Abstract
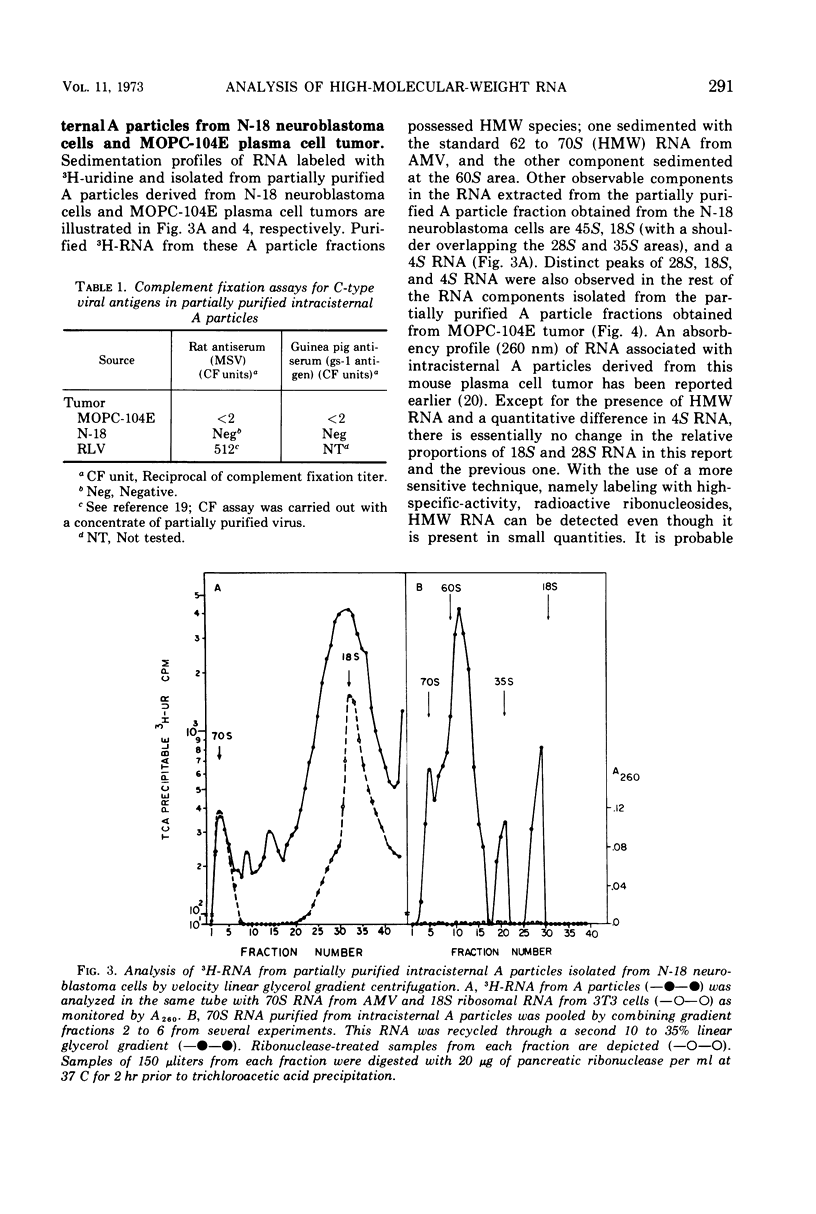
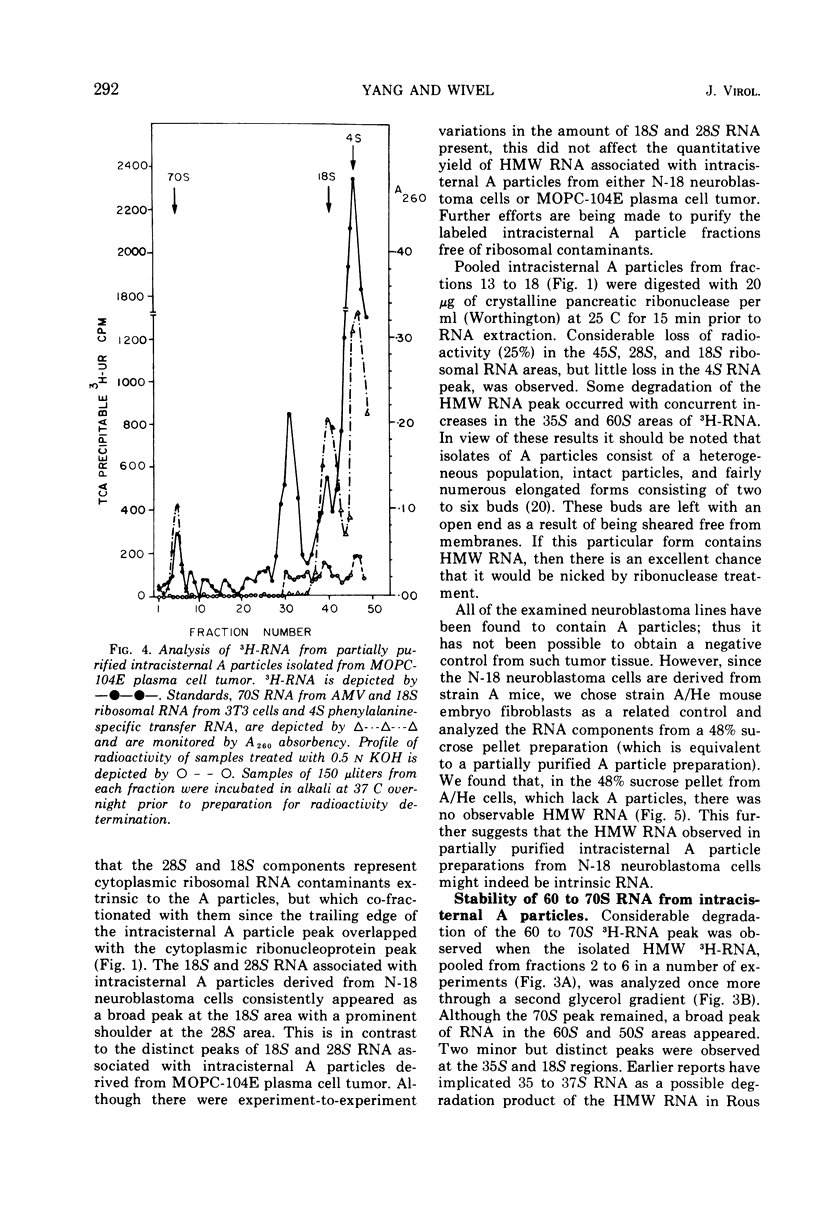
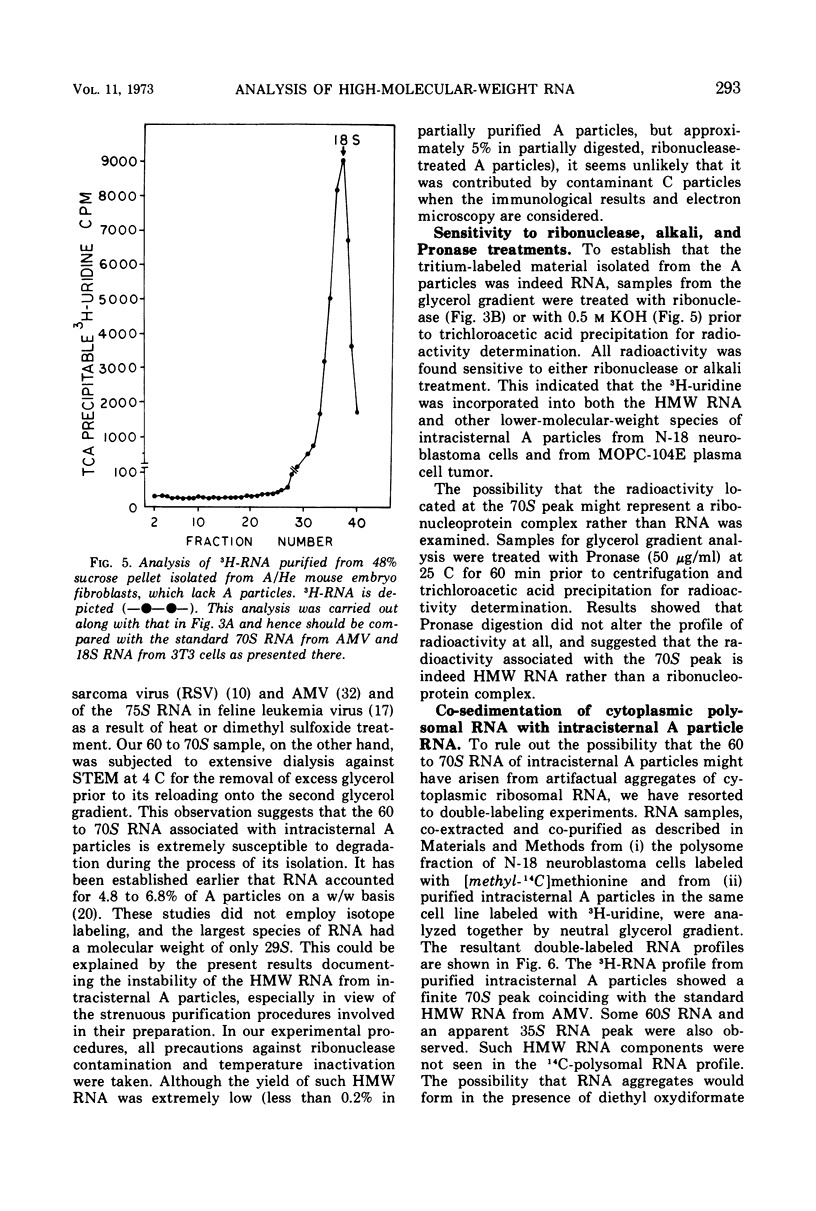
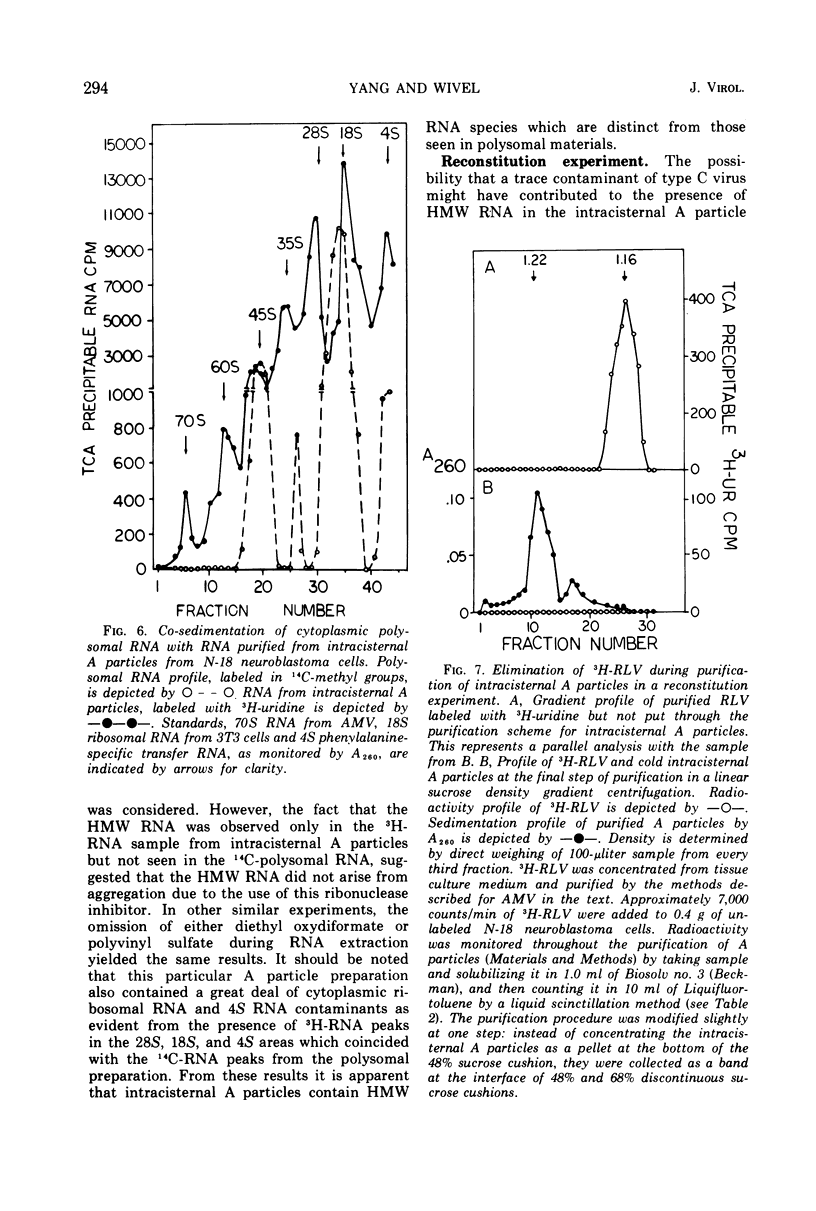
Intracisternal A particles, known primarily for their association with various tumors, have been shown to contain high-molecular-weight (HMW) ribonucleic acid (RNA) by velocity centrifugation, using linear glycerol gradients. This HMW RNA is sensitive to ribonuclease digestion and alkali treatment but is resistant to Pronase treatment. By a double-labeling experiment, HMW RNA was shown to be intrinsic to intracisternal A particles and not to have resulted from cytoplasmic polysomal RNA aggregation. By a reconstitution experiment, it was determined that the results were not due to C-type virus contamination. The synthesis of HMW RNA in intracisternal A particles is inhibited by actinomycin D and ethidium bromide. These observations emphasize that there are probably some taxonomic relationships between intracisternal A particles and oncogenic RNA viruses.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BADER J. P. THE ROLE OF DEOXYRIBONUCLEIC ACID IN THE SYNTHESIS OF ROUS SARCOMA VIRUS. Virology. 1964 Apr;22:462–468. doi: 10.1016/0042-6822(64)90067-4. [DOI] [PubMed] [Google Scholar]
- Bishop J. M., Levinson W. E., Quintrell N., Sullivan D., Fanshier L., Jackson J. The low molecular weight RNAs of Rous sarcoma virus. I. The 4 S RNA. Virology. 1970 Sep;42(1):182–195. doi: 10.1016/0042-6822(70)90251-5. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Bauer H. Polypeptides of avian RNA tumor viruses. 1. Isolation and physical and chemical analysis. Virology. 1970 Dec;42(4):1097–1112. doi: 10.1016/0042-6822(70)90357-0. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Gelderblom H., Bauer H., Mölling K., Hüper G. Polypeptides of avian RNA tumor viruses. V. Analysis of the virus core. Virology. 1972 Mar;47(3):567–578. doi: 10.1016/0042-6822(72)90546-6. [DOI] [PubMed] [Google Scholar]
- DALTON A. J., FELIX M. D. The electron microscopy of normal and malignant cells. Ann N Y Acad Sci. 1956 Mar 30;63(6):1117–1140. doi: 10.1111/j.1749-6632.1956.tb32127.x. [DOI] [PubMed] [Google Scholar]
- DALTON A. J., POTTER M., MERWIN R. M. Some ultrastructural characteristics of a series of primary and transplanted plasma-cell tumors of the mouse. J Natl Cancer Inst. 1961 May;26:1221–1267. [PubMed] [Google Scholar]
- DE HARVEN E., FRIEND C. Electron microscope study of a cell-free induced leukemia of the mouse: a preliminary report. J Biophys Biochem Cytol. 1958 Mar 25;4(2):151–156. doi: 10.1083/jcb.4.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DMOCHOWSKI L., GREY C. E. Electron microscopy of tumors of known and suspected viral etiology. Tex Rep Biol Med. 1957;15(3):704–753. [PubMed] [Google Scholar]
- Duesberg P. H. Physical properties of Rous Sarcoma Virus RNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1511–1518. doi: 10.1073/pnas.60.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDLAENDER M., MOORE D. H. Occurrence of bodies within endoplasmic reticulum of Ehrlich ascites tumor cells. Proc Soc Exp Biol Med. 1956 Aug-Sep;92(4):828–831. doi: 10.3181/00379727-92-22627. [DOI] [PubMed] [Google Scholar]
- GRANBOULAN N., RIVIERE M. R., BERNHARD W. [Presence of particles of viral appearance in a transplantable mouse sarcoma induced by methylcholanthrene]. Bull Assoc Fr Etud Cancer. 1960 Apr-Jun;47:291–307. [PubMed] [Google Scholar]
- Gardner M. B., Officer J. E., Rongey R. W., Estes J. D., Turner H. C., Huebner R. J. C-type RNA tumour virus genome expression in wild house mice. Nature. 1971 Aug 27;232(5313):617–620. doi: 10.1038/232617a0. [DOI] [PubMed] [Google Scholar]
- Hall W. T., Hartley J. W., Sanford K. K. Characteristics of and relationship between C particles and intracisternal A particles in cloned cell strains. J Virol. 1968 Mar;2(3):238–247. doi: 10.1128/jvi.2.3.238-247.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H., Hanafusa T. Noninfectious RSV deficient in DNA polymerase. Virology. 1971 Jan;43(1):313–316. doi: 10.1016/0042-6822(71)90251-0. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Capps W. I., Huebner R. J. Isolation of naturally occurring viruses of the murine leukemia virus group in tissue culture. J Virol. 1969 Feb;3(2):126–132. doi: 10.1128/jvi.3.2.126-132.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett O., Pitts J. D., Whalley J. M., Clason A. E., Hay J. Isolation of the nucleic acid of feline leukemia virus. Virology. 1971 Jan;43(1):317–320. doi: 10.1016/0042-6822(71)90252-2. [DOI] [PubMed] [Google Scholar]
- Kakefuda T., Roberts E., Suntzeff V. Electron microscopic study of methylcholanthrene-induced epidermal carcinogenesis in mice: mitochondrial dense bodies and intracisternal A-particles. Cancer Res. 1970 Apr;30(4):1011–1019. [PubMed] [Google Scholar]
- Kuff E. L., Leuders K. K., Ozer H. L., Wivel N. A. Some structural and antigenic properties of intracisternal A particles occurring in mouse tumors (complement fixation-immunodiffusion-neuroblastoma-plasma-cell tumor). Proc Natl Acad Sci U S A. 1972 Jan;69(1):218–222. doi: 10.1073/pnas.69.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Wivel N. A., Lueders K. K. The extraction of intracisternal A-particles from a mouse plasma-cell tumor. Cancer Res. 1968 Oct;28(10):2137–2148. [PubMed] [Google Scholar]
- Nadel E., Banfield W., Burstein S., Tousimis A. J. Virus particles associated with strain 2 guinea pig leukemia (L2C/N-B). J Natl Cancer Inst. 1967 Jun;38(6):979–981. [PubMed] [Google Scholar]
- Nowinski R. C., Old L. J., Sarkar N. H., Moore D. H. Common properties of the oncogenic RNA viruses (oncornaviruses). Virology. 1970 Dec;42(4):1152–1157. doi: 10.1016/0042-6822(70)90367-3. [DOI] [PubMed] [Google Scholar]
- Obara T., Bolognesi D. P., Bauer H. Ribosomal RNA in avian leukosis virus particles. Int J Cancer. 1971 May 15;7(3):535–546. doi: 10.1002/ijc.2910070320. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Fisher C. L., Stanley T. B., Gilden R. V. Proteins of the murine C-type RNA tumour viruses: isolation of a group-specific antigen by isoelectric focusing. J Gen Virol. 1970 Jul;8(1):1–10. doi: 10.1099/0022-1317-8-1-1. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Schubert D., Humphreys S., Baroni C., Cohn M. In vitro differentiation of a mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):316–323. doi: 10.1073/pnas.64.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E., Rands E., Aaronson S. A., Todaro G. J. RNA-dependent DNA polymerase activity in five RNA viruses: divalent cation requirements. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1789–1796. doi: 10.1073/pnas.67.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. H., Andervont H. B., Dunn T. B. Attempts to detect nodule-inducing virus in strain RIII mice. J Natl Cancer Inst. 1970 Mar;44(3):657–671. [PubMed] [Google Scholar]
- Stephenson M. L., Wirthlin L. S., Scott J. F., Zamecnik P. C. The 3'-terminal nucleosides of the high molecular weight RNA of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1972 May;69(5):1176–1180. doi: 10.1073/pnas.69.5.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S. E., Kasnic G., Jr, Draycott C., Ben T. Activation of viruses in human tumors by 5-iododeoxyuridine and dimethyl sulfoxide. Science. 1972 Jan 14;175(4018):198–199. doi: 10.1126/science.175.4018.198. [DOI] [PubMed] [Google Scholar]
- TEMIN H. M. THE PARTICIPATION OF DNA IN ROUS SARCOMA VIRUS PRODUCTION. Virology. 1964 Aug;23:486–494. doi: 10.1016/0042-6822(64)90232-6. [DOI] [PubMed] [Google Scholar]
- Tumilowicz J. J., Cholon J. J. Intracisternal type A particles and properties of a continuous cell line originating from a gerbil fibroma. Proc Soc Exp Biol Med. 1971 Apr;136(4):1107–1110. doi: 10.3181/00379727-136-35439. [DOI] [PubMed] [Google Scholar]
- Wilson S. H., Kuff E. L. A novel DNA polymerase activity found in association with intracisternal A-type particles. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1531–1536. doi: 10.1073/pnas.69.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wivel N. A., Smith G. H. Distribution of intracisternal A-particles in a variety of normal and neoplastic mouse tissues. Int J Cancer. 1971 Jan 15;7(1):167–175. doi: 10.1002/ijc.2910070119. [DOI] [PubMed] [Google Scholar]