Abstract
The effect of antifungal hot-water treatments (AHWT) at 55 °C for 0, 3, 6 and 9 min on quality attributes and cell-wall enzymatic activity during storage at 25 °C was investigated in papaya fruit. The total soluble solids (TSS), pH, titratable acidity (TA), firmness and fresh weight loss were not affected, whereas color on skin was negatively affected by the treatments of 6- and 9-min. However, the skin color was not different between the 3-min treated fruit and the untreated fruit during the storage. Decay was delayed and reduced by AHWT. We observed that the 3-min treatment of 55 °C did not affect softening and quality of papaya cv Maradol when applied as a pesticide-free treatment at color-break stage of papaya. PME (Pectinmethylesterase) and PG (Polygalacturonase) activities were not significantly affected by AHWT. We concluded that the AHWT did not affect the softening process from papaya pulp since the cell-wall enzyme activity (PME and PG) was not altered by treatments.
Keywords: Carica papaya, Antifungal-hot-water-treatments, Pectinmethylesterase, Polygalacturonase, Decay, Postharvest quality
Introduction
Many chemical treatments have been banned or restricted as postharvest fungicide treatments of fruits in some countries, and the demand of pesticide-free produce has increased (Adaskaveg et al. 2002). So, it has been necessary to develop alternative treatments in order to avoid toxic and dangerous chemical compounds in food for human consumption. Heat treatments have been shown to be effective as a non-chemical mean of improving postharvest quality of a range of horticultural products. They are usually applied as hot water dips, vapor heat, or hot-air treatments (Lurie 1998). They may affect ripening and protect against physiological disorders (Klein and Lurie 1992), and have been used as an effective alternative treatment for decay control (Cantwell and Nie 1996). Furthermore, hot water immersion, humid vapor exposition and forced hot-air treatments are the main technologies of physical type used as pest and disease control in fruits and vegetables (Paull and Armstrong 1994). It is well known that heat treatments offer a viable alternative to the postharvest problems, since they produce a fungicidal and insecticidal action on papaya fruit (Paull and Armstrong 1994), and keep the good quality of some fruits (Klein and Lurie 1992). Furthermore, they alleviate some physiological disorders as chilling injury (McDonald et al. 1993; Chan et al. 1994). However, the excessive-heating effect on ripening (Paull 1994; Paull and Chen 1990) and on papaya-polygalacturonase activity has also been reported (Chan et al. 1981, Lazan et al. 1989). Chan et al. (1981) observed a significant PG-activity inhibition when the hot water (HW) treatments of 46 °C for, 40, 65 and 90 min were applied on Papaya cv Solo at their advanced ripeness stages (quarter and half ripe). Pectin Methyl Esterase (PME) (EC 3.1.11) and Polygalacturonase (PG) (EC 3.2.1.15) are two enzymes frequently associated with cell wall changes and fruit softening during ripening (Huber 1983; Carrillo-López et al. 2002). Both, PME and PG act in concert to increase cell wall solubilization (Pressey and Avants 1982). De-esterification of cell wall galacturonans by PME may be required prior to hydrolysis by PG (Awad and Young 1979). The activity of these enzymes account for textural changes that collectively contribute to fruit softening, increased pathogen susceptibility and ultimately to the deterioration of fruit tissue (Bennet 2001; Carrillo-López et al. 2003). Furthermore, fruits and vegetables are very susceptible to undesirable alterations as a consequence of injuries suffered during storage, handling and processing (Watada et al. 1990). Among such alterations are flavor and color which may decrease their quality and the market value of the produce may suffer economic losses (Almeida and Nogueira 1995). Papaya is a tropical fruit that possesses good nutritional quality attributes, but may suffer some postharvest handling problems due to its susceptibility to decay and insect pests (Salunkhe and Desai 1984; McGregor 1987). AHWT of 55 °C for several minutes could be applied to control decay with no deleterious effect on softening and quality of papaya. The aim of this paper is to know the effect of AHWT applied on Papaya cv Maradol at color-break stage on the activity of softening-related enzymes (PME and PG) and on postharvest quality attributes (TSS, pH, TA, fresh weight loss, skin color, firmness and decay) when stored at temperatures of marketing (25 °C).
Materials and methods
Papaya (Carica papaya cv. ‘Maradol’) fruit were harvested from Angostura, Sinaloa at physiological maturity stage (color-break stage) and immediately transported to the laboratory. Fruits were washed and chosen based on uniformity of skin color, free of physical damage and with an average weight of 1400 g ± 300 g. Fruits were randomly sorted to the planned treatments. Papaya fruit was immersed in hot water at 55 °C for 0, 3, 6 and 9 min, using a bath with a temperature controller Cole Parmer, Model 1266-02. Immediately after the AHWT the fruits were cooled with water at 25 °C during 20 min. Fruits were evaluated every 3 days during storage for 9 to 15 days at 25 ± 1 °C, 80–85% HR.
Quality parameters
Physical and Chemical analysis performed included, total soluble solids (TSS), pH, titratable acidity (TA), fresh weight loss, skin color, firmness and decay.
TSS, pH, and TA
These were determined following the methodology of the AOAC (1998). TSS was determined using a Refractometer Abbe Bellingham + Staley Limited and expressed as °Brix. The pH was determined using a pH-meter (Orion Research Inc. model 520.Boston, M.A, USA,). TA was expressed as % of malic acid.
Fresh weight loss
It was measured employing a scale OHAUS (Ohaus Corporation Florham Park, N.J. EUA, model TP2KS) by weighing periodically the whole fruit and calculating the percentage of weight loss in relation to the initial weight of the fruit.
Skin color (L*, a*, b*, C * and hue)
It was evaluated using a colorimeter (Minolta Chromameter model CR-210, Japan) in three different points on the fruit (McGuire 1992).
Firmness
It was measured according to Barca et al. (2000) as the maximum compression force in Newtons (N) reached during the rupture of the fleshy portion of the fruit on six points of every fruit, employing a plunger of 8-mm diameter, using a digital firmness-tester (Chatillon model DF150, John Chatillon & Sons, Inc. NY, N.Y, EUA)
Decay on skin
It was evaluated subjectively according to McDonald et al. (1998), measuring on fruit the rotted area in relation to the skin total area expressed as percentage.
Enzyme extraction
PME (EC 3.1.11) extraction was carried out as described by Fayyaz et al. (1993). Twenty grams of fresh tissue were homogenized in a blender (Model Osterizer Lo-20 Mexico) with 30 mL 2 M NaCl at 4 °C. The homogenate was adjusted to pH 7.5 with 0.1 N NaOH or 0.1 N HCl, previously and during a period of stirring for 30 min at 4 °C. Then it was centrifuged (Centrifugal Ivan Sorball Inc. Super model Speed RC-2, Connecticut, USA) at 24000 × g during 30 min at 4 °C. The supernatant was used as the enzymatic extract. PG extraction was carried out as described by Roe and Bruemmer (1981) and by Aina and Oladunjoye (1993). Twenty five grams of fresh tissue were homogenized in a blender (Osterizer Model Lo-20 Mexico) with 25 mL of a solution containing 12% polyethylene glycol (VWR Scientific Einecs, Switzerland) and 0.2% sodium bisulfite pH 5.0, at 4 °C. The suspension was centrifuged (Ivan centrifuges Sorball Inc. Super model Speed RC-2, Connecticut, USA) at 5000 × g during 20 min. The pellet was washed twice with cold distilled water and centrifuged again at 5000 × g during 20 min. The pellet was resuspended in 25 mL of 0.5 M NaCl for 30 min and centrifuged again at 5000 × g during 10 min. The supernatant was used as the enzymatic extract.
Enzyme assays
PME activity was determined according to Hagerman and Austin (1986) based on the color change of a pH indicator during the PME-catalyzed reaction. As the ester bonds from pectin are hydrolyzed, acid groups are produced and the pH is lowered, causing the indicator dye to change its color. This change is continuously monitored spectrophotometrically. In a cuvette, 2 mL of 0.5% citric pectin (Sigma Chemical Co. St. Louis. MO, USA) was mixed with 0.5 mL of 0.01% bromothymol blue solution (Sigma from Mexico, D.F., Mexico) (in 0.003 M potassium phosphate buffer) (Sigma Chemical Co. St. Louis. MO, USA) and 0.4 mL of distilled water. The mix was adjusted to pH 7.5 and the reaction was started by adding 100 μL of PME-enzymatic extract. The reaction was carried out at 30 °C and monitored during 5 min at 620 nm. (Spectrophotometer UV-visible model Australian Varian Cary 1E). A standard curve of galacturonic acid was used (Sigma Chemical Co. St. Louis. MO, USA) (Hagerman and Austin 1986). The units of PME activity are expressed as μmoles of H+ released/min × 100 g fresh tissue at 30 °C at pH 7.5. PG activity was assayed spectrophotometrically according to Gross (1982) on the basis of the hydrolytic release of reducing groups from polygalacturonic acid. Four milliliter of PG-enzymatic extract and 4 mL of 0.8% polygalacturonic acid (Sigma Chemical Co. St. Louis. MO, USA) were mixed with 1.6 mL of 0.2 M sodium acetate buffer (Baker Analyzed, J.T. Baker, CORP. of C.V. Xalostoc, Mexico) at pH 4.6 and incubated for 2 h at 37 °C. 0.8 mL were taken from the incubated reaction and mixed with 4 mL of 0.1 M borate buffer (pH 9) (VWR Scientific Einecs, Switzerland) to stop the reaction and then 0.8 mL of 0.1% 2-cyanoacetamide (Aldrich Chemical Company, Inc. Milwaukee Wis, USA) was added. Samples previously mixed were immersed in boiling water for 10 min. After equilibration to 25 °C, the absorbance at 276 nm was measured to quantify the released reducing groups (Spectrophotometer UV-visible model Australian Varian Cary 1E). A standard curve of reducing groups from galacturonic acid was used. (Sigma Chemical Co. St. Louis. MO, USA). The units of PG activity are expressed as μmoles of reducing groups (as galacturonic acid) released/h × 100 g fresh tissue at 37 °C, pH 4.6.
Statistical analysis
Data were analyzed using a completely randomized design (Montgomery 1991). Analysis of variance with at least four replicates was done and comparison of means using the least significant difference (LSD) test with a significance level of 5% was carried out. The SG-Plus (1992) program was used.
Results and discussion
TSS, pH and TA on pulp
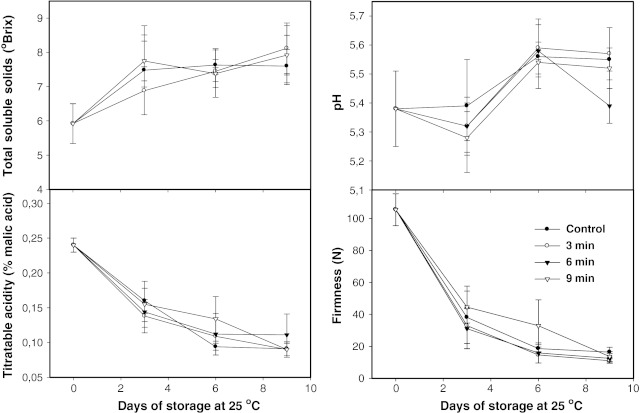
TSS, pH and TA were measured on the edible portion of the fruit. The results are shown in Fig. 1. During ripening of untreated papaya fruit stored at 25 °C, TSS increased from 5.92 to 7.6. The pH increased from 5.38 to 5.55, while TA decreased from 0.24 to 0.09. The behavior of these three quality parameters did not show significant differences (p ≤ 0.05) between untreated and treated fruits during storage, being this indicative that, the taste quality of papaya fruit was not affected by the applied AHWT because of these chemical parameters are good fruit-taste and ripeness indicators (Cámara et al. 1993). Jagtiani et al. (1988) reported pH in papaya varying between 5.5 and 5.9, and TSS between 5.65 and 7.1%. Also, Lazan et al. (1989) observed that hot water treatments at 48 °C for 20 min, did not affect TA in papaya cv ‘Backcross Solo’ stored to 24–28 °C.
Fig. 1.
TSS (ºBrix), pH, TA (% malic acid) and Firmness (N) on Papaya treated with hot water at 55 °C for 0, 3, 6, and 9 min. Vertical bars are the standard error of the mean of at least four replicates
Firmness
The untreated fruit showed an initial firmness of 106 N at its color-break stage. However, during the 9-day postharvest life, the flesh firmness diminished to 16 N (losing the 85% of its initial firmness). Papaya flesh lost the 64% of its firmness during the first 3 days of storage (Fig. 1). Gaete-Eastman et al. (2009) reported that firmness of untreated fruit Cv ‘mountain’ fell quickly during the first days of shell life, with minimum firmness values reached after 7 days of storage at 20 °C. Similar behavior of loss of firmness was observed in our results. Hot-water treated fruit did not show significant differences in firmness during storage in relation to the unheated fruit. Our treated fruits did not show heat-injured mesocarp tissue as seen in Papaya cv ‘Solo’ by Chan et al. (1981) who reported that hot-water treatments of 46 °C for 65 and 90 min caused a delayed and uneven softening in one-quarter and half-ripe papaya fruit cv ‘Solo’, but not in color-break stage fruits. Also, Paull and Chen (1990), observed that ripening was impaired by heating the papaya cv ‘Sunset’ at 42 °C for 30 min followed by 49 °C for 70 min, with areas of flesh failing to soften. The higher temperature (55 °C) together with the shorter times along with the ripeness stage in our study could explain this difference.
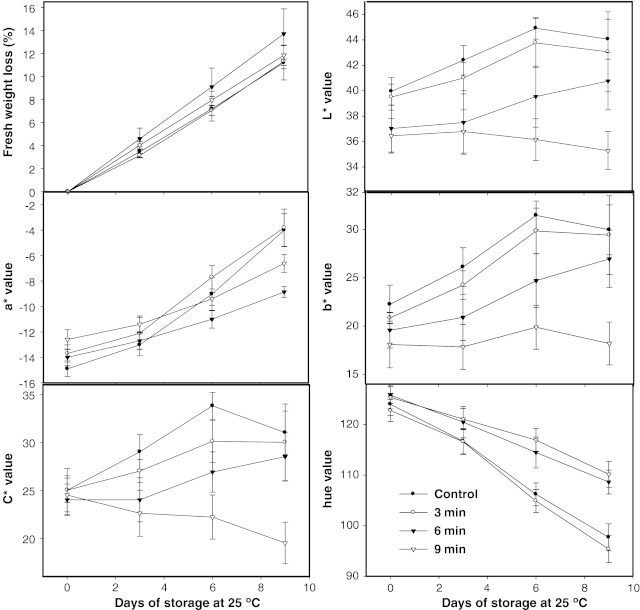
Fresh weight loss
Weight loss in papaya cv Maradol increased during storage (Fig. 2). At the ninth day, the untreated (control) fruit had lost a level of 11.2% (±2.59) of its weight. Similar weight loss was reported by Ramakrishna et al. (2002) for untreated Papaya Cv ‘Co-2’ during storage at 30 °C. Our treated fruit did not show significant differences in weight loss (P < 0.05) during all the storage period in relation to the control fruit.
Fig. 2.
Fresh weight loss (in%) and color on skin (L*, a*, b*, C* and h values) from papaya fruit treated with hot water at 55 °C for 0, 3, 6, and 9 min. Vertical bars are the standard error of the mean of at least four replicates
Skin color
Since papaya peel turns from green to yellow during ripening in ‘Maradol’ variety, L* (lightness), a* (green to red axis), b* (blue to yellow axis) and C* (chromaticity) values of skin papaya fruit, normally increases while h value (hue angle) decreases as seen in untreated fruit (control) (Fig. 2). However, AHWT significantly affected skin color, depending on the exposure time at 55 °C. While the 3-min treatment did not show any noticeable effect on skin color, the 6- and 9-min treatments negatively affected (p < 0.05) the peel color as can be seen in every measured parameter of color (L*, a*, b*, C* and h values) being the effects on skin color noticeably shown at the 6 and 9 days of storage at 25 °C (Fig. 2). Papaya fruit from the 6- and 9-min treated lots showed an irregular development on skin color, exhibiting abnormal degreening/yellowing during ripening in storage being the fruit color quality seriously damaged. At the end of ripening process the fruit exhibited a clearly browning on peel showing remaining-green areas. Similar results were observed in papaya cv ‘Solo’ by Akamine and Hundtoft (1971). Also, Paull (1994) reported the rupture of the normal color development as a heat damage manifestation.
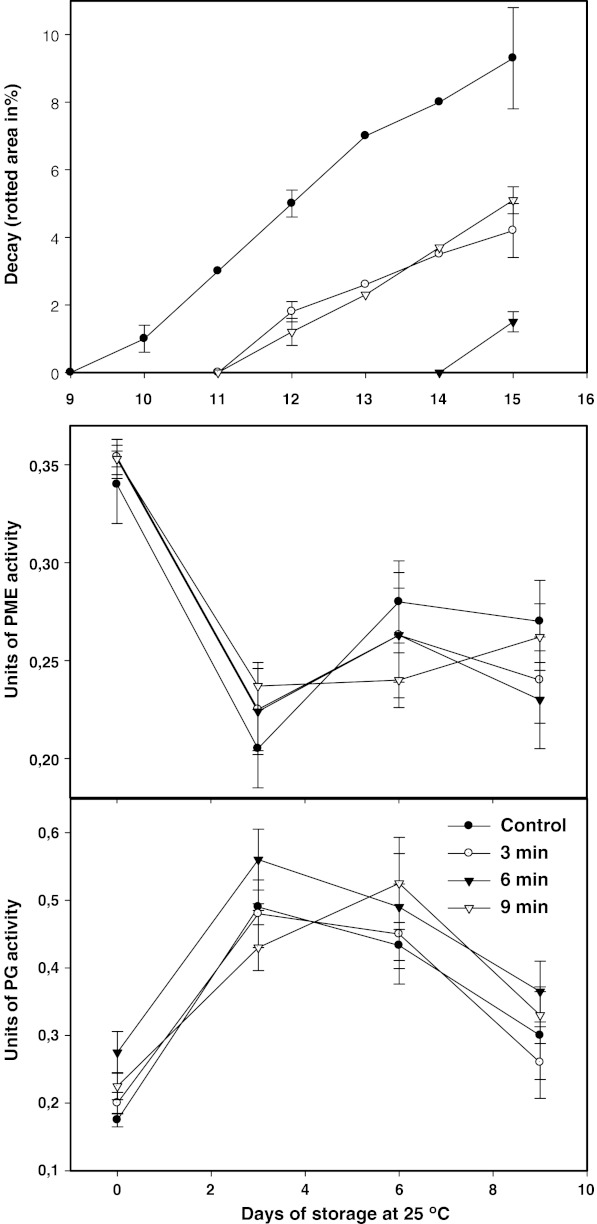
Decay on skin
The earliest fruit showing decay was the untreated one after the 9th day of storage (Fig. 3). AHWT delayed decay appearance on fruit for several days stored at 25 °C. The 3- and 9-min hot-water treated fruit showed decay development after the 11th day of storage while the 6-min treated fruit exhibited decay after the 14th day of storage. Furthermore, AHWT reduced the fungi growth as noticed by a lower rotted area on treated fruits. Decay on treated fruit was lower than untreated fruit during all the storage period. However, although the 6- and 9-min AHWT delayed and decreased the fungi growth on papaya peel, the effect was neglected due to the color-quality damage provoked on peel, although the flesh quality was unaffected. However, the 3-min AHWT delayed the appearance of decay without negatively affecting the peel color quality on papaya cv ‘Maradol’. This delaying of decay can be satisfactory for marketing conditions handling the papaya fruit at 25 °C. Similar results of decay control on papaya fruit cv ‘Solo’, specially anthracnose (Colletotrichum gloesosporioides Penz) and stem-end rots were observed by Akamine and Hundtoft (1971), and Couey et al. (1984) applying hot-water-spray treatments of 54 °C by 3 min. In addition, Perez-Carrillo and Yahia (2004) observed a decrease in fungi development applying dry hot-air treatments (48.5 °C, 50% RH).
Fig. 3.
Decay and enzymatic activity of PME and PG in papaya fruit pulp treated with hot water at 55 °C for 0, 3, 6, and 9 min. Vertical bars are the standard error of the mean of at least four replicates
Cell wall enzymatic activity
PME from untreated fruit had a 43% activity loss (from 0.35 to 0.20 Units) during the first 3 days of storage and remained constant thereafter. PME from treated fruit had a similar behavior throughout the storage period and significant differences in relation to untreated fruits were not detected at any time (Fig. 3). PG from untreated fruit showed an increased activity of about 3 fold (from 0.17 to 0.49) in the first 3-day period of storage and thereafter decreased to 0.3. PG activity from treated fruits was not different than untreated one at any time (Fig. 3). Chan et al. (1981) reported that PG activity was unaffected when hot water treatments of 46 °C for, 40, 65 and 90 min were applied on Papaya cv Solo at the color-break stage. However, they observed a significant PG-activity inhibition when the hot water treatments were applied at more advanced ripeness stages (quarter and half ripe). In addition, Lazan et al. (1989) reported that the effect of heat treatments in suppressing PG activity was relatively greater in inner than in outer mesocarp, suggesting that sensitivity of the enzyme to heat treatments may vary with stage of ripeness of the tissue. It is well known that the ripening of papaya initiates at the inner area of the pulp and it is followed for the outer area. Also, Paull and Chen (1990) suggested that the riper-inner mesocarp of papaya fruit may be more susceptible to heat stress than the less-ripe outer mesocarp. For papaya cv ‘Solo’, the PG-activity inhibition has been correlated to delayed softening by Chan et al. (1981) suggesting that PG activity is important during its softening process, although Lazan et al. (1989) reported that the extent of tissue softening in cv ‘Backcross Solo’ during tissue ripening may not be dependent entirely on PG action. Because of the measured-in-pulp physico-chemical parameters (TSS, pH, TA, Firmness), and PME-, PG- activity were not significantly affected, it is observed that the tested AHWT had no effect on the overall ripening process of papaya fruit. And, due to the skin color and decay were seriously affected by 6- and 9-min treatments, it is suggested that these treatments can be applied at 55 °C for about 3 min at the color-break stage of papaya fruit cv “Maradol” for fresh market. However, because the inner quality was not affected for the treatments of 6 and 9 min, these treatments can be applied when the papaya fruit is destined to processing and the skin is discarded or the skin color quality is not an important factor. Papaya peel possesses lower thermal conductivity than pulp (Espinoza-Guevara et al. 2010), and due to this fact, peel is established as a heat-transfer barrier limiting the heat transfer inward the pulp and avoiding excessive temperature elevation at the more heat-sensitive-inner-mesocarp. Furthermore, short exposure treatments of 55 °C for 3 to 9 min rather than the longer commercial treatments of 46–47.5 °C for 40–90 min, makes faster the packinghouse operations.
Conclusions
We concluded that the AHWT of 55 °C for 3, 6 and 9 min. did not affect the softening process from papaya pulp because of the cell wall enzymes (PME and PG) were not altered at their activity by these applied treatments. The AHWT of 55 °C for 3 min., did not affect negatively the quality parameters of pulp and skin of papaya at their color-brake stage of ripeness. So, this treatment can be applied in order to delay decay development during the papaya marketing at non-refrigerated temperatures (25 °C).
References
- Adaskaveg JE, Forster H, Sommer NF (2002) Principles of postharvest pathology and management of decays of edible horticultural crops. Ch. 17. In: Kader AA (ed) Postharvest technology of horticultural crops. University of California. Agriculture and Natural Resources. Publication 3311, pp 163–195
- Aina JO, Oladunjoye OO. Respiration, pectolytic activity and textural changes in ripening African mango (Irvinga gabonensis) fruits. J Sci Food Agric. 1993;63:451–454. doi: 10.1002/jsfa.2740630412. [DOI] [Google Scholar]
- Akamine EK, Hundtoft EB. Establishing the effects of post-harvest treatment on fresh market papayas by response surface methodology. J Agric Eng Res. 1971;16:343–352. doi: 10.1016/S0021-8634(71)80033-1. [DOI] [Google Scholar]
- Almeida MEM, Nogueira JN. The control of polyphenol oxidase activity in fruits and vegetables. A study of the interactions between the chemical compounds used and heat treatment. Plant Foods Hum Nutr. 1995;47:245–256. doi: 10.1007/BF01088333. [DOI] [PubMed] [Google Scholar]
- AOAC (1998) Official methods of analysis. 16th ed, Association of Official Analytical Chemists. Guithersburg, MD. USA
- Awad M, Young RE. Postharvest variation in cellulase, polygalacturonase and pectin methylesterase in avocado (Persea americana Mill., cv. Fuertes) fruits in relation to respiration and ethylene production. Plant Physiol. 1979;64:306–308. doi: 10.1104/pp.64.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barca EA, Kalantari S, Markhlouf J, Arul J. Impact of UV-C irradiation on cell wall degrading enzymes during ripening of tomato (Lycopersicon esculentum L.) fruit. J Agric Food Chem. 2000;48:667–671. doi: 10.1021/jf9906174. [DOI] [PubMed] [Google Scholar]
- Bennet AB. Biochemical and genetic determinants of cell wall disassembly in ripening fruit: a general model. HortScience. 2001;37:447–450. [Google Scholar]
- Cámara MM, Díez C, Torija ME. Changes during ripening of papaya fruit in different storage systems. Food Chem. 1993;46:81–84. doi: 10.1016/0308-8146(93)90080-Y. [DOI] [Google Scholar]
- Cantwell M, Nie X (1996) Use of heat treatments to control postharvest pathogens on tomatoes and melons. In: Organica‘92. Proc. Organic Farming Symp. Univ. California Div. Agr. Natl. Res. Publ. 3356. pp 96–101
- Carrillo-López A, Cruz-Hernández A, Cárabez-Trejo A, Guevara-Lara F, Paredes-López O. Hydrolytic activity and ultrastructural changes in fruit skins from two prickly pear (Opuntia sp.) varieties during storage. J Agric Food Chem. 2002;50:1681–1685. doi: 10.1021/jf011195c. [DOI] [PubMed] [Google Scholar]
- Carrillo-López A, Cruz-Hernández A, Guevara-Lara F, Paredes-López O. Physicochemical changes during ripening in storage of two varieties of prickly pear stored at 18 °C. J Food Sci Technol. 2003;40:461–464. [Google Scholar]
- Chan HT, Jr, Tam SYT, Seo ST. Papaya polygalacturonase and its role in thermally injured ripening fruit. J Food Sci. 1981;46:190–192. doi: 10.1111/j.1365-2621.1981.tb14561.x. [DOI] [Google Scholar]
- Chan HT, Jr, Sanxter SS, Nishijima KA. Heat-treating “Sharwil” avocado for cold tolerance in quarantine cold treatments. HortScience. 1994;29:1166–1168. [Google Scholar]
- Couey HM, Alvarez AM, Nelson MG. Comparison of hot-water spray and inmersion treatments for control of postharvest decay of papaya. Plant Dis. 1984;68:436–437. [Google Scholar]
- Espinoza-Guevara R, Caro-Corrales J, Ordorica-Falomir C, Zazueta-Morales J, Vega-Garcia M, Cronin K. Thermophysical properties of pulp and rind of papaya Cv Maradol. Int J Food Prop. 2010;13:65–74. doi: 10.1080/10942910802180166. [DOI] [Google Scholar]
- Fayyaz A, Asbi BA, Ghazali HM, Che Man YB, Jinap S. Pectinesterase extraction from papaya. Food Chem. 1993;47:183–185. doi: 10.1016/0308-8146(93)90241-7. [DOI] [Google Scholar]
- Gaete-Eastman C, Figueroa CR, Balbontín C, Moya M, Atkinson RG, Herrera R, Moya-León MA. Expression of an ethylene-related expansin gene during softening of mountain papaya fruit (Vasconcellea pubescens) Postharvest Biol Technol. 2009;53:58–65. doi: 10.1016/j.postharvbio.2009.03.007. [DOI] [Google Scholar]
- Gross KC. A rapid and sensitive spectrophotometric method for assaying polygalacturonase using 2-cyanoacetamide. HortScience. 1982;17:993–994. [Google Scholar]
- Hagerman AE, Austin PJ. Continuous spectrophotometric assay for plant pectin methyl esterase. J Agric Food Chem. 1986;34:440–444. doi: 10.1021/jf00069a015. [DOI] [Google Scholar]
- Huber D. The role of cell wall hydrolases in fruit softening. Hort Rev. 1983;5:169–219. [Google Scholar]
- Jagtiani J, Chan HT, Jr, Sakai WS. Papaya. In: Jagtiani J, Chan HT Jr, Sakai WS, editors. Tropical fruit processing. USA: Academic Press Inc; 1988. pp. 105–145. [Google Scholar]
- Klein JD, Lurie S. Heat treatments for improved postharvest quality of horticultural crops. HortTechnol. 1992;2:316–320. [Google Scholar]
- Lazan H, Ali ZM, Liang KS, Yee KL. Polygalacturonase activity and variation in ripening of papaya fruit with tissue depth and heat treatment. Physiol Plant. 1989;77:93–98. doi: 10.1111/j.1399-3054.1989.tb05983.x. [DOI] [Google Scholar]
- Lurie S. Postharvest heat treatments of horticultural crops. Hort Rev. 1998;22:91–121. [Google Scholar]
- McDonald RE, McCollum TG, D’Aquino S. Heat treatment inhibits mango chilling injury. HortScience. 1993;28:197–198. [Google Scholar]
- McDonald RE, McCollum TG, Baldwin EA. Heat treatment of mature-green tomatoes: differential effects of ethylene and partial ripening. J Amer Soc Hort Sci. 1998;123:457–462. [Google Scholar]
- McGregor BM (1987) Manual de Transporte de Productos Tropicales. Departamento de Agricultura de los Estados Unidos. Manual de Agricultura. Washington, D.C. USA, No. 668. 103 p. (In Spanish)
- McGuire RG. Reporting of objective color measurements. HortScience. 1992;27:1254–1255. [Google Scholar]
- Montgomery DC (1991) Diseño y Análisis de Experimentos. Grupo editorial Iberoamericana, S.A. de C.V. pp 175–222 México, D.F. (In Spanish)
- Paull RE. Response of tropical horticultural commodities to insect disinfestation treatments. HortScience. 1994;29:988–991. [Google Scholar]
- Paull RE, Armstrong JW (1994) Insect pests and fresh horticultural products: Treatments and Responses. 360 p. Wallingford, UK, Cab International
- Paull RE, Chen NJ. Heat shock response in field-grown, ripening papaya fruit. J Amer Soc Hort Sci. 1990;115:623–631. [Google Scholar]
- Perez-Carrillo E, Yahia EM. Effect of postharvest hot air and fungicide treatments on the quality of “Maradol” Papaya (Carica papaya L.) J Food Qual. 2004;27:127–139. doi: 10.1111/j.1745-4557.2004.tb00643.x. [DOI] [Google Scholar]
- Pressey R, Avants JK. Solubilization of cell walls by tomato polygalacturonase: effects of pectinesterase. J Food Biochem. 1982;6:57–74. doi: 10.1111/j.1745-4514.1982.tb00296.x. [DOI] [Google Scholar]
- Ramakrishna M, Haribabu K, Purushotham K. Rffect of Post-harvest application of growth regulators on storage behavior of papaya (Carica papaya L.) Cv. ‘Co-2’. J Food Sci Technol. 2002;39:657–659. [Google Scholar]
- Roe B, Bruemmer JH. Changes in pectin substances and enzymes during ripening and storage of “Keitt” mangos. J Food Sci. 1981;46:186–189. doi: 10.1111/j.1365-2621.1981.tb14560.x. [DOI] [Google Scholar]
- Salunkhe DK, Desai BB. Postharvest biotechnology of fruits. Ch. 3. Vol. II. Boca Raton: CRC Press, Inc; 1984. pp. 13–25. [Google Scholar]
- SG PLUS (1992) Statgraphics plus version 6.0. Statistical graphics system. Rockville, MA, USA. Statistical Graphics Co
- Watada AE, Abe K, Yamuchi N. Physiological activities of partially processed fruits and vegetables. Food Technol. 1990;44:116–122. [Google Scholar]