogens. One specific group of oligosaccharides that has attracted much commercial interest as prebiotic is the fructooligosaccharides (FOS) (Handan and Robert 2000). Hence, FOS was selected as the source of carbohydrate for the growth of LAB. The antagonistic activity of the LAB is essentially due to its ability to produce organic acids as metabolic end products as result of FOS utilization, leading to a decrease in pH of a medium which in turn inhibits the growth of many food borne pathogens (Lee and Salminen 1995).
The organism under study as a potential source of cyanocobalamin (vitamin B12), an essential vitamin which needs to be supplemented in human diet has already been reported (Madhu et al. 2010). Its recommended daily requirement is 1 μg for adults. Supplementation of milk and related dairy products with vitamin B12 producing probiotic LAB may be beneficial. Isolation and characterization of Lactobacillus strains from breast fed infants fecal flora was attempted. L. fermentum CFR 2195 was found to be a potent probiotic strain (Girishkumar and Prapulla 2010) based on in vitro probiotic properties (acid tolerance, bile tolerance, synthetic stomach juice test, antimicrobial activity, antibiotic test and cholesterol reduction). The present study is focused on a few other important functional aspects of L. fermentum CFR 2195 like adherence to human intestinal cell lines, antimicrobial properties of synbiotic preparation, production of free amino acids and vitamin B12.
Material and methods
Bacterial strains and culture conditions
Fresh fecal samples were obtained from the healthy breast-fed infants born in Mysore, India, aged from of 1–6 months. Samples were collected in sterile plastic tubes, stored at 4 ± 1 °C for further microbiological analysis. Within an hour of collection, 1 g of faecal matter was homogenized and diluted with 0.85% NaCl, 0.1% peptone and 0.01% cysteine; pH 7.0. 100 μL of different dilutions were spread on to de Man Rogosa Sharpe (MRS) agar for the isolation of lactobacilli. Plates were incubated aerobically at 37 °C for 24–48 h. Based on the colony color and/or colony morphology individual colonies were randomly picked up and purified. Purity of the isolates was established by streaking on MRS agar plates and microscopic examination and maintained at −20 °C in MRS broth supplemented with 20% sterile glycerol.
L. fermentum CFR 2195, isolated from the 4 month old infant feces as mentioned above and was further characterized. The pathogenic indicator bacterial strains used for the study of antimicrobial activity were Escherichia coli MTCC 108, Bacillus cereus F 4810, Listeria monocytogenes Scott A, Yersinia enterocolitica MTCC 859, Staphylococcus aureus F 722, Enterotoxigenic Escherichia coli (procured from local hospital, Mysore) and Salmonella Typhi. The strains were maintained at −20 °C in respective broth supplemented with 20% sterile glycerol and subcultured twice prior to analysis/assay.
Cell-lines
The HT-29 and Caco-2 cell-lines were procured from NCCS, Pune, India. Cells were grown routinely in Dulbecco’s modified Eagle’s minimal essential medium (MEM enriched with Glutamax and HEPES); Gibco Bethesda Laboratories [BRL] supplemented with 10% heat inactivated (30 min at 56 °C) fetal bovine serum (Gibco BRL), 0.1 mM nonessential amino acids (Gibco BRL), and 0.5 ml of Gentamycin (50 mg/ml) (Gibco BRL) and incubated at 37 °C in a water-jacketed incubator with 5% carbon dioxide. Cells were used for adherence assay at post confluence. Concentration of HT-29 and Caco-2 cells in the monolayer was determined by trypsinizing the cells for 10 min at 37 °C and counting them using a heamocytometer. For adhesion experiments, HT-29 and Caco-2 cells were cultured separately. Cell suspension (3 ml) containing 105cells/ml were transferred to 35 mm diameter dishes (Nuncon) and incubated until a complete monolayer was obtained. Medium was changed every 48 h.
Adherence assays
The adherence of lactobacilli to HT-29 and Caco-2 cells was examined as previously described (Coconnier et al. 1992). Briefly, the monolayers were washed twice with phosphate buffered saline (PBS), pH 7.3. For each adhesion assay, 1 ml of Lactobacillus suspension (108cfu/ml in PBS) was added to each well of the tissue culture plate, incubated 37 °C in 5% CO2. After 90 min of incubation, the monolayer was washed five times with sterile PBS, fixed with methanol, stained with Giemsa stain and examined microscopically. Each adherence assay was conducted in duplicate over three successive passages of the intestinal cells. For each monolayer on a glass coverslip placed in 6 well tissue culture plates, the number of adherent bacteria was counted in 20 random microscopic areas. Adhesion of lactobacilli was expressed as the number of bacteria adhering to 100 of each cell-line. Bacterial adhesion was scored as non-adhesive when fewer than 40 bacteria were present in 20 fields, 41–100 bacteria in 20 fields as adhesive and strongly adhesive with more than 100 bacteria in 20 fields.
Growth curve of L. fermentum CFR 2195
MRS basal medium (without carbohydrates) was used as control. MRS broth enriched with FOS was prepared by adding varying concentration of filter sterilized FOS syrup (0.5, 1.0, 1.5, 2.0 and 2.5% v/v) to the pre sterilized basal MRS broth. MRS broth containing glucose as carbohydrate source was used as control. L. fermentum was grown in 10 ml of modified MRS broth containing a range of FOS and also grown in MRS broth. L. fermentum was incubated at 37 °C for 36 h under microaerophilic conditions. OD was recorded at 600 nm during the entire fermentation period. Plate counts on MRS agar were used to determine the initial live bacterial counts, in the exponential phase after 10 h and at the end of the growth curve experiments after 24 h. The pH of the culture medium was recorded at the end of the fermentation. All the experiments were carried out in triplicates.
Synbiotic effect on pathogens
The effect of synbiotic (FOS) on the selected food borne pathogens (viz. Bacillus cereus F 4810, Listeria monocytogenes Scott A, Escherichia coli MTCC 108, Enterotoxigenic Escherichia coli, Staphylococcus aureus F 722, Salmonella Typhi and Yersinia enterocolitica MTCC 859) was carried out according to Fooks and Gibson (2002) with some modifications. L. fermentum was grown in carbon limiting MRS medium (Devoid of glucose). FOS was added to a final concentration of 2% as the sole carbon source to MRS broth (100 ml). Pathogenic bacteria were grown in the nutrient broth medium. Sterilized MRS agar was poured into the sterile Petri dishes and allowed to set. One ml of an exponential culture of the various pathogenic strains was mixed with 7 ml of soft agar (0.7%) and poured immediately over the surface of the MRS agar plates.
Overnight culture (108cfu/ml) of LAB was centrifuged at 10,000 rpm for 10 min at 4 °C. The pH of the supernatant was recorded. Absence of the cells in the supernatant was confirmed by viable plate count method. The pH of the supernatant was neutralized using 1 M NaOH (Neutralized supernatant). The cell pellet was washed and re-suspended in the phosphate buffer (pH 7.4; 1 M). Wells were made on the agar surface using sterile borer (Diameter: 3 mm) and 50 μl of the cell pellets, supernatant and neutralized supernatant were added to separate wells. The plates were incubated microaerophically at 37 °C for 24–48 h and the zone of inhibition (diameter) was measured in mm. Each test was performed in triplicate.
Assay for amino acid
The free amino acids were analyzed according to the protocol described by Zhao et al. (2007). Sterilized skim milk was inoculated with 10% (v/v) liquid culture of L. fermentum CFR 2195 aseptically, and then incubated at 37 °C for 36 h. About the 5 ml of fermented skim milk mixed with 10 ml 10% (v/v) metaphosphate, centrifuged at 10,000 rpm for 15 min. Subsequently, 1 ml of supernatant was mixed with 1 ml of citric acid buffer (pH 2.2). The mixture (50 μl) was collected and nineteen free amino acids were quantitatively determined by an Amino Acid Analytic Facility (Pico-Tag™ WORKSTATION, Waters).
Column specification & other parameters
Column: Pico Tag, 3.9 × 150 nm, Solvent A: 11.46 g Sodium acetate (anhydrous)/L Mili Q water containing 500 μl of Triehtylamine (TEA). The pH of the solution was adjusted to 6.4, using acetic acid, filtered and degassed. The degassed solution (940 ml) was taken with 60 ml of acetonitrile (gradient controlled). Solvent B: 60% acetonitrile (gradient controlled), Detector: PDA (Photodiode array) at 254 nm, Injection volume: 5 μl.
Analysis of vitamin B12: quantification and bioavailability
The production of vitamin B12 from L. fermentum was done according to the method described by Madhu et al. 2010. Vitamin B12 was quantified using a commercial kit (R-Biopharm AG, Darmstadt, Germany) by competitive ELISA. An agar cup method was also carried out to confirm the results using E. coli ATCC 11105 (Poonawalla and Iyengar 1965). The mutant strain that was grown in maintenance medium at 37 °C to OD 600 0.28 (1 ml) was mixed with B12 assay agar (25 ml) and pour plated. Wells, 5 mm in diameter, were bored in the solid agar media. Standard and test solutions (50 μl) were inoculated into the wells. Sterile water used as negative control and the plates were incubated at 37 °C for 24 h.
Statistical analysis
All the values are expressed as mean ± standard deviation (SD). Statistical analysis was performed using one way ANOVA followed by Duncan multiple comparison tests using the software-statistical package for social sciences (SPSS Inc, Version 10.0.5) to obtain the significant difference. A value of P < 0.05 was considered to indicate a significant difference between the parameters.
Results and discussions
Adherence to HT-29 and Caco-2 cell-lines
One of the important criteria for a potentially probiotic strain is the ability to adhere to mucosal surfaces of the human gastrointestinal tract. Bacterial adhesion to the intestinal epithelium influences the residence time and the ability of probiotic strains to modulate the immune responses and, thereby, to exert health effects in the gut (Servin and Coconnier 2003).
Several reports have been published on the usefulness of human intestinal cell-lines, e.g. HT-29, Caco-2 and HT29-MTX, as in vitro model systems for assessing the colonization potential of a bacterial strain (Bernet et al. 1993; Tuomola and Salminen 1998; Wang et al. 2008). In the present investigation, the relative adhesion of L. fermentum CFR 2195 to HT-29 and Caco-2 intestinal epithelial cell-lines was analyzed and were 197.66 ± 15.62 and 100.33 ± 15.69, respectively. As can be seen from the results, L. fermentum CFR 2195 showed strong and moderate adhesion towards HT-29 and Caco-2 cell-lines respectively.
Growth curve of L. fermentum CFR 2195: carbohydrate effect
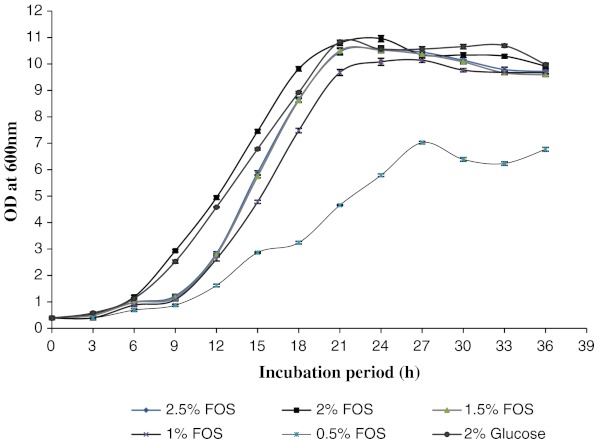
The effect of different concentration of FOS on the growth of L. fermentum was studied and the results are presented in Fig. 1. Optimal growth was observed in the media containing 2.0% FOS as the carbon and energy source. To ascertain that the resultant growth was due to the selective utilization of FOS, strain was also inoculated into MRS basal broth containing 2% glucose as the carbohydrate source. As can be seen from the results, the growth in medium containing glucose was much lower than that of FOS enriched medium. Of the different concentrations of FOS studied, it was observed that the growth was highest at 2.0% level and hence was selected for further studies.
Fig. 1.
Growth curve of L. fermentum. Results are expressed as the mean value of three trials ± standard deviation (SD)
Synbiotic effect on pathogens
The microbiota of the human large intestine influences health and well-being. Whereas it has long been accepted that gut bacteria play a role in host pathogenesis, current opinion is that certain microflora components can have beneficial effects on gastroenteritis resistance, blood lipids, antitumor properties, lactose tolerance, and gastrointestinal immunity (Collins and Gibson 1999). Another possibility in microflora management procedures is the use of synbiotics, in which probiotics and prebiotics are used in combination (Gibson and Roberfroid 1995). In this study, the live microbial additions (probiotic—L. fermentum) was used in conjunction with specific substrate (prebiotic—FOS). This combination could improve the survival of the probiotic organism, because its specific substrate is readily available for utilization, and results in advantages to the host. L. fermentum when tested using well diffusion assay exhibited some inhibition against the following pathogenic strains; B. cereus, L. monocytogenes, E. coli, Enterotoxigenic E Coli, S. aureus, S. Typhi, and Y. enterocolitica. The inhibition of E. coli by the organism was most pronounced in comparison with that of other tested pathogens. No inhibition was observed in case of uninoculated culture medium, which served as negative control. The major end products of lactobacilli are acetate and lactate leading to a decrease in the culture pH (Macfarlane and Gibson 1997). It was observed that the pH of the culture filtrate was slightly lower when FOS was used as the sole carbohydrate source. The zone of inhibition by the supernatant obtained from L. fermentum grown in FOS enriched medium was found to be higher in comparison with that of glucose, suggesting a possible mechanism of antimicrobial action could be attributable to the lower culture pH. The culture fluid obtained by the fermentation of FOS (2%), at the end 18 h of incubation effectively inhibited E. coli, B. cereus, L. monocytogenes and S. Typhi (Table 1). The supernatant consistently conferred a significant inhibitory effect than either the cells or neutralized supernatant fractions. Neutralized supernatant (N/SN) showed a moderate inhibitory effect against all tested organisms when compared with cells and supernatant (SN). This was tested by Duncan Multiple Range Test (p < 0.05). The pH of the supernatant was recorded (Supplementary data); a slight decrease in pH was observed when 2% FOS was used as the carbohydrate source.
Table 1.
Plate assay inhibition of pathogens by L. fermentum grown in broth culture with a different percentage of FOS
| Test organism | Inhibition zone (mm) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cells | S/N | N. S/N | ||||||||||||||||
| 2% Glu | 2.5% FOS | 2% FOS | 1.5% FOS | 1% FOS | 0.5% FOS | 2% Glu | 2.5% FOS | 2% FOS | 1.5% FOS | 1% FOS | 0.5% FOS | 2% Glu | 2.5% FOS | 2% FOS | 1.5% FOS | 1% FOS | 0.5% FOS | |
| BCE | 4.0 ± 0.20c | 4.0 ± 0.33bc | 4.6 ± 0.28d | 4.0 ± 0.28c | 3.3 ± 0.26ab | 2.8 ± 0.42a | 7.9 ± 0.30c | 8.4 ± 0.35cd | 8.8 ± 0.20d | 7.3 ± 0.26b | 7.3 ± 0.25b | 6.6 ± 0.30a | 4.4 ± 0.40b | 4.7 ± 0.26b | 4.8 ± 0.20b | 4.5 ± 0.26b | 4.3 ± 0.43b | 3.7 ± 0.15a |
| LM | 3.7 ± 0.25bc | 3.7 ± 0.30bc | 4.2 ± 0.25c | 3.5 ± 0.30b | 3.0 ± 0.45a | 2.7 ± 0.30a | 8.2 ± 0.20de | 7.8 ± 0.20cd | 8.7 ± 0.17e | 7.1 ± 0.65bc | 6.6 ± 0.52ab | 5.9 ± 0.55a | 3.6 ± 0.20bc | 3.2 ± 0.25b | 4.0 ± 0.25c | 3.5 ± 0.50bc | 3.3 ± 0.30b | 2.3 ± 0.35a |
| EC | 3.8 ± 0.20bc | 4.0 ± 0.30c | 4.5 ± 0.46d | 3.4 ± 0.40bc | 3.3 ± 0.30b | 2.6 ± 0.25a | 8.2 ± 0.20d | 7.6 ± 0.25c | 8.8 ± 0.26e | 7.4 ± 0.15c | 6.5 ± 0.30b | 5.8 ± 0.15a | 4.0 ± 0.30c | 4.2 ± 0.20cd | 4.7 ± 0.26d | 3.7 ± 0.41bc | 3.2 ± 0.32b | 2.5 ± 0.41a |
| ETEC | 3.9 ± 0.10cd | 3.9 ± 0.30cd | 4.4 ± 0.47d | 3.6 ± 0.15c | 3.1 ± 0.11b | 2.3 ± 0.41a | 7.2 ± 0.25c | 7.2 ± 0.55c | 7.8 ± 0.20c | 6.3 ± 0.50b | 5.5 ± 0.50ab | 4.9 ± 0.55a | 3.4 ± 0.40b | 3.4 ± 0.43b | 3.8 ± 0.40b | 3.5 ± 0.43b | 3.1 ± 0.52ab | 2.4 ± 0.30a |
| SEA | 3.0 ± 0.20b | 3.7 ± 0.30b | 3.5 ± 0.30b | 3.5 ± 0.41b | 3.1 ± 0.68b | 2.3 ± 0.28a | 8.2 ± 0.25e | 7.3 ± 0.26d | 8.9 ± 0.41e | 6.2 ± 0.35c | 5.5 ± 0.56b | 4.2 ± 0.56a | 3.8 ± 0.25b | 3.5 ± 0.30b | 4.6 ± 0.40c | 3.5 ± 0.37b | 2.6 ± 0.20a | 2.2 ± 0.25a |
| ST | 3.8 ± 0.20cd | 3.2 ± 0.25b | 4.3 ± 0.30d | 3.5 ± 0.30bc | 2.7 ± 0.41a | 2.2 ± 0.25a | 7.3 ± 0.30c | 6.9 ± 0.45c | 8.5 ± 0.41d | 6.8 ± 0.26c | 5.9 ± 0.26b | 4.8 ± 0.45a | 4.0 ± 0.20b | 4.2 ± 0.25bc | 4.7 ± 0.40c | 3.7 ± 0.41b | 2.9 ± 0.41a | 2.5 ± 0.20a |
| YE | 3.8 ± 0.20b | 3.4 ± 0.20b | 4.3 ± 0.35c | 3.3 ± 0.30b | 2.6 ± 0.32a | 2.2 ± 0.28a | 7.4 ± 0.50d | 8.2 ± 0.20e | 8.3 ± 0.30e | 6.8 ± 0.25c | 5.5 ± 0.30b | 4.4 ± 0.36a | 2.9 ± 0.30a | 3.6 ± 0.32b | 4.3 ± 0.41c | 3.7 ± 0.35b | 3.5 ± 0.15b | 3.33 ± 0.30ab |
Cells viable cell pellets, S/N supernatant, N.S/N neutralized supernatant, BCE Bacillus cereus F 4810, LM Listeria monocytogenes Scott A, EC Escherichia coli MTCC 108, ETEC Enterotoxigenic Escherichia coli (procured from local hospital, Mysore, India), SEA Staphylococcus aureus F 722, YE: ST Salmonella Typhii and Yersinia enterocolitica MTCC 859
The values are represented as mean ± SD, n = 3. The level of significance was tested by Duncan multiple range test at P < 0.05. Values were significantly different among different concentrations of FOS when compared with 2% glucose in either cells, supernatant or neutralized supernatants of L. fermentum against respective tested organisms. Letters a–e in each row in either cells, SN or N/SN represents the level of significant difference in increasing order at P < 0.05
The antimicrobial potential exhibited by L. fermentum, under study, appears to depend on the carbohydrate source used; the inhibition was slightly higher when grown on FOS than when grown on glucose, perhaps suggesting a structure to function relationship in terms of the prebiotics used. The type of bond linking the component monomers, in view of specific cleavage enzymes being required for the fermentation of carbohydrate may affect the fermentation rate and thereby determine the rate at which inhibitory metabolic end products are released. Chain length of the carbohydrate is also likely to be a contributory factor, since long chain oligosaccharides, with multiple branching, are required to be completely hydrolyzed by the organisms prior to its complete utilization (Fooks and Gibson 2002).
Production of free amino acids in fermented milk
Fermented milks are the outcome of intense bacterial activity of the starter cultures, leading to production of lactic acid and biologically active compounds, adding nutritional and physiological value, as well as enhancing flavour formation. Therefore, it is essential for LAB to be able to grow steadily in milk. LAB are fastidious microorganisms with regards to nutritional substances. Furthermore, they have (in most cases) limited biosynthetic ability, requiring an exogenous source of amino acids or peptides for optimum growth (Chopin 1993). Since milk is deficient in such low-molecular weight components, the growth of the starter bacteria depends on their proteolytic systems to hydrolyze caseins (Thomas and Mills 1981). The amino acids released by the bacteria accumulate in the milk and affect the nutritional potential and biological value of the fermented product. Amino acids may not be directly contributory to the flavour and aroma of fermented milks; however, they act as precursors of a number of reactions that produce carbonyl compounds. The spectrum and level of free amino acids in fermented milks depend on several variables such as type of milk, composition of the starter, method of preparation and storage conditions.
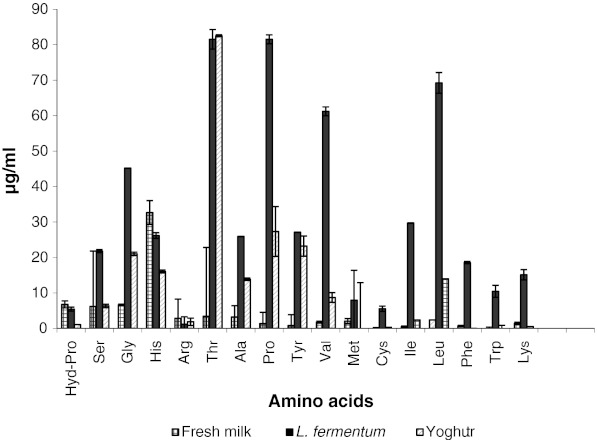
In the present investigation the amount of amino acids in fermented skim milk increased significantly in comparison with that of unfermented skim milk (Fig. 2). In particular, the amount of glycine, threonine, proline, valine and leucine were found to be increased after fermentation. The total amino acids of the fermented skim milk by L. fermentum were 555 mg/l. The total amino acid content in yogurt prepared by conventional methods using standard culture of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus was found to be 233 mg/l only (Zhao et al. 2007). The amino acid content of the milk used for the preparation of yogurt was found to be only 104 mg/l .The results obtained from the present investigation clearly indicates the ability of L. fermentum to hydrolyze milk proteins efficiently leading to release of free amino acids to a greater extent. This also indicated that hydrolysis of milk protein was strain dependent. Evaluation of the free amino acid content in the milk is an indicator of the protein availability. The results positively indicate the potential of L. fermentum to improve availability and functional properties of milk protein.
Fig. 2.
Content of free amino acids in fermented milk. Results are expressed as the mean value of three trials ± standard deviation. Note: Asp: Aspartic acid, Glu: Glutamic acid, Hyd-Pro: Hydroxy proline, Ser: Serine, Gly: Glycine, His: Histidine, Arg: Arginine, Thr: Threonine, Ala: Alanine, Pro: Proline, Tyr: Tyrosine, Val: Valine, Met: Methionine, Cys: Cysteine, Ile: Isoleucine, Leu: Leucine, Phe: Phenylalanine, Trp: Tryptophan, Lys: Lysine
Analysis of vitamin B12: quantification and bioavailability
Vitamin B12 by definition is cyanocobalamin, the most stable form, and is produced industrially by a batch fermentation process (Heudi et al. 2006). In humans, intestinal synthesis of vitamin B12 is not sufficient and must be obtained from food, most often from the flesh of other animals (Stabler and Allen 2004). The consumption of probiotic LAB cultures may also exert a protective effect against pathogenic infections. The study has distinctly shown the potential of the probiotic isolate for the production of vitamin B12. The fermentation parameters like, combination of anaerobic and aerobic conditions and optimization of media component (ZnCl2) resulted in better yield of vitamin B12. LAB strains which are cobalamin producers isolated from fecal matter, like the isolate in the present study facilitate the possibility of exploiting their generally regarded as safe’ (GRAS) status. The intracellular nature of the vitamin is the major stumbling block and can be overcome by developing autolytic mutants and metabolic engineering strategies for transferring the B12 production capability to other bacteria (Madhu et al. 2010).
In the present study results of competitive ELISA techniques indicated a yield of 29.45 ng vitamin B12/g of the dry biomass. Growth of mutant E. coli ATCC 11105 (vitamin B12 auxotroph derivative of the wild-type strain ATCC 9637), in the presence of cell free lysate of L. fermentum indicated the production of vitamin B12 and its bioavailability. A zone of growth was observed surrounding the wells containing standard vitamin B12 for mutant E. coli, as expected. Appearance of zone of growth surrounding the well containing cell free lysate confirmed the potential of L. fermentum to produce vitamin B12. There was no growth observed surrounding the wells containing sterile water.
Conclusion
L. fermentum CFR 2195, an isolate from fecal matter of healthy infants showed potent probiotic characteristics and thus, could be considered as a promising candidate for probiotic supplement in dairy products. It exhibited significant adherence to HT-29 and Caco-2 cell lines and tolerance to low pH conditions and bile acids under in vitro conditions. In addition assays, L. fermentum showed inhibitory properties towards selected food borne pathogens, in combination with a prebiotic (FOS). This antagonism was influenced by the carbohydrate (FOS) provided for growth. The study also has shown the potential of the probiotic isolate for the production of amino acids and vitamin B12 as additional functional properties. Efficacy of the organism is being investigated under in vivo conditions.
Electronic supplementary material
(DOCX 11 kb)
Acknowledgement
The authors are thankful to Director, CFTRI, Mysore, India for supporting the work. Mr. Girishkumar B is grateful to CSIR for the Senior Research Fellowship. Dr. Vijayan, HOD, Department of Biotechnology, JSS College of Pharmacy, Ooty is kindly acknowledged for his help in cell-lines studies.
References
- Bernet MF, Brassart D, Neeser JR, Servin AL. Adhesion of human bifidobacterial strains to cultured human intestinal epithelial cells and inhibition of enteropathogens-cell interactions. Appl Environ Microbiol. 1993;59:4121–4128. doi: 10.1128/aem.59.12.4121-4128.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopin A. Organization and regulation of genes for amino acid biosynthesis in lactic acid bacteria. FEMS Microbiol Rev. 1993;12:21–37. doi: 10.1111/j.1574-6976.1993.tb00011.x. [DOI] [PubMed] [Google Scholar]
- Coconnier MH, Klaenhammer T, Kerneis S, Bernet MF, Servin A. Protein-mediated adhesion of Lactobacillus acidophilus BG2FO4 on human enterocyte and mucus-secreting cell lines in culture. Appl Environ Microbiol. 1992;58:2034–2039. doi: 10.1128/aem.58.6.2034-2039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MD, Gibson GR. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr. 1999;69:1052S–1057S. doi: 10.1093/ajcn/69.5.1052s. [DOI] [PubMed] [Google Scholar]
- Fooks LJ, Gibson GR. In vitro investigations of the effect of probiotics and prebiotics on selected human intestinal pathogens. FEMS Microbiol Ecol. 2002;39:67–75. doi: 10.1111/j.1574-6941.2002.tb00907.x. [DOI] [PubMed] [Google Scholar]
- Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota—introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- Girishkumar B, Prapulla SG. Beneficial properties of lactic acid bacteria isolated from the breast fed infants fecal flora: in vitro evidences. Asian J Microbiol Biotechnol Environ Sci. 2010;12:887–897. [Google Scholar]
- Handan K, Robert WH (2000) Fermentation of Fructooligosaccharides by lactic acid bacteria and bifidobacteria. Appl Environ Microbiol 2682–2684 [DOI] [PMC free article] [PubMed]
- Heudi O, Kilinc T, Fontannaz P, Marleyc E. Determination of vitamin B12 in food products and in premixes by reverse phase high performance liquid chromatography and immunoaffinity extraction. J Chromatogr A. 2006;1101:63–68. doi: 10.1016/j.chroma.2005.09.059. [DOI] [PubMed] [Google Scholar]
- Joshi VK, Sharma S. Preparation and evaluation of sauces from lactic acid fermented vegetables. J Food Sci Technol. 2010;47:214–218. doi: 10.1007/s13197-010-0037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Salminen S. The coming of age of probiotics. Trends Food Sci Technol. 1995;6:241–245. doi: 10.1016/S0924-2244(00)89085-8. [DOI] [Google Scholar]
- Lenaerts K, Bouwman FG, Lamers WH, Renes J, Mariman EC. Comparative proteomic analysis of cell lines and scrapings of the human intestinal epithelium. BMC Genomics. 2007;8:1–14. doi: 10.1186/1471-2164-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane GT, Gibson GR. Carbohydrate fermentation, energy transduction and gas metabolism in the human large intestine. In: Mackie RI, White BA, editors. Gastrointestinal microbiology, vol 1: gastrointestinal ecosystems and fermentations. London: Chapman and Hall; 1997. pp. 269–318. [Google Scholar]
- Madhu AN, Giribhattanavar P, Narayan MS, Prapulla SG. Probiotic lactic acid bacterium from kanjika as a potential source of vitamin B12: evidence from LC-MS, immunological and microbiological techniques. Biotechnol Lett. 2010;32:503–506. doi: 10.1007/s10529-009-0176-1. [DOI] [PubMed] [Google Scholar]
- Madhukumar MS, Muralikrishna G. Fermentation of xylooligosaccharides obtained from wheat bran and Bengal gram husk by lactic acid bacteria and bifidobacteria. J Food Sci Technol. 2011 doi: 10.1007/s13197-010-0226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poonawalla FM, Iyengar MRS. Microbiological assay of vitamin B12 in presence of tetracycline. Appl Microbiol. 1965;13:755–756. doi: 10.1128/am.13.5.755-756.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen S, Isolauri E, Salminen E. Clinical uses of probiotics for stabilizing the gut mucosal barrier: successful stains and future challenges. Antonie Leeuwenhoek. 1996;70:251–262. doi: 10.1007/BF00395941. [DOI] [PubMed] [Google Scholar]
- Servin AL, Coconnier MH. Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract Res Clin Gastroenterol. 2003;17:741–754. doi: 10.1016/S1521-6918(03)00052-0. [DOI] [PubMed] [Google Scholar]
- Sridevi J, Halami PM, Vijayendra SVN. Selection of starter cultures for idli batter fermentation and their effect on quality of idlis. J Food Sci Technol. 2010;47:557–563. doi: 10.1007/s13197-010-0101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabler SP, Allen RH. Vitamin B12 deficiency as a worldwide problem. Annu Rev Nutr. 2004;24:299–326. doi: 10.1146/annurev.nutr.24.012003.132440. [DOI] [PubMed] [Google Scholar]
- Thomas TD, Mills OE. Proteolytic enzymes of starter bacteria. Neth Milk Dairy J. 1981;35:255–273. [Google Scholar]
- Tuomola E, Salminen SJ. Adhesion of some probiotic and dairy Lactobacillus strains to Caco-2 cell cultures. Int J Food Microbiol. 1998;41:45–51. doi: 10.1016/S0168-1605(98)00033-6. [DOI] [PubMed] [Google Scholar]
- Wang B, Wei H, Yuan J, Li Q, Li Y, Li N, Li J. Identification of a surface protein from Lactobacillus reuteri JCM1081 that adheres to porcine gastric mucin and human enterocyte-like HT-29 cells. Curr Microbiol. 2008;57:33–38. doi: 10.1007/s00284-008-9148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Sun J, Mo H, Zhu Y. Analysis of functional properties of Lactobacillus acidophilus. World J Microbiol Biotechnol. 2007;23:195–200. doi: 10.1007/s11274-006-9209-2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 11 kb)