Abstract
Black mulberry juice was concentrated by different heating methods, including conventional heating and microwave heating, at different operational pressures (7.3, 38.5 and 100 kPa). The effects of each method on evaporation rate, quality attributes of concentrated juice were investigated. The final juice concentration of 42° Brix was achieved in 140, 120, and 95 min at 100, 38.5, and 7.3 kPa respectively by using a rotary evaporator. Applying microwave energy decreased required times to 115, 95, and 60 min. The changes in color, anthocyanin content during the concentration processes were investigated. Hunter parameters (L, a, and b) were measured to estimate the intensity of color loss. All Hunter color parameters decreased with time. Results showed that the degradation of color and consequently anthocyanins, was more pronounced in rotary evaporation compared to microwave heating method.
Keywords: Microwave heating, Rotary evaporation, Evaporation rate, Total Anthocyanin Content, Browning index
Introduction
The mulberry belongs to the genus Morus of the family Moraceae. There are 24 species of Morus and one subspecies, with at least 100 known varieties. Mulberry is found from the temperate to subtropical regions of the Northern hemisphere, through to the tropics of the Southern hemisphere, and can grow in a wide range of climatic, topographical, and soil conditions. These conditions are widely spread throughout all regions from the tropics to the sub-arctic and from sea level to altitudes as high as 4,000 m. There are three main species of mulberry for fruit production: black (Morus nigra), red (Morus rubra), and white (Morus alba) (Ercisli and Orhan 2007).
The black mulberry, which originates in Iran, was introduced to Britain more than 500 years ago and cultivated as food for silkworms. Although this venture did not prove successful, its fruit is still eaten in various forms today. It is cultivated for its fruits in southern Europe and southwest Asia, and is the most important species in the Mediterranean countries (Ercisli and Orhan 2008). The total production of black mulberry in Iran was about 160,000 tons in 2008, the majority of which was converted to juice and juice concentrate (Anonymous 2008).
Black mulberry (Morus nigra) is a fruit known not only for its nutritional qualities and its flavor, but also for its traditional use in natural medicine, as it has a high content of active therapeutic compounds. For example, the fruit has been used for the treatment of mouth, tongue, and throat inflammations (Martin et al. 2003). M. nigra fruit is a good source of several phytonutrients and contains high amounts of total phenolics, total flavonoids, and ascorbic acid. Also, the fruit has a pleasant taste with a slightly acidic flavor and an attractive dark red color (Koyuncu 2004; Ozgen et al. 2009a, b).
The fruits can be eaten raw, dried, or processed. Black mulberry is mostly used for making processed food such as pekmez, marmalades, juices, liquors, natural dyes, and frozen fruits for ice cream. Mulberries have a brief harvest season of about two months, after which fresh mulberries can only be stored refrigerated for a maximum of six weeks. Therefore further processing is desirable to extend shelf- life. As reported by several authors, thermal processing is one of the most important methods of food preservation; it is primarily intended to inactivate enzymes and deteriorative microorganisms, and to reduce water activity by dehydration (Maskan et al. 2002).
The concentration of fruit juices requires the part-removal of water without changes in the composition of solids, leaving all the original solid components, such as fruit sugars, minerals, and vitamins, to the more concentrated solution. Concentration of fruit juices, a major unit operation in the fruit-processing industry, is of critical importance as it determines the quality of the final product such as flavor, color, aroma, appearance and mouth feel (Maskan 2006).
Color kinetics is the most important aspect of the successful processing of mulberry fruit, color is usually the first property the consumer observes. Color deterioration reactions such as non-enzymatic browning reactions, pigment destruction, and polymerization could change the visual properties of concentrated fruit juices during thermal processing (Maskan 2006). Color is an important sensory property that plays a role in determining product quality; therefore, minimizing pigment losses during processing is of primary concern to the processor (Bridle and Timberlake 1997).
Mulberry fruits contain one main class of non-nutrient active constituents: the anthocyanins. The major compounds identified were cyanidin-3-glucoside and cyanidin-3-rutinoside, which contributes the red pigment that gives the fruit a red to purple color (Butkhup and Samappito 2008). Mulberry fruits contain reducing sugars and amino acids that enhance nonenzymatic browning during thermal processing (Suh et al. 2003).
During recent years, microwave heating and other emerging technologies have received considerable attention due to the consumer interest in minimally processed products.
Microwave heating can be compared with the other techniques used to concentrate fruit juices such as, membrane concentration and conventional heating.
Although membrane concentration can be used to remove water without heating, it is not useful as a standard concentration method for fruit juice products due to higher cost and limitations in obtaining high solid contents.
Compared to conventional heating process, microwave treatment has well-known advantages, particularly because foods are heated directly and rapidly without contact with hot surfaces. Another advantage of using microwave treatment is the shorter heat-up time, which makes it more energy-efficient than conventional heating, and allows a greatly increased heating rate while largely maintaining the nutritional value, color, and original flavor.
The present study was carried out to investigate the effect of microwave heating of black mulberry juice on quality parameters and to compare it with a conventional heating system under similar processing conditions.
Materials and methods
Sample preparation
Fruits of black mulberry (Morus nigra) at a commercially mature stage were purchased from a local market (Karaj, Iran). The fruit was homogeneously and carefully selected in terms of shape and ripeness, and then pulped in a blender. A sieve was used to eliminate the seeds, and the extract was then pressed softly in order to increase the yield. Finally, fresh juice was clarified using a spiral ultrafiltration system with a molecular weight cutoff equal to 40 KD (Osmonic, USA). This step was also necessary to avoid a heat load, and is commonly used for sterilization and native enzyme inactivation. The cold, sterile, single-strength clarified juice with 16% of total soluble solid (TSS) was rapidly cooled and frozen at −25 °C to use for further experiments.
Juice concentration
Clarified juice was concentrated using two different thermal methods from an initial concentration of 16° Brix to a final concentration of 42° Brix as follows:
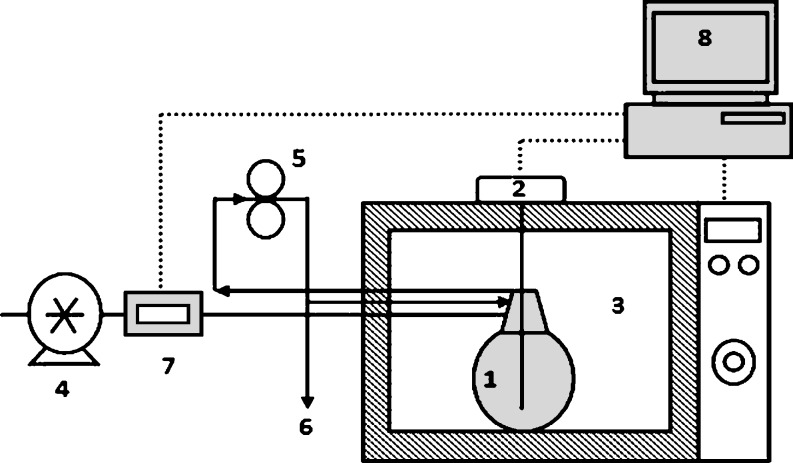
Microwave heating: a programmable domestic microwave oven (Butane MR-1, Iran, with a maximum output of 900 W at 2,450 MHz) was modified for microwave evaporation. A hermetic jar (600 mL) of juice sample was placed at the center of the microwave, which was connected to a vacuum pump (Robin-air, USA), and microwave energy was applied. Some undesirable results such as foaming and sample charring were observed with power above 300 W, and increasing the length of concentration time was observed by heating below 300 W power level, so the study was carried out at 300 W (Fig. 1).
Fig. 1.
Schematic diagram of the microwave evaporator unit, (1) Airtight jar; (2) Thermometer; (3) Microwave heating chamber; (4) Vacuum pump; (5) Recirculation pump; (6) Sampling point; (7) Pressure controller; (8) PC with data acquisition card
Operational parameters such as microwave power and pressure were controlled using a PC, and the temperature was recorded periodically. Samples were taken periodically and replaced after measurements were taken. Microwave heating was conducted at three operational pressures: 100 (atmospheric), 38.5, and 7.3 kPa, and their effects were investigated.
Conventional heating: A rotary vacuum evaporator (Heidolph, Heizbad HB Contr, Germany) was used for the 38.5, and 7.3 kPa pressures, and atmospheric evaporation. Soybean oil was used as operative liquid, (because of its high boiling temperature, 120 °C). Sampling was carried out by the same method as for microwave heating, and data were recorded during concentration without any interruption in the process.
Analytical methods
Total soluble solid (TSS) content measurement
During concentration processes, the soluble-solids content of the juice samples was measured by an Abbe refractometer (Atago Rx-7000a, Tokyo, Japan) at 20 °C and expressed in ºBrix.
The concentration rate constant (k) was calculated by the following equation:
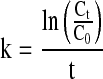 |
1 |
where C0 is the initial soluble solid content and Ct is the soluble solid content after t minutes of concentrating at a given pressure (or temperature).
Total anthocyanins content (TAC)
Total anthocyanins content of black mulberry juice was determined by the pH differential method using two buffer systems: potassium chloride buffer, pH 1.0 (0.025 M), and sodium acetate buffer, pH 4.5 (0.4 M) (Lako et al. 2007; Cam et al. 2009). Briefly, 0.4 mL of black mulberry juice sample was mixed with 3.6 mL of corresponding buffers and read against water as a blank at 510 and 700 nm. Absorbance (A) was calculated as:
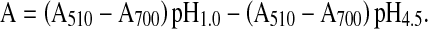 |
2 |
The total anthocyanin content of the samples (mg cyanidin-3-glucoside/ 100 mL of mulberry juice) was calculated by following equation:
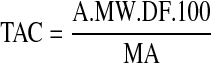 |
3 |
Where A is absorbance; MW is molecular weight (449.2); DF is the dilution factor (10); and MA is the molar absorptivity of cyanidin-3-glucoside (26,900).
The visible spectra of samples were determined by scanning the absorbance between 350 and 700 nm using a Cecil CE 2502 UV–vis. spectrophotometer (Cecil Ins., England). Quartz cuvettes with a 1 cm path length were used, and all measurements were carried out at room temperature (~22 °C). Absorbance readings were made against distilled water as a blank.
Color measurement
Sample color was measured before concentrating and at pre-specified time intervals using a HunterLab Colorflex (A-60-1010-615 Model Colorimeter, HunterLab, Reston, VA). The instrument was standardized each time with a black and a white (L = 91.10, a = −1.12, b = 1.26) tile. The difference between the color parameters of concentrated and fresh juice was calculated. At least five measurements were performed for each sample, and the measured values (mean values) were calculated. The L, a, and b values of the measurements are reported, where L represents the light–dark spectrum with a range from 0 (black) to 100 (white); a represents the green–red spectrum with a range from−60 (green) to +60 (red) (in other words, a measures redness when positive and greenness when negative); and b represents the blue–yellow spectrum with a range from−60 (blue) to +60 (yellow) (in other words, b measures yellowness when positive and blueness when negative).
The three measured color parameters were reported as the browning index (BI). BI represents the purity of the brown color and is considered an important parameter associated with browning (Diamante et al. 2010).
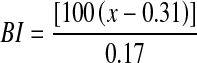 |
4 |
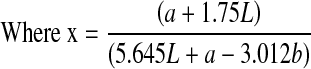 |
5 |
Statistical analysis
All experiments were conducted in triplicate, and an analysis of variance (ANOVA) was performed. The least significant difference (LSD) at p < 0.05 was calculated using Duncan’s Multiple Range Test to determine the significant differences in results using Minitab software (Minitab 15; Minitab Inc., Minneapolis, USA).
Results and discussion
Effect of operational pressure on concentration rate
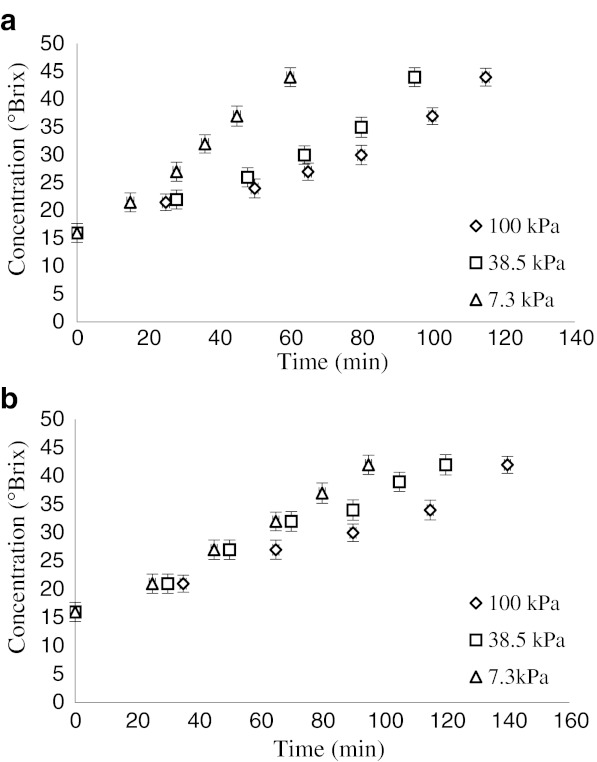
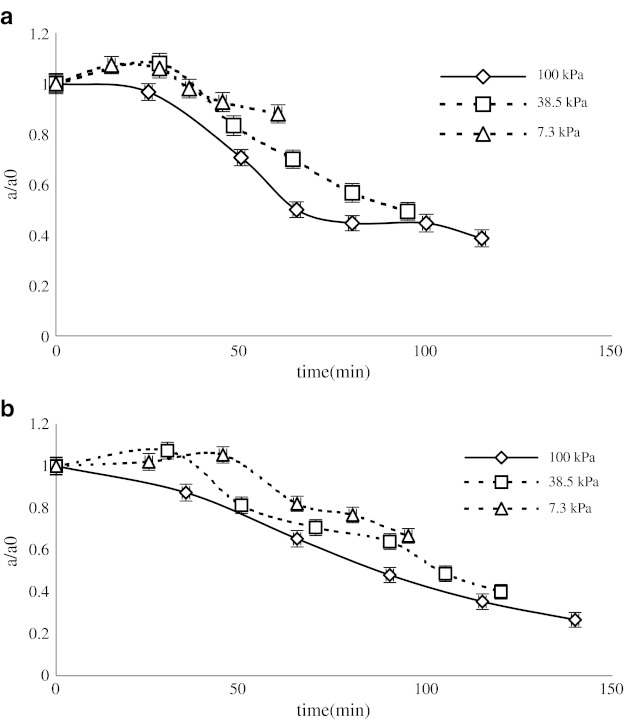
Figure 2 (a, b) illustrates the total soluble solid (TSS) concentrations of black mulberry juice versus time using two methods under different operational pressures. These results show that the elapsed time to reach the final concentration (42° Brix) is shorter for the samples processed at lower operational pressures.
Fig. 2.
Changes in juice concentration during processing by microwave a and rotary b heating methods at different pressures (7.3, 38.5 and 100 kPa)
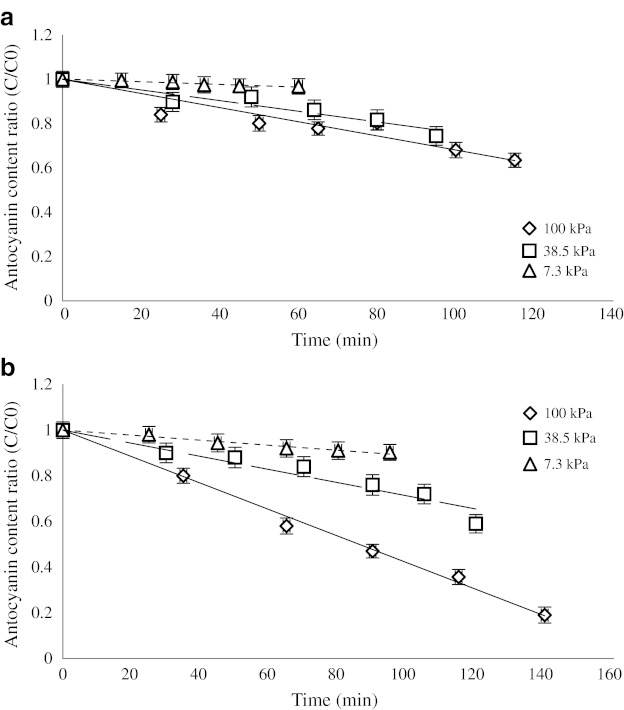
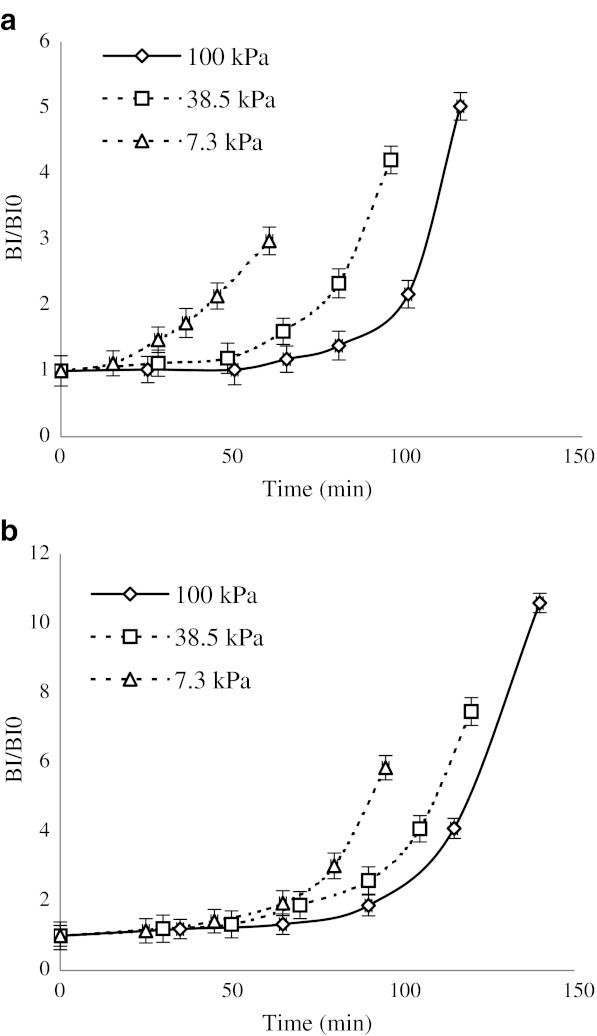
The changes in the soluble solid concentration of the samples followed a first-order reaction model (Fig. 3); Table 1 shows the concentration rate constant (k).
Fig. 3.
Degradation kinetics of the anthocyanins from the mulberry juice concentrated by microwave (a) and rotary (b) heating
Table 1.
Rate constant of thermal concentration of black mulberry juice calculated by eq. 1
| Heating method | Pressure (kPa) | Rate constant (min−1) | R2 |
|---|---|---|---|
| Rotary | 7.3 | 0.010 ± 0.0005 | 0.994 |
| 38.5 | 0.008 ± 0.0004 | 0.969 | |
| 100 | 0.006 ± 0.0003 | 0.986 | |
| Microwave | 7.3 | 0.018 ± 0.0006 | 0.987 |
| 38.5 | 0.010 ± 0.0004 | 0.993 | |
| 100 | 0.008 ± 0.0005 | 0.983 |
The desired level of concentration was obtained using a rotary evaporator after 140, 120, and 95 min at 100, 38.5, and 7.3 kPa respectively. This result shows that the operational time can be reduced depending on pressure. In the case of microwave heating, the required times were 115, 95, and 60 min respectively for the three operational pressures. Similar results were found in the literature (Maskan 2006).
The larger elapsed time and the smaller effect of pressure in the case of conventional heating could be related to the common rotary evaporator procedure used here. The difference between the boiling point of processed samples and the operative liquid could not be more than 20 °C; this would induce significance differences in heat transfer at different temperatures.
An increase in the temperature of the juice samples was observed, during evaporation. It can be explained by an increase in the soluble solid concentration, which increases the boiling point of liquids. However, the boiling point depends on the heating method, as the boiling points of microwave-heated products are evidently higher than conventionally heated ones. This is due to the superheating phenomenon that takes place during microwave heating of some kinds of liquids such as water.
Superheating is the phenomenon in which a liquid is heated to a temperature higher than its boiling point, without boiling. For a vapor bubble to expand, the temperature must be high enough that the vapor pressure exceeds the ambient pressure–the atmospheric pressure, primarily. Below that temperature, a water vapor bubble will shrink and vanish. Superheating is an exception: a liquid is sometimes observed not to boil even though its vapor pressure does exceed the ambient pressure. The cause is an additional force, the surface tension, which suppresses the growth of bubbles. Surface tension makes the bubble act a bit like a rubber balloon (more precisely, one that is under-inflated so that the rubber is still elastic). The pressure inside is raised slightly by the “skin” attempting to contract. For the bubble to expand-to boil-the temperature must be raised slightly above the boiling point to generate enough vapor pressure.
Another reason for superheating may be the intermittent heating that takes place in microwave ovens, which periodically apply an electromagnetic field. The phenomenon is more evident towards the end of the evaporation process, when there is a higher soluble-solid concentration and lower water content. This reduces the specific heat of the products, and the temperature of the concentrated juice undergoes a greater change.
Total anthocyanins content
Cyanidin-3-glucoside belongs to the flavonoid class of molecules and is a member of the anthocyanin family, the largest group of pigments present in many edible berries, dark grapes, cabbages, and other pigmented foods (Ding et al. 2006; Ozgen et al. 2009a, b).
The stability of anthocyanins during thermal processing has been well documented by several researchers (Scalzo et al. 2008; Kirca et al. 2007; Wang and Xu 2007).
The use of different dehydration processing methods for black mulberry juice resulted in variable retention of anthocyanin concentrations. Dehydration processes often affect color quality in foods, which reflects a loss of anthocyanins and other phytochemicals specific to fruits and vegetables (Kwok et al. 2004; Krifi et al. 2000).
The anthocyanin content (expressed in cyanidin-3-glucoside) of raw black mulberry juice was calculated to be 16.4 mg/100 mL. The results from Ozgen et al. (Ozgen et al. 2009a, b) showed total monomeric anthocyanin of some accessions of M.nigra (μg cyanidin-3-glucoside/ g fresh weight) range between 253–830 μg/g fw.
Thermal degradation of the anthocyanins from the black mulberry juice followed first-order reaction kinetics at 7.3, 38.5, and 100 kPa (Fig. 3), and the degradation of the anthocyanins over the whole pressure range is also well described using the analogue of Eq. 1.
These results are in agreement with those from previous investigations, which reported a first-order reaction model for the degradation of monomeric anthocyanins from the different products (Culpepper and Caldwell 1927; Kirca et al. 2007; Yang et al. 2008).
The different heating methods lead to different results for anthocyanin degradation, as shown in Fig. 3. There is an obvious difference between the degradation rates of the anthocyanins depending on the heating method (Table 2).
Table 2.
Thermal degradation of anthocyanins using different concentration methods
| Heating method | Pressure (kPa) | Rate constant (h−1) | R2 |
|---|---|---|---|
| Rotary | 7.3 | 0.066 ± 0.006 | 0.972 |
| 38.5 | 0.173 ± 0.008 | 0.931 | |
| 100 | 0.348 ± 0.01 | 0.994 | |
| Microwave | 7.3 | 0.034 ± 0.005 | 0.972 |
| 38.5 | 0.144 ± 0.007 | 0.957 | |
| 100 | 0.191 ± 0.009 | 0.961 |
As shown in Table 2, the degradation of black mulberry anthocyanins increased with pressure and temperature. Since the anthocyanin content was not reduced to 50% during this experiment, the t0.5 (the time required for the degradation of 50% of the total anthocyanins, which is usually used for the definition of anthocyanin loss) could not be calculated for the samples during concentration, except for the samples processed at atmospheric pressure using the conventional method. The degradation rate of anthocyanins in a microwave is smaller than for conventional heating method. These results are in agreement with those reported by Scalzo et al. (2008). These results demonstrate the time dependency of anthocyanin.
Color parameters
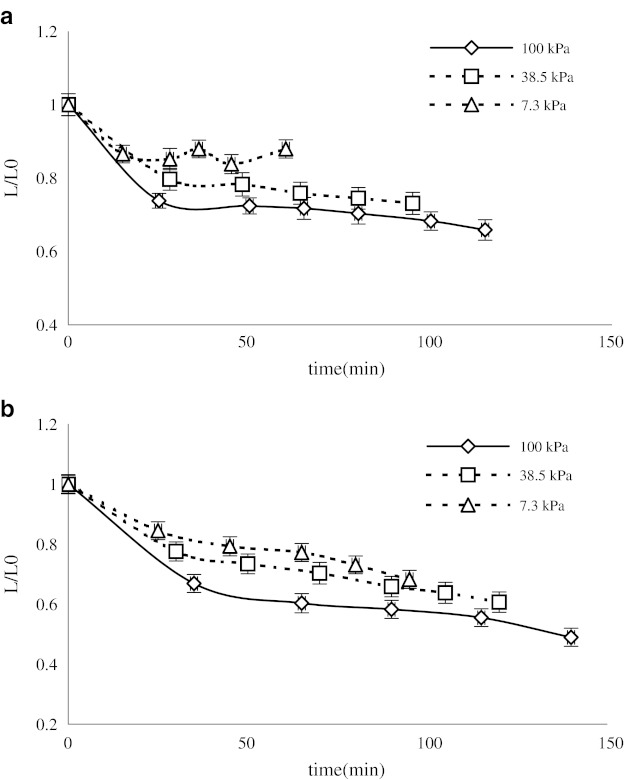
The study of the color change of black mulberry juice during concentration with both microwave and conventional methods showed that all the Hunter color parameters (L, a, and BI) changed significantly. The results for Hunter L, a, and BI value are presented in Figs. 4, 5, and 6 respectively.
Fig. 4.
Variation of the Hunter color L values during concentration of mulberry juice using microwave a and rotary b heating
Fig. 5.
Changes in the Hunter color a values during concentration of mulberry juice using microwave a and rotary b heating
Fig. 6.
Variation of the Browning Index during concentration of mulberry juice using microwave a and rotary b heating
There were obvious changes in the Hunter L and a, values, confirming the degradation of visual color components of the juice samples. The value of Hunter L decreased in all cases during concentration, but the final values were affected by the operational pressure, especially in the case of conventional heating (Fig. 4). The results show that higher pressure leads to a longer process time and lower L values. Concentration of mulberry juices using microwave power leads to a decrease in the L value, but there is an obvious difference between the samples produced using the different heating methods.
Different authors have reported that decreases in L value correlated well with increases in the browning of foods (Maskan et al. 2002).
The final L values of the samples concentrated using conventional heating were about 19.8, 17.6, and 14.2 for 7.3, 38.5, and 100 kPa respectively. However, in the case of microwave heating, the final L values were about 25.5, 21.2, and 19.1 for 7.3, 38.5, and 100 kPa.
Figure 5 shows the changes in the Hunter a value during the process of concentrating black mulberry juice by conventional and microwave heating. At atmospheric pressure, this parameter decreased with time for both methods. At lower pressure, a different trend over time was observed. The a value increased in the initial stages due to the concentration of pigments. At higher concentrations the pigment degradation was dominant, and the a value rapidly decreased. As a result of changes in the Hunter color parameters, the BI was changed during the process. Two different behaviors were observed: small increases in the BI were observed at lower concentrations (TSS <30%); after this point, the BI increased rapidly and reached its final value. This indicates that a browning reaction, such as the Maillard (or caramelization) reaction, is enhanced at lower moisture content and higher temperatures. This effect was more evident for the conventional heating method. Figure 6 shows that the final BI values obtained by the conventional method were higher than those from microwave heating, and that the extent of color degradation increased with soluble-solid concentration.
The complexity of fruit derivatives implies a wide range of nonenzymatic browning reactions caused by thermal treatments. The color changes in fruit extract can be due not only to the browning reaction, but also to the thermal destruction of pigments present in the extracts. Heating of juice containing anthocyanins causes the discoloration of deep red color, and further heat treatment causes an increase of the brown color. There have been some reports of the effects of sugar and its degradation products, including the acceleration of anthocyanins breakdown and enhancement of non-enzymatic browning reactions during thermal concentration (Cemeroglu et al. 1994; Suh et al. 2003).
Conclusion
This study investigated the effects of concentration using conventional and microwave methods under various operational pressures on the anthocyanin content and color of black mulberry juice. The results showed that the heating method and conditions (temperature or pressure) affect the samples’ total anthocyanin content. In this case, applying microwave instead of conventional heating method decreases the degradation of anthocyanins and it was less evident under low-pressure operation. Therefore, microwave heating at 7.3 Kpa pressure is the final recommended conditions for the concentration of black mulberry juice with better retention of anthocyanins. Investigation of the visual parameters of the samples showed that the lightness was decreased in all cases, but this effect was more obvious in conventional heating. On the other hand, the redness of the samples was well-preserved under microwave heating and lower operational pressure. The BI of the samples was increased during all treatments, but conventional heating and total soluble solids increased the BI more rapidly.
Acknowledgements
The authors thank the vice chancellor for Research of University of Tehran, Iran, for supporting the research reported in this article (Grant number: 7106014/1/01).
References
- Statistical book of agricultural of Iran. Tehran, Iran: Iranian Statistical Centre; 2008. [Google Scholar]
- Bridle P, Timberlake CF. Anthocyanins as natural food colors-selected aspects. Food Chem. 1997;58(2):103–109. doi: 10.1016/S0308-8146(96)00222-1. [DOI] [Google Scholar]
- Butkhup L, Samappito S. Analysis of anthocyanin, flavonoids, and phenolic acids in tropical bignay berries. J Fruit Sci. 2008;8(2):15–34. doi: 10.1080/15538360802365913. [DOI] [Google Scholar]
- Cam M, Hisil Y, Durmaz G. Classification of eight pomegranate juices based on antioxidant capacity measured by four methods. Food Chem. 2009;112:721–726. doi: 10.1016/j.foodchem.2008.06.009. [DOI] [Google Scholar]
- Cemeroglu B, Velioglu S, Isik S. Degradation kinetics of anthocyanins in sour cherry juice and concentrate. J Food Sci. 1994;59:1216–1218. doi: 10.1111/j.1365-2621.1994.tb14680.x. [DOI] [Google Scholar]
- Culpepper CW, Caldwell JS. The behavior of the anthocyanin pigments in canning. J Agri Res. 1927;35(2):107–132. [Google Scholar]
- Diamante L, Durand M, Savage G, Vanhanen L. Effect of temperature on the drying characteristics, colour and ascorbic acid content of green and gold kiwifruits. Int Food Res J. 2010;17:441–451. [Google Scholar]
- Ding M, Feng R, Wang S, Bowman L, Lu Y, Qian Y, Castranova V, Jiang B, Shi X. Cyanidin-3-glucoside, a natural product derived from blackberry, exhibits chemopreventive and chemotherapeutic activity. J Biol Chem. 2006;281(25):17359–17368. doi: 10.1074/jbc.M600861200. [DOI] [PubMed] [Google Scholar]
- Ercisli S, Orhan E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem. 2007;103:1380–1384. doi: 10.1016/j.foodchem.2006.10.054. [DOI] [Google Scholar]
- Ercisli S, Orhan E. Some physico-chemical characteristics of black mulberry (Morus nigra L.) genotypes from Northeast Anatolia region of Turkey. Sci Hortic. 2008;116:41–46. doi: 10.1016/j.scienta.2007.10.021. [DOI] [Google Scholar]
- Kirca A, Ozkan M, Cemeroglu B. Effects of temperature, solid content and pH on the stability of black carrot anthocyanins. Food Chem. 2007;101:212–218. doi: 10.1016/j.foodchem.2006.01.019. [DOI] [Google Scholar]
- Koyuncu F. Organoleptic acid composition of native black mulberry fruit. Chem Nat Compd. 2004;40(4):301–302. doi: 10.1023/B:CONC.0000048249.44206.e2. [DOI] [Google Scholar]
- Krifi B, Chouteau F, Boudrant J, Metchel M. Degradation of anthocyanins from blood orange juices. Int J Food Sci Technol. 2000;35:275–283. doi: 10.1046/j.1365-2621.2000.00330.x. [DOI] [Google Scholar]
- Kwok BHL, Hu C, Durance T, Kitts DD. Dehydration techniques affect phytochemical contents and free radical scavenging activities of saskatoon berries (Amelanchier alnifolia Nutt.) J Food Sci. 2004;69:122–126. [Google Scholar]
- Lako J, Trenerry VC, Wahlqvist M, Wattanapenpaiboon N, Sotheeswaran S, Premier R. Phytochemical flavonols, carotenoids and the antioxidant properties of a wide selection of Fijian fruit, vegetables and other readily available foods. Food Chem. 2007;101:1727–1741. doi: 10.1016/j.foodchem.2006.01.031. [DOI] [Google Scholar]
- Martin JD, Rodrigo GL, Cordero JH, Dia ED, Romero CD. Alcoholic beverages obtained from black mulberry. Food Technol Biotechnol. 2003;41(2):173–176. [Google Scholar]
- Maskan M. Production of pomegranate (Punica granatum L.) juice concentrate by various heating methods: colour degradation and kinetics. J Food Eng. 2006;72:218–224. doi: 10.1016/j.jfoodeng.2004.11.012. [DOI] [Google Scholar]
- Maskan A, Kaya S, Maskan M. Effect of concentration and drying processes on color change of grape juice and leather (pestil) J Food Eng. 2002;54:75–80. doi: 10.1016/S0260-8774(01)00187-X. [DOI] [Google Scholar]
- Ozgen M, Gunes M, Akca Y, Turemis N, Ilgin M, Kizilci G, Erdogan U, Serce S. Morphological Characterization of Several Morus Species from Turkey. Hort Environ Biotechnol. 2009;50(1):1–5. [Google Scholar]
- Ozgen M, Serc ZS, Kaya C. Phytochemical and antioxidant properties of anthocyanin-rich Morus nigra and Morus rubra fruits. Sci Hortic. 2009;119:275–279. doi: 10.1016/j.scienta.2008.08.007. [DOI] [Google Scholar]
- Scalzo R, Genna A, Branca F, Chedin M, Chassaigne H. Anthocyanin composition of cauliflower (Brassica oleracea L. var. botrytis) and cabbage (B. oleracea L. var. capitata) and its stability in relation to thermal treatments. Food Chem. 2008;107:136–144. doi: 10.1016/j.foodchem.2007.07.072. [DOI] [Google Scholar]
- Suh HJ, Noh DO, Kang CS, Kim JM, Lee SW. Thermal kinetics of color degradation of mulberry fruit extract. Food Nahrung. 2003;47(2):132–135. doi: 10.1002/food.200390024. [DOI] [PubMed] [Google Scholar]
- Wang WD, Xu SHY. Degradation kinetics of anthocyanins in blackberry juice and concentrate. J Food Eng. 2007;82:271–275. doi: 10.1016/j.jfoodeng.2007.01.018. [DOI] [Google Scholar]
- Yang Z, Zhenxin Y, Fan G, Chen Z. Thermal degradation kinetics of aqueous anthocyanins and visual color of purple corn (Zea mays L.) cob. Innov Food Sci Emerg Tech. 2008;9:341–347. doi: 10.1016/j.ifset.2007.09.001. [DOI] [Google Scholar]