Abstract
Free amino acids are important chemical components which impact the taste of green tea infusion. The hydrolysis of water-insoluble protein in the green tea residue helps to increase the contents of free amino acids components except theanine. Studies indicate that the hydrolysis of the tea protein could be restricted due to interaction of polyphenols with protein. The experiment indicates that the hydrolysis of tea protein by protease is the main trend when the polyphenols concentration is lower than 5 mg ml−1, however, the proteins (including tea protein and protease) would interact with polyphenoles instead of hydrolysis when the concentration of polyphenols is higher than 5 mg ml−1. The hydrolysis of tea protein is absolutely restrained when concentration comes to 10 mg ml−1.
Keywords: Free amino acids, Protease, Tea protein, Green tea residue, Hydrolysis
Introduction
Tea is one of the most popular beverages, and widely consumed in the world. Green tea is popular in most Asian areas, especially in China and Japan. Green tea was found to have important physiological properties and potential health benefits due to the presence of many compounds such as catechins, amino acids, caffeine and minerals (Cabrera et al. 2006). It contains about 1–4% free amino acids on dry basis (Alczar et al. 2007). It has been demonstrated that there was a good positive correlation between the green tea quality and the content of amino acids (Chu et al. 1997). The brothy, sweet, umami taste of green tea is due to amino acids (Thippeswamy et al. 2006), especially theanine. Theanine is the most important free amino acids in tea, accounting for about 40% of total free amino acids by weight (Li et al. 2008), and existing only in the free (non-protein) form (Lekh et al. 1999). However, the contribution of amino acids is limited because the content of free amino acids in green tea is low.
Green tea contains about 21–28% proteins on dry basis (Gu et al. 2002). However, protein in plant cells is hardly water soluble because of its hydrophobic nature and the disulphide bonding between protein molecules. Proteolytic enzyme helps to catalyze the hydrolysis of tea protein, releasing peptides and free amino acids, which help to advance the taste quality of tea infusion and restrain the formation of tea cream. Zheng and Zeng (2003) reported that, after the hydrolysis of green tea extract by pectinase and papain, the membrane flux and aminonitrogen of tea extract were increased, while the viscosity of tea extract was decreased. At the same time, protease could be easily interacted by polyphenols, and the proteolytic activity would be inhibited. Huang et al. (2002) found that poplyphenols interacted with papain, bromelain and trypsin, and then inhibited their proteolytic activities. Polyphenols were also found to be capable of binding and precipitating some digestive enzymes such as pepsin, alpha-amylase and lipase, suggesting that they may possess antinutritional properties (He et al. 2006). Many other enzymes, such as decarboxylase (Bertoldi et al. 2001), squalene epoxidase (Abe et al. 2000) and ribonuclease (Ghosh et al. 2004) were found to be denatured by polyphenols.
However, there is little information on how to avoid the interfering of polyphenols and to increase free amino acids contents by hydrolyzing tea protein with protease to improve the taste quality of tea infusion. The objective of this study is to investigate the effect of polyphenols on the hydrolysis of green tea residue protein catalyzed by protease, which is derived from a selected strain of Aspergillus oryzae.
Materials and methods
Materials
Yulu green tea, steam processed in Yuhang County of Zhejiang Province in China, was bought from market and used as tea material. Polyphenols (98% purity) was bought from Taiyo Green Power Co., Ltd. (Wuxi, China), which was extracted from green tea and contained about 70% catechins. Free amino acids components and other chemical reagents were bought from Sigma-Aldrich China (Shanghai, China). Protease G (Amano Enzyme Inc., Nagoya, Japan) is a proteolytic enzyme developed for protein hydrolyzates rich in amino acids, which is manufactured by a unique fermentation process with a selected strain of Aspergillus oryzae. Protease G has high proteinase activity, and the proteolytic combination system is possible to hydrolyze various proteins at high level. The peptidase activity is not less than 1,250 unit g−1 by LNA method, which was assayed using L-leucyl-α-napthylamide as substrate according to the method of Elleman (1974). The optimum temperature and pH were 45°C and 6.0–9.0, while the stable pH range was 4.0–9.0.
Preparation of green tea infusion and residue
Three grams of tea samples (20–60 mesh) were used for extracting, added with 120 ml distilled water (1:40, w/w) at 90°C for 10 min, and then quickly cooled down to 45°C by a glass condenser with tap water (15°C). The green tea extract was separated by four-layer muslin cloth (200 mesh). The clarified extract was labeled as infusion A, and the residue was labeled as residue A. And the green tea extract before separating was the mixture of infusion A and residue A.
Comparison of two hydrolysis approaches
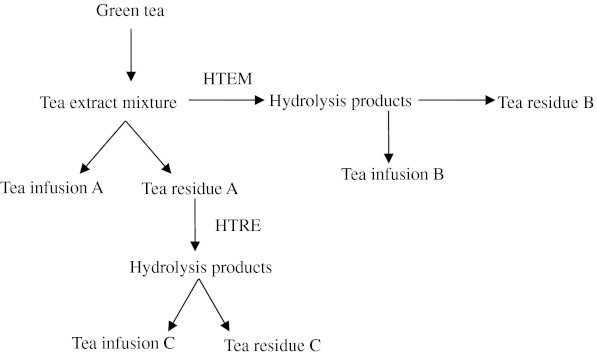
Two different hydrolysis approaches (Fig. 1), with or without tea extract, for the hydrolysis of tea protein in the residue were used in this experiment. Firstly, the mixture of the infusion A and the residue A were hydrolyzed with 0.05% (w/v) Protease G at 45°C for 5 h, defined as the hydrolysis of tea extracting mixture (HTEM). Secondly, the residue A added with 120 ml distilled water were also hydrolyzed with 0.05% (w/v) Protease G at 45°C for 5 h, defined as the hydrolysis of tea residue after extracting (HTRE). After hydrolysis, the protease was inactivated by heating treatment (90°C, 10 min), and then the hydrolysis products were separated by four-layer muslin cloth (200 mesh). The products of the first approach hydrolysis were labeled as infusion B and residue B, and the products of the second time hydrolysis were labeled as infusion C and residue C. Each treatment had a corresponding control, in which the protease was inactivated with heating treatment (90°C, 10 min) before hydrolysis.
Fig. 1.
Two hydrolysis approaches of green tea residue protein
Effect of polyphenols concentration on the hydrolysis
Green tea residue (residue A) added with 120 ml solution of different polyphenols concentrations (0, 1, 3, 5, 10 and 20 mg ml−1) were hydrolyzed with 0.05% (w/v) Protease G at 45°C for 5 h. After hydrolysis, the protease was inactivated by heating treatment (90°C, 10 min), and then the hydrolysis products were separated with four-layer muslin cloth (200 mesh). Each treatment had a corresponding control, in which the protease was inactivated with heating treatment (90°C, 10 min) before hydrolysis.
Analysis of total amino acids and total nitrogen
The content of total amino acids in the tea infusion was determined with a spectrophotometer (UV-2550, Shimadzu (Suzhou) Instruments Manufacturing, Co., Ltd., Suzhou, China) by ninhydrin dye method (Zhao et al. 2008). The content of total nitrogen in the tea residue was determined using the Kjeldahl method (Rai et al. 2010).
Analysis of polyphenols
The content of polyphenols in the tea infusion was determined by the spectrophotometric method with FeSO4, 3.5x10−3 M potassium sodium tartrate and buffer (Liang et al. 2003). Tartrate solution: 1 FeSO4 g and 5 g KNaC4H4O6 were dissolved in distilled water and made up to 1,000 ml. One ml tea extract (non-inoculated), 4 ml distilled water and 5 ml tartrate solution were added in a volumetric flask. Absorbance (E1) at 540 nm of the reaction solution was determined in a 1 cm photometer cuvette by Shimadzu UV-2550 spectrophotometer. Absorbance (E2) at 540 nm of a control reaction solution (containing 5 ml distilled water, 5 ml dyeing solution and 15 ml buffer) was determined as earlier. The content of tea polyphenols was calculated by the following equation: TP (mg •l−1) = (E1 – E2) × 3.9133 × 103.
Analysis of free amino acids components
Five ml tea infusion was evaporated and the dried sample was dissolved in 0.02 N HCl. Free amino acids components were determined with an amino acid analyzer (Hitachi 835-50, Japan). The relevant configurations were a single microbore stainless steel column (2.6 mm inside diameter by 15 cm in length) with a maximum of 5 programmable eluting buffers and one regenerant. The column material was Hitachi Custom Cation-Exchange #2619 F resin. Buffers and ninhydrin flow rates 0.25 and 0.30 ml/min respectively. Briefly, amino acids, separated by cation-exchange chromatography, were detected spectrophotometrically after postcolumn reaction with ninhydrin reagent. The contents of free amino acids components in samples were calculated according to the area normalization method (Zhao et al. 2008).
Sensory evaluation
Nine evaluators were randomly selected to undertake the preference test, aiming at the mouth feeling, taste and the overall acceptance of the green tea infusions. The Hedonic 9-point scale was used (9 = like extremely, 5 = neutral, 1 = dislike extremely). The results were statistically analyzed to show the acceptance of the sample evaluation (Ibanoglu et al. 2006).
Statistical analysis
Results are presented as mean value (at least three replicates). One-way ANOVA was performed to analyze the variance and significance amongst the means using SPSS (Version 13.0, SPSS Inc., Chicago, USA).
Results and discussion
Comparison of two hydrolysis approaches
Both of the two hydrolysis approaches help to increase the contents of free amino acids in the tea infusions, compared to the controls (Table 1). It is found that the increase of free amino acids content in infusion B is much lower than that in infusion C, compared to the corresponding controls. The total nitrogen contents of the corresponding tea residues are reduced. Protein is the main nitrogenous component of tea leaves, which contributes about 21–28% of tea leaf weight. Most of the tea protein is water-insoluble, and only a part of the tea protein is hydrolyzed by the protease in this study. The total nitrogen content of the residue C is lower than that of the residue B. The results indicate the hydrolysis degree of the tea residue protein by HTRE is larger than that of HTEM.
Table 1.
Contents of chemical components in the tea residues and infusions (mean ± s.d.)
| Treatments | Total nitrogen | Free amino acids | Polyphenols |
|---|---|---|---|
| In tea residue (%) | Content in tea infusion (mg ml−1) | ||
| A | 33.2 ± 0.30a | 0.45 ± 0.01a | 4.3 ± 0.07a |
| B | 30.9 ± 0.12b | 0.56 ± 0.01b | 4.4 ± 0.09a |
| B control | 31.9 ± 0.75c | 0.48 ± 0.02a | 4.3 ± 0.05a |
| C | 28.7 ± 0.85d | 0.22 ± 0.03c | 0.50 ± 0.09c |
| C control | 31.6 ± 0.09bc | 0.06 ± 0.01d | 0.32 ± 0.07d |
The means (n = 3) with the same letter in the same column are not significantly different (P ≥ 0.05)
The contents of free amino acids components are different in the hydrolysis products of different hydrolysis approaches (Table 2). Compared to the controls, the two hydrolysis approaches both help to increase the contents of most free amino acids components. And there are many free amino acids components, of which the increase is larger than 10 mg l−1 in the hydrolysis products, such as asparagine (Asp), serine (Ser) and lysine (Lys) in the infusion B, and cysteine (Cys), tyrosine (Tyr), valine (Val), Lys, isoleucine (Ile) and leucine (Leu) in the infusion C. Scharbert et al. (2004) reported that Asp, Ser, glutamic (Glu), proline (Pro) and alanine (Ala) tasted sweet or umami in the black tea infusion, while Tyr, Val, Ile, Leu and phenylalanine (Phe) tasted bitter. Table 3 shows the sensory evaluation in mouth feeling, taste and the overall acceptance of green tea infusion with or without protease treatment. The results reveal that the quality attributes of the infusion B are all better than those of the control, while there are only the mouth feeling and overall acceptance of the infusion C better than those of the control. Theanine exists only in the free (non-protein) form (Lekh et al. 1999). In this study, it is also found that theanine could be easily extracted and it can not be hydrolyzed from tea protein. The different increases of free amino acids contents in the hydrolysis products hydrolyzed by different approaches may be due to the different concentrations of the polyphenols that can be interacted with protease and inhibit the proteolytic activities, which was also observed by Huang et al. (2002).
Table 2.
Contents of free amino acids components in the tea infusions (mg l−1, mean ± s.d.)
| Free amino acids components | A | B Control | B | C Control | C |
|---|---|---|---|---|---|
| Asp | 69.3 ± 3.42a | 75.2 ± 3.00a | 91.2 ± 2.58b | 1.4 ± 0.08c | 1.6 ± 0.83c |
| Ser | 60.7 ± 3.58a | 59.6 ± 4.75a | 73.0 ± 3.58b | 7.8 ± 1.25c | 9.5 ± 1.75c |
| Glu | 14.5 ± 0.75a | 21.2 ± 1.83b | 20.7 ± 1.00b | 0.17 ± 0.08c | 3.92 ± 0.00d |
| Gly | 4.1 ± 1.25a | 3.9 ± 0.92a | 7.8 ± 1.50b | 2.6 ± 0.33c | 3.9 ± 0.50d |
| His | 14.2 ± 1.92a | 18.5 ± 1.67b | 18.4 ± 1.92b | 1.6 ± 0.00c | 5.7 ± 1.83d |
| Arg | 12.8 ± 1.50a | 13.9 ± 1.08a | 19.0 ± 3.08b | 0.17 ± 0.00c | 8.3 ± 0.00d |
| Thr | 9.5 ± 2.50a | 8.6 ± 1.08a | 10.8 ± 0.92a | 1.0 ± 0.17b | 1.7 ± 1.17b |
| Ala | 7.5 ± 2.58a | 9.3 ± 2.33ab | 11.7 ± 1.50b | 1.5 ± 0.17c | 1.4 ± 0.42c |
| Pro | 16.3 ± 1.25a | 14.7 ± 0.58a | 17.2 ± 2.33a | 0.42 ± 0.17b | 2.4 ± 0.75c |
| Cys | 0.94 ± 0.10a | 5.0 ± 2.58b | 7.9 ± 1.67b | 7.3 ± 0.33b | 57.6 ± 2.25c |
| Tyr | 10.2 ± 0.92a | 10.8 ± 1.58a | 12.3 ± 2.33a | 3.1 ± 0.50b | 17.9 ± 1.08c |
| Val | 8.8 ± 0.58a | 12.3 ± 2.08bd | 15.5 ± 2.17b | 1.4 ± 0.00c | 11.1 ± 1.67d |
| Met | 5.3 ± 1.17a | 8.6 ± 1.33b | 13.1 ± 0.67c | 3.3 ± 1.08a | 7.1 ± 0.33b |
| Lys | 15.3 ± 1.83a | 14.6 ± 0.63a | 28.7 ± 1.00b | 3.5 ± 0.33c | 35.6 ± 2.50d |
| Ile | 7.5 ± 0.75a | 8.0 ± 0.42a | 11.4 ± 1.58b | 1.6 ± 0.25c | 17.7 ± 1.92d |
| Leu | 9.5 ± 1.08a | 9.2 ± 0.72a | 13.7 ± 2.00b | 4.1 ± 0.58c | 20.8 ± 3.00d |
| Phe | 5.7 ± 1.25a | 14.1 ± 1.17b | 18.9 ± 1.25c | 2.5 ± 0.58d | 10.0 ± 2.17e |
| Thea | 89.2 ± 2.92a | 84.2 ± 4.75a | 88.3 ± 1.17a | 0.57 ± 0.16b | 0.71 ± 0.11b |
| Total | 361.1 ± 25.17a | 391.5 ± 17.00a | 479.4 ± 19.23b | 43.9 ± 2.4c | 216.8 ± 17.17d |
The means (n = 3) with the same letter in the same column are not significantly different (P ≥ 0.05)
Table 3.
Sensory scores of green tea infusions (mean ± s.d.)
| Green tea infusion | Mouth feeling | Taste | Overall acceptance |
|---|---|---|---|
| A | 6.2 ± 1.22ab | 6.4 ± 1.31ab | 7.3 ± 0.32a |
| B | 6.7 ± 1.14a | 6.9 ± 1.37a | 8.1 ± 0.44b |
| B control | 6.0 ± 1.41ab | 6.3 ± 1.22ab | 7.0 ± 0.52a |
| C | 4.7 ± 0.59b | 4.2 ± 1.30bc | 5.2 ± 0.41c |
| C control | 3.2 ± 0.52c | 3.8 ± 1.03c | 4.1 ± 0.53d |
The means (n = 9) with the same letter in the same column are not significantly different (P ≥ 0.05)
Polyphenols and protein were both found as the main components of tea cream in semifermented tea infusion (Chao and Chiang 1999) and green tea infusion (Liang et al. 2002), and they could be interacted with each other. Proteases are proteins, which can interact with polyphenols. When the protease hydrolyzes the protein and releases free amino acids, it also reduces the chance of the bonding between polyphenols and protein.
Effect of polyphenols concentration on the hydrolysis of tea protein
The effect of polyphenols concentration on the hydrolysis of tea protein is shown in Table 4. With the increasing of polyphenols concentration, the increase of free amino acids content in the hydrolysis products is decreased, compared to the controls. It reflects that the hydrolysis of tea protein is affected by the polyphenols concentration. And when the polyphenols concentration comes to 10 mg ml−1, the hydrolysis of tea protein is absolutely restrained. Polyphenols and protein were both found to be the main components of tea cream (Yin et al. 2009). Enzymes are also proteins, which can be interacted with polyphenols. And the polyphenols can form ionic and hydrogen bonds with amino-, hydroxyl- and carboxyl groups of proteins (Fickel et al. 1999). It was observed that the ester bond-containing catechins, such as EGCG and ECG, possess greater ability to inhibit enzymes (Bertoldi et al. 2001). It was also observed that there were many hydrophobic amino acids present in enzyme, such as proline, phenylalanine and tyrosine, and polyphenols would strongly bind enzymes through hydrophobic association (He et al. 2006). The occurrence of hydrogen bond and hydrophobic association would change the enzyme molecular configuration, resulting in an impact on the enzyme activities. From this study, it is suggested that the tea protein tend to be hydrolyzed by protease and free amino acids are released when the concentration of polyphenols is lower than 5 mg ml−1. However, the polyphenols would tend to interact with tea protein and protease when the concentration of polyphenols is higher than 5 mg ml−1.
Table 4.
Effect of polyphenols on the hydrolysis of the protein in green tea residue (mg ml−1, mean ± s.d.)
| Polyphenols (mg ml−1) | Free amino acids | Polyphenols | ||
|---|---|---|---|---|
| Hydrolysis | Control | Hydrolysis | Control | |
| 0 | 0.35 ± 0.02a | 0.06 ± 0.02a | 0.36 ± 0.04a | 0.18 ± 0.00a |
| 1 | 0.29 ± 0.01b | 0.07 ± 0.02a | 1.1 ± 0.01b | 0.70 ± 0.01b |
| 3 | 0.22 ± 0.01c | 0.08 ± 0.01a | 2.6 ± 0.07c | 1.8 ± 0.24c |
| 5 | 0.17 ± 0.01d | 0.08 ± 0.02a | 4.0 ± 0.12d | 3.4 ± 0.01d |
| 10 | 0.06 ± 0.00e | 0.06 ± 0.01a | 8.3 ± 0.04e | 7.3 ± 0.08e |
| 20 | 0.06 ± 0.00e | 0.06 ± 0.01a | 16.3 ± 0.11f | 15.0 ± 0.12f |
The means (n = 3) with the same letter in the same column are not significantly different (P ≥ 0.05)
Conclusion
Free amino acids are important chemical components which impact the taste of green tea infusion. The contents of the free amino acids are increased after hydrolyzing the protein in green tea residue. Experiments based on the hydrolysis with different concentrations of polyphenols indicate that the tea extract interfere the hydrolysis of tea protein, mainly due to the polyphenols. With the increase of polyphenols concentration, it becomes stronger to restrain the hydrolysis of tea protein, and when the polyphenols concentration increases to 10 mg ml−1, the hydrolysis of tea protein is absolutely restrained. To increase the free amino acids content by hydrolyzing the protein in green tea residue, it is suggested to limit the polyphenols concentration in the hydrolysis system, and the concentration of polyphenols should be lower than 5 mg ml−1.
Acknowledgements
The authors thank Lin-Chun Mao An-Di Zhang and Yun-Zhu Jiang for their revision, and Jian-Xin Chen, Hai-Bo Yuan, Wei-Wu Jiang and Da-Jiang Du for offering their kind help. Contract/grant sponsor: Natural Science Foundation of Zhejiang Province (R3090394) and Industry-Specific Program of Ministry of Agriculture (3-35-15).
References
- Abe I, Seki T, Umehara K, Miyase T, Noguchi H, Sakakibara J, Ono T. Green tea polyphenols: novel and potent inhibitors of squalene epoxidase. Biochem Biophys Res Commun. 2000;268:767–771. doi: 10.1006/bbrc.2000.2217. [DOI] [PubMed] [Google Scholar]
- Alczar A, Ballesteros O, Jurado JM, Pablos F, Martn MJ, Vilches JL, Navaln A. Differentiation of green, white black, oolong, and pu-erh teas according to their free amino acids content. J Agric Food Chem. 2007;55:5960–5965. doi: 10.1021/jf070601a. [DOI] [PubMed] [Google Scholar]
- Bertoldi M, Gonsalvi M, Voltattorni CB. Green tea polyphenols: novel irreversible inhibitors of dopa decarboxylase. Biochem Biophys Res Commun. 2001;284:90–93. doi: 10.1006/bbrc.2001.4945. [DOI] [PubMed] [Google Scholar]
- Cabrera C, Artacho R, Gimĕnez R. Beneficial effects of green tea - A review. J Am Coll Nutr. 2006;25:79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- Chao YC, Chiang BH. Cream formation in a semifermented tea. J Sci Food Agric. 1999;79:1767–1774. doi: 10.1002/(SICI)1097-0010(199910)79:13<1767::AID-JSFA433>3.0.CO;2-8. [DOI] [Google Scholar]
- Chu DC, Kobayashi K, Juneja LR, Yamamoto T. In: Chemistry and application of green tea. Yamamoto T, Juneja LR, Chu DC, Kim M, editors. USA: CRC Press; 1997. pp. 8–20. [Google Scholar]
- Elleman TC. Aminopeptidases of pea. Biochem J. 1974;141:113–118. doi: 10.1042/bj1410113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickel J, Pitra Ch, Joest BA, Hofmann RR. A novel method to evaluate the relative tannin-binding capacities of salivary proteins. Comp Biochem Physiol C. 1999;122:225–229. doi: 10.1016/s0742-8413(98)10112-3. [DOI] [PubMed] [Google Scholar]
- Ghosh KS, Maiti TK, Dasgupta S. Green tea polyphenols as inhibitor of ribonuclease A. Biochem Biophys Res Commun. 2004;325:807–811. doi: 10.1016/j.bbrc.2004.10.116. [DOI] [PubMed] [Google Scholar]
- Gu L, Lu J, Ye B. Tea chemistry. China: Chinese University of Science and Technology Press; 2002. pp. 25–43. [Google Scholar]
- He Q, Lv Y, Yao K. Effects of tea polyphenols on the activities of α-amylase, pepsin, trypsin, and lipase. Food Chem. 2006;101:1178–1182. doi: 10.1016/j.foodchem.2006.03.020. [DOI] [Google Scholar]
- Huang H, Wang S, Wang Z, Liang H. Study on inter-complexes and sediments between tea polyphenols and different protein recoveries. Food Sci. 2002;23:26–30. [Google Scholar]
- Ibanoglu S, Ainsworth P, Ozer EA, Plunkett A. Physical and sensory evaluation of a nutritionally balanced gluten-free extruded snack. J Food Eng. 2006;75:469–472. doi: 10.1016/j.jfoodeng.2005.04.060. [DOI] [Google Scholar]
- Lekh RJ, Djong-Chi C, Tsutomu O, Yukiko N, Hidehiko Y. L-theanine-a unique amino acid of green tea and its relaxation effect in humans. Trends Food Sci Tech. 1999;10:199–204. doi: 10.1016/S0924-2244(99)00044-8. [DOI] [Google Scholar]
- Li J, Li P, Liu F. Production of theanine by Xerocomus badius (mushroom) using submerged fermentation. LWT – Food Sci Technol. 2008;41:883–889. doi: 10.1016/j.lwt.2007.05.020. [DOI] [Google Scholar]
- Liang YR, Lu JL, Zhang LY. Comparative study of cream in infusions of black tea and green tea [Camellia sinensis (L.) O. Kuntze] J Food Sci Tech. 2002;37:627–634. doi: 10.1046/j.1365-2621.2002.00589.x. [DOI] [Google Scholar]
- Liang Y, Lu J, Zhang L, Wu S, Wu Y. Estimation of black tea quality by analysis of chemical composition and colour difference of tea infusions. Food Chem. 2003;80:283–290. doi: 10.1016/S0308-8146(02)00415-6. [DOI] [Google Scholar]
- Rai KP, Zhang C, Xia WS. Effects of pure starter cultures on physico-chemical and sensory quality of dry fermented Chinese-style sausage. J Food Sci Technol. 2010;47:188–194. doi: 10.1007/s13197-010-0030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharbert S, Holzmann N, Hofmann T. Identification of the astringency taste compounds in black tea by combining instrumental analysis and human bioresponse. J Agri Food Chem. 2004;52:3498–3508. doi: 10.1021/jf049802u. [DOI] [PubMed] [Google Scholar]
- Thippeswamy R, Mallikarjun Gouda KG, Rao DH, Martin A, Gowda R. Determination of theanine in commercial tea by liquid chromatography with fluorescence and diode array ultraviolet detection. J Agric Food Chem. 2006;54:7014–7019. doi: 10.1021/jf061715+. [DOI] [PubMed] [Google Scholar]
- Yin JF, Xu YQ, Yuan HB, Luo LX, Qian XJ. Cream formation and main chemical components of green tea infusions processed from different parts of new shoots. Food Chem. 2009;114:665–670. doi: 10.1016/j.foodchem.2008.10.004. [DOI] [Google Scholar]
- Zhao W, Yang R, Lu R, Wang M, Qian P, Yang W. Effect of PEF on microbial inactivation and physical-chemical properties of green tea extracts. LWT – Food Sci Technol. 2008;41:425–431. doi: 10.1016/j.lwt.2007.03.020. [DOI] [Google Scholar]
- Zheng B, Zeng S. Effects of enzymatic treatment on components of tea extracts and membrane flux. Trans Chin Soc Agric Eng. 2003;19:212–214. [Google Scholar]