Abstract
Marigold flower (Tagetes erecta L) is one of the richest sources of xanthophylls. An enzymatic pretreatment method was developed for improved extraction of pigments from marigold flowers. Pretreatment with enzyme solution increased the diffusion coefficient from 1.56 x 10-9 m2/s to 4.02 x 10-9 m2/s and mass transfer coefficient from 0.14 h-1 to 0.36 h-1 coefficients. At the same time, dry yield, resin yield and pigment yield were also found to increase along with increased retention of colour. Sodium hydroxide or citric acid pretreatments increased the diffusion coefficient during drying, but resulted in lower dry yield due to loss of soluble compounds whereas, pigment yield was higher as compared to control. The enzyme treated and air dried sample, stored at 4 °C was found to be the most stable, as indicated by a low (0.0006 day−1) degradation constant. Pretreatment of marigold flowers with an aqueous enzyme solution (0.2%) results in improved resin, pigment yield and retention of pigment during storage. Pretreatment of marigold flowers with sodium hydroxide citric acid followed by hydraulic pressing resulted in a significant reduction of water and also indicated improved dry yield, resin yield and pigment yield as compared to control sample.
Keywords: Marigold flowers, Oleoresin, Enzyme, Degradation index, Diffusion coefficient, Pretreatment
Introduction
‘Marigold’ (Tagetes erecta L) is an ornamental plant native to Mexico and Gautemala and it is reported to be used in traditional Mexican medicine Nehr (1968). The marigold flowers are of different shades like yellow, red, orange, dark orange and orange brown. African Aztec (Tagetes erecta) and French marigold (Tagets patula) are the two commonly found species. The African Aztec has large yellow or orange flower head, while French marigold has smaller single or double head of red and yellow or orange (Wealth of India 1976).
Lutein (C40H56O2) is the major pigment present in the marigold flower. About 95% of lutein present in the flowers is in the form of esters out of which lutein palmitate is the major pigment (Gau et al. 1983). Dimyristate, myristate, palmitate, stearate and distearate are the other esters of lutein present in marigold flowers. Lutein ester concentrations in fresh marigold flowers vary from 4.0 mg/g in greenish yellow flowers to 800 mg/g in orange brown flowers.
The extract from marigold flowers is used as an additive in poultry feed, to impart bright color to egg yolk, skin, and fatty tissues as the poultry pigmentation is often associated with good health and premium quality (Hencken 1992; Levi 2001; Winstead et al. 1985). Extraction and purification of marigold flower extract is reported to result in enriched concentrate Levi (2001).
Reported potential benefits of marigold include its role in cancer prevention and enhanced immune function (Chew et al. 1996), inhibition of the autooxidation of cellular lipids (Zhang et al. 1991), protection against oxidant induced cell damage (Martin et al. 1996) and prevention of age related macular degeneration (Fullmer and Shao 2001). These attributes of marigold xanthophylls have sparked interest in the development of new methods for xanthophylls production, such as chemical synthesis (Kreienbuhl et al. 2000) or fermentation technology (Gierhart 1994). These methods suffer from the limitation of removal of toxic solvents used in chemical synthesis as well as lower yield in fermentation process (Kreienbuhl et al. 2000). Therefore, the development of the alternative process may be desirable, which may lead to higher yield of xanthophylls in their native ester form by extraction and purification of marigold flower (Levi 2001). Solid state fermentation of marigold flowers for xanthophyll extraction from flowers has been studied by Navarrete-Bolanos et al. (2004).
There are many methods reported in literature for the extraction of lutein and esters of lutein from marigold flowers (Modad et al. 2000; Barzana et al. 2002; Navarrete-Bolanos et al. 2005). Generally, lutein is extracted from marigold flowers by solvent (hexane) extraction of dried flowers followed by the removal of solvent to obtain oleoresin, which is subjected to further purification steps to obtain a mixture of lutein and xanthophylls that is suitable for human consumption as a food additive or as nutritional supplement (Breithaupt and Schlatter 2005). The advantages of drying flowers are reduction in the bulk, lower water activity and ease of extraction of pigment. Extraction kinetics of lutein from marigold flowers with alkali treatment has been studied by Hojnik et al. (2008). Effect of enzyme pretreatment in comparison with acid and alkali pretreatment of fresh marigold flowers on kinetics of extraction of pigment has been reported in the present study. The objective of the present work was to assess the effect of various pretreatment methods such as sodium hydroxide solution, citric acid solution and enzyme solution on drying, dry yield, resin and pigment yield from marigold flowers.
Materials and methods
Raw material
Fresh marigold flowers were procured from the local market. The flowers had a moisture content of 93% and dark orange in colour. The flowers were cleaned and sound flowers were taken for the experiment.
Pretreatment of the flowers
The flowers were divided in to 4 batches of 10 kg each. First batch was used as a control i.e. without any pretreatment. The flowers were soaked in the pretreatment solution for 48 h at room temperature. In all the pretreatments flowers to solution ration was maintained at 1:1 weight to volume. During soaking the samples were mixed at manually at 1 h interval.
Sodium hydroxide pretreatment
A batch of fresh marigold flowers was soaked in 0.5% NaOH solution (0.125 M, pH 8.5) for 48 h at room temperature. The ratio of flower to soaking solution was maintained at 1:1.
Citric acid pretreatment
The marigold flowers (10 kg) were soaked in 1% citric acid solution (0.05 M, pH 4.0) for 48 h at room temperature (28 °C). The ratio of flower to solution was maintained at a ratio of 1:1.
Enzyme pretreatment
An aqueous solution of 0.2% commercial enzyme mixture (Viscozyme from M/s Novozyme, Netherland) was used to treat a batch of marigold flowers. Based on different trials with different concentration of enzyme from 0.05% to 2%, the optimum concentration of enzyme (0.2%) was selected. The enzyme was a mixture of cellulase, hemicellulase, protease, arabinase and xylanase enzymes. The pectin-solubilising activity and β-glucanase activity of enzyme mixture was in the range of 5,000–12,000 U/g and 50–120 U/g, respectively. The ratio of flower to enzyme solution was maintained at a ratio of 1:1. The pH of the soaking solution was adjusted to 4.5 with citric acid.
Drying of marigold flowers
The fresh marigold flowers dried without any pretreatment was taken as control sample. Pretreated flowers were taken out from solution, water was drained by placing the flowers on a fine cloth. Free water was removed using a hydraulic press (M/s B. Sen Barry and Co., India) operating at 1 to 2 ton pressure for 10 min. The pressed pretreated flowers were dried in a hot air dryer (Precision Products, India) by spreading 1.5 kg flowers on each tray of dimension 30 × 60 inches. Drying was done at 45 ± 2 ° C with the hot air velocity of 1.2 m/s. Samples were withdrawn at the interval of 1 h for the determination of moisture content. The dried samples were ground in a hammer mill (M/s Batliboi Pvt. Ltd., India) to obtain powdered, material passing through 40 mesh (B.S.). This material was used for the resin extraction, pigment estimation and storages studies.
Scanning Electron microscopy (SEM) studies
The samples of fresh and pretreated flowers were freeze dried and mounted on aluminium strobes and coated with thin layer of gold using polaron SEM coating system and observed with a LEO 435 VP Scanning Electron microscope (SEM) at 20 kV.
Determination of moisture diffusivity
The solution of Fick’s second law for diffusion from an infinite flat plate (thickness 2a) results in the following well known equations for the transfer of water (Crank (1975; Rastogi et al. 2002):
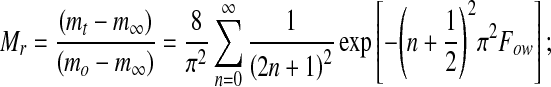 |
1 |
where Mr is the moisture ratio; m represents the moisture content; the subscripts o, ∞ and t represent the moisture content initially, at equilibrium, and at any given time. For an infinite flat sheet, Fourier numbers for moisture diffusion (Fow) is defined as (Dewt/a2) Rastogi et al. (2002), where Dew is the effective diffusivity of water; ‘a’ is the half thickness of infinite slab. An approximate form of Eq. 1 (for an infinite flat sheet) was graphically represented by plotting log (Mr) against the Fourier number (Fig. not shown). The slope of these line gives d (logMr)/d(Fow).
The slopes of the experimental lines for moisture diffusion [d(logMr)/dt] were obtained by fitting the experimental data to the solution of mass transfer equation for moisture mass transfer during drying process. The rate of change of moisture content was plotted against average moisture content, and the equilibrium moisture contents (m∞) as well as moisture mass transfer coefficients (km) were estimated from these plots (Rastogi et al. 2000).
Considering the equilibrium approach to mass transfer, the following equations for moisture mass transfer can be written (Rastogi et al. 2000):
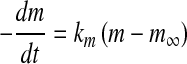 |
2 |
where km is the moisture mass transfer coefficients. Integration of Eq. 2, with the appropriate initial condition, resulted in the following equations:
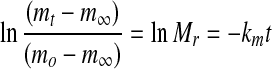 |
3 |
The experimental data (Mr versus t) were fitted to Eq. 3 to yield km, which in turn gave the slopes:
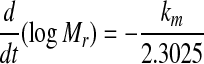 |
4 |
Dew values, considering an infinite flat sheet, were estimated from the following equation (Perry et al. 1984):
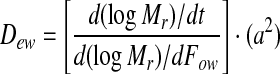 |
5 |
Analyses
Determination of moisture content
The moisture content of the marigold flower was determined by oven drying method as per AOAC procedure Method No. 930.15 AOAC (2006). Mean values of 5 replicates were expressed as kg of water per kg of dry solids. The control and pretreated flowers were dried to a moisture content of 10% (0.11 kg/kg). Yield of dried flowers was expressed as percentage of fresh flowers.
Estimation of resin yield and pigment yield
Dried sample (15 g) was taken in a glass column and extracted with hexane maintaining a material to solvent ratio of 1:6. Extractions were done in 5 batches. Extracts were pooled and evaporated in a flash evaporator under vacuum (22″ mercury) until the solvent was removed completely. The resin (residue) obtained was expressed as % resin on the basis of dried marigold powder.
Total pigment content in fresh flowers and dried powders was estimated by AOAC method AOAC (2006). For the estimation of pigment content in fresh flower, the petals were dried at low temperature (58 ± 2 °C), powdered and used for estimation. Marigold powder sample (1 g) was taken in a round bottom flask and 2 ml of 40% methanolic KOH was added and refluxed in a water bath at 56 °C for 20 min. After refluxing, the flask was cooled under tap and 30 ml of hexane was added and the flask was kept in dark for 2 h. Top layer of separated hexane was diluted appropriately with hexane and absorbance was read at 474 nm in a spectrophotometer. Total xanthophylls content was calculated in terms of lutein using E 1%1 cm value of trans lutein. The specific absortivity of trans lutein is 2360.
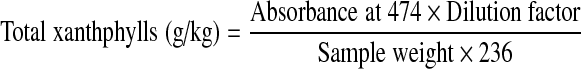 |
6 |
Measurement of color
Colour of dried and powdered samples was measured by Commission Internationale de l’Eclairage (CIE) method using an UV-Visible spectrophotometer with reflectance attachment (Model 2100, Shimadzu, Japan). D65 illuminant was used as a source of light and BaSO4 as a standard white. The marigold powders were directly placed in the sample holder to obtain the tristimulus values (X, Y, Z) (Hutchings 1994). The values were expressed as mean ± S.D. of 5 replicates.
Storage studies
Samples were packed in aluminum foil pouches and stored at 3 conditions of storage viz., cold (4 °C), ambient (27 °C) and accelerated condition (45 °C). Samples were withdrawn periodically once in a month and analyzed for resin and pigment content. The retention of pigment concentration was studied considering first order degradation kinetics.
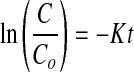 |
7 |
where K is the color degradation constant, C/Co is the color retention ratio and t is the time of storage.
Statistical analysis
Data were statistically analyzed by student t-test for comparison of means using Microsoft Excel for significance (p ≤ 0.05).
Result and discussion
Effect of pretreatments on drying behavior
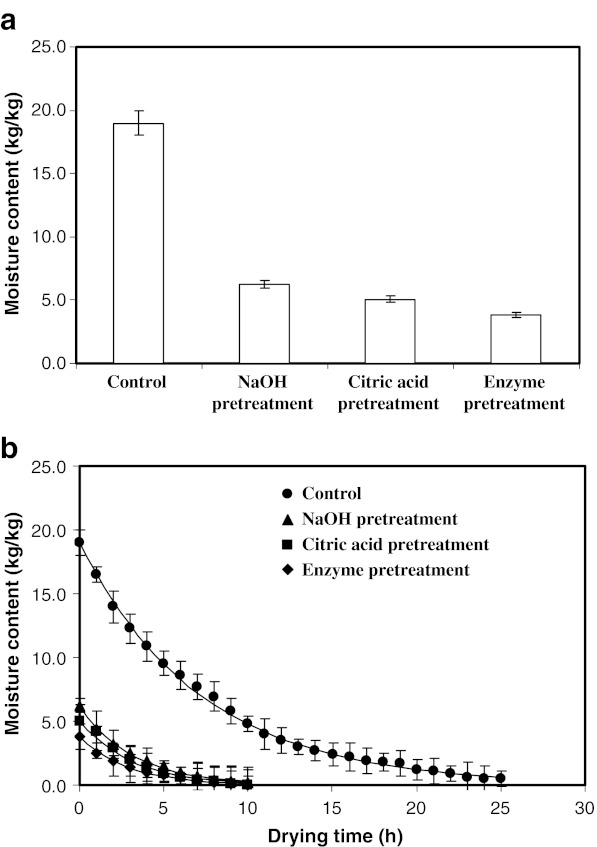
Moisture contents of marigold flowers immediately after the pretreatments are presented in Fig. 1a. Moisture content was found to vary as a function of pretreatments. The moisture content of fresh flowers was found to decrease from 19.0 kg/kg to 6.25, 5.04 and 3.83 kg/kg in samples treated with NaOH solution, citric acid solution or enzyme solution, respectively (p < 0.05, Fig. 1a). This may be attributed to the permeabilization of cell due to pretreatments, which resulted in decreased water holding capacity (Delgado-Vargas and Paredes-Lopez 1997; Barzana et al. 2002).
Fig. 1.
a Effect of various pretreatments and hydraulic pressing on moisture content of marigold flowers; b Variation of moisture content with drying time. Values are average of triplicate analysis
The change in moisture content with drying time is presented in Fig. 1b. The moisture content of test samples at the end of various pretreatment varied as a function of pretreatments. Therefore, a simple plot of moisture content versus time (Fig. 1b) could not be directly used to compare mass transfer characteristics. Hence, the effective diffusivities of moisture were determined. The relevant values (Table 1) indicated that the diffusion coefficient for moisture increased for the pretreatments as compared to control (p < 0.05). The highest diffusion coefficient was obtained for the enzyme treated sample, which may by due to the changes in cell permeabilization and structure. Pretreatment was found to reduce the drying time from 25 h to approximately 10 h (Fig 1b).
Table 1.
Effective diffusion coefficients of water (Dew) during dehydration for different pretreatments
| Pretreatments | Km (h−1) | Dew × 10−9 (m2/s) | m∞ (kg/kg) |
|---|---|---|---|
| Control | 0.138 | 1.555 | 0.009 |
| NaOH pretreatment | 0.320 | 3.598 | 0.020 |
| Citric acid pretreatment | 0.323 | 3.636 | 0.022 |
| Enzyme pretreatment | 0.357 | 4.023 | 0.013 |
Degradation of cell wall components mainly cellulose and hemicellulose, brought about by pretreatment with hydrolytic enzymes increased the permeability of cell wall resulting in decreased water holding capacity and increased mass transfer during extraction. Delgado-Vargas and Paredes-Lopez (1997) as well as Barzana et al. (2002) have indicated the increased extraction of xanthophylls content due to enzymatic pretreatment during extraction of marigold flower. The use of enzymes was reported to result in enhanced extraction of color and capsaicin from chilli (Santamaria et al. 2000), chlorophyll and protein from alfalfa (Weinberg et al. 1990) and vanillin from vanilla beans (Teran et al. 2001).
Sodium hydroxide and citric acid pre-treatments were demonstrated to be effective on the surface, but did not permeabilize the tissue (Ade-Omowaye et al. 2001). These pretreatments were shown to increase the mass transfer coefficients due to partial removal of the waxy layer from the skin surface in case of dehydration of paprika (Ade-Omowaye et al. 2001) as well as in case of osmotic dehydration of tomato (Shi et al. 1997).
Dry yield, resin yield and pigment yield
Enzyme pretreated sample showed the dry yield (5.58%), resin yield (10.5%) and pigment yield (2.48%) compared to control (5.60%), (8.65%) and (1.66%) respectively (Table 2). Cellulase, hemicellulase and xylanase act upon the cell wall, which in turn increase the permeability leading to increase in extraction of cell components. Whereas, protease and arabinase act upon protein and starch, respectively. Since cell wall is made up of cellulose, hemicellulose and lignins, the use of commercial mixture of enzymes was used to extract pigment from marigold flowers instead of single enzyme. The study reported by Delgado-Vargas (1997) also shown a similar trend, enzymatic pretreatment of marigold flowers with different commercial enzymes at concentrations of 0.01–0.1% has been studied. It has been reported that with enzyme treatment resulted in enhanced pigment extraction 1.7–7.4 g/kg which is in par with the results of the present study (1.66–2.48%). Sodium hydroxide and citric acid pretreated samples resulted in lower yields of dry matter and the resin (p < 0.05) as compared to control, however, the pigment yield was significantly higher (p < 0.05, Table 2), which may be due to leaching of the resinous solids which in turn results in higher pigment yield during these pretreatments (Sowbhagya et al. 2008). Effect of temperature and solvents of different polarity on extraction kinetics of pigment have been studied Hojnik et al. (2008). Diffusion coefficient values of lutein in different solvents ranged form 0.012 × 10−8 to 0.30 × 10−8 cm2/s.
Table 2.
Yields of dried flower petals, resin and pigment from 100 g of fresh flower petals and color values for different pretreatments
| Pretreatment | Dried flower petals (g/100 g) | Resin yield (g/100 g) | Pigment yield (g/100 g) | L * | a* | b* |
|---|---|---|---|---|---|---|
| Control | 5.60 ± 0.02 | 0.48 ± 0.03 (8.7) | 0.09 ± 0.01 (1.7) | 47.9 ± 0.50 | 25.2 ± 0.65 | 50.5 ± 0.24 |
| Citric acid | 4.40 ± 0.01 | 0.33 ± 0.02 (7.5) | 0.09 ± 0.01 (2.0) | 42.5 ± 0.12 | 16.9 ± 0.90 | 37.3 ± 0.60 |
| Sodium Hydroxide | 3.20 ± 0.02 | 0.27 ± 0.04 (8.4) | 0.07 ± 0.01 (2.1) | 40.9 ± 0.18 | 13.4 ± 0.32 | 34.0 ± 0.50 |
| Enzyme | 5.58 ± 0.01 | 0.59 ± 0.01 (10.5) | 0.14 ± 0.02 (2.5) | 39.8 ± 0.12 | 13.1 ± 0.23 | 51.2 ± 0.35 |
All the values are the average of triplicate analysis. The data in the parenthesis are the percentage yield of resin and pigment on the basis of dried flower petals.
Visual color measurement
Table 2 gives the L*, a* and b* values of pretreated and control samples. There was no significant difference (p < 0.05) between the ‘b*’ values (yellow color) of control and enzyme treated sample. Whereas the ‘b*’ values were less for the sodium hydroxide or citric acid treated and air dried samples as compared to control sample. Control sample was brighter compared to all the pretreated samples as seen by the L* values. Sodium hydroxide and enzyme treated samples were darker in appearance than the control and citric acid treated samples. Values of a* was highest in case of control followed by citric acid treated and lowest in case of enzyme and sodium hydroxide treatment.
SEM studies
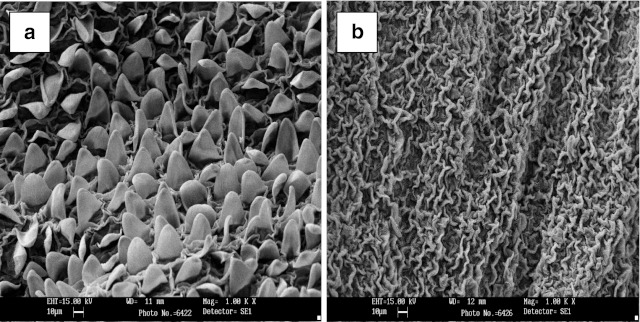
Scanning electron micrograph studies indicated that fresh marigold flower had well organized undulated structure, which found to distorted due to the pretreatment with enzyme (Fig. 2). The increased yield in pigment and resin in case of enzyme pretreated flowers could be related to the change in the structure of the flower. In case of alkali and acid treatments, similar structural changes were also observed.
Fig. 2.
Scanning electron micrograph of marigold petals A = control, B = enzyme
Storage studies
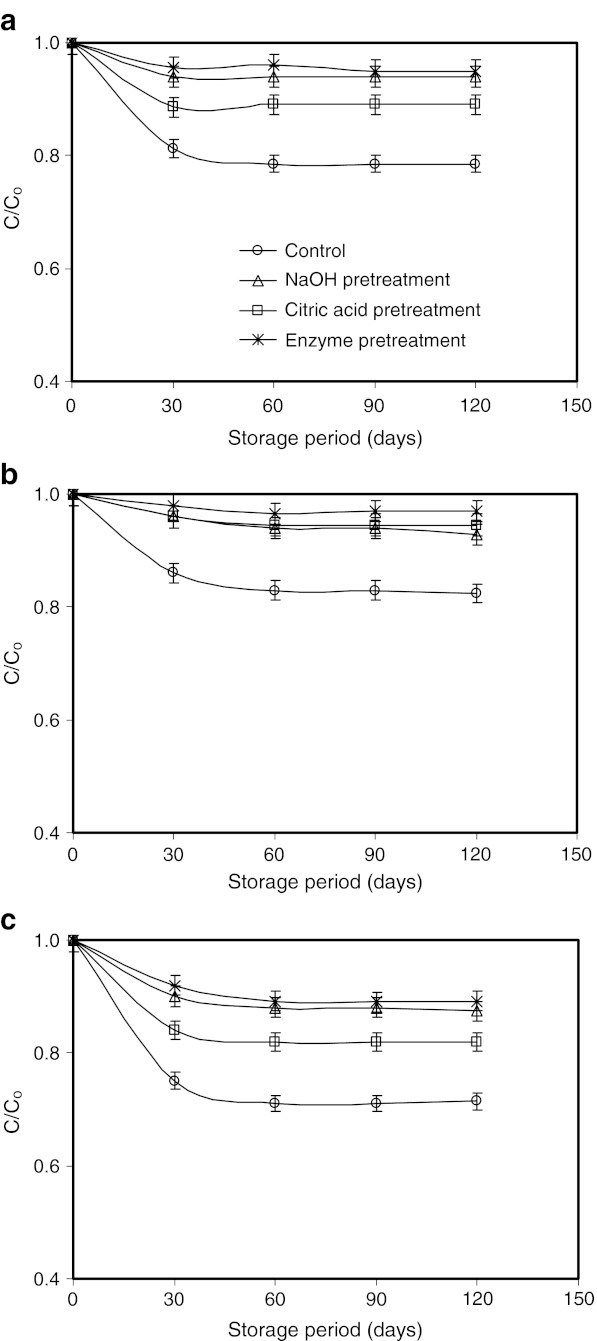
The retention of pigment during storage was analyzed considering first order degradation kinetics (Fig. 3). The degradation coefficients (Table 3) indicate that the degradation of enzyme pretreated and air dried sample was minimum (degradation constant being the minimum) when it was stored in cold storage (4 °C).
Fig. 3.
Retention of pigment concentration (C*/Co) considering first order degradation kinetics of dried marigold flowers stored at a 4 °C b 27 °C and c 45 °C. Values are average of triplicate analysis
Table 3.
Degradation coefficients of marigold pigment for different pretreatments
| Pretreatments | Ambient (day−1) | Cold storage (day−1) | Accelerated (day−1) |
|---|---|---|---|
| Control | 0.0046 | 0.0035 | 0.0065 |
| NaOH pretreatment | 0.0024 | 0.0011 | 0.0038 |
| Citric acid pretreatment | 0.0012 | 0.0010 | 0.0024 |
| Enzyme pretreatment | 0.0008 | 0.0006 | 0.0021 |
Conclusions
Pretreatment of fresh marigold flowers with sodium hydroxide, citric acid or enzyme followed by hydraulic pressing resulted in a significant reduction of water content of pressed flowers and increased rate of drying. Pretreatment of marigold flower with hydrolytic enzyme solution increased the yield of dry matter, resin and pigment. Though the pretreatment with sodium hydroxide or citric acid resulted in lower dry yield and resin yield, the pigment yield was found to increase which may be due to solubilization of resinous solids and better retention of pigment. During storage, the pigment loss was the least in enzyme treated samples stored at 4 °C. Since marigold oleoresin with higher pigment content will fetch high price, pretreatments of flowers resulting in enhanced extraction of pigment is highly valuable from commercial point of view.
Acknowledgement
Authors thank Dr. V. Prakash, Director, CFTRI for his encouragement and keen interest. The authors would like to thank Department of Biotechnology, New Delhi for the financial support to carry out this work.
References
- Ade-Omowaye BI, Rastogi NK, Angersbach A, Knorr D. Effect of high pressure or high electrical field pulse pretreatment on dehydration characteristics of paprika. Inno Food Sci Emerg Technol. 2001;2:1–7. doi: 10.1016/S1466-8564(00)00022-9. [DOI] [Google Scholar]
- AOAC (2006) Official methods of analysis, 18th edn, Method No. 930.71 Washington, DC: Association of Official Analytical Chemists, Washington DC
- Barzana E, Rubio D, Santamaria RI, Garcia-Correa F, Garcia VE, Ridaura-Sanz Enzyme-mediated solvent extraction of carotenoids from marigold flower (Tagetes erecta) J Agric Food Chem. 2002;50:4491–4496. doi: 10.1021/jf025550q. [DOI] [PubMed] [Google Scholar]
- Breithaupt DE, Schlatter J. Lutein and Zeaxanthin in new dietary supplements-analysis and quantification. Euro Food Res Technol. 2005;220:648–652. doi: 10.1007/s00217-004-1075-2. [DOI] [Google Scholar]
- Chew BP, Wong MW, Wong TS. Effects of lutein from marigold extract on immunity and growth of mammary tumours in mice. Anticancer Res. 1996;16(16B):3689–3694. [PubMed] [Google Scholar]
- Crank J. The mathematics of diffusion. London: Oxford University Press; 1975. [Google Scholar]
- Delgado-Vargas F (1997) Enzymatic pretreatment to enhance carotenoid content in dehydrated marigold. Plant Foods Hum Nutr 50(2):163–169 [DOI] [PubMed]
- Delgado-Vargas F, Paredes-Lopez O. Effects of enzymatic treatments on carotenoid extraction from marigold flowers (Tagetes erecta) Food Chem. 1997;58:255–258. doi: 10.1016/S0308-8146(96)00163-X. [DOI] [Google Scholar]
- Fullmer LA, Shao A. The role of lutein in eye health and nutrition. Am Asso Cereal Chem. 2001;46:408–413. [Google Scholar]
- Gau W, Plosche HJ, Wunsche C. Mass spectrophotometric identification of xanthophylls fatty acid esters from marigold flowers (Tagetes erecta) obtained by high performance liquid performance liquid chromatography. J Chromatogr. 1983;262:277–284. doi: 10.1016/S0021-9673(01)88106-1. [DOI] [Google Scholar]
- Gierhart DL (1994) Production of Zeaxanthin and Zeaxanthin containing compositions. US patent No. 5308759
- Hencken H. Chemical and physiological behaviour of feed carotenoids and their effects on pigmentation. Poult Sci. 1992;71:711–717. doi: 10.3382/ps.0710711. [DOI] [PubMed] [Google Scholar]
- Hojnik M, Skerget M, Knez Z. Extraction of lutein from Marigold flower petals-experimental kinetics and modeling. LWT Food Sci Technol. 2008;41:2008–2016. doi: 10.1016/j.lwt.2007.11.017. [DOI] [Google Scholar]
- Hutchings JB. Food colour and appearance. London: Blackie Academic and Professional; 1994. [Google Scholar]
- Kreienbuhl P, Rudin P, Rudolph W (2000) Method of making Carotenoids. US Patent 6,150,561
- Levi LW (2001) Trans-Xanthophyll ester concentrates of enhanced purity and methods of making same. US Patent 6,191,293
- Martin KR, Eailla ML, Smith JC (1996) β-Carotene and lutein protect HepG2 human liver cells against oxidant-induced damage. J Nutr 126:2098–2106 [DOI] [PubMed]
- Modad NA, Lopez-Munguia VAG, Barzana E. Solubility of purified lutein esters from Tagetes erecta in supercritical CO2 and the effect of solvent modifiers. J Agric Food Chem. 2000;48:5640–5642. doi: 10.1021/jf000121i. [DOI] [PubMed] [Google Scholar]
- Navarrete-Bolanos JL, Jimenez-Islas H, Botello-Alvare E, Rico-martinez R, Paredes-lopez O. An optimization study of solid- state fermentation: xanthophylls extraction from marigold flowers. Appl Microbiol Biotechnol. 2004;65:383–390. doi: 10.1007/s00253-004-1615-5. [DOI] [PubMed] [Google Scholar]
- Nehr NT. The ethnobotany of tagetes. Econ Bot. 1968;22:317–324. doi: 10.1007/BF02908126. [DOI] [Google Scholar]
- Navarrete-Bolanos JL, Claudia Rangel-Cruz L, Jimenez-Islas H, Botello-Alvarcz E, Rico-martinez R. Pretreatment effects on the extraction efficiency of xanthophylls from marigold flower (Tagetes erecta) using hexane. Food Res Intl. 2005;38:159–165. doi: 10.1016/j.foodres.2004.09.007. [DOI] [Google Scholar]
- Perry RH, Green DW, Maloney JO. Perry’s chemical engineer’s handbook. 6. New York: McGraw Hills; 1984. pp. 20.11–20.14. [Google Scholar]
- Rastogi NK, Angersbach A, Niranjan K, Knorr D. Rehydration kinetics of high-pressure pretreated and osmotically dehydrated pineapple. J Food Sci. 2000;65:838–841. doi: 10.1111/j.1365-2621.2000.tb13597.x. [DOI] [Google Scholar]
- Rastogi NK, Raghavarao KSMS, Niranjan K, Knorr D. Recent developments in osmotic dehydration: methods to enhance mass transfer. Trends Food Sci Technol. 2002;13:58–69. doi: 10.1016/S0924-2244(02)00032-8. [DOI] [Google Scholar]
- Teran FR, Amador IP, Munguia LA. Enzymatic extraction and transformation of glucovanillin to vanilla pods. J Agric Food Chem. 2001;49:5207–5209. doi: 10.1021/jf010723h. [DOI] [PubMed] [Google Scholar]
- Santamaria RI, Durate MD, Barzana E, Fernando D, Lopez M. Selective enzyme mediated extraction of capsaicinoids and carotenoids from chilli (Gujillo puya) (capscicum annum) using ethanol as solvent. J Agric Food Chem. 2000;48:3063–3067. doi: 10.1021/jf991242p. [DOI] [PubMed] [Google Scholar]
- Sowbhagya HB, Madhava Naidu M, Sampathu SR (2008) A process for the preparation of carotenoid enriched marigold oleoresin. (Indian Patent application No.2829/DEL/2008)
- Shi JX, Maguer ML, Wang SL, Liptay A. Application of osmotic treatment in tomato processing effect of skin treatments on mass transfer in osmotic dehydration of tomatoes. Food Res Intl. 1997;30:669–674. doi: 10.1016/S0963-9969(98)00031-3. [DOI] [Google Scholar]
- Publication and Information Directorate, vol X. New Delhi: CSIR; 1976. pp. 109–112. [Google Scholar]
- Weinberg ZG, Szakacs G, Linden JC, Tengerdy RP. Recovery of protein and chlorophyll from Alfalfa by simultaneous lactic acid fermentation and enzyme hydrolysis. Enz Micro Technol. 1990;12:921–925. doi: 10.1016/0141-0229(90)90110-C. [DOI] [Google Scholar]
- Winstead CS, Meinecke CF, Miller A, Beasley JN, Skeles K, Stephenson EL. Factors related to the incidence of the malabsorbtion syndrome. Poult Sci. 1985;64:499–501. doi: 10.3382/ps.0640499. [DOI] [PubMed] [Google Scholar]
- Zhang LX, Coney RV, Betram JS. Carotenoids enhance gap functional communication and inhibit lipid peroxidation in C3H/10T/2 cells: relationship to their cancer chemo preventive action. Carcinogenesis. 1991;12:2109–2114. doi: 10.1093/carcin/12.11.2109. [DOI] [PubMed] [Google Scholar]