Abstract
Designer foods are normal foods fortified with health promoting ingredients. These foods are similar in appearance to normal foods and are consumed regularly as a part of diet. In this article we have reviewed the global regulatory status and benefits of available designer foods such as designer egg, designer milk, designer grains, probiotics, designer foods enriched with micro and macronutrients and designer proteins. Designer foods are produced by the process of fortification or nutrification. With the advances in the biotechnology, biofortification of foods using technologies such as recombinant DNA technology and fermentation procedures are gaining advantage in the industry. The ultimate acceptability and extensive use of designer foods depend on proper regulation in the market by the regulatory authorities of the country and by creating consumer awareness about their health benefits through various nationwide programs.
Keywords: Designer food, Fortification, Nutrification, Micronutrients, Probiotics, Designer egg, Designer milk, Designer proteins
Introduction
Designer food refers to the food that is designed to have some health benefits other than its traditional nutritional value. ‘Designer food’, ‘functional food’ and ‘fortified food’ are synonym, which refers to the food fortified or enriched with nutrient content already present in them or other complementary nutrient. The term was introduced in Japan in 1980s for referring processed food containing nutrient conferring of some additional health benefits apart from its own nutritional value (Arai 1996), whereas in China, designer food (referred to as health foods) is used in their traditional medicine. About 3000 varieties of health foods are available in China and widely accepted among the consumers due to their long history. Health Canada, department of the government of Canada with responsibility for national public health defines functional food as “A functional food is similar in appearance to, or may be, a conventional food that is consumed as part of a usual diet, and is demonstrated to have physiological benefits and/or reduce the risk of chronic disease beyond basic nutritional functions, i.e. they may contain bioactive compounds” (Health Canada 1998). The Institute of Medicine’s Food and Nutrition Board (IOM/NAS 1994) defined functional foods as “any food or food ingredient that may provide a health benefit beyond the traditional nutrients it contains”.
Functional foods included a wide variety of foods and food components believed to improve overall health and well-being, reduce the risk of specific diseases, or minimize the effects of other health concerns (IFIC 2011). It can be produced by fortification or nutrification of conventional food. Genetically engineered foods containing higher than normal amounts of health promoting nutrients and fermented foods with live cultures are considered functional foods. Infant formula may be the first designer food as it contains nutrients for the development of brain and immune system. The addition of docosahexaenoic acid (DHA) to health drinks for improving brain and visual development, the alteration or reduction of allergenic components in food, the use of probiotics and nucleotides to enhance immune response and sports nutrition are important examples of designer foods. Table 1 summarizes the health benefits of various designer foods. Fermentation is also a form of food modification. Folk medicine in various countries like China, Japan and India has the tradition of using fermented food for its health benefits, which includes red wine, yogurt, tempeh, red yeast rice etc.
Table 1.
Summary of designer foods and their health benefits
| S No | Micro/Macro nutrient | Designer foods | Health benefits |
|---|---|---|---|
| 1 | Omega 3 fatty acid | Omega 3 fatty acid enriched egg, oil and milk | Management of Cardiovascular disease, hypertension, autoimmune, allergic, neurological disorders, maternal health (Hargis and Van Elswyk 1993), osteoarthritis (Roush et al. 2010) and rheumatoid arthritis (Kjeldsen-Kragh et al. 1992) |
| 2 | Conjugated linoleic acid (CLA) | CLA enriched egg and milk | Antiadipogenic, anti-carcinogenic, anti-atherogenic and anti-inflammatory (Magdalena et al. 2008) |
| 3 | Selenium (Se) | Se enriched egg, broccoli and milk | Prevents cardiac muscle degeneration, muscular dystrophy (Beale et al. 1990), reduce the risk and prevalence of prostate and colon cancer and antioxidant activity (Navarro-Alarcon and Cabrera-Vique 2008) |
| 4 | Glucoraphanin | Glucoraphanin enriched broccoli sprouts | Reduce the risk of cancer (Latté et al. 2011) |
| 5 | Probiotics | Probiotic yoghurt | Produces pro-inflammatory cytokines (Meyer et al. 2007), eliminates enterotoxigenic Bacteroides fragilis, H. Pylori, prevents gastrointestinal (Odamaki et al. 2012) and lower respiratory tract infections (Jayakanthan et al. 2011), improves defecation frequency and abdominal pain due to constipation in pediatric patients (Guerra et al. 2011), improves antioxidant status in type 2 diabetic patients (Ejtahed et al. 2011) |
| 6 | Vitamin D and calcium | Vitamin D and calcium fortified milk | Lowers PTH levels, reduce bone turnover, prevents the occurrence of overweight and obesity among postmenopausal women (Bonjour et al. 2009; Kruger et al. 2010) |
| 7 | Micronutrients | Micronutrient fortified milk, salt fortified with iodine, iron and vitamin A | Improves anemic status and reduces anemia in children and pregnant women (De-Regil et al. 2011; Sunawang et al. 2009) |
| 8 | Docosahexaenoic acid (DHA) | DHA enriched milk | Reduces the level of blood lipids, improves composition of red blood cell membranes (Atalah et al. 2009) and intelligence in infants when consumed by pregnant and lactating mother (Gale et al. 2010) |
| 9 | Monacolin, Gamma amino butyric acid (GABA) | MFR (Expand) | Anti-diabetic and anti-cholesterol property (Rajasekaran and kalaivani 2009; 2011), promotes bone formation and immunomodulation (Tseng et al. 2012) |
| 10 | Phytosterols | Phytosterols enriched oil | Reduces total cholesterol, very low density lipoproteins and RLP cholesterol (Lugasi 2009) |
| 11 | Folic acid | Folic acid fortified grains | Reduces the risk of neural tube defects in newborns (Hertrampf et al. 2003) |
| 12 | Vitamins | Golden rice | Management of vitamin deficiencies (Potrykus 2003) |
Global regulatory status on designer foods
Most of the countries have most stringent regulations for food manufactured and imported for its sale, which include United States Food and Drug Administration (USFDA) in the USA (Anon 2010), Health Canada for Canada (Health Canada 1998), European Food Safety Authority for European Union (EFSA 2002), The State Food and Drug Administration for China (SFDA), Food Safety and Standards Authority of India (FSSAI) and Ministry of Food Processing Industry (MOFPI) (FSSAI 2006) for India and Ministry of Health, Labour and Welfare for Japan (MHLW). Only Japan is having specific regulatory approval process for designer foods i.e. Foods for Specified Health Use (FOSHU) approved from the Japanese Ministry of Health, Labour and Welfare (Arai 1996). Currently, 100 products are licensed as FOSHU foods in Japan. FOSHU approved products should be in the form of ordinary food not pills or sachets and are for regular consumption as a part of the diet.
Designer food or functional foods are gaining greater importance in USA due to their role in disease prevention and health promotion. USFDAs Dietary Supplement Health and Education Act of 1994 (DSHEA) regulates only the dietary supplement or dietary ingredient but not designer food but health claims may be made for foods and dietary supplements in accordance with the 1990 Nutrition Labelling and Education Act (NLEA) and the 1994 DSHEA, an amendment to the Food, Drug and Cosmetic Act. The legitimate basis for health claims were expanded in 1997 with the passage of the Food and Drug Administration Modernization Act. Under this act health claims may be approved on the basis of recommendation from certain federal agencies in the Department of Health and Human Services and the Department of Agriculture, and from the National Academy of Sciences or any of its subdivisions (USFDA). According to NLEA health claim represents the relationship between a nutrient and a disease or medical condition that is related to the diet.
In Canada there is no specific regulation for neutraceuticals, but the Food and Drugs Act and Regulations regulates the quality and safety of all foods and drugs. Under the Act, the “food” includes “any article manufactured, sold or represented for use as food or drink by man, chewing gum and any ingredient that may be mixed with food for any purpose whatever.” As per this regulation currently permit only ‘food as part of healthy eating and claiming that a nutrient or nutritive substance is generally recognized as an aid or factor in maintaining the functions of the body, or necessary for the maintenance of good health and normal growth and development (also known as “biological role claims” and nutrient function claims)’. Because of the dichotomy of designer food between foods and drugs, manufacturers of nutraceuticals and functional foods are left with two choices, either they can market their product without health claims or they have to follow more stringent regulatory requirements necessary for drugs (Food and Drug Regulations).
The Europoean regulatory has broad category as nutraceuticals and the definition of a nutraceutical is, “any food or food ingredient which is considered to have a beneficial effect on health”. Nutraceuticals are required to comply with food law but are not under the provision of s130(2) Medicines Act 1968 due to its health claim not medicinal claim. The Food Safety Act 1990 (FSA), subsequent primary and secondary legislation and codes of practice ensure that food placed on the market is safe and that any information provided about the product is not misleading. European regulatory includes food for specific health benefit rather than to enhance physiologic function, may include infant formula, processed baby foods (weaning foods), low-calorie foods for weight reduction, high-calorie foods for weight gain, ergogenic foods for athletes, and foods for special medical purposes like the treatment of diabetes or hypertension.
India has Food Safety and Standards Act, 2006, Food Safety and Standards Rules, 2011, Food Safety and Standards Regulations, 2011 and the Food Safety and Standards Authority of India (FSSAI), established under the Food Safety and Standards Act, 2006 as a statutory body for laying down science based standards for articles of food and regulating manufacturing, processing, distribution, sale and import of food so as to ensure safe and wholesome food for human consumption. In India, normal food, nutraceuticals, designer food/functional food etc. are not categorized separately (FSSAI 2006).
A brief account of selected designer foods is given below.
Designer eggs
Regular intake of well balanced diet plays an important role in maintaining good health. Among various foods, egg is an important and easily available food delivering balanced essential nutrients to the body and egg is the best medium for incorporating health components in it. The designer food approach has been explored widely using egg in providing various essential nutrients to the human body, which are not usually present in required quantity (Sim 1998). Designer egg approach was started in 1934 by Cruickshank, who reported the modification of fatty acid composition in egg yolk by making feed interventions. Omega-3 fatty acids are proved to be beneficial in various disorders such as cardiovascular disease, hypertension, autoimmune, allergic, and neurological disorders and it is also essential for normal functioning of the human physiology not only in normal adult and also in pregnant and lactating women. A diet balanced in omega-3 and omega-6 fatty acids is important during human evolution (Hargis and Van Elswyk 1993; Simopoulos 2000). Dennehy (2011) stated in his review on omega-3 fatty acid that there are sufficient data from randomized controlled trials on omega-3 fatty acids and ginger that their pharmacologic properties, efficacy, and safety data for specific indications in maternal health. Omega-3 fatty acids benefit gestation, infant vision, and neurodevelopment and ginger is efficacious for nausea in pregnancy but is limited in its safety data. It is also recommended to take omega-3 fatty acid supplement daily during pregnancy (Abdel-Nour and Ngadi 2011). Gheita et al. (2012) in their study stated that add on supplementation with omega-3 fatty acid along with non steroidal anti-inflammatory drugs (NSAIDs) in juvenile idiopathic arthritis was proved to be effective as it reduces the daily requirements of NSAIDs and the risk of related side effects.
Sim and Sunwoo (2002) developed designer egg rich in omega-3 fatty acids and antioxidants by feeding hen with flax seed and patented as Professor Sim’s Designer Egg, in which saturated fatty acid in yolk was replaced with 3-poly unsaturated fatty acid (PUFA) i.e. the yolk triglyceride is replaced by linolenic acid and yolk phospholipids are replaced by longer chain omega-3 fatty acids, such as eicosapentaenoic (EPA), dososapentaenoic and docosahexaenoic (DHA) acids (Jiang et al. 1991). To overcome the instability due to 3-PUFA the authors incorporated natural antioxidants like vitamin E, selenium and carotenoid pigments. Caston and Leeson (1990) also reported that the increase in the concentration of omega-3 fatty acid in hens egg by adding flax seed or fish oil in hens diet. Bourre and Galea (2006) produced designer egg fortified with omega-3 fatty acid by feeding hens with linseed, minerals, vitamins and lutein. The nutritional value of 100 g of these eggs contains 6 times more of the omega-3 fatty acid alpha-linolenic acid (ALA), 3 times more DHA, 3 times more vitamin D, 4 times more folic acid, 6 times more vitamin E, 6 times more lutein and zeaxanthine, 2.5 times more iodine and 4 times more selenium. These eggs also contain a little amount of cholesterol and, like standard eggs, are rich in B—complex vitamins and vitamin A, phosphorus and proteins. The consumption of these eggs improved the blood concentration of omega-3 fatty acids, high density lipoprotein (HDL)—cholesterol, low density lipoproteins (LDL)—cholesterol and triglycerides. These omega-3 fatty acids enriched designer eggs showed better stability of PUFA during egg storage and cooking, high availability of such nutrients as vitamin E and carotenoids, which improves antioxidant and omega-3 status of people consuming these eggs (Surai and Sparks 2001).
Fortification of omega-3 fatty acid not only increases the health benefits of designer egg but also reduces the cholesterol content of the egg by replacing saturated fatty acid in egg yolk. The dietary cholesterol and fatty acids plays an important role in various cardiovascular diseases. The scientific attempt to reduce cholesterol content in diet is the promising approach for the management of cholesterol (Hargis 1988). Designer eggs were also developed by replacing yolk cholesterol with conjugated linoleic acid (CLA). CLA is studied for its various health related properties such as anti-adipogenic, anti-carcinogenic, anti-atherogenic and anti-inflammatory (Magdalena et al. 2008). Raes et al. (2002) produced designer egg enriched with CLA by feeding hens with CLA rich diet and found that adding CLA to layers diets rich in omega-3 fatty acids produces CLA enriched eggs. Cook et al. (2000) had patented a method of production of CLA enriched designer eggs by feeding poultry a diet enriched in CLA. Dietary addition of CLA to hens diet decreased lipid content and concentrations of monounsaturated fatty acids in egg yolk, but increased CLA and saturated FA. CLA supplementation of egg also increased yolk moisture content, firmness and impaired the sensory quality of eggs.
Magdalena et al. (2008) studied the anti-cholesterol and anti-inflammatory effect of CLA-enriched eggs in animal models. The authors found that CLA-enriched eggs significantly reduce total plasma cholesterol as compared with CLA-supplemented eggs and it also reduces the size of atherosclerotic plaque, number of atherogenic macrophages and increases the area occupied by smooth muscle cells in atherosclerotic. Cholesterol content of the egg yolk can also be reduced by supplementing hen’s feed with chromium at 200–800 ppb concentration (13.9 to 33.7 % reduction) (Anderson et al. 1989; Lien et al. 1996). The pharmacological approach was also studied for reducing cholesterol level in egg by administering egg laying hens with cholesterol lowering drugs. US patent was issued to Meier and Wilson (1997) for the method of reducing the cholesterol content of egg by supplementing hen with L-dihydroxyphenylalanine (L-DOPA).
Selenium (Se), a micronutrient is essential for preventing cardiac muscle degeneration. Supplementation of Se will enhance Se-specific antioxidant status and improve the development of embryos in gilts (Fortier et al. 2012). Se has an essential role in thyroid hormone metabolism and iodine (I) deficiency (Kandhro et al. 2011). Se supplementation is used globally for preventing and treating muscular dystrophy and other Se deficiency syndromes (Beale et al. 1990). Se deficiency is a global problem, to treat this deficiency regular intake of Se rich food is important. Organic source of Se is better absorbed than inorganic source (Fortier et al. 2012). Compared to plant source, egg and meat are the good source of Se. Recommended daily requirement of Se is 55 μg for human adults (Bennett and Cheng 2010). Regular dietary intake at recommended level may reduce the risk and prevalence of prostate and colon cancer and it also proved to have a role in prevention of cardiovascular diseases through antioxidant property (Navarro-Alarcon and Cabrera-Vique 2008; Bennett and Cheng 2010). Among the food sources of selenium egg is the most important and it is easy to fortify the egg with increasing amount of Se. Se concentration in egg had increased from 0.044 to 0.243 μg/g for eggs from hens fed with organic Se (Se yeast product) (Cantor and Scott 1974). Se enriched eggs are marketed in various countries like UK, Ireland, Mexico, Columbia, Malaysia, Thailand, Australia, Turkey, Russia and the Ukraine (Fisinin et al. 2009). Bennett and Cheng (2010) in their study on the production of Se enriched egg concluded that feeding hen up to 5.1 μg/g of Se will increase the concentration of Se in egg without affecting egg production. Se-enriched chicken, pork and beef can also be produced by feeding organic Se in the diet of poultry and farm animals (Fisinin et al. 2009). Increased levels of Se are excreted in liver, kidney and eggs (Beale et al. 1990).
Apart from Se, other nutritional quality of the egg was also improved by enhancing levels of antioxidants such as vitamin E, omega-3 fatty acids such as DHA, carotenoids and Se (Surai and Sparks 2001). In a double-blind, placebo-controlled trial by Surai et al. (2000) to evaluate the ability of designer eggs enriched in vitamin E, lutein, Se and DHA in delivering micronutrients to the human found that consumption of designer eggs enriched in vitamin E, lutein, Se and DHA significantly increased the levels of α-tocopherol, lutein and DHA in plasma as compared to the normal table eggs, whereas it did not change Se concentration in plasma, blood pressure and plasma lipid profile. From the results the authors concluded that dietary intake of designer eggs enriched in vitamin E, lutein, Se and DHA will be a beneficial approach in supplying those nutrients. Designer egg with enhanced vitamin A and β—carotene concentration was also developed (Jiang et al. 1994). Sookwong et al. (2008) developed Tocotrienol (T3)-fortified eggs (0.62 mg of T3/egg) by adding rice bran scum oil to the feed, whereas normal egg contains 0.11 mg of T3/egg.
Helicobacter pylori are one of important cause of gastritis and gastric ulcer and leads to gastric cancers in most of the developed and developing countries (Satoh et al.1996; Malekshahi et al. 2011). H. pylori has been classified as a category 1 carcinogen by WHO (Ruggiero et al. 2002; Prinz et al. 2003). Development of resistance to antibiotic leads to failure of antibiotic therapy (Malekshahi et al. 2011). Roe et al. (2002) developed a egg-yolk immunoglobulin (IgY) against H. pylori whole-cell lysate, which reduces gastric inflammation due to H. pylori-infection in Mongolian gerbils. Later various researchers found that administration of anti-urease specific IgY was effective passive immunization against H. pylori infection and its related gastrointestinal disorders (Shin et al. 2003, 2004; Nomura et al. 2005). Malekshahi et al. (2011) in their study developed recombinant IgY from by using hens immunized with recombinant UreC and found that UreC-induced IgY is specifically successful in inhibition of H. pylori infection. Genetic immunization of ducks with a plasmid expressing H. pylori UreB produced UreB induced IgY polyclonal and monospecific antibodies against recombinant H. pylori urease (Kazimierczuk et al. 2005). Consumer acceptance of these designer eggs are similar to that of normal eggs (Scheideler et al. 1997).
Designer oil with omega 3 fatty acid
Omega 3 fatty acids are unsaturated fatty acids found in green leafy vegetables, vegetable oils, nuts and fish and fish oil. The most common omega-3 fatty acids found in the diet include long-chain PUFA, EPA and DHA. Dietary consumption of omega 3 fatty acid reduces the incidence of cardiovascular disease (CVD), osteoarthritis (Roush et al. 2010), and rheumatoid arthritis (Kjeldsen-Kragh et al. 1992). Designer diet based approach will be effective for increasing omega-3 fatty acid (Penny et al. 2002). Riediger et al. (2008) studied the impact of the source of n-3 fatty acid on cardiovascular benefits using C57BL/6 mice. From the study the authors suggested that the health benefits may be achieved by lowering dietary omega-6: omega-3 fatty acid even in a high fat diet medium. Riediger et al. (2009) studied cardiovascular and metabolic benefits of ‘designer oils’ containing a lower ratio of omega-6: omega-3 fatty acids. Three groups of C57BL/6 mice were fed for 6 weeks with an atherogenic diet supplemented with either a fish oil- or flaxseed oil-based ‘designer oil’ with an approximate omega-6: omega-3 fatty acid ratio of 2:1 or with a safflower oil-based formulation with omega-6: omega-3 fatty acids ratio of 25:1. From the observation of food intake, body weight, and blood lipid levels it was concluded that lowering dietary ratio of omega-6: omega-3 fatty acids may significantly reduce cardiovascular and metabolic risks in mice. Napier and Graham (2010) have reported the promising approach of transgenic metabolic engineering in developing transgenic plant producing designer oil enriched with omega-3 long chain PUFA equivalent to the level found in marine organisms.
Designer oil based approach for rapeseed oil enriched with micronutrients such as polyphenols, tocopherols and phytosterols may be effective in the prevention of atherogenesis (Xu et al. 2011). Phenol enrichment of olive oils (Suárez et al. 2010) and palmitic acid-fortified vegetable oil produced synergistic effects on calcium absorption and it is beneficial for baby foods including infant formula, with regard to increasing absorption of calcium by higher soluble calcium in the small intestinal content (Lee et al. 2008). Eshigina et al. (2007) investigated the influence of dietary therapy containing sunflower oil with phospholipids (PL) on the lipid profile of plasma and composition of fatty acids of red blood cells in patients with hypertension and obesity and observed the reduction in serum total cholesterol, low density lipoprotein (LDL), apolipoprotein A 1, apoB and fibrinogen.
Designer broccoli
Broccoli is a highly valued vegetable due to its chemopreventive property. Latté et al. (2011) reported that the benefit from consumption of broccoli in modest quantities and in processed form outweighs potential risks. Sulforaphane is a chemopreventive isothiocyanate (ITC) derived from glucoraphanin (GRP) hydrolysis by myrosinase, a thioglucoside present in broccoli. Due to lack of myrosinase in commercially available glucoraphanin supplement bioavailability of sulforaphane is not achieved. Cramer et al. (2011) showed that combining broccoli sprouts with the glucoraphanin powder synergistically enhanced the early bioavailability of sulforaphane, proved that regular intake of designer broccoli sprouts enriched with glucoraphanin powder reduces the risk of cancer compared the GRP powder or sprouts alone. Designer broccoli fortified with Se is effective in cancer prevention, due to its high glucosinolate (GSL) content and Se accumulation, which can be developed by fertilizing broccoli with Se (Hsu et al. 2011). Abdulah et al. (2009) achieved Se enrichment by using a sodium selenite solution, which showed potential anticancer properties in human prostate cancer cell lines, as compared with those of a control broccoli sprout extract.
Probiotics
Probiotics are live microorganisms such as Lactobacilli Sp., Bifidobacteria Sp. and Streptococcus thermophilus, which provide various health benefits upon ingestion. These probiotics are commercially available as spores or in lyophilized forms or in the form of probiotic fortified fermented dairy products. Probiotics are potential in the treatment for eczema (Gore et al. 2011), pediatric antibiotic-associated diarrhea (Johnston et al. 2011), acute upper respiratory tract infections (Hao et al. 2011), chronic and acute enteric infections and their associated diarrheal complexes (Sleator 2010). Daily dietary intake of probiotics reduces the incidence and severity of acute and chronic infection, prevents and reduces the recurrence of cancer and incidence of several atopic conditions (Sleator and Hill 2008) and also increases the level of liver aminotransferases in patients with non - alcoholic fatty liver disease (NAFLD) (Aller et al. 2011). Manipulation of cellular system using recombinant technology in probiotic bacteria is easier, which can facilitate survival in stressful conditions, and can also lead to production of designer strains (Corcoran et al. 2008). ‘Designer probiotics’—strains are specifically tailored to target certain pathogens and/or toxins in vivo (Sleator and Hill 2008), for controlling human immunodeficiency virus (HIV) and as novel mucosal vaccine delivery vehicles (Elson 2006; Sleator 2010) and which interacts ligand-receptor binding of toxins released by microbial pathogens causing enteric infections (Paton et al. 2006).
Designer drinking yogurt
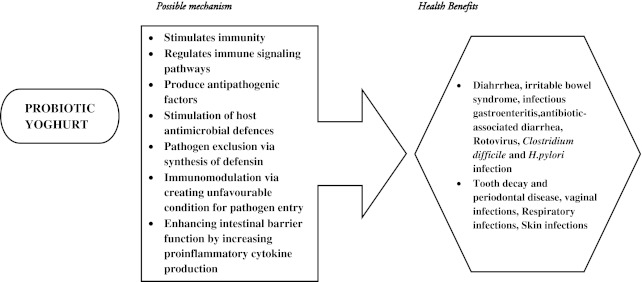
Fresh yogurt is an example for naturally available probiotics; it contains live cultures of lactic acid-producing bacteria that can prevent traveler’s diarrhea, antibiotic-induced diarrhea, rotavirus infection, and vaginal yeast infection (Fig. 1). Regular dietary intake of conventional and probiotic yogurt stimulated the production of pro-inflammatory cytokines in young healthy women (Meyer et al. 2007) and anti-inflammatory activity in parallel with the expansion of peripheral pool of putative T(reg) cells in inflammatory bowel disease patients (Lorea Baroja et al. 2007). Probiotic yogurts potentially eliminate enterotoxigenic Bacteroides fragilis, which causes acute and persistent diarrheal disease, inflammatory bowel disease and colorectal cancer (Odamaki et al. 2012). Designer yoghurt containing Bifidobacterium lactis Bb12(R) prevents gastrointestinal and lower respiratory tract infections (Jayakanthan et al. 2011) and also improves defecation frequency and abdominal pain due to constipation in pediatric patients (Guerra et al. 2011).
Fig. 1.
Possible mechanism of action and health benefits of probiotic yoghurt
Regular consumption of probiotic yogurt improves fasting blood glucose and antioxidant status in type 2 diabetic patients (Ejtahed et al. 2011), apart from anti-diabetic activity vitamin D or vitamin D - calcium fortified yoghurt ameliorated compromised vitamin D status and improved lipid profile and endothelial biomarkers in type 2 diabetic patients (Nikooyeh et al. 2011; Shab-Bidar et al. 2011). Curcumin mixed into yoghurt exhibited anti-diabetic activity and improved physiological and biochemical markers of experimental diabetes (Gutierres et al. 2011). The gut-associated lymphoid tissue (GALT) is a major site of HIV activity and significantly influences disease prognosis. Reducing immune activation at this site helps slow disease progression. Studies also showed that probiotic microorganisms have considerable immunomodulatory effects at the level of the GALT. Irvine et al. (2011) in Mwanza, Tanzania evaluated the ability of probiotic yogurt in reducing the incidence and severity of opportunistic infections among people with HIV and concluded that yogurt supplemented with L. rhamnosus may effectively alleviate GI symptoms and improve productivity, nutritional intake and tolerance to antiretroviral treatment among people with HIV.
Various in vitro studies indicated that yoghurt bacteria generates water-soluble vitamins such as vitamin B1 and B2 and daily intake of 200 g of probiotic or conventional yoghurt will be a good source of these micronutrients (Fabian et al. 2008), when compared to the specific intake of probiotic bacteria. Horie et al. (2004) designed drinking yogurt containing Lactobacillus acidophilus and Bifidobacterium sp. and fortified with 1 % egg yolk IgY-urease, consumption of which may lead to suppression of H. pylori infection in humans. Estrada et al. (2011) developed a fortified strawberry yogurt containing microencapsulated salmon oil, which help to increase intake of long-chain n-3 fatty acids. McCowen et al. (2010) studied that ω-3 fatty acid fortified yoghurt increases ω-3 fatty acid content of plasma lipids, and reduces arachidonic acid concentrations on plasma lipids. Increase in storage of fat during pregnancy leads to elevated lipid levels. Consumption of probiotic yogurt among pregnant women could not affect normal serum lipid profile as compared to the conventional yogurt (Asemi et al. 2011). Apart from health benefits designer yoghurts are useful in maintaining healthy skin. Antioxidant potential of yoghurt face pack using natural ingredients (F-YOP) improved the moisture, brightness and elasticity of treated skin (Yeom et al. 2011).
Designer milk
Designer milk may have modification in the primary structure of casein, alteration in the lipid profile to include more healthy fatty acids such as conjugated linoleic acid (CLA) and omega-fats, improved amino acid profiles, more protein, less lactose and absence of beta-lactoglobulin (beta-LG) and increased protein recovery. Other milk containing nutraceuticals are the important aspects in designer milk achieved through transgenic technology. Cow milk allergy in children could also be reduced by eliminating the beta-LG gene from bovines (Sabikhi 2007). The genetic manipulation of dairy cattle is also a feasible and has significant impacts on milk quality, attributes of novel dairy products and human health (Karatzas and Turner 1997).
Hernández et al. (2007) studied the effect of 11.2 % sunflower seed supplemented diet for cows on the chemical composition of milk and dairy products. The results of the study showed that the contents of CLA and transvaccenic acid (TVA) were increased from 0.54 to 1.6 g/100 g total fatty acid, respectively in control products to 2 and 6.4 g/100 g total fatty acid, respectively in diet supplemented group without affecting lactose content in milk, total fat, protein and ash contents in the dairy products, which is approximately 4 fold higher. Moreover, CLA-rich products showed considerably low atherogenicity index and thrombogenicity index i.e. 38.4 and 25 % less than those from control products. The study also demonstrated that fatty acid profiles were unaffected during processing. From these studies Hernández et al. (2007) concluded that designer milk produced by supplementing cow with 11.2 % sunflower seed diet may contribute to the reduction of the risk of CVDs in humans.
Various forms of designer milks and milk products were evaluated by researchers for the health benefits of human, which include milk-based beverages fortified with added apple or grape seed polyphenols (Axten et al. 2008), lutein-fortified fermented milk (Granado-Lorencio et al. 2010), milk fortified with phenolic compounds from olive vegetable water and fermented with γ-amino butyric acid (GABA)-producing (Lactobacillus plantarum C48) and autochthonous human gastro-intestinal (Lactobacillus paracasei 15 N) lactic acid bacteria (Servili et al. 2011), prebiotic and probiotic fortified milk (Sazawal et al. 2010), lactoferrin-enriched fermented milk, which ameliorates acne vulgaris (Kim et al. 2010), folic acid fortified milks for better absorption of folic acid (Achón et al. 2011), milk fortified with EPA (Martin-Bautista et al. 2010). Fermented milk was produced by fermenting Chingshey purple sweet potato (CPSP) substrate/media milk with lactic acid bacteria strains possessing high GABA concentrations, organic acid contents, anthocyanin contents, and antioxidant activity (Wu et al. 2011), among which calcium and vitamin D fortified milk and cheese are important for bone health of children and women. Calcium and vitamin D fortified milk along with magnesium and zinc improves vitamin D status, lower parathyroid hormone levels and reduce bone turnover (Bonjour et al. 2009; Kruger et al. 2010) Regular consumption of vitamin D-fortified milk provides a mean intake of nearly 4 μg/d in children (Green et al. 2010; Houghton et al. 2011; Rich-Edwards et al. 2011) and study also showed that high-calcium enriched milk prevents the occurrence of overweight and obesity among postmenopausal women (Angeles-Agdeppa et al. 2010). Good bone health can be achieved through appropriate diet and lifestyle, which protects from osteoporesis and bone fracture in later stage of life. Optimal intake of calcium and vitamin D in early adulthood is essential and should be achieved through diet. Van der Hee et al. (2009) developed designer icecream fortified with calcium. Calcium bioavailability in the two calcium-fortified ice cream formulations as high as milk indicates that ice cream will be a good vehicle for delivery of calcium.
Anemia during infancy impairs neurodevelopment; iron-fortified milk improves anemic status (Rivera et al. 2010; Semba et al. 2010). Multiple micronutrient deficiencies are highly prevalent among preschool children and often lead to anemia and growth faltering. Milk provides an acceptable and effective vehicle for delivery of specific micronutrients, especially zinc and iron. Micronutrient fortified milk providing micronutrient bundle, improved growth and iron status and reduced anemia in children 1–4 years old (Sazawal et al. 2010). Designer milk produced by enriching milk with fish oil, oleic acid, minerals and vitamins reduces indices of endothelial cell activation in children (Romeo et al. 2011). EPA and DHA enriched milk reduces the level of blood lipids, mainly cholesterol, LDL-cholesterol and triglycerides (Lopez-Huertas 2010). Consumption of DHA fortified milk during pregnancy and lactation improved composition of red blood cell membranes as well as in human milk (Atalah et al. 2009) and showed improved intelligence in infants (Gale et al. 2010). Selenium enriched milk may improve Se status and reduce the risk of cancer (Hu et al. 2010).
Human milk is the best food for neonates; however, in very low birth weight pre-term infants it may not provide sufficient protein and energy. Various studies reported the use of fortified human milk in preterm infants, which produces adequate growth and satisfies the specific nutritional requirements (Arslanoglu et al. 2009; Maggio et al. 2009; Martins and Krebs 2009; Reali et al. 2010; Di Natale et al. 2011; Zachariassen et al. 2011). α-Lactalbumin-enriched infant formula containing 12.8 μg/l protein was found to be safe and supported age-appropriate growth; weight gain (Trabulsi et al. 2011). Due to unawareness among consumers and other issues, demand for fortified human milk is less as compared to that of infant formulas (Arslanoglu et al. 2010). Early neonatal intake of cow’s milk protein may sensitize immune system and lead to allergic conditions for cow’s milk (Høst 1991).
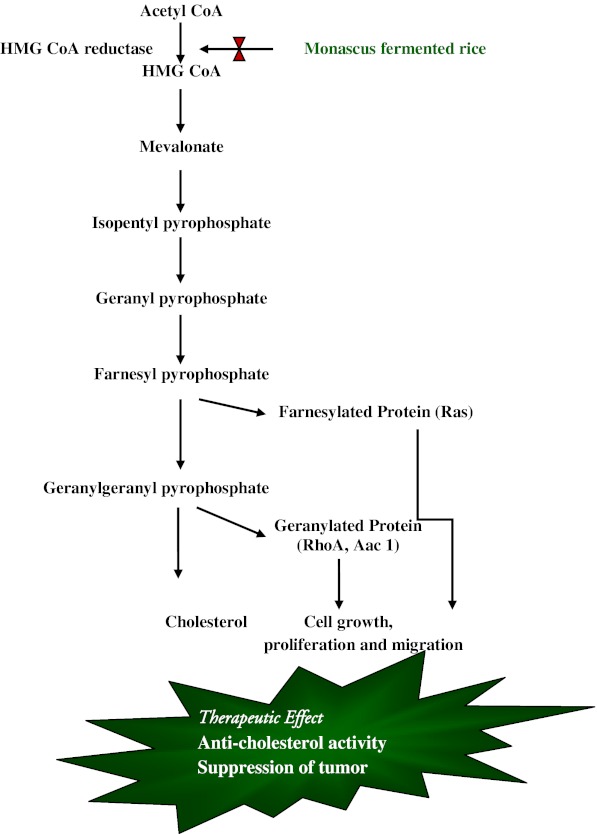
Monascus fermented rice
Monascus fermented products have featured in Chinese cuisine for thousands of years and are widely used as food colourants and dietary materials in many Asian countries. It is produced by fermenting rice with fungi Monascus sp, which leads to fortification of rice with active constituents such as monacolins and γ - amino butyric acid (GABA). Tseng et al. (2012) reported in vitro antioxidant and immunomodulatory activity in RAW 264.7 cells. Various studies also proved its health benefits for human with regard to diabetes, cholesterol, cancer, alcohol induced liver disease and inflammation (Kalaivani et al. 2010). It is also proved to have potential for promoting bone formation and immunomodulation. In our laboratory, we have developed Monascus fermented rice using Indian variety of rice and reported its anti-diabetic (Rajasekaran and Kalaivani 2009) and anti-cholesterol (Rajasekaran and Kalaivani 2011) activity in animal models. The benefits of the Monascus fermented rice may be due to the presence of monacolins (Fig. 2).
Fig. 2.
Schemetic representation of mechanism of action of Monacolins present in Monascus fermented rice
Phytosterols enriched designer food
Phytosterols are isoprene compounds present in different food products, among which the most important are beta-sitosterol, campesterol and stigmasterol. Plant sources of phytosterols are oily seeds, nuts, plant oils, grains, and pulses. Studies have reported the reduction in total cholesterol and LDL cholesterol level on regular consumption of 2–3 g phytosterols fortified foods per day (Lugasi 2009), which in turn reduces the risk of cardiovascular diseases. Polagruto et al. (2006) and Escurriol et al. (2009) also reported the reduction in total chloesterol and LDL—cholesterol on consumption of food products fortified with phytosterols. Intake of phytosterols enriched designer oil (0.45 g/day (as free sterol) (Seki et al. 2003) significantly reduced total cholesterol, very low density lipoprotein (VLDL) cholesterol and remnant-like lipoprotein (RLP) cholesterol compared with the control vegetable oil, which is helpful in reducing the risk of coronary heart disease (CHD) in the population. Clifton et al. (2004) reported that phytosterol ester-enriched milk and yoghurt significantly reduce LDL and total cholesterol. Plasma sitosterol was increased by 17%–23 % and campesterol by 48%–52 % with phytosterol-enriched milk and bread. Phytosterols, fat-soluble fractions of plants are consumed at levels of 200–400 mg/day in Western diets. Phytosterol chemically resembles cholesterol, inhibits the absorption of cholesterol. Addition of phytosterol in diet is effective in reducing the risk of CHD (Jones et al. 1997).
Phytosterol ester-enriched margarine (Mussner et al. 2002) also significantly reduces total cholesterol, LDL—cholesterol, HDL—cholesterol, apolipoprotein B and LDL/HDL cholesterol ratio compared with the control margarine (Cerrato 1999).
Designer grains
Wheat germ-enriched bread has been prepared by al-Hooti et al. (2002) using white flour, 20 % raw wheat germ, 0.5 % sodium stearoyl-2-lactylate, 30 ppm potassium bromate and 50 ppm ascorbic acid. Maternal supplementation of folic acid in early pregnancy reduces the risk of severe language delay in children at 3 years of age (Roth et al. 2011). Sittig et al. (2011) recommended dose of folate at 400 ug/day for adolescents and nonpregnant adults. US had implemented mandate of folic acid fortification of grains (Johnston and Tamura 2004). Due to lack of stringent regulations, there are reports on higher than expected fortification. Over dose of folate supplementation in adolescence may lead to motivational and spatial memory deficits (Johnston and Tamura 2004; Sittig et al. 2011). Chilean Ministry of Health recommended the fortification of wheat flour with folic acid at a concentration of 2.2 mg of folic acid/kg for women of child bearing age in order to reduce the risk of neural tube defects in newborns (Hertrampf et al. 2003). Folic acid fortification of grains are recommended also in Iran (Abdollahi et al. 2008). Vitamin deficiency diseases are common in the countries where rice is the staple food. Golden rice to combat vitamin deficiency was developed by fortifying rice with vitamins (Potrykus 2003). Apart from biotechnological approach there are maize and wheat with higher levels of iron, zinc, β—carotene, lysine and tryptophan are available due to natural variation in its germplasm (Hoisington 2002).
Designer foods: role in cancer prevention
There are numerous anti-carcinogens available naturally in food or herbs. The effective use of those constituents is important in preventing cancer. The “designer foods” approach is one of the best approaches, by which the constituents having anti-cancer potential can be fortified into the regular diet (Pariza 1992). Various studies have proved the designer food approach for the prevention of cancer. Dietary administration of bovine milk lactoferrin and black tea polyphenols combination significantly reduced the tumor incidence, development of hamster buccal pouch carcinomas, carcinogen-metabolizing enzymes and cellular redox status (Chandra Mohan et al. 2005, 2006; Mohan et al. 2008).
Polyphenolic compounds such as anthocyanins and flavonoids in red grape wine were proved to have inhibitory effect on breast cancer cells. A study by Hakimuddin et al. (2004) also supported that red wine polyphenolic fractions have anticancer property against breast cancer cell lines (MCF-7) and the authors also reported relatively low cytotoxicity towards normal human mammary epithelial cells (HMEC) and a non-tumorigenic MCF-10A cells, which is contrast to the authentic flavonoids such as quercetin, naringenin and catechin which inhibited the growth of HMEC much more than that of MCF-7 cancer cells. In another study, Hakimuddin et al. (2006) reported the effect of red grape wine polyphenol in gene expression and biochemical pathways. The polyphenols induced calcium release by disrupting mitochondrial function through membrane damage, which results in selective cytotoxicity toward MCF-7 cells. Apart from its anticancer activity, the polyphenolic fractions showed discrete antioxidant action on cancer cell lines (Damianaki et al. 2000). The above studies suggested that consumption of wine or other polyphenol-rich foods and beverages, could have a beneficial antiproliferative effect on breast cancer cell growth.
Tea is consumed as beverage in many countries in the world. Studies have also showed beneficiary effect of tea in reducing the risk of a variety of illnesses such as cancer and coronary heart disease. Tea plant, Camellia sinesis is cultivated globally and contains polyphenols as one of the active constituent. Among tea, both black tea and green tea were proved to have potential in preventing lung, stomach, esophagus, duodenum, pancreas, liver, breast, colon (Weisburger et al. 1998) and skin cancers and also have preventive effect on atherosclerosis and coronary heart disease, high blood cholesterol concentrations and high blood pressure. The health benefits of polyphenols from tea can be extended by combining them with other food in the form of designer food (Mukhtar and Ahmad 2000). Among flavonoids, flavone, flavonol, flavanone and isoflavone classes possess antiproliferative effects on various cancer cell lines LLC-PK1 (renal tubular cell line), MCF-7 (human breast cancer cell line), Caco-2 (human colon cancer cell lines) and HT-29 (resembling colonic crypt cells) (Kuntz et al. 1999).
Designer food enriched with macro and micronutrients
In a survey by Wagner et al. (2005) on fortified foods in Austria showed that about 470 fortified products are available commercially. The most frequently added nutrients were vitamin C (73 %), vitamin B6 (43 %), niacin (37 %) and among mineral and trace elements calcium (23 %) was the most added. Among people who are buying fortified foods the contribution of vitamins and minerals increased up to 74 and 19 %, respectively and no risk due to overdose was found. To address micronutrient deficiency in European population, micronutrients are added safely to foods at levels recommended by European Commission Recommended Daily Intake (EC RDA): the micronutrients includes vitamin B12, vitamin C, vitamin E, riboflavin, panthothenic acid, niacin, thiamine, vitamin B6, vitamin D, folic acid, biotin, copper, iodine, selenium, iron, zinc, calcium, phosphorus and magnesium (Flynn et al. 2003). Dietary micronutrient deficiencies lead to diseases such as iodine deficiency disorders, iron-deficiency anemia, and vitamin A deficiency, and other serious public health problems in the developing world. Fortification of salt with iodine, iron, and vitamin A is an easier approach and is followed in most of the developing countries including India (Raileanu and Diosady 2006).
Micronutrient-fortified, cereal-based infant foods are recommended for reducing multiple micronutrient deficiencies in infants (Gibson et al. 2011). Home fortification of foods with multiple micronutrient powders is an effective intervention to reduce anemia and iron deficiency in children of 6 to 23 months age (De-Regil et al. 2011). Micronutrient deficiency in pregnant women leads to low birth weight, lower cognition and reproductive performance and anemia. Deficiencies of micronutrients (zinc, iron, folic acid and iodine) is highly prevalent in pregnant women in South Asia, India and other developing countries (Seshadri 2001; Pathak et al. 2004; Haider and Bhutta 2006), which may be due to poor dietary intake of food and low frequency of consumption of foods rich in micronutrients. UNICEF/United Nations University/World Health Organization jointly proposed a formulation for a multiple micronutrient supplement for pregnant women. Use of multivitamin supplementation among pregnant women is effective in improving anemia status (Sunawang et al. 2009). Multiple micronutrient supplements increases hemoglobin and improves micronutrient status in pregnant women better than iron supplements alone or iron with folic acid (Allen and Peerson 2009). Micronutrient fortified flour reduced both iron and zinc deficiency postpartum among breast-feeding women (Stuetz et al. 2011)
Designer protein
The synthesis of proteins with high essential amino acid content has potential applications in animal nutrition (Gagnon et al. 2000; Doucet et al. 2002). Cysteine-rich proteins, such as keratin, may have advantages over the simple amino acid or its derivatives, which improves antioxidant status in health and disease management (McPherson and Hardy 2011).
Biotechnology in designer food
Gene technology had created a platform for genetic manipulation in farming and the use of plants as ‘pharma’ factories to manufacture therapeutics (Chang 2001). Transgenic technology can improve functional properties of dairy milk by altering major component of milk in high producing dairy cows (Karatzas 2003). In a study by Brophy et al. (2003), casein concentration in the milk was enhanced by introducing additional copies of the genes encoding bovine beta- and kappa-casein (CSN2 and CSN3, respectively) into female bovine fibroblasts. In another study by Hyvönen et al. (2006) human lactoferrin content was increased in cow’s milk. Fastest growth in dairy biotechnology, particularly for altering the milk composition paved a path for designer milk (Sabikhi 2007). To address vitamin A deficiency, an important nutritional problem in India, advances in biotechnology had developed genetically modified mustard (Brassica juncea) to express high levels of β—carotene, the precursor of vitamin A (Chow et al. 2010).
Biofortification
To meet the nutritional needs of fast growing global population at the rate of 1.4 % per year, i.e. 8 billion by 2030, there is a need for 50 % more food grains with higher and more stable yields (Khush 2002; Yan and Kerr 2002). Macronutrient and micronutrient deficiencies are prevalent in most of the developing countries and there is a decline in natural resources such as arable land and water. To meet these challenges biotechnology is a valuable tool to improve nutritional value in plants and crops (Yan and Kerr 2002). Plant biotechnology has made important contributions in developing designer grains enriched with vitamins, amino acids and micronutrients. The use of conventional breeding techniques and biotechnology to improve the quality of staple crops is a new strategy to address nutrient deficiencies in developing countries, which is referred to as “biofortification”. Potential of biofortification is proved in improving iron, zinc, and vitamin A status in low-income populations (Hotz and McClafferty 2007). Biofortification is the cost effective way as it does not require a change in dietary habits. In the year 1992 International Rice Research Institute, Manila, Philippines, had initiated a project to improve the iron and zinc content of rice, which was followed by many other researchers and developed lines of rice with increased iron, zinc and β—carotene content. Rice lines with improved iron contents were developed (Sautter et al. 2006). The biofortification with nutrients was extended to wheat, maize, cassava, sweet potatoes and beans. Maize with improved amino acid balance was developed and grown in several African countries (Khush 2002; Friedrich 1999). The rapidity of research in food biotechnology, regulatory issues, legislation and intellectual property rights will enhance the discovery and innovation, but public education on awareness about biotechnologically produced products should be continually enhanced for its acceptance among the people (Chang 2001).
Conclusion
Advantages of designer food approach are that it does not require change in dietary habit/pattern of the population and it can deliver recommended amount of nutrients regularly. It can be easily merged with existing system of food production and distribution. In developed countries designer foods played a major role in improving the diet and eliminating nutritional deficiencies. For example, elimination of vitamin A deficiency leading to night blindness was achieved by vitamin A fortified margarine in Denmark and vitamins A and D fortified milk eliminated vitamin D deficiency and rickets in Europe and North America. In the developing countries, food fortification has gained importance since 1990s. Fortification of wheat flour with iron, vitamin A, folic acid and other B vitamins in Asian countries such as India, Indonesia and the Philippines was successful in eliminating these micronutrient deficiencies, whereas in Thailand, foods such as noodles and fish were fortified with micronutrients.
Designer foods approach is one of the major strategies to reduce micronutrient deficiency in developing countries, which can be done systematically to reach entire population by public and private partnership. Best example for commonly used designer food was iodized salt, which is widely used by the population and had eliminated iodine deficiency and its related disorders. The success of the drive for universal iodization of salt shows that the diets of children, women and families world-wide can be changed in small but very beneficial ways in just a few years as a result of concerted global, national and local action (UNICEF 2005).
Malnutrition is substantial in India. Data from National family Health Survey-3 (NFHS 2007) in India showed children upto the age of five are under weight due to malnutrition, which ranges from <25 % in states like Kerala, Sikkim, Punjab, Manipur, Mizoram to >40 % Madhya Pradesh, Bihar and Jharkhand (Fig. 3). The success of iodine fortification of salt leads to supply of other nutrient through designer food approach such as vitamin D and calcium fortified milk for bone health, folic acid fortified cereals, sterols fortified yogurt, margarine, chocolate, cheese and juice in the management of cholesterol, iron fortified milk, salt and condiments for management of anemia. In India, there is a cultural relationship between food and health, which is well recognized and used in day to day life. Food is considered as medicine in Indian systems of medicine. Regularly used foods in India such as tea, green tea, oil, sugar, dhal, etc. can be positively explored for designer foods approach in improving health of the society. Prior experience in designer foods indicate that fortification of food is completely safe but fortification should be done rationally i.e. it is usually less than one third of the total Recommended Daily Allowance (RDA). It should be strictly regulated with stringent quality control measures to ensure that there is no excessive intake of specific nutrients.
Fig. 3.

Percentage of underweight in children due to malnutrition in different states of India. Source: NFHS (2007)
References
- Abdel-Nour N, Ngadi M. Detection of omega-3 fatty acid in designer eggs using hyperspectral imaging. Int J Food Sci Nutr. 2011;62(4):418–422. doi: 10.3109/09637486.2010.542407. [DOI] [PubMed] [Google Scholar]
- Abdollahi Z, Elmadfa I, Djazayeri A, Sadeghian S, Freisling H, Mazandarani FS, Mohamed K. Folate, vitamin B12 and homocysteine status in women of childbearing age: baseline data of folic acid wheat flour fortification in Iran. Ann Nutr Metab. 2008;53(2):143–150. doi: 10.1159/000170890. [DOI] [PubMed] [Google Scholar]
- Abdulah R, Faried A, Kobayashi K, Yamazaki C, Suradji EW, Ito K, Suzuki K, Murakami M, Kuwano H, Koyama H. Selenium enrichment of broccoli sprout extract increases chemosensitivity and apoptosis of LNCaP prostate cancer cells. BMC Cancer. 2009;9:414–425. doi: 10.1186/1471-2407-9-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achón M, Arrate A, Alonso-Aperte E, Varela-Moreiras G. Plasma folate concentrations after a single dose ingestion of whole and skimmed folic acid fortified milks in healthy subjects. Eur J Nutr. 2011;50(2):119–125. doi: 10.1007/s00394-010-0121-z. [DOI] [PubMed] [Google Scholar]
- al-Hooti SN, Sidhu JS, al-Saqer JM, al-Othman A. Effect of raw wheat germ addition on the physical texture and objective color of a designer food (pan bread) Nahrung. 2002;46(2):68–72. doi: 10.1002/1521-3803(20020301)46:2<68::AID-FOOD68>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Allen LH, Peerson JM. Maternal Micronutrient Supplementation Study Group. Impact of multiple micronutrient versus iron-folic acid supplements on maternal anemia and micronutrient status in pregnancy. Food Nutr Bull. 2009;30(4 Suppl):S527–S532. doi: 10.1177/15648265090304S407. [DOI] [PubMed] [Google Scholar]
- Aller R, De Luis DA, Izaola O, Conde R, Gonzalez Sagrado M, Primo D, De La Fuente B, Gonzalez J. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci. 2011;15(9):1090–1095. [PubMed] [Google Scholar]
- Anderson RA, Bryden NA, Planski MM, Richards MP. Chromium supplementation of turkeys: Effects on tissue chromium. J Agric Food Chem. 1989;37:131–134. doi: 10.1021/jf00085a031. [DOI] [Google Scholar]
- Angeles-Agdeppa I, Capanzana MV, Li-Yu J, Schollum LM, Kruger MC. High-calcium milk prevents overweight and obesity among postmenopausal women. Food Nutr Bull. 2010;31(3):381–390. doi: 10.1177/156482651003100301. [DOI] [PubMed] [Google Scholar]
- Anon (2010) USFDA Center for Food Safety and Applied Nutrition http://www.cfsan.fda.gov/list.html. Accessed on 22 December 2011
- Arai S. Studies on functional foods in Japan. Bioscie Biotechnol Biochemi. 1996;60:9–15. doi: 10.1271/bbb.60.9. [DOI] [PubMed] [Google Scholar]
- Arslanoglu S, Moro GE, Ziegler EE. Optimization of human milk fortification for preterm infants: new concepts and recommendations. J Perinat Med. 2010;38(3):233–238. doi: 10.1515/jpm.2010.073. [DOI] [PubMed] [Google Scholar]
- Arslanoglu S, Moro GE, Ziegler EE. Preterm infants fed fortified human milk receive less protein than they need. J Perinatol. 2009;29(7):489–492. doi: 10.1038/jp.2009.50. [DOI] [PubMed] [Google Scholar]
- Asemi Z, Samimi M, Tabasi Z, Talebian P, Azarbad Z, Hydarzadeh Z, Esmaillzadeh A (2011) Effect of daily consumption of probiotic yoghurt on lipid profiles in pregnant women: a randomized controlled clinical trial. J Matern Fetal Neonatal Med 1–5 Ahead of Print. [DOI] [PubMed]
- Atalah SE, Araya BM, Rosselot PG, Araya LH, Vera AG, Andreu RR, Barba GC, Rodriguez L. Consumption of a DHA-enriched milk drink by pregnant and lactating women, on the fatty acid composition of red blood cells, breast milk, and in the newborn] Arch Latinoam Nutr. 2009;59(3):271–277. [PubMed] [Google Scholar]
- Axten LG, Wohlers MW, Wegrzyn T. Using phytochemicals to enhance health benefits of milk: impact of polyphenols on flavor profile. J Food Sci. 2008;73(6):H122–H126. doi: 10.1111/j.1750-3841.2008.00808.x. [DOI] [PubMed] [Google Scholar]
- Beale AM, Fasulo DA, Craigmill AL. Effects of oral and parenteral selenium supplements on residues in meat, milk and eggs. Rev Environ Contam Toxicol. 1990;115:125–150. doi: 10.1007/978-1-4612-3416-6_4. [DOI] [PubMed] [Google Scholar]
- Bennett DC, Cheng KM. Selenium enrichment of table eggs. Poult Sci. 2010;89(10):2166–2172. doi: 10.3382/ps.2009-00571. [DOI] [PubMed] [Google Scholar]
- Bonjour JP, Benoit V, Pourchaire O, Ferry M, Rousseau B, Souberbielle JC. Inhibition of markers of bone resorption by consumption of vitamin D and calcium-fortified soft plain cheese by institutionalised elderly women. Br J Nutr. 2009;102(7):962–966. doi: 10.1017/S0007114509371743. [DOI] [PubMed] [Google Scholar]
- Bourre JM, Galea F. An important source of omega-3 fatty acids, vitamins D and E, carotenoids, iodine and selenium: a new natural multi-enriched egg. J Nutr Health Aging. 2006;10(5):371–376. [PubMed] [Google Scholar]
- Brophy B, Smolenski G, Wheeler T, Wells D, L’Huillier P, Laible G. Cloned transgenic cattle produce milk with higher levels of beta-casein and kappa-casein. Nat Biotechnol. 2003;21(2):157–162. doi: 10.1038/nbt783. [DOI] [PubMed] [Google Scholar]
- Cantor AH, Scott ML. The effect of selenium in the hen’s diet on egg production, hatchability, performance of progeny and selenium concentrations in eggs. Poultry Sci. 1974;53(5):1870–1880. doi: 10.3382/ps.0531870. [DOI] [PubMed] [Google Scholar]
- Caston L, Leeson S. Research note: Dietary flaxseed and egg composition. Poultry Sci. 1990;69:1617–1620. doi: 10.3382/ps.0691617. [DOI] [Google Scholar]
- Cerrato PL. Using designer margarines to control lipid levels. Registered Nurse. 1999;62(10):67–68. [PubMed] [Google Scholar]
- Chandra Mohan KV, Devaraj H, Prathiba D, Hara Y, Nagini S. Antiproliferative and apoptosis inducing effect of lactoferrin and black tea polyphenol combination on hamster buccal pouch carcinogenesis. Biochim Biophys Acta. 2006;1760(10):1536–1544. doi: 10.1016/j.bbagen.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Chandra Mohan KV, Hara Y, Abraham SK, Nagini S. Comparative evaluation of the chemopreventive efficacy of green and black tea polyphenols in the hamster buccal pouch carcinogenesis model. Clin Biochem. 2005;38(10):879–886. doi: 10.1016/j.clinbiochem.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Chang SK. Biotechnology–updates and new developments. Biomed Environ Sci. 2001;14(1–2):32–39. [PubMed] [Google Scholar]
- Chow J, Klein EY, Laxminarayan R. Cost-effectiveness of “golden mustard” for treating vitamin A deficiency in India. PLoS One. 2010;5(8):e12046. doi: 10.1371/journal.pone.0012046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton PM, Noakes M, Sullivan D, Erichsen N, Ross D, Annison G, Fassoulakis A, Cehun M, Nestel P. Cholesterol-lowering effects of plant sterol esters differ in milk, yoghurt, bread and cereal. Eur J Clin Nutr. 2004;58(3):503–509. doi: 10.1038/sj.ejcn.1601837. [DOI] [PubMed] [Google Scholar]
- Cook; Mark E. (Madison, WI), Aydin; Rahim (Madison, WI), Pariza; Michael W. (Madison, WI). Eggs enriched with conjugated linoleic acid. United States Patent, Appl. No.:09/060, 588
- Corcoran BM, Stanton C, Fitzgerald G, Ross RP. Life under stress: the probiotic stress response and how it may be manipulated. Curr Pharm Des. 2008;14(14):1382–99. doi: 10.2174/138161208784480225. [DOI] [PubMed] [Google Scholar]
- Cramer JM, Teran-Garcia M, Jeffery EH. Enhancing sulforaphane absorption and excretion in healthy men through the combined consumption of fresh broccoli sprouts and a glucoraphanin-rich powder. Br J Nutr. 2011;13:1–6. doi: 10.1017/S0007114511004429. [DOI] [PubMed] [Google Scholar]
- Cruickshank EM. Studies in fat metabolism in fowl. I. Composition of egg fat and depot fat of thr fowl as affected by the ingestion of large amount of different fats. Biochem J. 1934;28:965–977. doi: 10.1042/bj0280965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damianaki A, Bakogeorgou E, Kampa M, Notas G, Hatzoglou A, Panagiotou S, Gemetzi C, Kouroumalis E, Martin PM, Castanas E. Potent inhibitory action of red wine polyphenols on human breast cancer cells. J Cell Biochem. 2000;78(3):429–441. doi: 10.1002/1097-4644(20000901)78:3<429::AID-JCB8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Dennehy C. Omega-3 fatty acids and ginger in maternal health: Pharmacology, Efficacy and Safety. J Midwifery & Women’s Health. 2011;56(6):584–590. doi: 10.1111/j.1542-2011.2011.00120.x. [DOI] [PubMed] [Google Scholar]
- De-Regil LM, Suchdev PS, Vist GE, Walleser S, Peña-Rosas JP. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age. Cochrane Database of Systematic Reviews. 2011;9:CD008959. doi: 10.1002/14651858.CD008959.pub2. [DOI] [PubMed] [Google Scholar]
- Di Natale C, Coclite E, Di Ventura L, Di Fabio S. Fortification of maternal milk for preterm infants. J Matern Fetal Neonatal Med. 2011;1:41–43. doi: 10.3109/14767058.2011.607569. [DOI] [PubMed] [Google Scholar]
- Doucet A, Williams M, Gagnon MC, Sasseville M, Beauregard M. Engineering nutritious proteins: improvement of stability in the designer protein MB-1 via introduction of disulfide bridges. J Agric Food Chem. 2002;50(1):92–98. doi: 10.1021/jf010839d. [DOI] [PubMed] [Google Scholar]
- EFSA (2002) http://www.efsa.europa.eu. Accessed on 22 December 2011
- Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V (2011) Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. doi:10.1016/j.nut.2011.08.013 [DOI] [PubMed]
- Elson CO. From cheese to pharma: a designer probiotic for IBD. Clin Gastroenterol Hepatol. 2006;4(7):836–867. doi: 10.1016/j.cgh.2006.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escurriol V, Cofán M, Serra M, Bulló M, Basora J, Salas-Salvadó J, Corella D, Zazpe I, Martínez-González MA, Ruiz-Gutiérrez V, Estruch R, Ros E. Serum sterol responses to increasing plant sterol intake from natural foods in the Mediterranean diet. Eur J Nutr. 2009;48(6):373–82. doi: 10.1007/s00394-009-0024-z. [DOI] [PubMed] [Google Scholar]
- Eshigina S, Gapparov MM, Mal’tsev GIu, Kulakov SN. Influence of dietary therapy containing sunflower oil fortified with phospholipids on the lipid metabolism in patients with hypertension and obesity. Vopr Pitan. 2007;76(1):58–62. [PubMed] [Google Scholar]
- Estrada JD, Boeneke C, Bechtel P, Sathivel S. Developing a strawberry yogurt fortified with marine fish oil. J Dairy Sci. 2011;94(12):5760–5769. doi: 10.3168/jds.2011-4226. [DOI] [PubMed] [Google Scholar]
- Fabian E, Majchrzak D, Dieminger B, Meyer E, Elmadfa I. Influence of probiotic and conventional yoghurt on the status of vitamins B1, B2 and B6 in young healthy women. Ann Nutr Metab. 2008;52(1):29–36. doi: 10.1159/000114408. [DOI] [PubMed] [Google Scholar]
- Fisinin VI, Papazyan TT, Surai PF. Producing selenium-enriched eggs and meat to improve the selenium status of the general population. Crit Rev Biotechnol. 2009;29(1):18–28. doi: 10.1080/07388550802658030. [DOI] [PubMed] [Google Scholar]
- Flynn A, Moreiras O, Stehle P, Fletcher RJ, Müller DJ, Rolland V. Vitamins and minerals: a model for safe addition to foods. Eur J Nutr. 2003;42(2):118–130. doi: 10.1007/s00394-003-0391-9. [DOI] [PubMed] [Google Scholar]
- Fortier ME, Audet I, Giguère A, Laforest JP, Bilodeau JF, Quesnel H, Matte JJ. Effect of dietary organic and inorganic selenium on antioxidant status, embryo development and reproductive performance in hyperovulatory first-parity gilts. J Anim Sci. 2012;90(1):231–40. doi: 10.2527/jas.2010-3340. [DOI] [PubMed] [Google Scholar]
- Friedrich MJ. Genetically enhanced rice to help fight malnutrition. J Am Med Assoc. 1999;282(16):1508–1509. doi: 10.1001/jama.282.16.1508. [DOI] [PubMed] [Google Scholar]
- FSSAI (2006).Food Safety Satandards Authority of India, New Delhi, India. http://www.fssai.gov.in Accessed on 22 December 2011
- Gagnon MC, Williams M, Doucet A, Beauregard M. Replacement of tyr62 by trp in the designer protein milk bundle-1 results in significant improvement of conformational stability. FEBS Lett. 2000;484(2):144–148. doi: 10.1016/S0014-5793(00)02142-6. [DOI] [PubMed] [Google Scholar]
- Gale CR, Marriott LD, Martyn CN, Limond J, Inskip HM, Godfrey KM, Law CM, Cooper C, West C, Robinson SM. Group for Southampton women’s survey study: Breastfeeding, the use of docosahexaenoic acid-fortified formulas in infancy and neuropsychological function in childhood. Arch Dis Child. 2010;95(3):174–179. doi: 10.1136/adc.2009.165050. [DOI] [PubMed] [Google Scholar]
- Gheita T, Kamel S, Helmy N, El-Laithy N, Monir A. Omega 3 fatty acids in juvenile idiopathic arthritis: effect on cytokines (IL-1 and TNF-α), disease activity and response criteria. Clin Rheumatol. 2012;31(2):363–366. doi: 10.1007/s10067-011-1848-5. [DOI] [PubMed] [Google Scholar]
- Gibson RS, Kafwembe E, Mwanza S, Gosset L, Bailey KB, Mullen A, Baisley K, Filteau S. A micronutrient-fortified food enhances iron and selenium status of Zambian infants but has limited efficacy on zinc. J Nutr. 2011;141(5):935–943. doi: 10.3945/jn.110.135228. [DOI] [PubMed] [Google Scholar]
- Gore C, Custovic A, Tannock GW, Munro K, Kerry G, Johnson K, Peterson C, Morris J, Chaloner C, Murray CS, Woodcock A (2011) Treatment and secondary prevention effects of the probiotics Lactobacillus paracasei or Bifidobacterium lactis on early infant eczema: randomized controlled trial with follow-up until age 3 years. Clin Exp Allergy. doi:10.1111/j.1365-2222.2011.03885.x [DOI] [PubMed]
- Granado-Lorencio F, Herrero-Barbudo C, Olmedilla-Alonso B, Blanco-Navarro I, Pérez-Sacristán B. Lutein bioavailability from lutein ester-fortified fermented milk: in vivo and in vitro study. J Nutr Biochem. 2010;21(2):133–139. doi: 10.1016/j.jnutbio.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Green TJ, Skeaff CM, Rockell JE. Milk fortified with the current adequate intake for vitamin D (5 microg) increases serum 25-hydroxyvitamin D compared to control milk but is not sufficient to prevent a seasonal decline in young women. Asia Pac J Clin Nutr. 2010;19(2):195–199. [PubMed] [Google Scholar]
- Guerra PV, Lima LN, Souza TC, Mazochi V, Penna FJ, Silva AM, Nicoli JR, Guimarães EV. Pediatric functional constipation treatment with Bifidobacterium-containing yogurt: a crossover, double-blind, controlled trial. World J Gastroenterol. 2011;17(34):3916–3921. doi: 10.3748/wjg.v17.i34.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierres VO, Pinheiro CM, Assis RP, Vendramini RC, Pepato MT, Brunetti IL. Curcumin-supplemented yoghurt improves physiological and biochemical markers of experimental diabetes. Br J Nutr. 2011;9:1–9. doi: 10.1017/S0007114511005769. [DOI] [PubMed] [Google Scholar]
- Haider BA, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev. 2006;4:CD004905. doi: 10.1002/14651858.CD004905.pub2. [DOI] [PubMed] [Google Scholar]
- Hakimuddin F, Paliyath G, Meckling K. Treatment of mcf-7 breast cancer cells with a red grape wine polyphenol fraction results in disruption of calcium homeostasis and cell cycle arrest causing selective cytotoxicity. J Agric Food Chem. 2006;54(20):7912–7923. doi: 10.1021/jf060834m. [DOI] [PubMed] [Google Scholar]
- Hakimuddin F, Paliyath G, Meckling K. Selective cytotoxicity of a red grape wine flavonoid fraction against MCF-7 cells. Breast Cancer Res Treat. 2004;85(1):65–79. doi: 10.1023/B:BREA.0000021048.52430.c0. [DOI] [PubMed] [Google Scholar]
- Hao Q, Lu Z, Dong BR, Huang CQ, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database of Systematic Reviews. 2011;9:CD006895. doi: 10.1002/14651858.CD006895.pub2. [DOI] [PubMed] [Google Scholar]
- Hargis PS. Modifying egg cholesterol in the domestic fowl. World Poult Sci J. 1988;44:17–29. doi: 10.1079/WPS19880002. [DOI] [Google Scholar]
- Hargis PS, Van Elswyk ME. Manipulating the fatty acid composition of poultry meat and eggs for the health conscious consumer. World’s Poultry Sci J. 1993;49:251–264. doi: 10.1079/WPS19930023. [DOI] [Google Scholar]
- Health Canada (1998) Health Canada policy paper. http://www.hc-sc.gc.ca. Accessed on 22 December 2011.
- Hernández ER, Jácome MM, Lee RG, Nakano T, Ozimek L, Guzmán IV. High conjugated linoleic acid (CLA) content in milk and dairy products using a dietary supplementation of sunflower seed in cows. Thrombogenic/atherogenic risk issues. Arch Latinoam Nutr. 2007;57(2):173–178. [PubMed] [Google Scholar]
- Hertrampf E, Cortés F, Erickson JD, Cayazzo M, Freire W, Bailey LB, Howson C, Kauwell GP, Pfeiffer C. Consumption of folic acid-fortified bread improves folate status in women of reproductive age in Chile. J Nutr. 2003;133(10):3166–3169. doi: 10.1093/jn/133.10.3166. [DOI] [PubMed] [Google Scholar]
- Hoisington D. Opportunities for nutritionally enhanced maize and wheat varieties to combat protein and micronutrient malnutrition. Food Nutr Bull. 2002;23(4):376–377. doi: 10.1177/156482650202300411. [DOI] [PubMed] [Google Scholar]
- Horie K, Horie N, Abdou AM, Yang JO, Yun SS, Chun HN, Park CK, Kim M, Hatta H. Suppressive effect of functional drinking yogurt containing specific egg yolk immunoglobulin on Helicobacter pylori in humans. J Dairy Sci. 2004;87(12):4073–4079. doi: 10.3168/jds.S0022-0302(04)73549-3. [DOI] [PubMed] [Google Scholar]
- Høst A. Importance of the first meal on the development of cow's milk allergy and intolerance. Allergy Proc. 1991;12(4):227–232. doi: 10.2500/108854191778879287. [DOI] [PubMed] [Google Scholar]
- Hotz C, McClafferty B. From harvest to health: challenges for developing biofortified staple foods and determining their impact on micronutrient status. Food Nutr Bull. 2007;28(2 Suppl):S271–279. doi: 10.1177/15648265070282S206. [DOI] [PubMed] [Google Scholar]
- Houghton LA, Gray AR, Szymlek-Gay EA, Heath AL, Ferguson EL. Vitamin D-fortified milk achieves the targeted serum 25-hydroxyvitamin D concentration without affecting that of parathyroid hormone in New Zealand toddlers. J Nutr. 2011;141(10):1840–1846. doi: 10.3945/jn.111.145052. [DOI] [PubMed] [Google Scholar]
- Hsu FC, Wirtz M, Heppel SC, Bogs J, Krämer U, Khan MS, Bub A, Hell R, Rausch T. Generation of Se-fortified broccoli as functional food: impact of Se fertilization on S metabolism. Plant Cell Environ. 2011;34(2):192–207. doi: 10.1111/j.1365-3040.2010.02235.x. [DOI] [PubMed] [Google Scholar]
- Hu Y, McIntosh GH, Le Leu RK, Young GP. Selenium-enriched milk proteins and selenium yeast affect selenoprotein activity and expression differently in mouse colon. Br J Nutr. 2010;104(1):17–23. doi: 10.1017/S0007114510000309. [DOI] [PubMed] [Google Scholar]
- Hyvönen P, Suojala L, Haaranen J, von Wright A, Pyörälä S. Human and bovine lactoferrins in the milk of recombinant human lactoferrin-transgenic dairy cows during lactation. Biotechnol J. 2006;1(4):410–412. doi: 10.1002/biot.200600016. [DOI] [PubMed] [Google Scholar]
- IFIC (2011) Background on functional foods. http://www.foodinsight.org. Accessed on 22 December 2011
- Thomas PR, Earl R, editors. Food and Nutrition Board. Washington: National Academy Press; 1994. Opportunities in the nutrition and food sciences: Research challenges and the next generation of investigators. [Google Scholar]
- Irvine SL, Hummelen R, Hekmat S. Probiotic yogurt consumption may improve gastrointestinal symptoms, productivity, and nutritional intake of people living with human immunodeficiency virus in Mwanza, Tanzania. Nutr Res. 2011;31(12):875–881. doi: 10.1016/j.nutres.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Jayakanthan K, Shobana Devi R, Regina Mary R, Prabhavathi D, Vidya R, John M, Mahendri NV, Srinivasan P, Ramakrishna BS. Effect of yoghurt containing Bifidobacterium lactis Bb12(R) on faecal excretion of secretory immunoglobulin A and human beta-defensin-2 in healthy adult volunteers. Nutr J. 2011;10:138–141. doi: 10.1186/1475-2891-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston BC, Goldenberg JZ, Vandvik PO, Sun X, Guyatt GH. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2011;9:CD004827. doi: 10.1002/14651858.CD004827.pub3. [DOI] [PubMed] [Google Scholar]
- Jiang YH, McGeachin RB, Bailey CA. a-tocopherol, ß-carotene and retinol enrichment of chicken eggs. Poultry Sci. 1994;73:1137–1143. doi: 10.3382/ps.0731137. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Ahn DU, Sim JS. Effect of feeding flaxseed and two types of sunflower seed on fatty acid composition of yolk lipid classes. Poultry Sci. 1991;70:2467–2475. doi: 10.3382/ps.0702467. [DOI] [PubMed] [Google Scholar]
- Jones PJ, MacDougall DE, Ntanios F, Vanstone CA. Dietary phytosterols as cholesterol-lowering agents in humans. Can J Physiol Pharmacol. 1997;75(3):217–227. doi: 10.1139/y97-011. [DOI] [PubMed] [Google Scholar]
- Johnston KE, Tamura T. Folate content in commercial white and whole wheat sandwich breads. J Agric Food Chem. 2004;52(20):6338–6340. doi: 10.1021/jf0494736. [DOI] [PubMed] [Google Scholar]
- Kazimierczuk K, Cova L, Ndeboko B, Szczyrk U, Targosz A, Brzozowski T, Sirko A. Genetic immunization of ducks for production of antibodies specific to Helicobacter pylori UreB in egg yolks. Acta Biochimica Polonica. 2005;52(1):261–266. [PubMed] [Google Scholar]
- Kalaivani M, Sabitha R, Kalaiselvan V, Rajasekaran A. Health benefits and clinical impact of major nutrient, Red yeast rice: A review. Food Bioprocess Technol. 2010;3(3):333–339. doi: 10.1007/s11947-009-0197-8. [DOI] [Google Scholar]
- Karatzas CN. Designer milk from transgenic clones. Nat Biotechnol. 2003;21(2):138–139. doi: 10.1038/nbt0203-138. [DOI] [PubMed] [Google Scholar]
- Karatzas CN, Turner JD. Toward altering milk composition by genetic manipulation: current status and challenges. J Dairy Sci. 1997;80(9):2225–2232. doi: 10.3168/jds.S0022-0302(97)76171-X. [DOI] [PubMed] [Google Scholar]
- Kandhro GA, Kazi TG, Sirajuddin KNF, Kazi N, Afridi HI, Baig JA, Shah AQ, Wadhwa SK, Khan S, Arain MB. Effects of selenium supplementation on iodine and thyroid hormone status in a selected population with goitre in Pakistan. Clin Lab. 2011;57(7–8):575–585. [PubMed] [Google Scholar]
- Raes K, Huyghebaert G, De Smet S, Nollet L, Arnouts S, Demeyer D. The deposition of conjugated linoleic acids in eggs of laying hens fed diets varying in fat level and fatty acid profile. J Nutr. 2002;132(2):182–189. doi: 10.1093/jn/132.2.182. [DOI] [PubMed] [Google Scholar]
- Khush GS. The promise of biotechnology in addressing current nutritional problems in developing countries. Food Nutr Bull. 2002;23(4):354–357. doi: 10.1177/156482650202300406. [DOI] [PubMed] [Google Scholar]
- Kim J, Ko Y, Park YK, Kim NI, Ha WK, Cho Y. Dietary effect of lactoferrin-enriched fermented milk on skin surface lipid and clinical improvement of acne vulgaris. Nutrition. 2010;26(9):902–909. doi: 10.1016/j.nut.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Kjeldsen-Kragh J, Lund JA, Riise T, Finnanger B, Haaland K, Finstad R, Mikkelsen K, Førre O. Dietary omega-3 fatty acid supplementation and naproxen treatment in patients with rheumatoid arthritis. J Rheumatol. 1992;19(10):1531–1536. [PubMed] [Google Scholar]
- Kruger MC, Schollum LM, Kuhn-Sherlock B, Hestiantoro A, Wijanto P, Li-Yu J, Agdeppa I, Todd JM, Eastell R. The effect of a fortified milk drink on vitamin D status and bone turnover in post-menopausal women from South East Asia. Bone. 2010;46(3):759–767. doi: 10.1016/j.bone.2009.10.036. [DOI] [PubMed] [Google Scholar]
- Kuntz S, Wenzel U, Daniel H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur J Nutr. 1999;38(3):133–142. doi: 10.1007/s003940050054. [DOI] [PubMed] [Google Scholar]
- Latté KP, Appel KE, Lampen A. Health benefits and possible risks of broccoli - An overview. Food Chem Toxicol. 2011;49(12):3287–3309. doi: 10.1016/j.fct.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Lee YS, Kang EY, Park MN, Choi YY, Jeon JW, Yun SS. Effects of sn-2 palmitic acid-fortified vegetable oil and fructooligosaccharide on calcium metabolism in growing rats fed casein based diet. Nutr Res Pract. 2008;2(1):3–7. doi: 10.4162/nrp.2008.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien TF, Chen SY, Shiau SP, Froman DP, Hu CY. Chromium picolinate reduces laying hen serum and egg yolk cholesterol. The Professional Anim Scientist. 1996;12:77–80. [Google Scholar]
- Lopez-Huertas E. Health effects of oleic acid and long chain omega-3 fatty acids (EPA and DHA) enriched milks. A review of intervention studies. Pharmacol Res. 2010;61(3):200–207. doi: 10.1016/j.phrs.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Lorea Baroja M, Kirjavainen PV, Hekmat S, Reid G. Anti-inflammatory effects of probiotic yogurt in inflammatory bowel disease patients. Clin Exp Immunol. 2007;149(3):470–479. doi: 10.1111/j.1365-2249.2007.03434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugasi A. Foods fortified with phytosterins: Their role in decreasing serum cholesterol level, their European community authorization and requirements for placing them on the market. Orv Hetil. 2009;150(11):483–496. doi: 10.1556/OH.2009.28572. [DOI] [PubMed] [Google Scholar]
- Maggio L, Costa S, Gallini F. Human milk fortifiers in very low birth weight infants. Early Hum Dev. 2009;85(10 Suppl):S59–S61. doi: 10.1016/j.earlhumdev.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Magdalena Franczyk-Z˙ aro’w, Renata B. Kostogrys, Beata Szymczyk, Jacek Jawien, Mariusz Gajda,Tadeusz Cichocki, Leszek Wojnar, Stefan Chlopicki and Paweł M. Pisulewski (2008) Functional effects of eggs, naturally enriched with conjugated linoleic acid,on the blood lipid profile, development of atherosclerosis and composition of atherosclerotic plaque in apolipoprotein E and low-density lipoprotein receptor double-knockout mice (apoE/LDLR2/2) British Journal of Nutrition 99: 49–58 [DOI] [PubMed]
- Malekshahi ZV, Gargari SL, Rasooli I, Ebrahimizadeh W. Treatment of Helicobacter pylori infection in mice with oral administration of egg yolk-driven anti-UreC immunoglobulin. Microb Pathog. 2011;51(5):366–372. doi: 10.1016/j.micpath.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Martins EC, Krebs VL. Effects of the use of fortified raw maternal milk on very low birth weight infants. J Pediatr (Rio J) 2009;85(2):157–162. doi: 10.2223/JPED.1878. [DOI] [PubMed] [Google Scholar]
- Martin-Bautista E, Muñoz-Torres M, Fonolla J, Quesada M, Poyatos A, Lopez-Huertas E. Improvement of bone formation biomarkers after 1-year consumption with milk fortified with eicosapentaenoic acid, docosahexaenoic acid, oleic acid, and selected vitamins. Nutr Res. 2010;30(5):320–326. doi: 10.1016/j.nutres.2010.05.007. [DOI] [PubMed] [Google Scholar]
- McCowen KC, Ling PR, Decker E, Djordjevic D, Roberts RF, Coupland JN, Bistrian BR. A simple method of supplementation of omega-3 polyunsaturated fatty acids: use of fortified yogurt in healthy volunteers. Nutr Clin Pract. 2010;25(6):641–645. doi: 10.1177/0884533610385699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson RA, Hardy G. Clinical and nutritional benefits of cysteine-enriched protein supplements. Curr Opin Clin Nutr Metab Care. 2011;14(6):562–568. doi: 10.1097/MCO.0b013e32834c1780. [DOI] [PubMed] [Google Scholar]
- Meier AH, Wilson JM (1997) Method of altering the contents of eggs. Patent:5665375. http://www.freepatentsonline.com/5665375.html
- Meyer AL, Elmadfa I, Herbacek I, Micksche M. Probiotic, as well as conventional yogurt, can enhance the stimulated production of proinflammatory cytokines. J Hum Nutr Diet. 2007;20(6):590–598. doi: 10.1111/j.1365-277X.2007.00807.x. [DOI] [PubMed] [Google Scholar]
- Mohan KV, Letchoumy PV, Hara Y, Nagini S. Combination chemoprevention of hamster buccal pouch carcinogenesis by bovine milk lactoferrin and black tea polyphenols. Cancer Invest. 2008;26(2):193–201. doi: 10.1080/07357900701511961. [DOI] [PubMed] [Google Scholar]
- Mukhtar H, Ahmad N. Tea polyphenols: prevention of cancer and optimizing health. Am J Clin Nutr. 2000;71(6):1698S–1702S. doi: 10.1093/ajcn/71.6.1698S. [DOI] [PubMed] [Google Scholar]
- Mussner MJ, Parhofer KG, Von Bergmann K, Schwandt P, Broedl U, Otto C. Effects of phytosterol ester-enriched margarine on plasma lipoproteins in mild to moderate hypercholesterolemia are related to basal cholesterol and fat intake. Metabolism. 2002;51(2):189–194. doi: 10.1053/meta.2002.29988. [DOI] [PubMed] [Google Scholar]
- Napier JA, Graham IA. Tailoring plant lipid composition: designer oilseeds come of age. Curr Opin Plant Biol. 2010;13(3):330–337. doi: 10.1016/j.pbi.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Navarro-Alarcon M, Cabrera-Vique C. Selenium in food and the human body: a review. Sci Total Environ. 2008;400(1–3):115–141. doi: 10.1016/j.scitotenv.2008.06.024. [DOI] [PubMed] [Google Scholar]
- International Institute for Population Sciences (IIPS) and ORC Macro (2007): National Family Health Survey-3, 2005–06. India: Volume-I, International Institute of Population Sciences, Mumbai; 2007. [Google Scholar]
- Nikooyeh B, Neyestani TR, Farvid M, Alavi-Majd H, Houshiarrad A, Kalayi A, Shariatzadeh N, Gharavi A, Heravifard S, Tayebinejad N, Salekzamani S, Zahedirad M. Daily consumption of vitamin D− or vitamin D + calcium-fortified yogurt drink improved glycemic control in patients with type 2 diabetes: a randomized clinical trial. Am J Clin Nutr. 2011;93(4):764–771. doi: 10.3945/ajcn.110.007336. [DOI] [PubMed] [Google Scholar]
- Nomura S, Suzuki H, Masaoka T, Kurabayashi K, Ishii H, Kitajima M, Nomoto K, Hibi T. Effect of dietary anti-urease immunoglobulin Y on Helicobacter pylori infection in Mongolian gerbils. Helicobacter. 2005;10(1):43–52. doi: 10.1111/j.1523-5378.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- Odamaki T, Sugahara H, Yonezawa S, Yaeshima T, Iwatsuki K, Tanabe S, Tominaga T, Togashi H, Benno Y, Xiao JZ. Effect of the oral intake of yogurt containing Bifidobacterium longum BB536 on the cell numbers of enterotoxigenic Bacteroides fragilis in microbiota. Anaerobe. 2012;18(1):14–18. doi: 10.1016/j.anaerobe.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Pariza MW. Designer foods: effects on development of cancer. J Natl Cancer Inst Monogr. 1992;12:105–107. [PubMed] [Google Scholar]
- Pathak P, Kapil U, Kapoor SK, Saxena R, Kumar A, Gupta N, Dwivedi SN, Singh R, Singh P. Prevalence of multiple micronutrient deficiencies amongst pregnant women in a rural area of Haryana. Indian J Pediatr. 2004;71(11):1007–1014. doi: 10.1007/BF02828117. [DOI] [PubMed] [Google Scholar]
- Paton AW, Morona R, Paton JC. Designer probiotics for prevention of enteric infections. Nat Rev Microbiol. 2006;4(3):193–200. doi: 10.1038/nrmicro1349. [DOI] [PubMed] [Google Scholar]
- Penny M, Kris-Etherton WilliamS Harris, Appel JL. Fish consumption, fish oil, omega-3 fatty acids and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.CIR.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- Polagruto JA, Wang-Polagruto JF, Braun MM, Lee L, Kwik-Uribe C, Keen CL. Cocoa flavanol-enriched snack bars containing phytosterols effectively lower total and low-density lipoprotein cholesterol levels. J Am Diet Assoc. 2006;106(11):1804–13. doi: 10.1016/j.jada.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Potrykus I. Nutritionally enhanced rice to combat malnutrition disorders of the poor. Nutr Rev. 2003;61(6 Pt 2):S101–104. doi: 10.1301/nr.2003.jun.S101-S104. [DOI] [PubMed] [Google Scholar]
- Prinz C, Hafsi N, Voland P. Helicobacter pylori virulence factors and the host immune response: implications for therapeutic vaccination. Trends Microbiol. 2003;11:134–138. doi: 10.1016/S0966-842X(03)00024-6. [DOI] [PubMed] [Google Scholar]
- Raileanu I, Diosady LL. Vitamin A stability in salt triple fortified with iodine, iron, and vitamin A. Food Nutr Bull. 2006;27(3):252–259. doi: 10.1177/156482650602700308. [DOI] [PubMed] [Google Scholar]
- Rajasekaran A, Kalaivani M. Anti-diabetic activity of aqueous extract of Monascus purpureus fermented rice in high cholesterol diet fed-streptozotocin-induced diabetic rats. Published online in Asian J Scientific Res. 2009;4:180–189. [Google Scholar]
- Rajasekaran A, Kalaivani M. Hypolipidemic and antioxidant activity of aqueous extract of Monascus purpureus fermented Indian rice in high cholesterol diet fed rats. Turk J Med Sci. 2011;41(1):25–32. [Google Scholar]
- Reali A, Greco F, Fanaro S, Atzei A, Puddu M, Moi M, Fanos V. Fortification of maternal milk for very low birth weight (VLBW) pre-term neonates. Early Hum Dev. 2010;86(1):33–36. doi: 10.1016/j.earlhumdev.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards JW, Ganmaa D, Kleinman K, Sumberzul N, Holick MF, Lkhagvasuren T, Dulguun B, Burke A, Frazier AL. Randomized trial of fortified milk and supplements to raise 25-hydroxyvitamin D concentrations in schoolchildren in Mongolia. Am J Clin Nutr. 2011;94(2):578–584. doi: 10.3945/ajcn.110.008771. [DOI] [PubMed] [Google Scholar]
- Riediger ND, Azordegan N, Harris-Janz S, Ma DW, Suh M, Moghadasian MH. Designer oils low in n-6:n-3 fatty acid ratio beneficially modifies cardiovascular risks in mice. Eur J Nutr. 2009;48(5):307–314. doi: 10.1007/s00394-009-0015-0. [DOI] [PubMed] [Google Scholar]
- Riediger ND, Othman R, Fitz E, Pierce GN, Suh M, Moghadasian MH. Low n-6:n-3 fatty acid ratio, with fish- or flaxseed oil, in a high fat diet improves plasma lipids and beneficially alters tissue fatty acid composition in mice. Eur J Nutr. 2008;47(3):153–60. doi: 10.1007/s00394-008-0709-8. [DOI] [PubMed] [Google Scholar]
- Rivera JA, Shamah T, Villalpando S, Monterrubio E. Effectiveness of a large-scale iron-fortified milk distribution program on anemia and iron deficiency in low-income young children in Mexico. Am J Clin Nutr. 2010;91(2):431–439. doi: 10.3945/ajcn.2009.28104. [DOI] [PubMed] [Google Scholar]
- Romeo J, Wärnberg J, García-Mármol E, Rodríguez-Rodríguez M, Diaz LE, Gomez-Martínez S, Cueto B, López-Huertas E, Cepero M, Boza JJ, Fonollá J, Marcos A. Daily consumption of milk enriched with fish oil, oleic acid, minerals and vitamins reduces cell adhesion molecules in healthy children. Nutr Metab Cardiovasc Dis. 2011;21(2):113–120. doi: 10.1016/j.numecd.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Roe IH, Nam SU, Yang MR, Myung NH, Kim JT, Shin JH. The promising effect of egg yolk antibody IgY on the treatment of Helicobacter pylori-associated gastric diseases. Kor J Gastroenterol. 2002;39:260–268. [Google Scholar]
- Roth C, Magnus P, Schjølberg S, Stoltenberg C, Surén P, McKeague IW, Davey Smith G, Reichborn-Kjennerud T, Susser E. Folic acid supplements in pregnancy and severe language delay in children. J Am Med Assoc. 2011;306(14):1566–1573. doi: 10.1001/jama.2011.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roush JK, Dodd CE, Fritsch DA, Allen TA, Jewell DE, Schoenherr WD, Richardson DC, Leventhal PS, Hahn KA. Multicenter veterinary practice assessment of the effects of omega-3 fatty acids on osteoarthritis in dogs. J Am Vet Med Assoc. 2010;236(1):59–66. doi: 10.2460/javma.236.1.59. [DOI] [PubMed] [Google Scholar]
- Ruggiero P, Peppoloni S, Berti D, Rappuoli R, Giudice GD. New strategies for the prevention and treatment of Helicobacter pylori infection. Expert Opin Investig Drugs. 2002;11:1127–1138. doi: 10.1517/13543784.11.8.1127. [DOI] [PubMed] [Google Scholar]
- Sabikhi L. Designer milk. Adv Food Nutr Res. 2007;53:161–198. doi: 10.1016/S1043-4526(07)53005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K, Kimura K, Taniguchi Y, Yoshida Y, Kihira K, Takimoto T, Kawata H, Saifuku K, Ido K, Takemoto T, Ota Y, Tada M, Karita M, Sakaki N, Hoshihara Y. Distribution of inflammation and atrophy in the stomach of Helicobacter pylori-positive and -negative patients with chronic gastritis. Am J Gastroenterol. 1996;91:963–969. [PubMed] [Google Scholar]
- Sautter C, Poletti S, Zhang P, Gruissem W. Biofortification of essential nutritional compounds and trace elements in rice and cassava. Proc Nutr Soc. 2006;65(2):153–159. doi: 10.1079/PNS2006488. [DOI] [PubMed] [Google Scholar]
- Sazawal S, Dhingra U, Dhingra P, Hiremath G, Sarkar A, Dutta A, Menon VP, Black RE. Micronutrient fortified milk improves iron status, anemia and growth among children 1–4 years: a double masked, randomized, controlled trial. PLoS One. 2010;5(8):e12167. doi: 10.1371/journal.pone.0012167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheideler SE, Froning G, Cuppett S. Studies of Consumer Acceptance of High Omega-3 Fatty Acid Enriched Eggs. J Appl Poultry Res. 1997;6:137–146. [Google Scholar]
- Seki S, Hidaka I, Kojima K, Yoshino H, Aoyama T, Okazaki M, Kondo K. Effects of phytosterol ester-enriched vegetable oil on plasma lipoproteins in healthy men. Asia Pac J Clin Nutr. 2003;12(3):282–291. [PubMed] [Google Scholar]
- Semba RD, Moench-Pfanner R, Sun K, de Pee S, Akhter N, Rah JH, Campbell AA, Badham J, Bloem MW, Kraemer K. Iron-fortified milk and noodle consumption is associated with lower risk of anemia among children aged 6–59 mo in Indonesia. Am J Clin Nutr. 2010;92(1):170–176. doi: 10.3945/ajcn.2010.29254. [DOI] [PubMed] [Google Scholar]
- Servili M, Rizzello CG, Taticchi A, Esposto S, Urbani S, Mazzacane F, Di Maio I, Selvaggini R, Gobbetti M, Di Cagno R. Functional milk beverage fortified with phenolic compounds extracted from olive vegetation water, and fermented with functional lactic acid bacteria. Int J Food Microbiol. 2011;147(1):45–52. doi: 10.1016/j.ijfoodmicro.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Seshadri S. Prevalence of micronutrient deficiency particularly of iron, zinc and folic acid in pregnant women in South East Asia. Br J Nutr. 2001;85(Suppl 2):S87–S92. doi: 10.1079/BJN2000299. [DOI] [PubMed] [Google Scholar]
- SFDA State Food and Drug Administration, China. http://www.sfda.com. Accessed on 22 December 2011
- Shab-Bidar S, Neyestani TR, Djazayery A, Eshraghian MR, Houshiarrad A, Gharavi A, Kalayi A, Shariatzadeh N, Zahedirad M, Khalaji N, Haidari H. Regular consumption of vitamin D-fortified yogurt drink (Doogh) improved endothelial biomarkers in subjects with type 2 diabetes: a randomized double-blind clinical trial. BMC Med. 2011;9:125–134. doi: 10.1186/1741-7015-9-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Roe IH, Kim HG. Production of anti-Helicobacter pylori urease-specific immunoglobulin in egg yolk using an antigenic epitope of H. pylori urease. J Med Microbiol. 2004;53(Pt 1):31–34. doi: 10.1099/jmm.0.05327-0. [DOI] [PubMed] [Google Scholar]
- Shin JH, Nam SW, Kim JT, Yoon JB, Bang WG, Roe IH. Identification of immunodominant Helicobacter pylori proteins with reactivity to H. pylori-specific egg-yolk immunoglobulin. J Med Microbiol. 2003;52(Pt 3):217–222. doi: 10.1099/jmm.0.04978-0. [DOI] [PubMed] [Google Scholar]
- Sim JS. Designer eggs and their nutritional and functional significance. World Rev Nutr Diet. 1998;83:89–101. doi: 10.1159/000059655. [DOI] [PubMed] [Google Scholar]
- Sim SJ, Sunwoo HH (2002) Designer eggs: Nutritional and functional significance. In : Watson RR (ed) Egg and health promotion, 1st edn.Wiley-Blackwell, pp19–35.
- Simopoulos AP. Human requirement for n-3 polyunsaturated fatty acids. Poult Sci. 2000;79:961–970. doi: 10.1093/ps/79.7.961. [DOI] [PubMed] [Google Scholar]
- Sittig LJ, Herzing LB, Xie H, Batra KK, Shukla PK, Redei EE (2011) Excess folate during adolescence suppresses thyroid function with permanent deficits in motivation and spatial memory. Genes Brain Behav Nov 3. doi:10.1111/j.1601-183X.2011.00749.x [DOI] [PMC free article] [PubMed]
- Sleator RD. Probiotics—a viable therapeutic alternative for enteric infections especially in the developing world. Discov Med. 2010;10(51):119–124. [PubMed] [Google Scholar]
- Sleator RD, Hill C. New frontiers in probiotic research. Lett Appl Microbiol. 2008;46(2):143–147. doi: 10.1111/j.1472-765X.2007.02293.x. [DOI] [PubMed] [Google Scholar]
- Sookwong P, Nakagawa K, Nakajima S, Amano Y, Toyomizu M, Miyazawa T. Tocotrienol content in hen eggs: its fortification by supplementing the feed with rice bran scum oil. Biosci Biotechnol Biochem. 2008;72(11):3044–3047. doi: 10.1271/bbb.80432. [DOI] [PubMed] [Google Scholar]
- Stuetz W, Carrara VI, McGready R, Lee SJ, Erhardt JG, Breuer J, Biesalski HK, Nosten FH (2011) Micronutrient status in lactating mothers before and after introduction of fortified flour: cross-sectional surveys in Maela refugee camp. Eur J Nutr. doi:10.1007/s00394-011-0226-z [DOI] [PMC free article] [PubMed]
- Suárez M, Romero MP, Motilva MJ. Development of a phenol-enriched olive oil with phenolic compounds from olive cake. J Agric Food Chem. 2010;58(19):10396–10403. doi: 10.1021/jf102203x. [DOI] [PubMed] [Google Scholar]
- Sunawang UB, Hidayat A, Kusharisupeni S. Preventing low birth weight through maternal multiple micronutrient supplementation: a cluster-randomized, controlled trial in Indramayu, West Java. Food Nutr Bull. 2009;30(4 Suppl):S488–S495. doi: 10.1177/15648265090304S403. [DOI] [PubMed] [Google Scholar]
- Surai PF, Sparks NHC. Designer eggs: from improvement of egg composition to functional food. Trends Food Sci Technol. 2001;12(1):7–16. doi: 10.1016/S0924-2244(01)00048-6. [DOI] [Google Scholar]
- Surai PF, MacPherson A, Speake BK, Sparks NH. Designer egg evaluation in a controlled trial. Eur J Clin Nutr. 2000;54(4):298–305. doi: 10.1038/sj.ejcn.1600939. [DOI] [PubMed] [Google Scholar]
- Trabulsi J, Capeding R, Lebumfacil J, Ramanujam K, Feng P, McSweeney S, Harris B, DeRusso P. Effect of an α-lactalbumin-enriched infant formula with lower protein on growth. Eur J Clin Nutr. 2011;65(2):167–174. doi: 10.1038/ejcn.2010.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KC, Fang TJ, Chiang S-S, Liu C-F, Wu C-L, Pan T-M (2012) Immunomodulatory activities and antioxidant properties of polysaccharides from Monascus-fermented products in vitro. J Sci Food Agric 92(7):1483–1489 [DOI] [PubMed]
- UNICEF (2005) Micronutrients - Iodine, Iron and Vitamin A. http://www.unicef.org/nutrition/index_iodine.html. Accessed on 22 December 2011
- Van der Hee RM, Miret S, Slettenaar M, Duchateau GS, Rietveld AG, Wilkinson JE, Quail PJ, Berry MJ, Dainty JR, Teucher B, Fairweather-Tait SJ. Calcium absorption from fortified ice cream formulations compared with calcium absorption from milk. J Am Diet Assoc. 2009;109(5):830–835. doi: 10.1016/j.jada.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KH, Blauensteiner D, Schmid I, Elmadfa I. The role of fortified foods–situation in Austria. Forum Nutr. 2005;57:84–90. doi: 10.1159/000083771. [DOI] [PubMed] [Google Scholar]
- Weisburger JH, Rivenson A, Aliaga C, Reinhardt J, Kelloff GJ, Boone CW, Steele VE, Balentine DA, Pittman B, Zang E. Effect of tea extracts, polyphenols, and epigallocatechin gallate on azoxymethane-induced colon cancer. Proc Soc Exp Biol Med. 1998;217(1):104–108. doi: 10.3181/00379727-217-44211. [DOI] [PubMed] [Google Scholar]
- Wu TY, Tsai CC, Hwang YT, Chiu TH (2011) Effect of antioxidant activity and functional properties of chingshey purple sweet potato fermented milk by Lactobacillus acidophilus, L. delbrueckii subsp. lactis, and L. gasseri Strains. J Food Sci Dec 19. doi:10.1111/j.1750-3841.2011.02507.x [DOI] [PubMed]
- Xu J, Zhou X, Deng Q, Huang Q, Yang J, Huang F. Rapeseed oil fortified with micronutrients reduces atherosclerosis risk factors in rats fed a high-fat diet. Lipids Health Dis. 2011;13(10):96. doi: 10.1186/1476-511X-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Kerr PS. Genetically engineered crops: their potential use for improvement of human nutrition. Nutr Rev. 2002;60(5 Pt 1):135–141. doi: 10.1301/00296640260093797. [DOI] [PubMed] [Google Scholar]
- Yeom G, Yun DM, Kang YW, Kwon JS, Kang IO, Kim SY. Clinical efficacy of facial masks containing yoghurt and Opuntia humifusa Raf. (F-YOP) J Cosmet Sci. 2011;62(5):505–514. [PubMed] [Google Scholar]
- Zachariassen G, Faerk J, Grytter C, Esberg BH, Hjelmborg J, Mortensen S, Thybo Christesen H, Halken S. Nutrient enrichment of mother’s milk and growth of very preterm infants after hospital discharge. Pediatrics. 2011;127(4):e995–e1003. doi: 10.1542/peds.2010-0723. [DOI] [PubMed] [Google Scholar]