Abstract
Defatted wheat germ peptides (DWGPs) were prepared by fermentation with Bacillus Subtilis B1 and the antioxidant activities of DWGPs were investigated. The fermentation condition was optimized by response surface method (RSM) with three factors and three levels according to Box-Behnken theory. A maximal yield of DWGPs was achieved 8.69 mg/mL under optimal conditions: inoculum size 8%, fermentation temperature 31 °C and time 48 h. The main portion in the hydrolysates after fermentation was not free amino acid but peptide. The main molecular weight distribution of DWGPs was lower than 1000 Da. A positive correlation (R2 = 0.9911) was found between concentration of DWGPs and total antioxidant capacity (T-AOC). DWGPs presented a significant does-dependent on scavenging activities of DPPH, hydroxyl and superoxide anion radicals. The EC50 values for the scavenging rates of DPPH, hydroxyl and superoxide anion radicals were 3.16 mg/mL, 6.04 mg/mL and 7.46 mg/mL, respectively. The results suggested that DWGPs produced by fermentation could be used as a promising antioxidant ingredient.
Keywords: Defatted wheat germ peptides, Fermentation, Bacillus Subtilis B1, Free radical scavenging activity
Introduction
Bioactive peptides with special physical function, such as antioxidant activity(Sun et al. 2011), ACE-inhibitory activity(Jia et al. 2010), antimicrobial activity(Thammasirirak et al. 2010), have attracted much attention. Until now, bioactive peptides have mainly been prepared from protein with commercial enzyme hydrolysis. Owing to the high cost of the commercial enzyme and the bitterness of hydrolysates, the industrial production of bioactive peptides in developing countries has been limited. Throughout history, fermentation has been successfully used to improve product properties (Jamdar et al. 2010; Lee et al. 2008; Lin et al. 2006). Bioactive peptides produced by fermentation (Rajapakse et al. 2005; Sun et al. 2004) from raw materials, particularly obtained from milk (Tsai et al. 2008) and soybean (Yu, et al. 2008), have been widely reported and proved to be an economical alternative method of bioactive peptides production. Meanwhile, the more complex proteinase secreted by microbial, may modify the exposed bitter taste of hydrophobic groups, making the peptides relatively mild flavor.
Defatted wheat germ, main byproduct obtained from the production of wheat germ oil, has relative high content of protein (~35%) and essential amino acids, such as lysine, methionine, and threonine, which are deficient in the many cereals (Ahmad et al. 2010; Ge et al. 2001; Zhu et al. 2006). Wheat germ protein contains many amino acid sequences with biological activity, which can be released by the specific proteinase, could be a good source of bioactive peptides. Therefore, production of peptides from defatted wheat germ can improve the utilization of defatted wheat germ and accelerate the industrial production process.
There are many studies on the production of wheat germ peptides by the method of commercial enzyme hydrolysis with wheat germ protein. Some peptides derived from wheat germ protein by enzyme hydrolysis displayed high bioactive, such as antioxidant activity(Zhu et al. 2006), ACE-inhibitory activity(Jia, et al. 2010; Matsui et al. 1999). However, the production of wheat germ peptides by fermentation has not been reported.
The objective of the present work was to produce bioactive peptides from defatted wheat germ by fermentation with Bacillus Subtilis B1. In order to obtain the highest yield of peptides, the fermentation condition was optimized according to RSM. Moreover, the antioxidant activities of fermented peptides were also investigated by means of in vitro free radicals scavenging tests.
Materials and methods
Materials
Bacillus Subtilis B1 used as fermentation strain throughout the work was screened by ourselves. The strain was stored on agar slant at 4 °C and sub-cultured monthly. Defatted wheat germ (DWG) was donated by Zhanyuan Co. (Hefei, China). T-AOC kit was purchased from Jiancheng Biological Engineering Institute (Nanjing, China). 1,1-diphenyl-2-pycrylhydrazyl (DPPH) was purchased from Sigma Chemical Co. (St. Louis, MO, USA). All other chemicals and reagents were of analytical grade and purchased from Sinopharm Chemical Reagent Co, Ltd (Shanghai, China).
Fermentation of defatted wheat germ (DWG)
Bacillus Subtilis B1 transferred from a slant and grown in broth media (3 g of beef extract, 10 g of peptone and 5 g of NaCl dissolved in 1000 mL of distilled water; pH 7.2) at 37 °C for 24 h was used as seed broth. The fermentation medium was composed of 5% (w/v) defatted wheat germ. After sterilization, every 50 mL fermentation medium in 250 mL flask was inoculated with seed broth.
Preparation of defatted wheat germ peptides (DWGPs)
After fermentation, the fermentation broth was incubated at 105 °C for 10 min to kill the microorganism and then was centrifuged at 1119 × g for 15 min. The supernatant was filtered through a 0.45 μm membrane filter and collected as hydrolysates. The hydrolysates were freeze-dried and stored at 4 °C before further analysis. The lyophilized hydrolysates were named defatted wheat germ peptides (DWGPs).
Determination of peptides contont in the fermentation broth
After fermentation, trichloracetic acid (TCA, 10%) solution was added to an equal volume of the hydrolysates to precipitate protein. The supernatant was collected by centrifuged at 1119 × g for 15 min. The content of TCA-N in the supernatants was determined by an automatic Kjeldahl analyzer (UDK152,VELP). The content of free amino acid (FAA) in the hydrolysates was determined by modified ninhydrin colorimetry (Guo 2000). The content of peptides in the fermentation broth was calculated by the equation below.
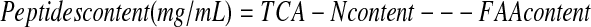 |
Experimental design for optimization of fermentation parameters
The fermented condition was optimized using a Box-Behnken design containing three levels for each parameter. Inoculum size (X1), fermentation time (X2) and temperature (X3) were chosen for independent variables and coded for the appraisals of factors. Uncoded and coded values of the variables were given in Table 1. The levels of the parameters were based on preliminary experimental results. All experiments were carried out in randomized order and done in triplicate. The average of the yield of DWGPs was selected as response (Y). A second-order polynomial regression model was used to express Y as a function of the independent variables as follows:
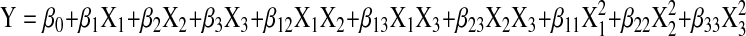 |
where Y is the yield of DWGPs (mg/mL), X1 is the inoculum size (%), X2 is the fermentation time (h) and X3 is the fermentation temperature (°C).
Table 1.
Uncoded and coded levels of independent variables used in the RSM design
| Variables | Symbols | Levels* | |||
|---|---|---|---|---|---|
| Coded | Uncoded | −1 | 0 | 1 | |
| Inoculum size (%) | X1 | x1 | 6 | 8 | 10 |
| Time (h) | X2 | x2 | 36 | 48 | 60 |
| Temperature (°C) | X3 | x3 | 28 | 31 | 34 |
*X1 = (x1 - 8)/2; X2 = (x2 - 48)/12; X3 = (x3 – 31)/3
DPPH radical scavenging activity
The DPPH radical-scavenging activity of DWGPs was measured according to the method described by Li et al. (Li et al. 2008) with some modifications. 0.5 mL of DWGPs was mixed with 2.0 mL of 0.004% DPPH in 95% ethanol. The mixture was shaken vigorously and immediately placed in dark for 30 min. The absorbance was monitored at 517 nm using a UV-Visible Beckman Coulter spectrophotometer (Fullerton, CA, USA).
 |
where AA is the absorbance value of the tested sample; AB is the absorbance value of the blank.
Superoxide anion radical scavenging activity
The superoxide anion scavenging activity of DWGPs was determined by the method described by Li et al. (Li, et al. 2008) with some modifications. The DWGPs were dissolved in Tris–HCl–EDTA buffer (0.1 M, pH 8.0) and then the mixtures were incubated at 25 °C for 10 min. Pyrogallol solution (3 mM) was added into the mixtures. The absorbance was measured at 320 nm every 10 s in 5 min. The regression equation was set according to the absorbance and time; the slope was the rate of pyrogallol autoxidation V.
 |
where V0 is the rate of pyrogallol autoxidation of control (ΔA0/s); VS is the rate of pyrogallol autoxidation of samples (ΔAs/s)
Hydroxyl radical scavenging activity
The hydroxyl radical scavenging activity of DWGPs was determined by the method described by Zhang et al. (Zhang, et al. 2009) with some modifications. Both 0.5 mL 1,10-phenanthroline (0.75 mM) and 0.5 mL FeSO4 (0.75 mM) were dissolved in 1 mL phosphate buffer (pH 7.4) and mixed thoroughly. 0.5 mL H2O2 (0.01%) and 0.5 mL sample were added. The mixture was incubated at 37 °C for 60 min, and the absorbance was measured at 536 nm.
 |
where AS is the absorbance value of the sample; A1 is the absorbance value of control solution containing 1,10-phenanthroline, FeSO4 and H2O2; A0 is the absorbance value of blank solution containing 1,10-phenanthroline and FeSO4.
Total antioxidant capacity
The total antioxidant capacity (T-AOC) of DWGPs was determined using a commercial kit (Jiancheng Biological Engineering Institute, Nanjing, China) and the result was calculated by the equation below.
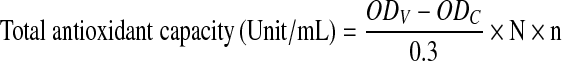 |
where ODV is the absorbance value of the sample; ODC is the absorbance value of the control; N is dilution of the reaction system; n is the dilution of the sample.
Analysis of amino acid composition
Pretreatments of the hydrolysates were done before amino acid analysis. For determination of free amino acid, an equivalent volume of TCA was added to the hydrolysate to precipitate proteins. The supernatant was obtained by centrifuged at 1119 × g for 15 min. For the total amino acids determination, the hydrolysate was hydrolyzed at 110 °C for 22 h with 6 M HCl. After pretreatments, the compositions of amino acid in the samples were determined with an automatic amino acid analyzer (835–50, Hitachi). Amino acid composition was reported as mg amino acid/mL.
Determination of molecular weight distribution
Molecular weight distribution profile of DWGPs was determined using an AKTA Purifier System (GE Healthcare, USA). The samples were loaded onto a gel column named Superdex Peptide 10/300 GL (10 i.d. ×300 mm), eluted with deionized water at a flow rate of 0.4 mL/min and monitored at 220 nm. A molecular weight calibration curve was obtained from the following standards from Sigma: cytochrome C (12,500 Da), aprotinin (6500 Da), bacitracin (1450 Da), tetrapeptide GGYR (451 Da), and tripeptide GGG (189 Da). Results were processed using UNICORN Version 5.1 software (GE Healthcare, USA).
Statistical analysis
All of the experiments were performed in triplicates. The average value and standard deviation were calculated. The data were analyzed using SPSS 13.0 statistical software. The response surface analysis procedure was performed using Design-Expert 7.0 statistical software.
Results and discussion
Optimization of fermentation condition
The yield of DWGPs obtained from all the experiments along with the predicted yield of DWGPs were listed in Table 2 according to RSM design.
Table 2.
Experimental scheme and results obtained from RSM
| Runs | Factors | Yield of DWGPs (mg/mL) | |||
|---|---|---|---|---|---|
| Inoculum size (X1,%) | Time (X2, h) | Temperature (X3,°C) | Exp. | Pred. | |
| 1 | 0 | −1 | −1 | 6.582 | 6.618 |
| 2 | 0 | −1 | 1 | 6.31 | 6.371 |
| 3 | 0 | 1 | −1 | 6.112 | 6.051 |
| 4 | 0 | 1 | 1 | 6.265 | 6.229 |
| 5 | −1 | 0 | −1 | 7.393 | 7.382 |
| 6 | −1 | 0 | 1 | 6.861 | 6.825 |
| 7 | 1 | 0 | −1 | 6.678 | 6.714 |
| 8 | 1 | 0 | 1 | 7.192 | 7.203 |
| 9 | −1 | −1 | 0 | 6.936 | 6.911 |
| 10 | −1 | 1 | 0 | 5.938 | 6.010 |
| 11 | 1 | −1 | 0 | 6.292 | 6.220 |
| 12 | 1 | 1 | 0 | 6.387 | 6.412 |
| 13 | 0 | 0 | 0 | 8.801 | 8.763 |
| 14 | 0 | 0 | 0 | 8.866 | 8.763 |
| 15 | 0 | 0 | 0 | 8.736 | 8.763 |
| 16 | 0 | 0 | 0 | 8.733 | 8.763 |
| 17 | 0 | 0 | 0 | 8.681 | 8.763 |
ANOVA for the response surface quadratic model was shown in Table 3. The P-value was used to identify the effect of each factor on yield of DWGPs. From statistical analysis, the model with the P-value less than 0.0001 was highly significant, which implied the model was suitable for this experiment. Meanwhile, the “lack of fit” was insignificant, the R-Squared was 0.997638, which indicated 99.7638% of the variability in the response on yield of DWGPs can be explained by the model.
Table 3.
ANOVA for response surface quadratic model
| Source | Sum of squares | Degree of freedom | Mean Square | F-value | P-valuea |
|---|---|---|---|---|---|
| Model | 18.99 | 9 | 2.11 | 328.55 | < 0.0001 |
| X1 | 0.042 | 1 | 0.042 | 6.52 | 0.0379 |
| X2 | 0.25 | 1 | 0.25 | 39.13 | 0.0004 |
| X3 | 0.002346 | 1 | 0.002346 | 0.37 | 0.5647 |
| X1 × X2 | 0.30 | 1 | 0.30 | 46.50 | 0.0002 |
| X1 × X3 | 0.27 | 1 | 0.27 | 42.58 | 0.0003 |
| X2 × X3 | 0.045 | 1 | 0.045 | 7.03 | 0.0329 |
| X1 × X1 | 2.91 | 1 | 2.91 | 452.34 | < 0.0001 |
| X2 × X2 | 10.04 | 1 | 10.04 | 1563.59 | < 0.0001 |
| X3 × X3 | −0.90 | 1 | −0.90 | 532.97 | < 0.0001 |
| Residual | 0.045 | 7 | 0.006423 | ||
| Pure Error | 0.02 | 4 | 0.005101 | ||
| Lack of Fit | 0.025 | 3 | 0.008186 | 1.60 | 0.3216 |
| R-Squared | 0.997638 |
aP < 0.01 highly significant; 0.01 ≤ P < 0.05 significant; P ≥ 0.05 not significant
Data also showed that fermentation time affected the yield of DWGPs highly significant, inoculum size affected the yield of DWGPs significant, but temperature affected the yield of DWGPs not significant. The interaction between inoculum size and temperature, inoculum size and time affected the yield of DWGPs highly significant, the interaction between temperature and time affected the yield of DWGPs significant. All the second-order terms (X21, X22, X23) were affected the yield of DWGPs highly significant.
The coefficients of independent variables determined for the second-order polynomial model for the yield of peptides was given below:
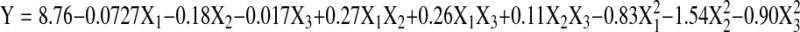 |
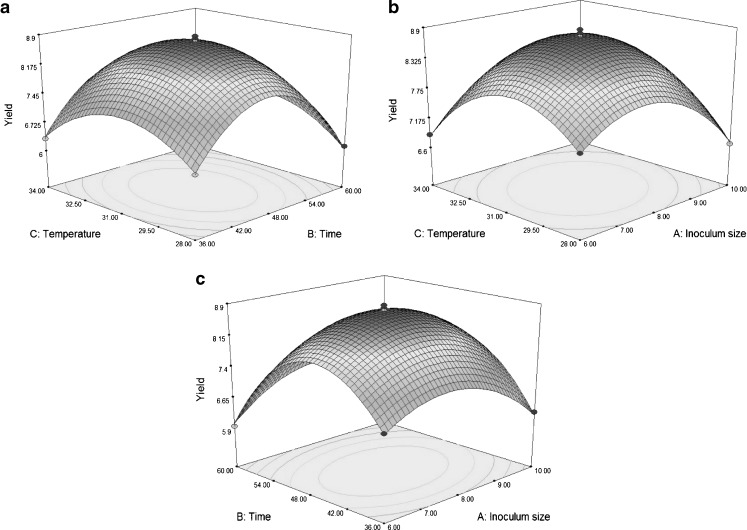
In order to determine the optimal levels of each variable for maximum yield of DWGPs and the interaction of the variables effect on the yield of DWGPs, 3D response surfaces plots were employed. As shown in Fig. 1(a), the yield of DWGPs was increased until the fermentation time and temperature reached an optimum point and then decreased. The interaction between temperature and time affected the yield of DWGPs significant. The maximal yield of DWGPs was observed at around 31 °C and 48 h. Figure 1(b) demonstrated the effect of inoculum size and fermentation temperature on DWGPs yield. The yield of DWGPs was affected by the inoculum size and fermentation temperature, and the interaction between inoculum size and temperature affected the yield highly significant. The effect of fermentation time and inoculum size on DWGPs yield was showed in Fig. 1(c). The yield of DWGPs was increased until the fermentation time and inoculum size reached an optimum point and then decreased. The maximal yield of DWGPs was observed at around 8% and 48 h.
Fig. 1.
3D-response surface plot of the effects of variables on DWGPs yield. (a)fermentation time and temperature; (b) inoculum size and fermentation temperature; (c)fermentation time and inoculum size. * All of the experiments were performed in triplicates
The optimization of the model was performed using auto-analysis software. A maximal yield of DWGPs was achieved 8.77 mg/mL under optimal conditions: fermentation temperature 31 °C, fermentation time 48 h, inoculum size 8%. Additional experiments in triplicates under these optimized fermentation condition were carried out. These triplicate experiments got an average 8.69 mg/mL, which concurred with the model prediction.
Amino acid composition of hydrolysates
With the hydrolysis by protease secreted from Bacillus Subtilis B1, the defatted wheat germ was gradually hydrolyzed into short peptides and free amino acids. The amino acid composition of peptides formed in hydrolysates at different fermentation time was shown in Table 4. Data showed that the main portion in the hydrolysates after fermentation was peptide. In general, glutamic acid, aspartic acid, leucine and lysine were abundant in DWGPs. Although the hydrophobic amino acids, such as tyrosine and alanine were increased after fermentation, they existed as peptides, not as free amino acids, which may reduce the bitterness of DWGPs. As shown in the composition of peptide in 48 h, there was no much difference from the DWG (Jiang and Niu 2011), suggesting that DWGPs may display the same function and value as DWG.
Table 4.
The amino acid composition of peptide in hydrolysates at different fermentation time
| Amino acids (mg/mL) | 0 h | 48 h |
|---|---|---|
| Asp | 0.094 | 0.578 |
| Glu | 0.058 | 1.21 |
| Ser | 0.612 | 0.29 |
| His | 0.124 | 0.23 |
| Gly | 0.062 | 0.509 |
| Thr | 0.1152 | 0.2742 |
| Arg | 0.424 | 0.412 |
| Ala | 0.142 | 0.788 |
| Tyr | 0.0278 | 0.794 |
| Cys | 0.00474 | – |
| Val | 0.0854 | 0.366 |
| Met | 0.04082 | 0.086 |
| Phe | 0.0642 | 0.48 |
| Ile | 0.087 | 0.35 |
| Leu | 0.1668 | 0.576 |
| Lys | 0.174 | 0.802 |
| Total amino acids | 2.28196 | 7.7452 |
- means no detected
Molecular weight distribution
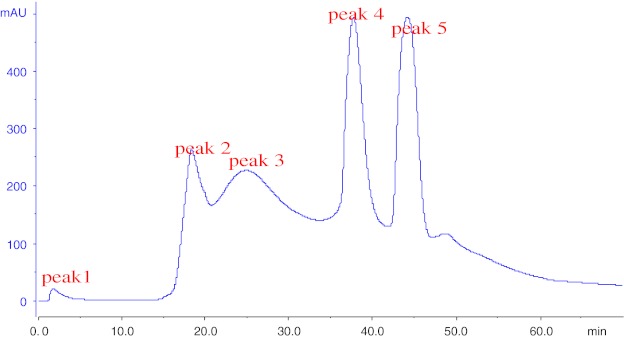
The molecular weight distribution of DWGPs was shown in Fig. 2. The peaks were located at > 2000 Da (1.6%), 2000–1000 Da (15.66%), 1000–500 Da (29.37%), 500–180 Da (25.96%) and < 180 Da (27.12%). Data indicated that many low molecular weight (1000–180 Da) peptides were formed (55.33%) after fermentation. A lot of studies have already shown that the bioactivities of peptides were depended on their molecular weight distribution. Zhu et al.(Zhu et al. 2006) reported that wheat germ protein hydrolysis with molecular weight ranging from 1050–180 Da had high free radical-savenging activities. In this study, peptides with a low molecular weight ranging from 1000–180 Da successfully obtained by fermentation probably associated with higher antioxidant activity.
Fig. 2.
Molecular weight distribution of DWGPs. * All of the experiments were performed in triplicates
T-AOC activity of hydrolysates during different fermentation time
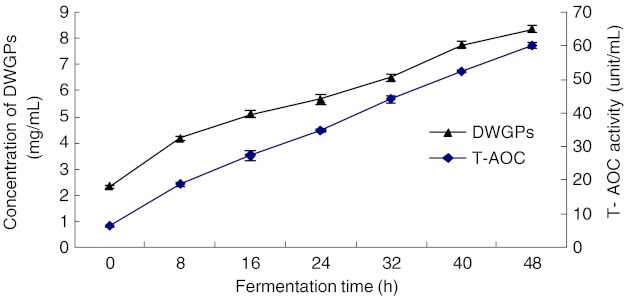
Figure 3 showed the interaction between concentration of DWGPs and T-AOC activity at different fermentation times. The concentration of DWGPs and T-AOC activity were increased with the fermentation time increasing. When the fermentation time was 48 h, the concentration of DWGPs came to 8.368 mg/mL, and the T-AOC activity reached 60.31 Unit/mL, which was about 10 times of the values at 0 h (6.33 Unit/mL). The antioxidant activity was increased highly by fermentation. Antioxidant activity has been detected in cereals(Sun and Ho 2005; Yu et al. 2002). Dordevic T. M. et al. (Dordevic et al. 2010) reported the effect of fermentation on antioxidant properties of some cereals, such as buck wheat, wheat germ, barley and rye. The presence of microorganisms (lactic acid bacteria Lactobacillus rhamnosus, and yeast Saccharomyces cerevisiae) was more or less important for enhanced levels of antioxidant activity. In this study, we used Bacillus Subtilis B1 as ferment strain, and similar result was also obtained.
Fig. 3.
The interaction between concentration of DWGPs and T-AOC activity during different fermentation time. * All of the experiments were performed in triplicates
There may be several ambiguous explanations of the antioxidant activity increasing, such as the presence of phenolics (Dordevic et al. 2010). However, in this study, although the total phenolics were increased after fermentation, there was no correlation between the total phenolics and T-AOC (data not shown). A positive correlation (R2 = 0.9911) was found between concentration of DWGPs and total antioxidant capacity (T-AOC). Many peptides, which displayed high antioxidant activities, have been obtained from fermentation recently. (Rajapakse, et al. 2005; Yu, et al. 2008). The peptides produced by enzymatic hydrolysis of wheat germ protein have been reported to be high antioxidant activities (Zhu, et al. 2006). Therefore, the wheat germ protein may contain some antioxidant squences of amino acids. DWGPs prepared by fermentation may have antioxidant activity. Different methods used for measuring antioxidant activity based on different mechanisms may lead to different observations. Besides T-AOC activity, other antioxidant activities were determined to confirm the antioxidant activity of DWGPs.
Antioxidant activity of DWGPs
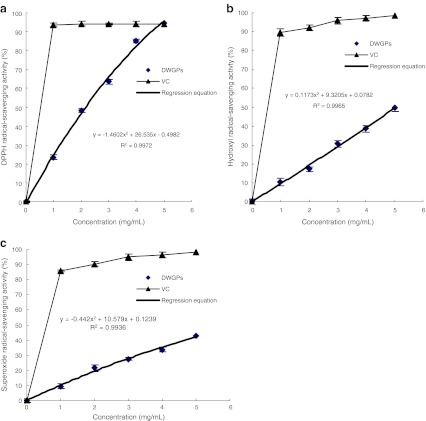
The relatively stable DPPH radical was widely used to investigate the scavenging activity of some antioxidants(Li, et al. 2008; Sachindra, et al. 2010; Zhu, et al. 2006). DPPH radical-scavenging activity of DWGPs at different concentrations was shown in Fig. 4a, VC was used as a positive control. In the range of 1–5 mg/mL, DWGPs displayed a significant does-dependent on the DPPH radical-scavenging activity. VC showed strong DPPH radical-scavenging activity at a lower concentration, while DWGPs needed high concentration to show the same activity. When the concentration was 5 mg/mL, the DPPH radical-scavenging activity was 94.61%, which was correspond to VC at the same concentration. The EC50 (concentration of samples required to scavenge 50% of free radicals) value calculated from the regression equation (y = −1.4602x2 + 29.455x – 28.493, R2 = 0.9972) was 3.16 mg/mL. The value was a little lower than the hydrolysates obtained by enzymatic hydrolysis (Zhu, Zhou et al. 2006), but this result showed that DWGPs had a potency to affect DPPH radical.
Fig. 4.
DPPH (a), hydroxyl (b) and superoxide (c) radical-scavenging activity of DWGPs at different concentrations. * All of the experiments were performed in triplicates
Hydroxyl radical-scavenging activity of DWGPs at different concentrations was shown in Fig. 4b, VC was used as a positive control. The hydroxyl radical-scavenging activity increased along with the concentration of DWGPs increased. The EC50 value calculated from the regression equation (y = 0.1173x2 + 9.0859x – 9.125, R2 = 0.9965) was 6.04 mg/mL. As shown in Fig. 4c, DWGPs displayed a significant does-dependent on the superoxide radical-scavenging activity. The EC50 value calculated from the regression equation (y = −0.442x2 + 11.463x – 10.897, R2 = 0.9936) was 7.46 mg/mL, while the EC50 value of VC was reported to be 1.33 mg/mL (Zhou et al. 2008). Although the hydroxyl radical and superoxide radical scavenge activities were much lower than these of VC at a low concentration, increasing the concentration of DWGPs may reach the same scavenge effect of VC. DWGPs will be used as a promising antioxidant component in functional food.
Conclusion
Fermentation could be a promising way to produce peptides and enhance product function. Fermentation of defatted wheat germ successfully provided peptides with antioxidant activities. The T-AOC activity highly increased after fermentation. DWGPs prepared by fermentation displayed an effect on scavenging activity of DPPH, hydroxyl and superoxide anion radicals. The results suggested that DWGPs could be used as a promising antioxidant ingredient in food industry. However, further studies on isolation and purification of a high antioxidant activity peptide from DWGPs, as well as the in vivo antioxidant activity tests are needed.
Acknowledgements
This work was financially supported by the National Key Technology Research and Development Program in the 11th 5 year Plan of China (2010BAD01B07), the High-Tech Research and Development Program of China (2010AA101503)
References
- Ahmad S, Rizawi J, Srivastava P. Effect of soy protein isolate incorporation on quality characteristics and shelf-life of buffalo meat emulsion sausage. J Food Sci Tech. 2010;47:290–294. doi: 10.1007/s13197-010-0045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dordevic TM, Siler-Marinkovic SS, Dimitrijevic-Brankovic SI. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 2010;119:957–963. doi: 10.1016/j.foodchem.2009.07.049. [DOI] [Google Scholar]
- Ge YQ, Sun AD, Ni YY, Cai TY. Study and development of a defatted wheat germ nutritive noodle. Eur Food Res Technol. 2001;212:344–348. doi: 10.1007/s002170000253. [DOI] [Google Scholar]
- Guo XF. Determination of hydrolysis degree of protein. China Oils Fats. 2000;25:176–177. [Google Scholar]
- Jamdar SN, Rajalakshmi V, Pednekar MD, Juan F, Yardi V, Sharma A. Influence of degree of hydrolysis on functional properties, antioxidant activity and ACE inhibitory activity of peanut protein hydrolysate. Food Chem. 2010;121:178–184. doi: 10.1016/j.foodchem.2009.12.027. [DOI] [Google Scholar]
- Jia JQ, Ma HL, Zhao WR, Wang ZB, Tian WM, Luo L, He RH. The use of ultrasound for enzymatic preparation of ACE-inhibitory peptides from wheat germ protein. Food Chem. 2010;119:336–342. doi: 10.1016/j.foodchem.2009.06.036. [DOI] [Google Scholar]
- Jiang ST, Niu LY. Optimization and evaluation of wheat germ oil extracted by supercritical CO2. Grasas Aceites. 2011 [Google Scholar]
- Lee IH, Hung YH, Chou CC. Solid-state fermentation with fungi to enhance the antioxidative activity, total phenolic and anthocyanin contents of black bean. Int J Food Microbiol. 2008;121:150–156. doi: 10.1016/j.ijfoodmicro.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Li YH, Jiang B, Zhang T, Mu WM, Liu J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH) Food Chem. 2008;106:444–450. doi: 10.1016/j.foodchem.2007.04.067. [DOI] [Google Scholar]
- Lin CH, Wei YT, Chou CC. Enhanced antioxidative activity of soybean koji prepared with various filamentous fungi. Food Microbiol. 2006;23(7):628–633. doi: 10.1016/j.fm.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Matsui T, Li CH, Osajima Y. Preparation and characterization of novel bioactive peptides responsible for angiotensin I-converting enzyme inhibition from wheat germ. J Pept Sci. 1999;5:289–297. doi: 10.1002/(SICI)1099-1387(199907)5:7<289::AID-PSC196>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Rajapakse N, Mendis E, Jung WK, Je JY, Kim SK. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res Int. 2005;38:175–182. doi: 10.1016/j.foodres.2004.10.002. [DOI] [Google Scholar]
- Sachindra N, Airanthi M, Hosokawa M, Miyashita K. Radical scavenging and singlet oxygen quenching activity of extracts from Indian seaweeds. J Food Sci Tech. 2010;47:94–99. doi: 10.1007/s13197-010-0022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Ho CH. Antioxidant activities of buckwheat extracts. Food Chem. 2005;90:743–749. doi: 10.1016/j.foodchem.2004.04.035. [DOI] [Google Scholar]
- Sun J, He H, Xie BJ. Novel antioxidant peptides from fermented mushroom Ganoderma lucidum. J Agr Food Chem. 2004;52:6646–6652. doi: 10.1021/jf0495136. [DOI] [PubMed] [Google Scholar]
- Sun Q, Shen H, Luo Y. Antioxidant activity of hydrolysates and peptide fractions derived from porcine hemoglobin. J Food Sci Tech. 2011;48:53–60. doi: 10.1007/s13197-010-0115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thammasirirak S, Pukcothanung Y, Preecharram S, Daduang S, Patramanon R, Fukamizo T, Araki T. Antimicrobial peptides derived from goose egg white lysozyme. Comp Biochem Phys C. 2010;151:84–91. doi: 10.1016/j.cbpc.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Tsai J, Chen T, Pan B, Gong S, Chung M. Antihypertensive effect of bioactive peptides produced by protease-facilitated lactic acid fermentation of milk. Food Chem. 2008;106:552–558. doi: 10.1016/j.foodchem.2007.06.039. [DOI] [Google Scholar]
- Yu L, Perret J, Davy B, Wilson J, Melby C. Antioxidant properties of cereal products. J Food Sci. 2002;67:2600–2603. doi: 10.1111/j.1365-2621.2002.tb08784.x. [DOI] [Google Scholar]
- Yu B, Lu ZX, Bie XM, Lu FX, Huang XQ. Scavenging and anti-fatigue activity of fermented defatted soybean peptides. Eur Food Res Technol. 2008;226:415–421. doi: 10.1007/s00217-006-0552-1. [DOI] [Google Scholar]
- Zhang JH, Zhang H, Wang L, Guo XN, Wang XG, Yao HY (2009) Antioxidant activities of the rice endosperm protein hydrolysate: identification of the active peptide. Eur Food Res Technol 4:709–719
- Zhou HM, Zhu KX, Qian HF. Antioxidant and free radical-scavenging activities of wheat germ protein hydrolysates (WGPH) prepared with alcalase. Process Biochem. 2006;41:1296–1302. doi: 10.1016/j.procbio.2005.12.029. [DOI] [Google Scholar]
- Zhou C, Wang Y, Ma H, He R. Effect of Ultrasonic Degradation on In Vitro Antioxidant Activity of Polysaccharides from Porphyra yezoensis (Rhodophyta) Food Sci Technol Int. 2008;14:479–486. doi: 10.1177/1082013208100665. [DOI] [Google Scholar]
- Zhu KX, Zhou HM, Qian HF. Proteins extracted from defatted wheat germ: Nutritional and structural properties. Cereal Chem. 2006;83:69–75. doi: 10.1094/CC-83-0069. [DOI] [Google Scholar]