Abstract
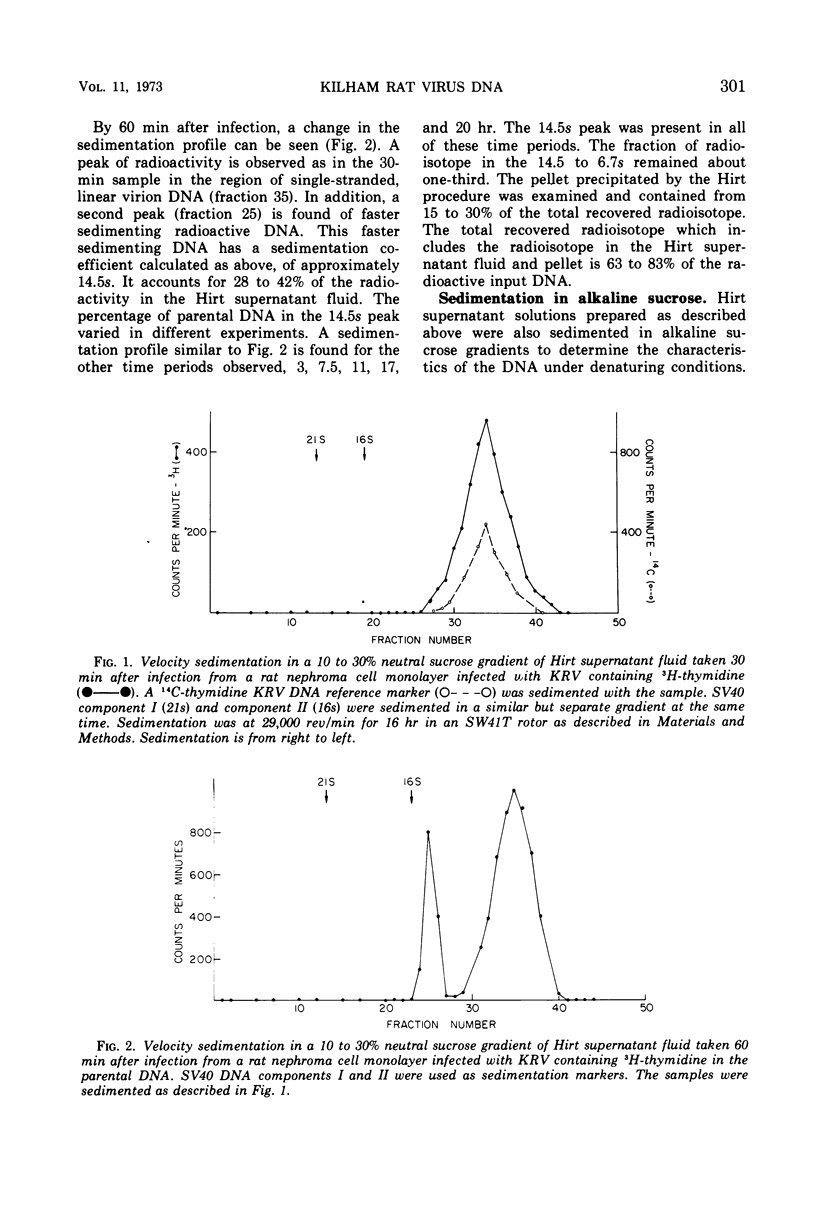
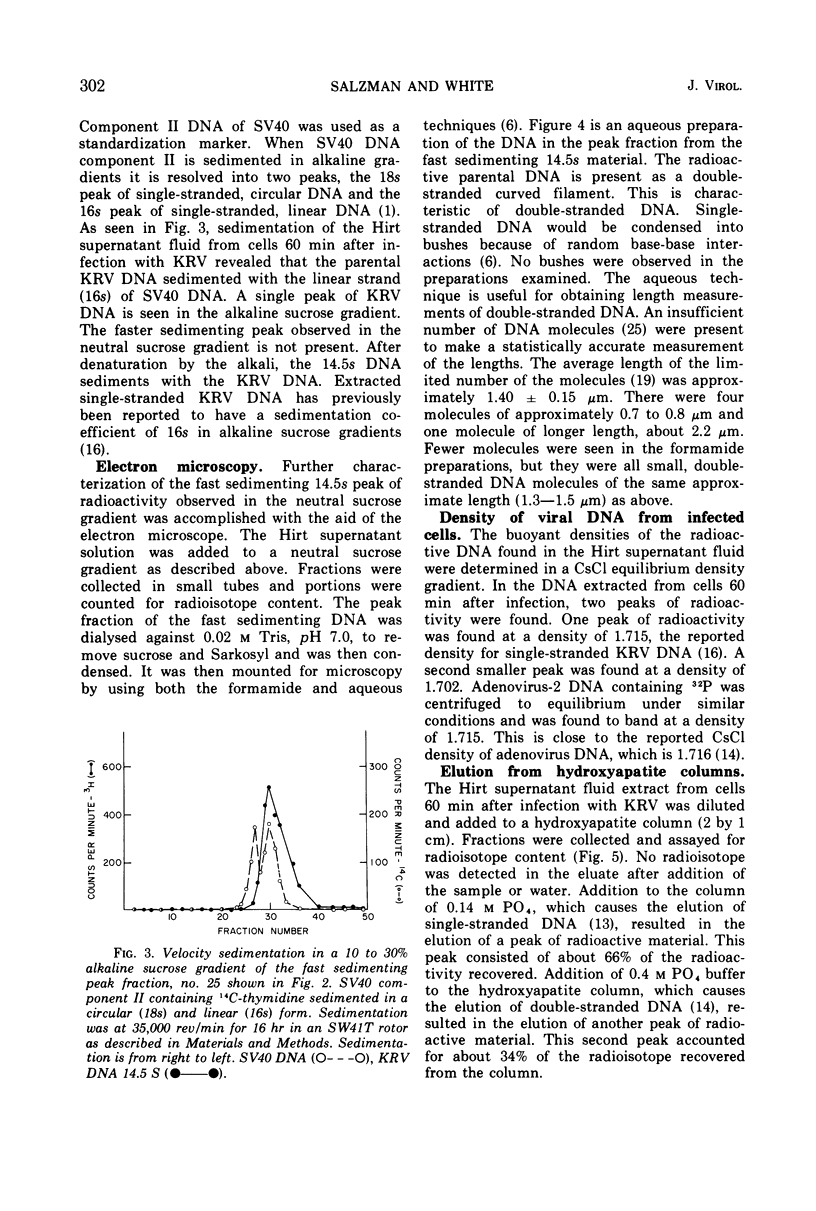
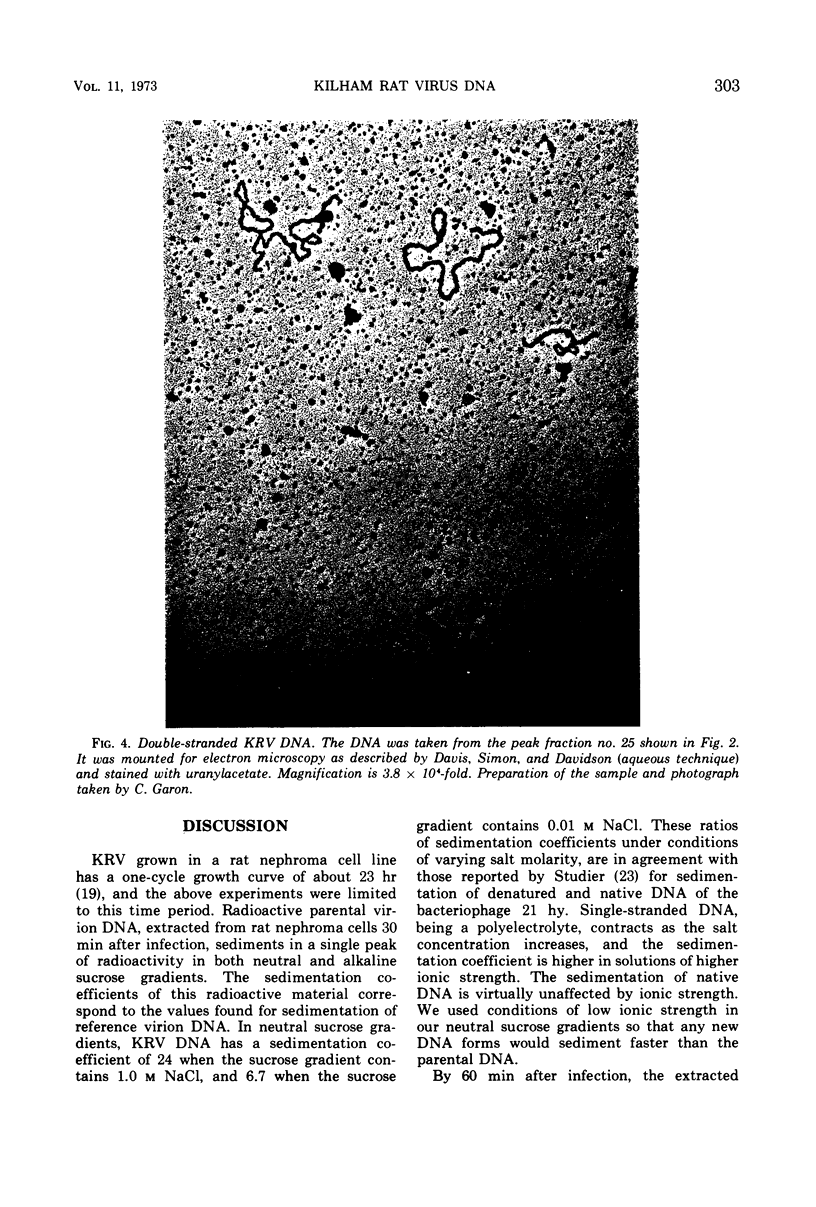
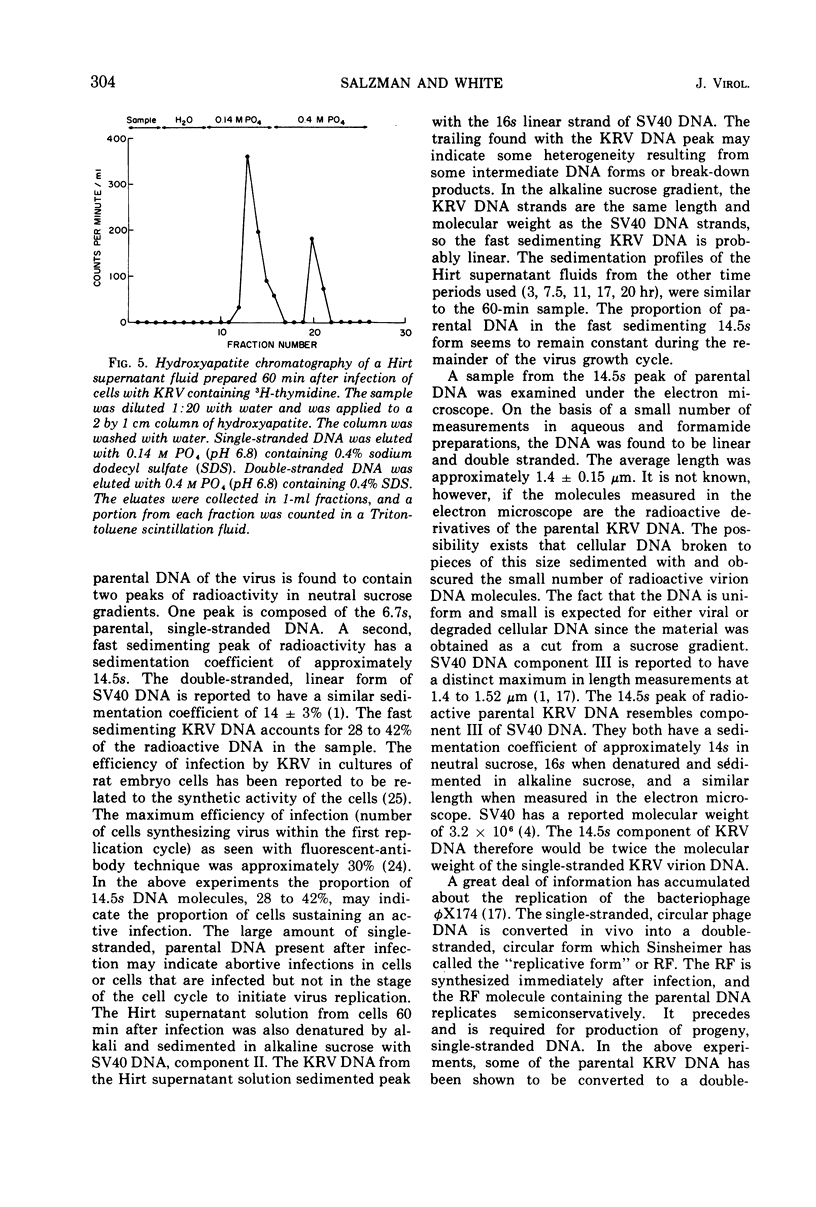
Kilham rat virus (KRV) contains linear, single-stranded DNA in the virion. The fate of radioactive viral DNA was followed after infection of monolayer cells. Within 60 min after infection of cells, 28 to 42% of the parental viral DNA is converted to a new form. This new DNA form is believed to be double stranded and linear on the basis of its sedimentation in neutral and alkaline sucrose gradients, elution from hydroxyapatite columns, its buoyant density in equilibrium CsCl density gradients, and appearance in the electron microscope. The double-stranded linear KRV DNA may be analogous to the replicative form of certain bacteriophages, including φX174, which contain single-stranded circular genomes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderer F. A., Schlumberger H. D., Koch M. A., Frank H., Eggers H. J. Structure of simian virus 40. II. Symmetry and components of the virus particle. Virology. 1967 Jul;32(3):511–523. doi: 10.1016/0042-6822(67)90303-0. [DOI] [PubMed] [Google Scholar]
- Archetti I., Bereczky E., Bocciarelli D. S. A small virus associated with the simian adenovirus SV11. Virology. 1966 Aug;29(4):671–675. doi: 10.1016/0042-6822(66)90291-1. [DOI] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAWFORD L. V., BLACK P. H. THE NUCLEIC ACID OF SIMIAN VIRUS 40. Virology. 1964 Nov;24:388–392. doi: 10.1016/0042-6822(64)90176-x. [DOI] [PubMed] [Google Scholar]
- Crawford L. V. A minute virus of mice. Virology. 1966 Aug;29(4):605–612. doi: 10.1016/0042-6822(66)90284-4. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- KILHAM L., FERM V. H. RAT VIRUS (RV) INFECTIONS OF PREGNANT, FETAL AND NEWBORN RATS. Proc Soc Exp Biol Med. 1964 Dec;117:874–879. doi: 10.3181/00379727-117-29723. [DOI] [PubMed] [Google Scholar]
- KILHAM L. Mongolism associated with rat virus (RV) infection in hamsters. Virology. 1961 Jan;13:141–143. doi: 10.1016/0042-6822(61)90043-5. [DOI] [PubMed] [Google Scholar]
- KILHAM L., OLIVIER L. J. A latent virus of rats isolated in tissue culture. Virology. 1959 Apr;7(4):428–437. doi: 10.1016/0042-6822(59)90071-6. [DOI] [PubMed] [Google Scholar]
- MIYAZAWA Y., THOMAS C. A., Jr NUCLEOTIDE COMPOSITION OF SHORT SEGMENTS OF DNA MOLECULES. J Mol Biol. 1965 Feb;11:223–237. doi: 10.1016/s0022-2836(65)80053-5. [DOI] [PubMed] [Google Scholar]
- Matsuo Y., Spencer H. J. Studies on the infectivity of rat virus (RV) in BALB-c mice. Proc Soc Exp Biol Med. 1969 Jan;130(1):294–299. doi: 10.3181/00379727-130-33541. [DOI] [PubMed] [Google Scholar]
- Mayor H. D., Jamison R. M., Jordan L. E., Melnick J. L. Structure and Composition of a Small Particle Prepared from a Simian Adenovirus. J Bacteriol. 1965 Jul;90(1):235–242. doi: 10.1128/jb.90.1.235-242.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piña M., Green M. Biochemical studies on adenovirus multiplication. IX. Chemical and base composition analysis of 28 human adenoviruses. Proc Natl Acad Sci U S A. 1965 Aug;54(2):547–551. doi: 10.1073/pnas.54.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Koczot F. Adenovirus-associated virus multiplication. VI. Base compostion of the deoxyribonucleic acid strand species and strand-specific in vivo transcription. J Virol. 1971 Nov;8(5):771–777. doi: 10.1128/jvi.8.5.771-777.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Salzman L. A., Jori L. A. Characterization of the Kilham rat virus. J Virol. 1970 Feb;5(2):114–122. doi: 10.1128/jvi.5.2.114-122.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman L. A., White W. L., Kakefuda T. Linear, single-stranded deoxyribonucleic acid isolated from Kilham rat virus. J Virol. 1971 Jun;7(6):830–835. doi: 10.1128/jvi.7.6.830-835.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman L. A., White W. L., McKerlie L. Growth characteristics of Kilham rat virus and its effect on cellular cellular macromolecular synthesis. J Virol. 1972 Oct;10(4):573–577. doi: 10.1128/jvi.10.4.573-577.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman L. A., White W. L. Structural proteins of Kilham rat virus. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1551–1556. doi: 10.1016/0006-291x(70)90564-4. [DOI] [PubMed] [Google Scholar]
- Sebring E. D., Kelly T. J., Jr, Thoren M. M., Salzman N. P. Structure of replicating simian virus 40 deoxyribonucleic acid molecules. J Virol. 1971 Oct;8(4):478–490. doi: 10.1128/jvi.8.4.478-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinsheimer R. L., Knippers R., Komano T. Stages in the replication of bacteriophage phi X174 DNA in vivo. Cold Spring Harb Symp Quant Biol. 1968;33:443–447. doi: 10.1101/sqb.1968.033.01.051. [DOI] [PubMed] [Google Scholar]
- TOOLAN H. W. Experimental production of mongoloid hamsters. Science. 1960 May 13;131(3411):1446–1448. doi: 10.1126/science.131.3411.1446. [DOI] [PubMed] [Google Scholar]
- Tennant R. W. Inhibition of mitosis and macromolecular synthesis in rat embryo cells by Kilham rat virus. J Virol. 1971 Oct;8(4):402–408. doi: 10.1128/jvi.8.4.402-408.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]




