Abstract
Central composite design of response surface methodology (RSM) was employed to optimize the extraction time (X1: 99.5–290.5 min) and temperature (X2: 30.1–54.9 °C) of Schizophyllum commune aqueous extract with high antioxidant activities and total phenolic content (TPC). Results indicated that the data were adequately fitted into four second-order polynomial models. The extraction time and temperature were found to have significant linear, quadratic and interaction effects on antioxidant activities and TPC. The optimal extraction time and temperature were: 290.5 min and 35.7 °C (DPPH• scavenging ability); 180.7 min and 41.7 °C (ABTS•+ inhibition ability); 185.2 min and 42.4 °C (ferric reducing antioxidant power, FRAP); 290.5 min and 40.3 °C (TPC). These optimum conditions yielded 85.10%; 94.31%; 0.74 mM Fe2+ equivalent/100 g; 635.76 mg gallic acid equivalent/100 g, respectively. The yields of antioxidant activities and TPC obtained experimentally were close to its predicted values. The establishment of such model provides a good experimental basis employing RSM for optimizing the extraction time and temperature on antioxidants from S. commune aqueous extract.
Keywords: Antioxidant activity, Extraction time, Temperature, Total phenolic content, Response surface methodology, Schizophyllum commune
Introduction
Mushrooms are known not only for their culinary value, but the medicinal properties found in certain species of mushrooms have generated vast interests amongst scientific communities. Some wild edible mushrooms are widely consumed that they are now cultivated commercially and one of those famous cultivated species is Pleurotus spp. (also known as oyster mushroom). Mushrooms are consumed as whole food and they have been used as flavouring materials in food such as soups and sauces due to their unique flavour (Mau et al. 2004). The consumption of wild edible mushrooms is increasing due to the good nutritional values (Chong et al. 2007). Mushrooms have been reported as therapeutic foods that are useful in preventing certain diseases such as hypertension, hypercholesterolemia, and cancer (Elmastas et al. 2007).
Antioxidants have been widely used as food additives to protect against oxidative degradation by free radicals, and also help to prevent harmful reactive oxygen species in human body. Many human diseases have been linked with oxidative stress generated an increased rate of oxidations which disturb the balance state between prooxidants and antioxidants in the body (Dubost et al. 2007, Li et al. 2009). Besides, free radicals react with food lipids and cause lipid peroxidation that deteriorates food quality, affecting the colour, flavour, taste, texture and nutritional value of foods (Ferreira et al. 2007, Biglari et al. 2008). Antioxidants are used in lipid-containing foods to minimize rancidity, preventing off-flavour, delay the formation of oxidation products, maintain nutritional quality and prolong the shelf life of food products (Maisuthisakul et al. 2007).
The search of potent antioxidant from natural sources has always remained as the mainstream of research. Mushrooms with delicate flavour and texture are recognised not only as nutritious food, but also an important source of biologically active compounds with medicinal values. Mushrooms have been reported to be an excellent source for antioxidant as they accumulate variety of secondary metabolites, including phenolic compounds, which are very competent scavengers of peroxy radicals (Cheung et al. 2003). High antioxidant capacity has been reported in wild growing mushrooms; among others are T. rutilans (Ribeiro et al. 2006); L. giganteus (Barros et al. 2007a) and B. edulis (Ramirez-Anguiano et al. 2007). Ferreira et al. (2007) reported that higher antioxidant capacity is found in caps compared to the stipes of the mushroom.
Schizophyllum commune is commonly known as “split gill” and normally associated with white rot decay of wood and a variety of other substrata in temperature, sub-tropical and tropical forest (Brady et al. 2005). The individual mature fruit bodies are elastic and tough, grey-white to brown in color, fan-shaped with short stripes and a wrinkled upper surface. Schizophyllan is a polysaccharide derived from S. commune and an important immune-modulator that demonstrated anti-tumor effects (Lindequist et al. 2005), and this polysaccharide is also believed to possess antioxidative properties. Dikin et al. (2007) reported that S. commune possesses anti-microbial substances which have broad spectrum to control pathogen fungi and suppress bacteria.
Solvent extraction is the most commonly used extraction method to recover a wide range of antioxidants and phenolic compounds (Abad-García et al. 2007, Chirinos et al. 2007). The efficacy of the extraction is influenced by factors such as storage time, solvent type, extraction method, the pH, extraction temperature, solvent-to-solid ratio, particle size and solvent concentration (Pinelo et al. 2005, Silva et al. 2007). Although solvent extraction is simple and easy, it is very time consuming, laborious and provide low extraction yields with large amount of organic solvents used (Herrero et al. 2005).
Response surface methodology (RSM) is an effective statistical technique for optimizing the process variables and a powerful tool which can present the optimal conditions that improve a process if it is used adequately (Silva et al. 2007, Fan et al. 2008). RSM helps to define the effect of the independent variables, whether it is alone or in combination in the process (Bas and Boyaci 2007). Thus, RSM is a useful tool for optimizing the chemical and biochemical process over the conventional one-factor-at-a-time approach, which is relatively time-consuming and expensive.
Report on antioxidant activity of wild grown S. commune found in Sabah, Malaysia is still scarce and no previous work was reported on optimizing the extraction conditions on antioxidant activity. Thus, the objective of the present study was to apply the RSM approach to optimize the extraction time and temperature in order to maximize the yield of antioxidant activity and total phenolic content from S. commune aqueous extract.
Materials and methods
Chemicals and reagents
All the chemicals and reagents were of analytical grade. Gallic acid, ethanol, glacial acetic acid, iron (III) chloride anhydrous, sodium carbonate anhydrous, potassium persulfate and 2,4,6-tripyridyl-s-triazine (TPTZ) were purchased from Fisher Scientific (Leicestershire, UK). Methanol, hydrochloric acid, Folin-Ciocalteu’s phenol reagent and 2,2′-azino-di[3-ethyl-benzthiazoline sulfonate] (ABTS) were from Merck (Darmstadt, Germany). Sodium acetate buffer (0.3 M) and 2,2-diphenyl-1-picryhydrazyl (DPPH) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Water used was of Millipore quality (Millipore, MA, USA).
Preparation of mushroom extract
The wild mushroom Schizophyllum commune was purchased from indigenous people who collect from forest and sell at local market in Kota Kinabalu, Sabah, Malaysia. The sample was washed, air dried followed by drying in an oven at 45 °C for 24 h. The dried sample was grounded to powder using a miller (MF 10 basic; IKA®-Werke, Staufen, Germany) with 0.5 mm mesh size and about 5 g of dried S. commune powder was extracted by 50 ml of distilled water. The mixture was shaken at required temperature and time using a water bath shaker at level 8 (Memmert, Schwabach, Germany). The extraction time and temperature were pre-determined using RSM software (Design Expert 6.0) as per the experimental design. Then, the mixture was centrifuged for 10 min at 4000 rpm (Universal 320R, Hettich Zentrifugen, Beverly, MA, USA). The supernatant was then filtered through a Whatman No. 1 filter paper to obtain clear extract.
DPPH radical scavenging activity
Radical scavenging activity of the mushroom extract was evaluated using DPPH radicals based on the method by Xu and Chang (2007) with slight modification. The DPPH• solution was prepared by dissolving 5.9 mg of DPPH• in ethanol (100 ml). Accurately, 3.8 ml of DPPH• ethanolic solution was added to 0.2 ml of mushroom extract. The mixture was shaken vigorously for 1 min and left to stand at room temperature in the dark for 30 min. Absorbance was measured against the blank reagent at 517 nm (XTD 5, Secomam, Alès Gard, France). All determinations were determined by replicate experiments with triplicate analysis. The radical scavenging activity was calculated according to the Eq. 1 below:
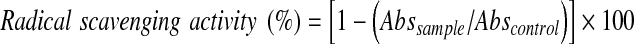 |
1 |
ABTS radical cation inhibition activity
Determination of ABTS radical cation inhibition activity of mushroom extract was performed according to the methods of Lo and Cheung (2005) and Biglari et al. (2008) with some modifications. The ABTS•+ reagent was prepared by mixing 5 ml of 7 mM ABTS•+ solution with 88 μl of 140 mM potassium persulfate (K2S2O8). The mixture was added into an amber bottle and kept in the dark at room temperature for 16 h to allow the completion of radical generation. After 16 h, 95% ethanol was used to adjust the absorbance of the ABTS•+ reagent to 0.70 ± 0.05 at 734 nm (XTD 5, Secomam, Alès Gard, France). Approximately 1 ml of ABTS•+ reagent was added to 10 μl of mushroom extract. The mixture was allowed to stand at room temperature for 6 min after the addition. Absorbance was measured against the blank reagent at 734 nm (XTD 5, Secomam, Alès Gard, France). All determinations were performed by replicate experiments with triplicate analysis. The radical inhibition activity was calculated according to the Eq. 2 below:
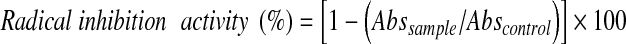 |
2 |
FRAP assay
The ferric reducing antioxidant power (FRAP) of mushroom extract was estimated based on the methods by Benzie and Strain (1996) and Xu and Chang (2007) with slight modification. The FRAP reagent was prepared by adding 2.5 ml of 10 mM TPTZ into 40 mM HCl. After dissolving TPTZ in HCl, 2.5 ml of 20 mM FeCl3·6H2O (ferric trichloride hexahydrate) was added followed by 25 ml of 0.3 M acetate buffer at pH 3.6. The freshly prepared FRAP working reagent was warmed to 37 °C. Then, approximately 3 ml of the FRAP reagent was added to 100 μl of mushroom extract and 300 μl of deionized water. The absorbance was measured at 593 nm against the blank (XTD 5, Secomam, Alès Gard, France) after 4 min. FRAP value was calculated and expressed as mM Fe2+ equivalent (FE) per 100 g sample using the calibration curve of Fe2+. All determinations were performed by replicate experiments with triplicate analysis. Linearity range of the calibration curve was 0.2–1 mM (R2 = 0.99).
Total phenolic content (TPC) analysis
The TPC analysis was performed using Folin-Ciocalteu method according to Barros et al. (2007b) and Zhao and Hall (2008) with slight modification. A 1 ml of sample was mixed with 1 ml of Folin-Ciocalteu’s solution. After 3 min, 1 ml of 7.5% sodium carbonate solution was added to the mixture and adjusted to 10 ml with deionized water. The mixture was allowed to stand at room temperature in the dark environment for 90 min. Absorbance was measured against the blank reagent at 725 nm using spectrophotometer (XTD 5, Secomam, Alès Gard, France). Gallic acid was used for the calibration curve with a concentration range of 50–1000 μg/ml (R2 = 0.99) and analyzed as above. Results were expressed as mg gallic acid equivalent (GAE)/100 g sample. All determinations were carried out by replicate experiments with triplicate analysis.
Experimental design
Before the development of the study by RSM, determination of experimental ranges for independent variables namely, solvent type, extraction time and temperature were carried out using total phenolic content as determinant factor as previously reported (Yim et al. 2009). Then, RSM was used to determine the optimum levels of extraction time (min) and temperature (°C) using water as extraction medium on four responses namely, DPPH• scavenging and ABTS•+ inhibition activities, FRAP and TPC in the S. commune extracts. These two factors, namely extraction time (X1) and temperature (X2) were coded into five levels (−1.414, −1, 0, 1, 1.414). The coded and uncoded independent variables used in the RSM design are shown in Table 1. Ranges of extraction time and temperature with water as the extraction solvent and the central points were selected based on preliminary experimental results. The experiments were designed according to central composite design (CCD) with a 22 factorial design consisting of four factorial points, four axial points and six central points (Table 2). The run of the experiments were conducted in a random order and the data were analyzed by multiple regressions using least-square method. The response function (Y) was partitioned into linear, quadratic, and interactive components and the experimental data were fitted to the second-order regression equation as shown in Eq. 3:
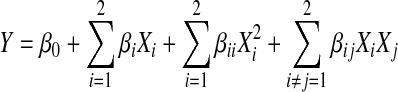 |
3 |
where Yk refers to the measured predicted responses, β0 is the intercept; βi, βii, and βij are the linear, quadratic and interaction coefficients, respectively of the model, and Xi and Xj are the levels of independent variables. The three-dimensional surface response plots were generated showing the relationship between the response and independent variables (Bas and Boyaci 2007). The experimental data were analyzed using MINITAB (Minitab Inc., State College, PA, USA) version 14 for Windows and Design Expert 6.0 (Stat-Ease Inc., Minneapolis, MN, USA). The significance level was based on a confidence level of 95.0%.
Table 1.
Coded and uncoded levels of independent variables used in the RSM design
| Symbols | Independent variables | Coded levels | ||||
|---|---|---|---|---|---|---|
| −1.414 | −1 | 0 | 1 | 1.414 | ||
| X1 | Extraction time (min) | 60.0 | 99.5 | 195.0 | 290.5 | 330.0 |
| X2 | Temperature (°C) | 25.0 | 30.1 | 42.5 | 54.9 | 60.0 |
Table 2.
Experimental design and responses of the dependent variables to extraction conditions
| No.a | Independent variables | Dependent variables (responses)b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Extraction time | Temperature | Y1 | Y2 | Y3 | Y4 | |||||
| X1 (min) | X2 (°C) | Expt. | Pred. | Expt. | Pred. | Expt. | Pred. | Expt. | Pred. | |
| 1 | 99.5 | 30.1 | 79.22 | 78.85 | 93.58 | 93.02 | 0.61 | 0.65 | 567.54 | 544.95 |
| 2 | 290.5 | 30.1 | 81.79 | 83.94 | 91.12 | 90.90 | 0.58 | 0.62 | 614.73 | 622.36 |
| 3 | 99.5 | 54.9 | 76.07 | 73.94 | 91.50 | 91.22 | 0.62 | 0.63 | 595.69 | 586.07 |
| 4 | 290.5 | 54.9 | 75.23 | 75.39 | 92.76 | 92.55 | 0.61 | 0.62 | 585.42 | 589.11 |
| 5 | 195.0 | 42.5 | 80.56 | 81.90 | 94.87 | 94.23 | 0.78 | 0.77 | 642.83 | 635.19 |
| 6 | 195.0 | 42.5 | 83.16 | 81.90 | 94.23 | 94.23 | 0.72 | 0.77 | 636.77 | 635.19 |
| 7 | 195.0 | 42.5 | 82.58 | 81.90 | 93.69 | 94.23 | 0.79 | 0.77 | 623.97 | 635.19 |
| 8 | 60.0 | 42.5 | 77.96 | 79.44 | 91.74 | 91.90 | 0.63 | 0.63 | 553.94 | 563.34 |
| 9 | 330.0 | 42.5 | 85.87 | 84.07 | 91.29 | 91.34 | 0.62 | 0.61 | 630.37 | 620.22 |
| 10 | 195.0 | 25.0 | 80.25 | 78.78 | 90.73 | 90.87 | 0.50 | 0.48 | 522.46 | 520.20 |
| 11 | 195.0 | 60.0 | 67.65 | 69.26 | 90.81 | 90.76 | 0.46 | 0.47 | 521.62 | 525.76 |
| 12 | 195.0 | 42.5 | 80.32 | 81.76 | 94.18 | 93.52 | 0.71 | 0.69 | 588.96 | 606.94 |
| 13 | 195.0 | 42.5 | 82.07 | 81.76 | 93.89 | 93.52 | 0.67 | 0.69 | 613.71 | 606.94 |
| 14 | 195.0 | 42.5 | 82.19 | 81.76 | 93.24 | 93.52 | 0.64 | 0.69 | 609.16 | 606.94 |
aNonrandomized order
bY1: 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging ability (%); Y2: 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) radical cation inhibition (%); Y3: Reducing power (FRAP) (mM of Fe2+ equiv. (FE)/100 g); Y4: Total phenolic content (TPC) (mg GAE/100 g)
Results and discussion
The selection of water as extracting solvent (out of other solvents such as methanol, ethanol, acetone and hexane) and the appropriate range of extraction time and temperature were determined using single factor experiments, the best extraction parameter was selected according to the value of TPC (mg GAE/100 g) as reported in Yim et al. (2009). Based on the results from single factor experiment, the ranges of extraction time (60–330 min) and temperature (25–60 °C) were optimized using RSM for maximal yield of antioxidant activity and TPC from S. commune aqueous extract as reported in the present study.
Fitting the models
The antioxidant activities namely DPPH• scavenging ability (Y1), ABTS•+ inhibition (Y2) and FRAP (Y3), and total phenolic content (Y4) in S. commune extracts obtained from the experiments are shown in Table 2. The experimental data were fitted into the second-order polynomial equations and the regression coefficients were calculated, the significance of the coefficients of the models were determined by analysis of variance (ANOVA) as summarized in Table 3. The adequacy of the model to fit the experimental data was verified by the lack of fit testing; ANOVA for the lack of fit test for all the responses were insignificant (p > 0.05) indicating that the model were adequately fitted the experimental data. The larger the regression coefficient in a model with significant p-value indicates a more significant effect on the respective response variables (Yang et al. 2009).
Table 3.
ANOVA for response surface quadratic model: estimated regression model of relationship between response variables (Y1, Y2, Y3, Y4) and independent variables (X1, X2)
| Source | Sum of square | DF | Mean square | F-value | p-value |
|---|---|---|---|---|---|
| Y1 | |||||
| Model | 231.66 | 5 | 46.33 | 12.73 | 0.0021 |
| Quadratic | 113.17 | 2 | 56.58 | 15.55 | 0.0027 |
| X1 | 20.85 | 1 | 20.85 | 5.73 | 0.0479 |
| X2 | 94.73 | 1 | 94.73 | 26.03 | 0.0014 |
| X22 | 112.06 | 1 | 112.06 | 30.79 | 0.0009 |
| Residual | 25.47 | 7 | 3.64 | ||
| Lack of fit | 19.55 | 3 | 6.52 | 4.41 | 0.0930 |
| Total | 257.51 | 13 | |||
| R2 (model) | 0.901 | ||||
| Y2 | |||||
| Model | 22.99 | 5 | 4.60 | 20.74 | 0.0005 |
| Quadratic | 19.10 | 2 | 9.55 | 43.06 | 0.0001 |
| X12 | 7.04 | 1 | 7.04 | 31.76 | 0.0008 |
| X22 | 13.44 | 1 | 13.44 | 60.60 | 0.0001 |
| X1X2 | 3.46 | 1 | 3.46 | 15.60 | 0.0055 |
| Residual | 1.55 | 7 | 0.22 | ||
| Lack of fit | 0.39 | 3 | 0.13 | 0.45 | 0.7318 |
| Total | 27.00 | 13 | |||
| R2 (model) | 0.937 | ||||
| Y3 | |||||
| Model | 0.088 | 5 | 0.018 | 15.88 | 0.0011 |
| Quadratic | 0.088 | 2 | 0.044 | 39.47 | 0.0002 |
| X12 | 0.0083 | 1 | 0.0083 | 7.47 | 0.0292 |
| X22 | 0.083 | 1 | 0.083 | 74.64 | <0.0001 |
| Residual | 0.0078 | 7 | 0.0011 | ||
| Lack of fit | 0.0025 | 3 | 0.0008 | 0.61 | 0.6413 |
| Total | 0.11 | 13 | |||
| R2 (model) | 0.919 | ||||
| Y4 | |||||
| Model | 15394.61 | 5 | 3078.92 | 18.35 | 0.0007 |
| Quadratic | 11940.08 | 2 | 5970.04 | 35.59 | 0.0002 |
| X1 | 2628.43 | 1 | 2628.43 | 15.67 | 0.0055 |
| X22 | 11909.97 | 1 | 11909.97 | 71.00 | <0.0001 |
| Residual | 1174.24 | 7 | 167.75 | ||
| Lack of fit | 641.72 | 3 | 213.91 | 1.61 | 0.3212 |
| Total | 20240.74 | 12 | |||
| R2 (model) | 0.929 | ||||
Y1: 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging ability (%)
Y2: 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) radical cation inhibition (%)
Y3: Reducing power (FRAP) (mM of Fe2+ equiv. (FE)/100 g)
Y4: Total phenolic content (TPC) (mg GAE/100 g)
Response surface analysis of DPPH• scavenging ability
The response surface analysis (RSA) of the experimental data as shown in Table 2 demonstrates that both extraction time and temperature have quadratic effect on DPPH• scavenging ability with a good regression coefficient (R2 = 0.901). The relationship between the DPPH• scavenging ability and the extraction parameters is shown in Eq. 4 as follows:
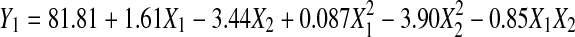 |
4 |
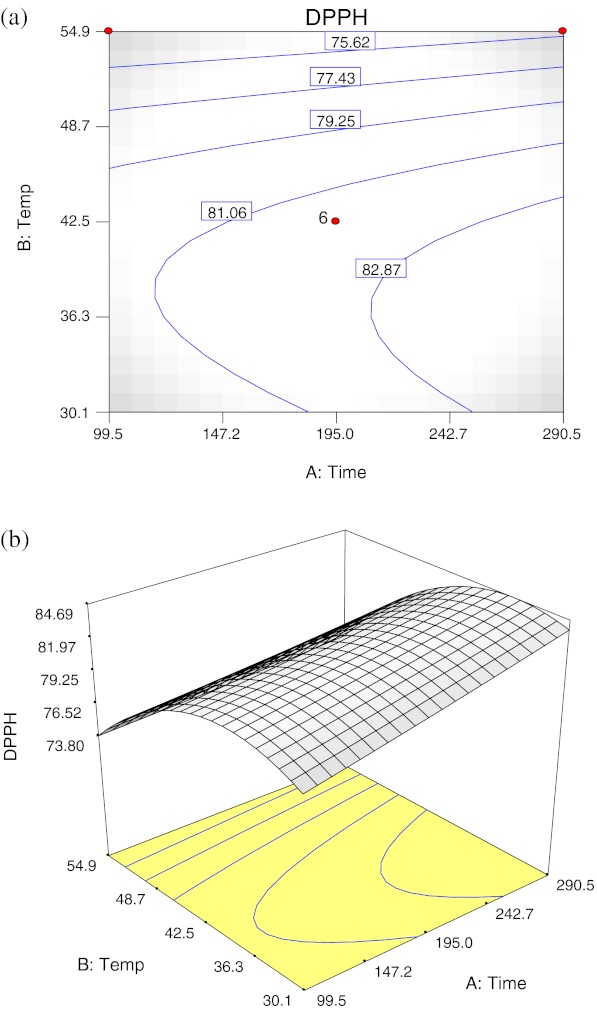
Extraction temperature had significant negative linear and quadratic effects (p < 0.01), and extraction time was shown to have a significant linear effect (p < 0.05) on DPPH• scavenging ability, and no significant interaction effect between extraction time and temperature was observed (Table 3). Figure 1 shows the (a) contour plot and (b) response surface plot of extraction time and temperature on DPPH• scavenging ability. The DPPH• scavenging ability increased when extraction time increased. As temperature increased from 30 °C to about 42.5 °C, higher DPPH• scavenging ability was detected and the DPPH• scavenging ability decreases drastically with increasing temperature after 42.5 °C region.
Fig. 1.
Contour plot (a) and response surface plot (b) of the DPPH• scavenging ability as a function of extraction time and temperature
A negative quadratic effect of temperature was observed for DPPH• scavenging ability, indicating that there is a maximum DPPH• scavenging ability at a certain temperature, and the DPPH• scavenging ability begins to decrease above this temperature. In order to obtain high DPPH• scavenging ability, the extraction temperature plays a more critical role in extends to extraction time. Generally, high DPPH• scavenging ability could be obtained with increasing extraction temperature, as according to Pinelo et al. (2005), increasing extraction temperature will enhance the solubility of solute and increased the extraction coefficient, but temperature above 50 °C will affect the stability of the phenolic compounds and the alteration of plant’s membrane integrity may affect the antioxidant capacity. Gan and Latiff (2010) revealed in their study that extraction temperature between 35 and 55 °C did not significantly increase the antioxidant activity of Parkia speciosa pod. Therefore, the decrease of the scavenging ability with the increase of extraction temperature above 42.5 °C region observed in the present study might possibly due to the decomposition of the antioxidative compounds that are heat-sensitive.
The maximal DPPH• scavenging ability predicted by RSA was 84.70% with optimum extraction time of 290.5 min and temperature of 35.7 °C.
Response surface analysis of ABTS•+ inhibition activity
The RSA as shown in Table 2 demonstrated that the relationship between ABTS•+ inhibition and extraction parameter is quadratic with a good regression coefficient (R2 = 0.937), and the Eq. 5 shows the relationship as follows:
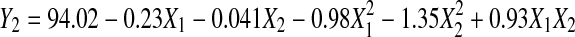 |
5 |
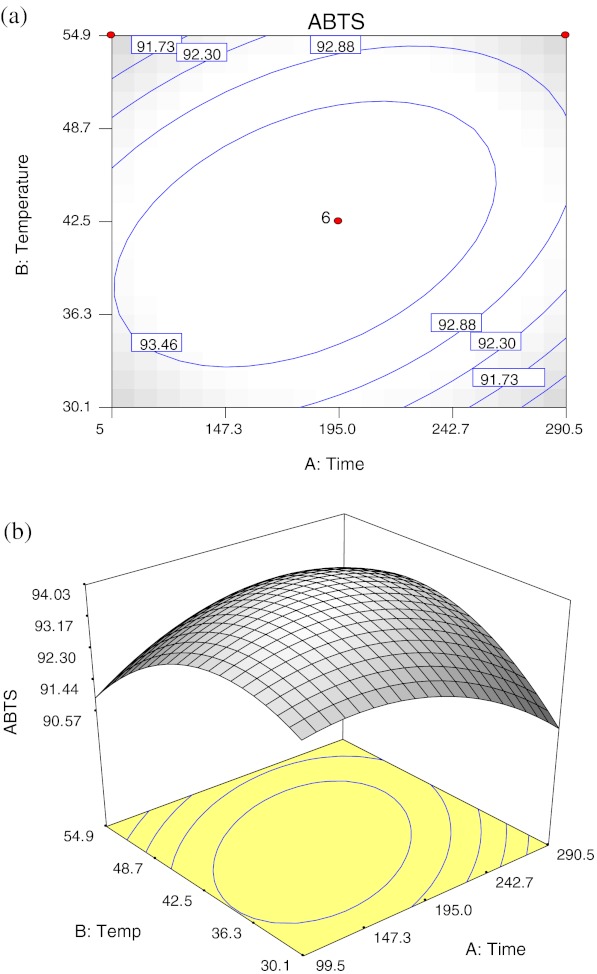
Extraction time and temperature had both significant negative quadratic effects (p < 0.01), and a significant interaction effect between extraction time and temperature was found (p < 0.01); no significant linear effect was observed for both extraction time and temperature (p > 0.05) on ABTS•+ inhibition (Table 3). Figure 2 shows the (a) contour plot and (b) response surface plot of ABTS•+ inhibition activity as a function of extraction time and temperature, the ABTS•+ inhibition activity increased from 99.5 min to 195 min and curve off thereafter. It was observed that as extraction temperature increases from 30 °C to 42.5 °C, the inhibition activity increases drastically. Beyond 42.5 °C, further increase in extraction temperature did not increase the inhibition activity but decreases drastically approaching 55 °C. This results a curvilinear effect response surface as observed in Fig. 2b.
Fig. 2.
Contour plot (a) and response surface plot (b) of the ABTS• + inhibition as a function of extraction time and temperature
The negative effects of both extraction time and temperature indicating that at a certain time and temperature deceleration of ABTS•+ inhibition occurs. Interestingly, a significant negative interaction between extraction time and temperature was obtained for the response of ABTS•+ inhibition, which may be attributed by decomposition of antioxidative compounds upon longer extraction time and higher temperature (Silva et al. 2007). According to practical cost-saving considerations, Xu et al. (2008) stated that the point representing possible combination of the lowest levels of factors within the optimum zone would be preferred over other combinations. It was important to obtain the highest inhibition of ABTS•+ extracted under moderate time (about 180 min) and temperature (40–42 °C).
The optimal extraction conditions were predicted to be the extraction time of 180.7 min and temperature of 41.7 °C for the maximal ABTS•+ inhibition activity of 94.04%.
Response surface analysis of ferric reducing antioxidant power
The RSA as shown in Table 2 demonstrated that the relationship between ferric reducing antioxidant power (FRAP) and extraction parameter is quadratic with a good regression coefficient (R2 = 0.919), and the Eq. 6 below shows the relationship:
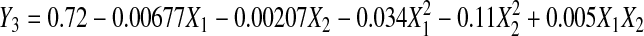 |
6 |
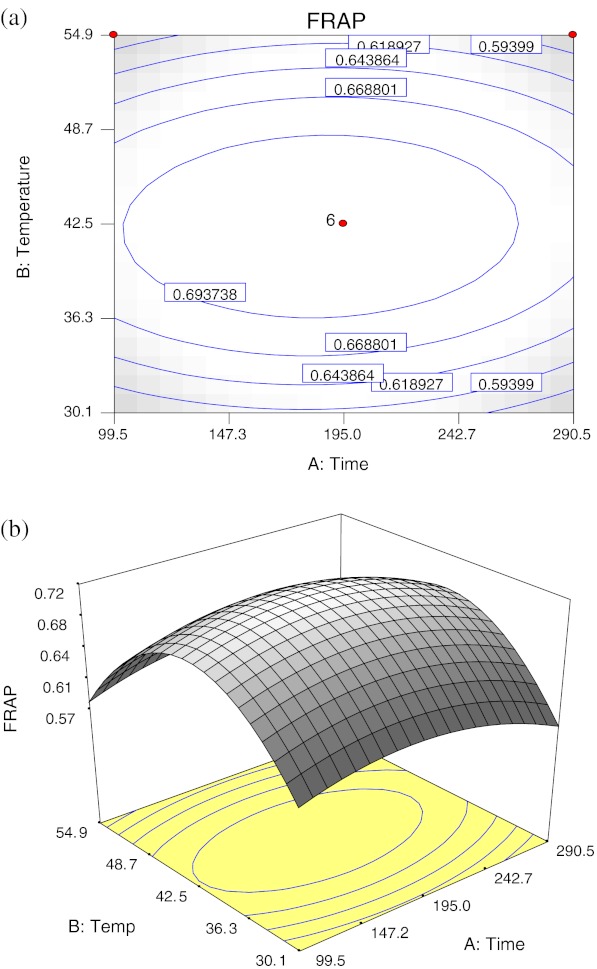
Extraction time (p < 0.05) and temperature (p < 0.01) had both significant negative quadratic effects; no significant linear effect and interaction between extraction time and temperature were observed (p > 0.05) on FRAP (Table 3). Figure 3 shows the (a) contour plot and (b) response surface plot of FRAP with the effect of extraction time and temperature, the FRAP was observed to increase in low extraction temperature and moderate extraction time. An increased of FRAP was observed with increase of extraction temperature from 30 °C to 42.5 °C, and extraction temperature above 42.5 °C decreases the FRAP value. In general, FRAP increases with moderate extraction time (180–190 min) and extraction temperature (40–42.5 °C).
Fig. 3.
Contour plot (a) and response surface plot (b) of the FRAP as a function of extraction time and temperature
The maximal FRAP predicted by RSA was 0.72 mM FE/100 g with the optimum extraction time of 185.2 min and extraction temperature of 42.4 °C.
Response surface analysis of total phenolic content
The RSA (Table 2) demonstrated a high regression coefficient (R2 = 0.929) and the Eq. 7 showed the relationship between TPC and extraction parameters of extraction time and temperature.
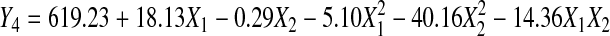 |
7 |
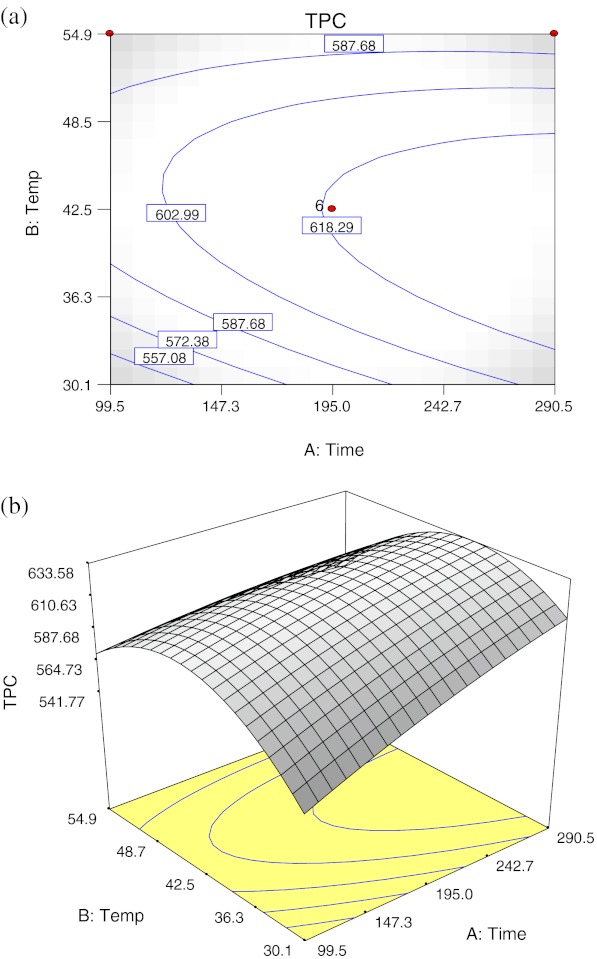
Extraction time had significant linear effect and temperature showed significant negative quadratic effects (p < 0.01) on TPC, and no significant interaction effect was observed between extraction time and temperature (Table 3). The contour plot (a) and response surface plot (b) with the effect of extraction time and temperature on TPC were shown in Fig. 4. It can be observed that TPC increases linearly with increases of extraction time from 99.5 to 290.5 min. Likewise, it was obvious that increasing extraction temperature from 30 °C to 42.5 °C promoted higher TPC level. From 42.5 °C to 55 °C, TPC level falls slightly as increased extraction time. As reported by Cacace and Mazza (2003), higher solubility and diffusion coefficient of polyphenols were observed with increased temperature, allowing more extraction rate. Nevertheless, an upper limit of temperature must be respected in order to prevent decomposition of thermo-sensitive compounds in particular flavonoids (Silva et al. 2007). The negative quadratic effect of temperature was observed for TPC, indicating that there is a maximum yield of TPC at a certain extraction temperature region, and the TPC starts to diminish above this region.
Fig. 4.
Contour plot (a) and response surface plot (b) of the total phenolic content as a function of extraction time and temperature
Extraction time and temperature are important parameters to be optimized in order to minimize energy cost of the process. The result revealed that the yield of TPC was highest at extraction time of about 290 min with moderate temperature (40 °C). Increase in the working temperature favours extraction, enhancing both the solubility of solute and the diffusion coefficient, but beyond a certain extend phenolic compounds could be decomposed. Compound stability may be affected due to chemical and enzymatic degradation or losses by thermal decomposition. This was the main mechanism causing the reduction in polyphenol content in onion as reported by Kiassos et al. (2009). Gan and Latiff (2010) reported that the temperature range of 35–55 °C did not significantly affect the TPC of Parkia speciosa pod in their study; however they suggested that this temperature range could be of optimum for the extraction at single parameter experiment. Moreover, phenols can react with other plant components, impeding their extraction. At this point extraction time becomes significant, since larger extraction periods might cause more extended polyphenol losses.
The optimal extraction conditions were predicted to be extraction time of 290.5 min and temperature of 40.3 °C. The maximal TPC value predicted by RSA was 633.59 mg GAE/100 g.
Verification of predictive model
Four individual verification experiments for DPPH• scavenging activity (Y1, %), ABTS•+ inhibition activity (Y2, %), FRAP (Y3, mM FE/100 g) and TPC (Y4, mg GAE/100 g) were carried out under respective optimal extraction time and temperature within the experimental range. Table 4 shows the suitability of the model equation for the prediction of maximum responses was verified using the respective responses’ optimal extraction. The experimental values of 85.10 ± 2.43% (Y1), 94.31 ± 0.41% (Y2), 0.74 ± 0.02 mM FE/100 g (Y3), and 635.76 ± 5.49 mg GAE/100 g (Y4), were found close to the predicted values derived from the respective regression models with the CV ranging from 0.29% to 2.78%.
Table 4.
Experimental data of the verification of predicted extraction parameters
| Responsesa | Extraction time (min) | Temperature (°C) | Predicted value | Experimental valueb | % Difference (CV) |
|---|---|---|---|---|---|
| DPPH (%) | 290.5 | 35.7 | 84.70 | 85.10 ± 2.43 | 0.47 |
| ABTS (%) | 180.7 | 41.7 | 94.04 | 94.31 ± 0.41 | 0.29 |
| FRAP (mM FE/100 g) | 185.2 | 42.4 | 0.72 | 0.74 ± 0.02 | 2.78 |
| TPC (mg GAE/100 g) | 290.5 | 40.3 | 633.59 | 635.76 ± 5.49 | 0.34 |
aDPPH: 2,2-diphenyl-1-picrylhydrazyl radical scavenging ability; ABTS: 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) radical cation inhibition; FRAP: Ferric reducing antioxidant power; TPC: Total phenolic content
bResults were expressed as mean ± standard deviation (n = 2)
Conclusion
RSM was employed to determine the optimum extraction time and temperature that yield high antioxidant activities (DPPH• scavenging ability, ABTS•+ inhibition activity and FRAP) and TPC from S. commune aqueous extract. ANOVA revealed that extraction temperature shows significant quadratic effects on all antioxidant activities and TPC, whereas extraction time had significant quadratic effects in ABTS•+ inhibition activity and FRAP only. The experimental values generated based on the optimized extraction parameters were well consistent with the predicted values. This study suggested that the models obtained can be utilized to optimize the extraction time and temperature for the maximal yield of antioxidant activities and TPC from S. commune aqueous extract.
Acknowledgements
This study was supported in part by the Science Fund under Agricultural Cluster (05-01-10-SF0082) by Malaysian Ministry of Agriculture and Agro-Based Industry; the authors gratefully acknowledge Universiti Malaysia Sabah and UCSI University for the laboratory supports.
References
- Abad-García B, Berrueta LA, López-Márquez DM, Crespo-Ferrer I, Gallo B, Vicente F. Optimization and validation of a methodology based on solvent extraction and liquid chromatography for the simultaneous determination of several polyphenolic families in fruit juices. J Chromatogr A. 2007;1154:87–96. doi: 10.1016/j.chroma.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Barros L, Calhelha RC, Vaz AJ. Antimicrobial activity and bioactive compounds of Portuguese wild edible mushrooms methanolic extracts. Eur Food Res Technol. 2007;225:151–156. doi: 10.1007/s00217-006-0394-x. [DOI] [Google Scholar]
- Barros L, Ferreira MJ, Queiros B, Ferreira CFR, Baptista P. Total phenols, beta-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem. 2007;103:413–419. doi: 10.1016/j.foodchem.2006.07.038. [DOI] [Google Scholar]
- Bas D, Boyaci IH. Modeling and optimization I: usability of response surface methodology. J Food Eng. 2007;78:836–845. doi: 10.1016/j.jfoodeng.2005.11.024. [DOI] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Biglari F, AlKarkhi AFM, Easa AM. Antioxidant activity and phenolic content of various date palm (Phoenix dactylifera) fruits for Iran. Food Chem. 2008;107:1636–1641. doi: 10.1016/j.foodchem.2007.10.033. [DOI] [Google Scholar]
- Brady KC, O’Kiely P, Forristal PD, Fuller H. Schizophyllum commune on big-bale grass silage in Ireland. Mycologist. 2005;19:30–35. doi: 10.1017/S0269915X05001059. [DOI] [Google Scholar]
- Cacace JE, Mazza G. Mass transfer process during extraction of phenolic compounds from milled berries. J Food Eng. 2003;59:379–389. doi: 10.1016/S0260-8774(02)00497-1. [DOI] [Google Scholar]
- Cheung LM, Cheung CK, Ooi EC. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem. 2003;81:249–255. doi: 10.1016/S0308-8146(02)00419-3. [DOI] [Google Scholar]
- Chirinos R, Rogez H, Camposa D, Pedreschi R, Larondelle Y. Optimization of extraction conditions of antioxidant phenolic compounds from mashua (Tropaeolum tuberosum Ruiz & Pavon) tubers. Separ Purif Technol. 2007;55:217–225. doi: 10.1016/j.seppur.2006.12.005. [DOI] [Google Scholar]
- Chong KS, Chye FY, Lee JS, Atong M. Nutritional properties of some edible wild mushrooms in Sabah. J Appl Sci. 2007;7(15):2216–2221. doi: 10.3923/jas.2007.2216.2221. [DOI] [Google Scholar]
- Dikin A, Sijam K, Kadir J, Seman IA. Effect of different carbon sources and peptones on the production of antimicrobial substances from bacteria against Schizophyllum commune FR. Int J Agric Biol. 2007;9(1):49–53. [Google Scholar]
- Dubost NJ, Ou B, Beelman RB. Quantification of polyphenols and ergothioneine in cultivated mushrooms and correlations to total antioxidant capacity. Food Chem. 2007;105:727–735. doi: 10.1016/j.foodchem.2007.01.030. [DOI] [Google Scholar]
- Elmastas M, Isidak O, Turkekul I, Temur N. Determination of antioxidant activity and antioxidant compounds in wild edible mushrooms. J Food Compos Anal. 2007;20:337–345. doi: 10.1016/j.jfca.2006.07.003. [DOI] [Google Scholar]
- Fan G, Han Y, Gu Z, Chen D. Optimization conditions for anthocyanins extraction from purple sweet potato using response surface methodology (RSM) LWT Food Sci Technol. 2008;41:155–160. doi: 10.1016/j.lwt.2007.01.019. [DOI] [Google Scholar]
- Ferreira ICFR, Baptista P, Vilas-Boas M, Barros L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: Individual cap and stipe activity. Food Chem. 2007;100:1511–1516. doi: 10.1016/j.foodchem.2005.11.043. [DOI] [Google Scholar]
- Gan C-Y, Latiff AA. Optimisation of the solvent extraction of bioactive compounds from Parkia speciosa pod using response surface methodology. Food Chem. 2010;124:1277–1283. doi: 10.1016/j.foodchem.2010.07.074. [DOI] [Google Scholar]
- Herrero M, Martin-Alvarez PJ, Senorans FJ, Cifuentes A, Ibanez E. Optimization of accelerated solvent extraction of antioxidants from Spirulina platensis microalga. Food Chem. 2005;93:417–423. doi: 10.1016/j.foodchem.2004.09.037. [DOI] [Google Scholar]
- Kiassos E, Mylonaki S, Makris DP, Kiassos PKE, Mylonaki S, Makris DP, Kefalas P. Implementation of response surface methodology to optimise extraction of onion (Allium cepa) solid waste phenolics. Innov Food Sci Emerg Technol. 2009;10:246–252. doi: 10.1016/j.ifset.2008.10.004. [DOI] [Google Scholar]
- Li H, Wang XY, Li Y, Li PH, Wang H. Polyphenolic compounds and antioxidant properties of selected China wines. Food Chem. 2009;112:454–460. doi: 10.1016/j.foodchem.2008.05.111. [DOI] [Google Scholar]
- Lindequist U, Niedermeyer THJ, Julich WD. The pharmacological potential of mushrooms. eCAM. 2005;2(3):285–299. doi: 10.1093/ecam/neh107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo KM, Cheung PCK. Antioxidant activity of extracts from the fruiting bodies of Agrocybe aegerita var. alba. Food Chem. 2005;89(4):533–539. doi: 10.1016/j.foodchem.2004.03.006. [DOI] [Google Scholar]
- Maisuthisakul P, Suttajit M, Pongsawatmanit R. Assessment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants. Food Chem. 2007;100:1409–1418. doi: 10.1016/j.foodchem.2005.11.032. [DOI] [Google Scholar]
- Mau JL, Chang CN, Huang SJ, Chen CC. Antioxidant properties of methonolic extracts from Grifola frondosa, Morchella esculenta and Termitomyces albuminosus mycelia. Food Chem. 2004;87:111–118. doi: 10.1016/j.foodchem.2003.10.026. [DOI] [Google Scholar]
- Pinelo M, Fabro PD, Manzocco L, Nunez MJ, Nicoli MC. Optimization of continuos phenol extraction from Vitis vinifera byproducts. Food Chem. 2005;92:109–117. doi: 10.1016/j.foodchem.2004.07.015. [DOI] [Google Scholar]
- Ramirez-Anguiano AC, Santoyo S, Reglero G, Soler-Rivas C. Radical scavenging activities, endogenous oxidative enzymes and total phenols in edible mushrooms commonly consumed in Europe. J Sci Food Agric. 2007;87:2272–2278. doi: 10.1002/jsfa.2983. [DOI] [Google Scholar]
- Ribeiro B, Rangel J, Valentao P, Baptista P, Seabra RM, Andrade PB. Contents of carboxylic acids and two phenolics and antioxidant activity of dried Portuguese wild edible mushrooms. J Agric Food Chem. 2006;54:8530–8537. doi: 10.1021/jf061890q. [DOI] [PubMed] [Google Scholar]
- Silva EM, Rogez H, Larondelle Y. Optimization of extraction of phenolics from Inga edulis leaves using response surface methodology. Separ Purif Technol. 2007;55:381–387. doi: 10.1016/j.seppur.2007.01.008. [DOI] [Google Scholar]
- Xu BJ, Chang SKC. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J Food Sci. 2007;72:S159–S166. doi: 10.1111/j.1750-3841.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- Xu X, Gao YX, Liu GM, Wang Q, Zhao J. Optimization of supercritical carbon dioxide extraction of sea buckthorn (Hippopha thamnoides L.) oil using response surface methodology. LWT Food Sci Technol. 2008;41:1223–1231. doi: 10.1016/j.lwt.2007.08.002. [DOI] [Google Scholar]
- Yang B, Liu X, Gao Y. Extraction optimization of bioactive compounds (crocin, geniposide and total phenolic compounds) from Gardenia (Gardenia jasminoides Ellis) fruits with response surface methodology. Innovat Food Sci Emerg Tech. 2009;10:610–615. doi: 10.1016/j.ifset.2009.03.003. [DOI] [Google Scholar]
- Yim HS, Chye FY, Ho SK, Ho CW. Phenolic profiles of selected edible wild mushrooms as affected by extraction solvent, time and temperature. As J Food Ag-Ind. 2009;2(3):371–380. [Google Scholar]
- Zhao B, Hall CA. Composition and antioxidant activity of raisin extracts obtained from various solvents. Food Chem. 2008;108(2):511–518. doi: 10.1016/j.foodchem.2007.11.003. [DOI] [PubMed] [Google Scholar]