Abstract
Pomegranate juice was diluted to 12° Brix and carriers (maltodextrin, gum Arabic, waxy starch) were added with varying concentrations of cellulose before being reduced to powder by spray drying. All carrier concentrations improved dryer yield, with gum Arabic being the most effective. The bulk density of the powder decreased when higher carrier concentrations were used. As cellulose concentration increased in solution, the solubility of the final product decreased. The optical properties of the powder were affected by the type and concentration of the carrier; powders produced with gum Arabic showed the greatest color change. Adding a carrier increased the Tg of the powder and its storage stability. Variation in the anthocyanin may be related to the type of carrier agent and its behavior during spray drying.
Keywords: Pomegranate juice, Spray drying, Physicochemical properties, Optical properties, Glass transition temperature (Tg)
Introduction
The pomegranate (Punica granatum, Punicaceae) is a native fruit of Iran, one of its biggest producers and exporters, producing over 0.67MT annually. Pomegranate juice has potential anti-atherogenic effects in healthy humans and atherosclerotic effects in mice along with other nutritional and health advantages, (Negi et al. 2005; Turk et al. 2008; Aviram et al. 2004). As a result, pomegranate juice has become popular worldwide.
Numerous studies on antioxidant activity have shown that pomegranate juice contains higher levels of antioxidants than most fruit juices, (Gil et al. 2000; Hong et al. 2008). Epidemiological studies have suggested that the consumption of red fruit juices, such as pomegranate, berry and grape, correlates with reduced risk of coronary heart disease, stroke, certain types of cancers and aging, (Hertog et al. 1997; Sumner et al. 2005). It has been reported that pomegranate juice is an important source of anthocyanins (cyanidin, delphinidin, pelargonidin), which gives the fruit and aril its red color, and phenolics and tannins (punicalin, pedunculagin, punicalagin, ellagic acid), (Kulkarni and Aradhya 2005).
The pomegranate is a seasonal fruit and not available year round. However, its high nutritional value makes it desirable to have a pomegranate product available throughout the year. Drying is an option. Dried products are used mainly as convenience foods and have long storage life at normal temperatures.
Dehydration by spray drying is used extensively in food-related industries for a wide range of products in dry particulate form as powders and agglomerates (Sagar and Suresh Kumar 2010). Economic considerations include the maintenance of hygienic conditions during processing, operational costs, and short contact time. However, the high sugar content of fruit juice gives it great economical potential, (Cano-Chauca et al. 2005).
The reconstitution quality of spray-dried powder is good because the product temperature is rarely elevated above 100 °C, (Adhikari et al. 2004). Spray-dried pomegranate juice powders can be added to food systems to provide a variety of functional benefits and nutritional properties. Ideally, spray-dried pomegranate powder should reconstitute instantly or serve as an anthocyanin-rich additive. However, control of the deposition of particles is a prevalent problem in spray drying, particularly for fruit and vegetable juices and purees, where low molecular-weight amorphous sugar particles and organic acids constitute more than 90% of the solids, (Bhandari et al. 1997).
Glass transition temperature (Tg) is defined as the temperature at which an amorphous system changes from a glassy to a rubbery state. Theoretically, in the glassy state, the high viscosity of the matrix (1012 Pa.s) does not allow the occurrence of diffusion-controlled reactions, (Schebor et al. 1999; Miao and Roos 2006). Fruit juices have a low Tg, due to their high content of sucrose (62 °C), Fructose (5 °C) and glucose (32 °C). They can stick on the dryer chamber wall during drying, leading to low product yield and operational problems.
The addition of high molecular weight additives to the product before atomizing is an widely-used alternative that increases Tg, (Bhandari and Howes 1999; Truong et al. 2005; Shrestha et al. 2007).Carrier agents such as maltodextrins, gum Arabic, waxy starch, and microcrystalline cellulose, when introduced into the feed solution, influence the properties and stability of the powder. Crystalline and amorphous forms of the same material powder show differences in particle size, particle shape, bulk density, physicochemical properties, chemical stability, water solubility, higroscopicity, flow properties and compatibility. However, there is limited scientific study specifically on the processing of pomegranate juice, (Maskan 2006; Magerramov et al. 2007; Gokoglu et al. 2009).
The present study investigates the spray drying of pomegranate juice and evaluates the bulk density, solubility, yield and Tg of the powder produced, as well as the color, anthocyanin content of the reconstituted juice. The influence of the carriers on the microstructure of the powder is analyzed and correlated with its functional properties.
Materials and methods
Pomegranates (cv. Malas) were purchased from a local market in Saveh, Iran. Only sweet, mature fruits were selected for these tests. The skin was removed and fruit juice extracted from the fleshy sacs using a hand-operated domestic press. The juice was stored at 4 °C overnight to allow the suspended particles to settle. The fresh juice was then clarified using a spiral ultrafiltration system with a molecular weight-cut off of 40 KD (Osmonic, USA). The cold sterile single strength clarified juice with 14.2% TSS (total suspended solid) was rapidly cooled and frozen at −25 °C.
Before dehydration, the juice was diluted and standardized with distilled water to 12°Brix TSS. Maltodextrin 20 DE (Glucosan Co., Iran), gum Arabic (Merck, Germany) and waxy starch (Merck, Germany) at 8% and 12% (w/w) concentrations were added after standardization. The solution was also treated with microcrystalline cellulose (Merck, Germany) at concentrations of 0%, 1.5%, 3% and 4.5% (w/w). All mixtures were homogenized using a laboratory homogenizer (UltraTrux, IKA, Germany) for 2 min.
Spray drying
Powder was produced using a mini spray dryer (BUCHI, B-191, Laboratory-Techniques LTD, Flawil, Switzerland). The spray dryer operates concurrently and has a spray nozzle, two-fluid atomizer with 0.7 mm diameter orifice. The inlet air temperature was 140 °C for all the solutions investigated. The outlet air temperature varied from 91 °C to 102 °C depending upon the sample.
Pomegranate juice was fed into the drying chamber using a peristaltic pump. The liquid feed rate to the dryer was 10% (0.000046 kg/s), the flow rate of the atomizing air was 600 mL min−1 and the aspirator rate was 60% of 100% (0.0149 kg/s). The testing was performed at constant process conditions. The product obtained was vacuum-sealed in polyethylene bags. The bags were then stored in a desiccator containing silica gel before quality evaluation. The physical properties of the powder measured were bulk density, solubility, dryer yield and Tg.
Bulk density
To determine the bulk density of 20 g of powder, it was weighed in a 100 mL graduated cylinder then gently dropped 10 times on a rubber mat from a height of 15 cm. The bulk density was calculated by dividing the mass of the powder by the volume that occupied the cylinder (Goula et al. 2008).
Solubility
Solubility was determined according to the Eastman and Moore method, Eastman and Moore (1984), with some modifications. First, 100 mL of distilled H2O was transferred into a blender jar. The powder sample (1 g, dry basis) was carefully added to the blender which operates at 15,000 rpm for 5 min. The solution was placed in a tube and centrifuged at 3,000× g for 5 min. An aliquot of 25 ml of the supernatant was then transferred to pre-weighed petri dishes and immediately oven-dried at 105 °C for 5 h. The solubility (%) was calculated as the weight difference (Cano-Chauca et al. 2005).
Yield
The weight of the dry material in the powder produced and the juice consumed was used to determine the spray-drying yield. This factor was calculated from following equation:
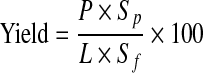 |
1 |
where P is the rate of powder production (g/min), SP is the percent of total solids of the powder, L is the feed flow rate (g/min), and SF is the percent of total solids of the feed (Chegini and Ghobadian 2007).
Tg point measurement
The Tg points of the powder was determined by differential scanning calorimetry (DSC) using a 2010 Modulated DSC (TA Instrument, New Castle, Del., USA). Indium and zinc (Perkin-Elmer standards) were used for temperature and heat flow calibration. The samples were cooled to desired temperature (−25 °C) by fast cooling to reach temperature equilibrium at this temperature. The purge gas used was dry nitrogen (25 mL/min).
Samples of 2–4 mg were sealed in a standard aluminum dish with an empty sealed aluminum dish as a reference sample. The tests were conducted −50 °C to 200 °C with a heating rate of 10 °C/min.
Color
The color of the fresh juice and reconstituted samples was analyzed using a spectrophotometer (with a program for color measuring, HACH, DR 4000U, USA) and the difference in their color parameters was calculated and the Hunter values (L, a, b) the optical parameters were compared.
Two grams of powder was dissolved in approximately 20 mL of distilled water and centrifuged at 4000 rpm for 15 min and the color of the supernatant was measured. Five measurements were recorded for each sample and their mean values calculated. The color values represented whiteness or brightness/darkness (L), redness/greenness (a) and yellowness/blueness (b). Another informative color attribute in the production of pomegranate juice is the total color difference (TCD) which is a combination of parameters L, a and b (Maskan 2006; Mirsaeedghazi et al. 2009), TCD is a colorimetric parameter commonly used to characterize the variation of color in foods during processing and is calculated as:
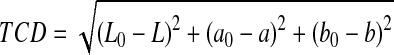 |
2 |
Browning index (BI) represents the purity of brown color and is calculated as (Askari et al., 2008):
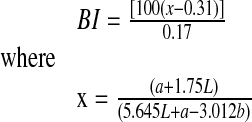 |
3 |
Anthocyanin determination
The total anthocyanin content (TAC) of the pomegranate juice was determined using the pH differential method with two buffer systems. Sample preparation was conducted as described for color measurement. The potassium chloride buffer was pH 1.0 (0.025 M) and sodium acetate buffer was pH 4.5 (0.4 M) (Lako et al. 2007).
Briefly, a 1 ml sample was mixed with 24 ml of corresponding buffers and read against water as a blank at 510 and 700 nm. Absorbance (A) was calculated as:
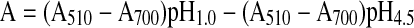 |
4 |
The total anthocyanin content of each sample (mg cyanidin-3-glucoside/100 ml) was calculated as:
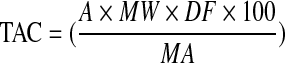 |
5 |
where MW is molecular weight of cyanidin-3- glucoside (4,492), DF is the dilution factor (25), and MA is the molar extinction coefficient of cyanidin-3-glucoside (26,900) (Cam et al. 2009).
Microstructure
The microstructure of the dehydrated pomegranate juice powdered was examined using a scanning electron microscope (XL-30, Philips, Amsterdam, The Netherlands). To obtain SEM images, small amount of powders were taken from well mixed powder samples and coated with very thin layer of gold under high vacuum conditions, to provide a reflective surface for the electron beam. Gold coating was carried out in a sputter coater BIO-RAD E-5200 (Bio-Rad Laboratories Ltd., London, UK) under a low vacuum in the presence of inert argon gas. The gold-coated samples were subsequently viewed under the microscope.
Statistical analysis
All experiments were conducted in triplicate and an analysis of variance was performed. The least significant difference at p < 0.05 was calculated using the Duncan Multiple Range Test on Minitab software (Minitab 15; Minitab Inc., Minneapolis, USA). The data were expressed as mean ± SD.
Results and discussions
Drying yield
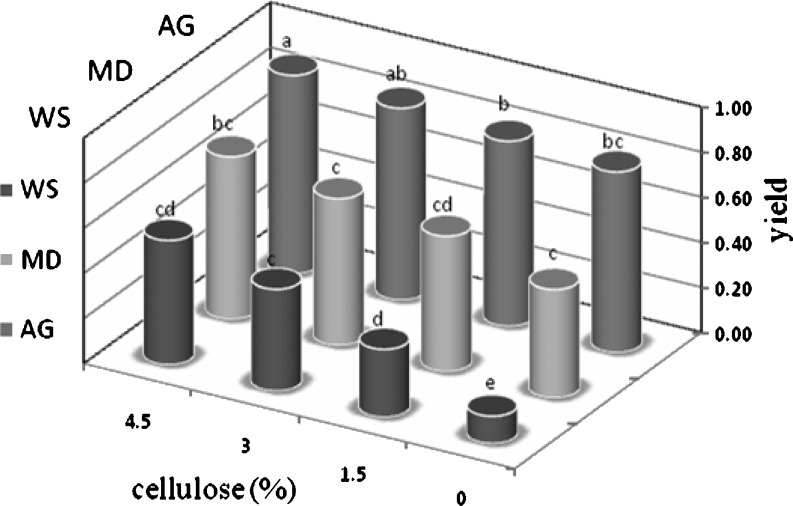
Drying yield testing was conducted with and without carrier substances. The effect of carrier on the dryer yield can be observed in Fig. 1. As seen, spray drying pomegranate juice without carriers causes the materials to adhere to the wall chamber and cyclone. As testing continued, a hard glass film formed on the walls. The addition of maltodextrin, gum Arabic, waxy starch or cellulose as a carrier changed the hygroscopic and thermoplastic character of powder. The results indicated that with the carriers’ concentration, yield increased and the deposits on the dryer walls decreased. The particles in the cyclone and chamber accumulated and measurement of the physical properties of the powder was possible. Carrier agents are necessary to produce of powders from juices with high sugar and acid content, due to their low Tg, which is similar to the results of previous studies (Chegini and Ghobadian 2007; Quek et al. 2007).
Fig. 1.
Effect of carrier and concentration on dryer yield. MD maltodextrin (12%); AG gum Arabic (12%); WS waxy starch (12%). The same letter for columns means that there is no significant difference (P < 0.05; n = 3)
The effect of carrier type on dryer yield is illustrated in Fig. 1 (with 12% of each carrier). Results showed that, at all concentrations, the addition of a carrier improved dryer yield, with gum Arabic being more effective than the other carriers. Dryer yield increased when the cellulose concentration increased. The effect of cellulose concentration was not consistent and was more evident when used with waxy starch, which was a poor carrier. The difference in the performance of the carriers may be related to the configuration of powders produced. When waxy starch was used as the main carrier, the powders had a crystalline configuration and wall deposition was enhanced, creating a predictably lower dryer yield.
Bulk density
Bulk density is the mass of the solid particles plus moisture divided by the total volume occupied by the particles, surface moisture and all pores, closed or open, in the surrounding atmosphere and is generally used to characterize the final product obtained by milling or drying (Johanson 2005; Barbosa-Cánovas and Juliano 2005).
The effect of the different carriers used to produce the juice powder on bulk density is shown in Figs. 2 and 3. As shown, there was no clear relationship between the concentration (8–12%) of the main carrier (maltodextrin: MD, gum Arabic: AG; waxy starch: WS) and bulk density. The bulk density of some samples powder decreased when carrier concentration increased. This phenomenon was observed when MD and AG were used without cellulose. Similar results were observed by (Goula et al.; Goula and Adamopoulos 2004; Abadio et al. 2004), when tomato and pineapple pulp were dried using maltodextrin as the carrier in a spray dryer. They stated that the particle size of the powder increased when the feed concentration increased. The lowest bulk density was observed with AG which may be related to the structure of the powder.
Fig. 2.
Effect of carrier and concentration on bulk density (n = 3)
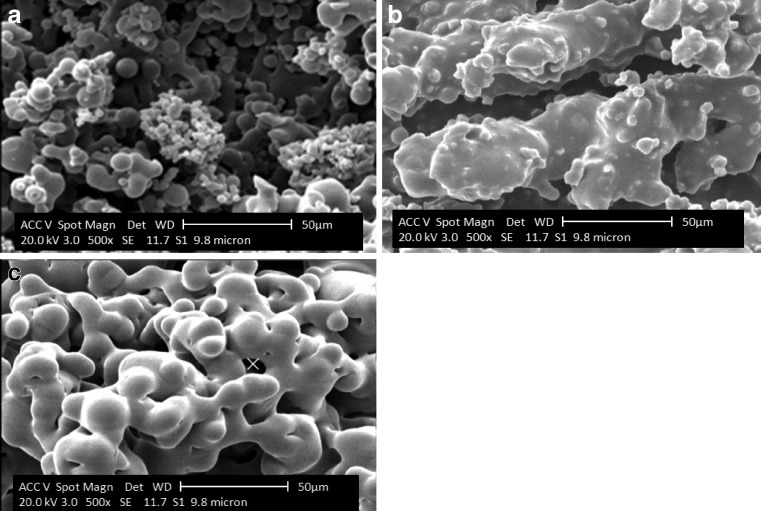
Fig. 3.
Effect of carrier type on microstructure of powder with gum Arabic (12%) (a), waxy starch (12%) (b) and maltodextrin (12%) (c) without cellulose
As seen in Fig. 3, the powders produced with maltodextrin and waxy starch presented non-spherical and smooth particles and more complex link bridges as a result of the higher hygroscopicity. When measured for bulk density, a less-packed structure formed in the measurement tube, with lower bulk density because of the fine structure of the powder particles containing Arabic gum. This can be explained by the molecular differentiation of Arabic gum with maltodextrin and waxy starch. Arabic gum has higher Tg point due to its larger molecule comparing two other carriers (Table 2) therefore powders produced by Arabic gum did not show amorphous behaviour during spray drying.
Table 2.
Glass-transition temperature (Tg) of spray dried pomegranate powders containing different carriers
| Composition of Carriers in Powder | Tg (°C) |
|---|---|
| Waxy starch (12%) | 25.1 ± 1.00 |
| Waxy starch (12%) + Cellulose (3%) | 48.0 ± 1.50 |
| Maltodextrin (12%) | 40.0 ± 3.30 |
| Maltodextrin (12%) + Cellulose (3%) | 59.9 ± 1.20 |
| Arabic Gum (12%) | 52.8 ± 1.00 |
| Arabic Gum (12%) + Cellulose (3%) | 77.0 ± 1.40 |
Using cellulose as an assisting carrier produced different effects on the bulk density at different concentrations. Branched and finer structured powder with lower bulk density formed when cellulose was added to the juice before spray drying. This was more evident when cellulose was used with MD and WS than with AG. However, a dissimilar effect was observed when more than 3% concentration was added. This may be from the formation of more packed powder in the measurement tube as cellulose collected in spaces between the branches, creating less internal space.
Solubility
The effect of carrier type and concentration on the solubility of the pomegranate juices powder is shown in Table 1. Testing showed that powder solubility was strongly affected by carrier type and, in some cases, by concentration. The crystalline configuration of the powders, especially those produced using waxy starch, were more stable than the more amorphous powder made with maltodextrin and gum Arabic as the main carrier.
Table 1.
Experimental characteristics of pomegranate juice powder
| Carrier | % | Cellulose (%) | Solubility (%) | L* | a* | b* | TCD | BI | Anthocyanins (mg/L) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MD | 8 | 0.0 | 92a | 98.3 | 1.79 | 1.69 | 0.77f | 3.01d | 75.2 ±1.27 |
| 2 | MD | 8 | 1.5 | 84b | 98.1 | 1.56 | 1.58 | 0.53f | 2.73d | 72.1 ± 1.24 |
| 3 | MD | 8 | 3.0 | 76c | 98.8 | 0.66 | 1.42 | 0.09 g | 1.89i | 71.4 ± 1.22 |
| 4 | MD | 8 | 4.5 | 68d | 98.6 | 0.65 | 1.99 | 0.57f | 2.47e | 69.2 ± 1.19 |
| 5 | MD | 12 | 0.0 | 92a | 98.5 | 1.50 | 1.38 | 0.36 g | 2.48e | 73.7 ± 1.26 |
| 6 | MD | 12 | 1.5 | 84b | 97.7 | 0.88 | 1.71 | 0.75f | 2.38e | 62.1 ± 1.06 |
| 7 | MD | 12 | 3.0 | 80bc | 98.7 | 1.21 | 1.22 | 0.07 h | 2.09gh | 74.6 ± 1.28 |
| 8 | MD | 12 | 4.5 | 76c | 98.7 | 1.21 | 0.89 | 0.09f | 1.77i | 75.8 ± 1.28 |
| 9 | AG | 8 | 0.0 | 92a | 96.7 | 0.60 | 5.05 | 4.14c | 5.68a | 48.7 ± 0.84 |
| 10 | AG | 8 | 1.5 | 84b | 97.0 | 0.99 | 3.70 | 4.91b | 4.54b | 56.7 ± 0.97 |
| 11 | AG | 8 | 3.0 | 80bc | 96.5 | 0.16 | 5.22 | 11.67a | 5.55a | 35.4 ± 0.61 |
| 12 | AG | 8 | 4.5 | 64d | 97.0 | 0.65 | 3.50 | 5.00b | 4.07b | 51.2 ± 0.87 |
| 13 | AG | 12 | 0.0 | 95a | 96.4 | 0.99 | 3.56 | 3.10d | 4.42b | 74.2 ± 1.27 |
| 14 | AG | 12 | 1.5 | 88ab | 96.9 | 0.92 | 3.15 | 3.90c | 3.91b | 72.2 ± 0.73 |
| 15 | AG | 12 | 3.0 | 84b | 97.1 | 0.78 | 2.82 | 3.13d | 3.46c | 54.6 ± 0.93 |
| 16 | AG | 12 | 4.5 | 66d | 97.3 | 0.75 | 2.82 | 2.94d | 3.43c | 67.5 ± 1.16 |
| 17 | WS | 8 | 0.0 | 52e | 97.6 | 1.02 | 2.43 | 1.45e | 3.22c | 47.5 ± 0.81 |
| 18 | WS | 8 | 1.5 | 45f | 97.3 | 1.21 | 1.50 | 1.21e | 2.41e | 62.9 ± 1.06 |
| 19 | WS | 8 | 3.0 | 40f | 97.9 | 1.10 | 1.23 | 0.49f | 2.05 h | 52.5 ± 0.91 |
| 20 | WS | 8 | 4.5 | 36 g | 97.3 | 0.93 | 1.34 | 1.41e | 2.05 h | 56.7 ± 0.97 |
| 21 | WS | 12 | 0.0 | 48f | 96.6 | 0.93 | 1.42 | 1.96e | 2.14 g | 67.1 ± 1.18 |
| 22 | WS | 12 | 1.5 | 36 g | 98.0 | 0.91 | 1.51 | 0.36f | 2.19 g | 55.4 ± 0.95 |
| 23 | WS | 12 | 3.0 | 32 h | 98.8 | 0.69 | 1.58 | 0.15f | 2.07 h | 51.2 ± 0.87 |
| 24 | WS | 12 | 4.5 | 28i | 98.7 | 0.84 | 1.19 | 0.03 g | 1.80i | 58.3 ± 0.97 |
The same letter in a column means that there is no significant difference (P < 0.05)
MD maltodextrin; AG gum Arabic; WS waxy starch
The low solubility of the starch powder in cold water should be noted. Table 1 shows no significant difference in powder solubility for maltodextrin and gum Arabic. However, when waxy starch was used in higher concentrations, the solubility of the powder decreased, as mentioned. When cellulose was used with the carriers, the solubility of the pomegranate juice powder decreased at all concentrations. A similar result has been reported for spray drying of mango powder (Cano-Chauca et al. 2005).
Effect of powder composition on Tg
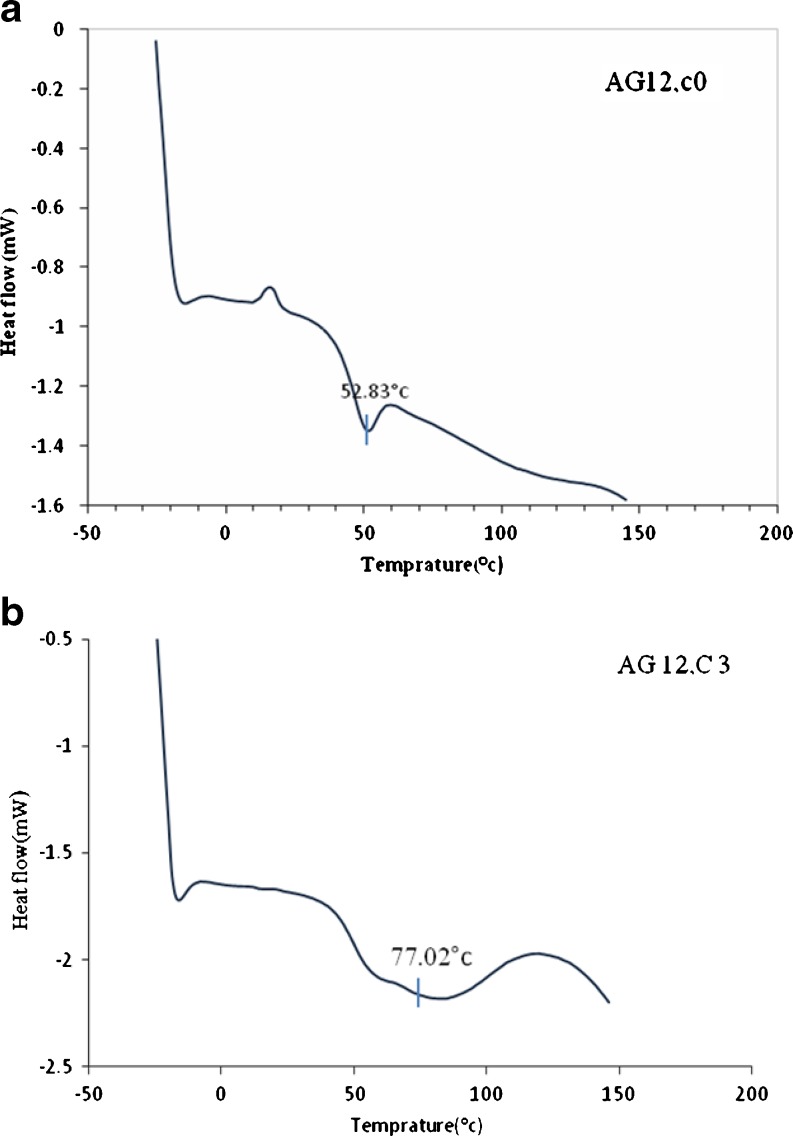
Figure 4 shows the Tg-DTA curves for spray-dried pomegranate juice containing gum Arabic with our without crystalline cellulose. The Tg of the compounds was difficult to detect using an ordinary DSC technique.
Fig. 4.
DSC profile for spray dried pomegranate juice produced using Arabic gum without cellulose (a) and with 3% cellulose (b)
Since powder could not be produced without the use of carriers, there is no DSC graph for such a powder. However, (Silva et al. 2006) observed lower values of Tg for powder without carriers produced by freeze drying. By comparing the curves obtained for the compounds with those of (Tonon et al. 2009). it is evident that the thermal decomposition of the powder changes with carrier concentration and type. Tg values for samples containing maltodextrin, waxy starch and gum Arabic were 39.96 °C, 25.12 °C and 52.83 °C, respectively. The higher Tg points to more stable powders during production and, especially, storage.
The effect of cellulose as a secondary carrier on Tg is demonstrated in Figs. 4a, b and Table 2. Crystalline cellulose was added in varying concentrations to the juice before spray drying. As seen, the Tg of the powders increased to 59.9, 48.07 and 77.02 for ones containing waxy starch, maltodextrin and Arabic gum respectively, as the concentration of cellulose increased.
Color measurement
The color parameters of the powders are shown in Table 1. To measure the optical properties of the reconstituted pomegranate juice, five measurement were made and the mean values calculated. (Laleh et al. 2006), reported that hydrolisis of pyrilium ring or 3-Glycoside structure and, in consequence, chalkon compound production, are the main factors responsible for the development of brown color, with temperature being the major determining factor.
All pomegranate powders were produced under the same operational conditions (feed and drying air temperature, flow and aspirator rate)., The differences in the optical properties of the powders and reconstituded juices depended upon carrier type and its concentration. This is evident in the SEM microraphs of the powders (Fig. 4). It can be seen that the more unwrapped structure was when gum Arabic was the main carrier. The mean TCD for the powders was 0.4, 4.8 and 0.88 for MD, AG and WS respectively.
The higher TCD for AG powder may be related to its structural properties. TCD was affected by the main carrier concentration. The mean TCD for 8% and 12% carrier concentrations were 0.49 and 0.31 for MD, 6.4 and 3.25 for AG, and 1.14 and 0.62 for WS, respectively. This demonstrates the reducing and preservative effect of the carrier on sample color. The coloring agent in the pomegranate may be absorbed into the carrier agent and protected from severe drying by high temperature during formation of the particles in the drying chamber.
The same behavior was observed for the browning index of the reconstituted juice. A higher BI was observed for AG. BI was affected by carrier concentration with the mean BI for 8% and 12% carrier concentrations were 2.5 and 2.17 for MD, 4.9 and 3.8 for AG and 2.4 and 2.05 for WS, respectively.
The effect of cellulose concentration on the color parametrs was also measured. Cellulose concentration did not have a clear effect on TCD, however BI was slightly affected. Higher concentrations of cellulose led to lower BI, with the mean BI values being 3.5, 3.02, 2.85 and 2.59 for 0%, 1.5%, 3% and 4.5%, respectively.
Anthocyanin
Anthocyanin concentration in the reconstituted juice is illustrated in Table 1. As reported, the retention of anyhocyanin is affected by temperature, so a consistent temperature was used for all experiments. The variation in anthocyanin content of the samples may be related to the carrier agent and its behavior during spray drying of the juice.
Microencapsulation of anthocyanin extracted from black carrot during spray drying was studied by (Ersus and Yurdagel 2007) at three drying temperatures (160 °C, 180 °C, 200 °C) and three types of maltodextrin (10, 20, 30 DE). The authors verified that powders containing maltodextrin with higher DE at 160 °C showed greater pigment retention than those produced at higher temperatures. Moreover, maltodextrin with higher DE increased the agglomeration of the powder and its exposure to oxygen, reducing oxidation and anthocyanin content.
The powder produced with gum Arabic had a fine and expanded structure with more surfaces exposed to oxygen, resulting in a lower anthocyanin content. The mean value of anthocyanin for samples using MD, AG and WS were 71.75, 57.56 and 56.44 mg/l, respectively. Greater agglomeration and stickiness, but the lowest anthocyanin content, was observed when powder was produced using WS as the main carrier. Because of the higher stickiness of these samples, they were collected from the connecting tubes, with greater air flow, which may be the cause of the lower anthocyanin content.
Conclusions
The use of carriers in pomegranate juice dehydration has an independent effect on the functional properties of the dehydrated material. The effect of carriers on the drying behaviour and quality are different for different parameters. Our results indicated that the highest yield was obtained when Arabic gum was used as carrier. And the finest powder and lowest bulk density was obtained using AG. The bulk density of some samples powder decreased when carrier concentration increased. Powder solubility was strongly affected by carrier type and concentration. Samples containing Arabic gum showed more solubility, however their optical properties were the poorest. In general it can be concluded that using Arabic gum as carrier leads to better physical properties (such as yield, bulk density, solubility and powder morphology). Better color properties and anthocyanin content can be obtained by using maltodextrin as carrier. Hence it seems better to use these carriers in combination.
Acknowledgements
The authors thank the vice chancellor for Research of University of Tehran, Iran, for supporting the research reported in this article (Grant number: 7106014/1/02).
References
- Abadio FDB, Domingues AM, Borges SV, Oliveira VM. Physical properties of powdered pineapple (Ananas Comosus) juice: effect of maltodextrin concentration an atomization speed. J Food Eng. 2004;64(3):285–287. doi: 10.1016/j.jfoodeng.2003.10.010. [DOI] [Google Scholar]
- Adhikari B, Howes T, Bhandari BR, Troung V. Effect of addition of maltodextrin on drying kinetics and stickiness of sugar and acid-rich foods during convective drying: experiments and modeling. J Food Eng. 2004;62:53–68. doi: 10.1016/S0260-8774(03)00171-7. [DOI] [Google Scholar]
- Askari GR, Emam-Djomeh Z, Mousavi SM. Investigation of the effects of microwave treatment on the optical properties of apple slices during drying. Drying Technol. 2008;26:1362–1368. doi: 10.1080/07373930802333502. [DOI] [Google Scholar]
- Aviram M, Rosenblat M, Gaitini D, Nitecki S, Hoffman A, Dornfeld L, Volkova N, Presser D, Attias J, Liker H, Hayek T. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin Nutr. 2004;23(3):423–433. doi: 10.1016/j.clnu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Barbosa-Cánovas GV, Juliano P. Physical and chemical properties of food powders. In: Onwulata C, editor. Encapsulated and powdered foods. Boca Raton: Taylor and Francis; 2005. pp. 39–71. [Google Scholar]
- Bhandari BR, Howes H. Implication of glass transition for the drying and stability of dried foods. J Food Eng. 1999;40:71–79. doi: 10.1016/S0260-8774(99)00039-4. [DOI] [Google Scholar]
- Bhandari BR, Datta N, Howes T. Problems associated with spray drying of sugar-rich foods. Drying Tech. 1997;15(2):671–684. doi: 10.1080/07373939708917253. [DOI] [Google Scholar]
- Cam M, Hısıl Y, Durmaz G. Classification of eight pomegranate juices based on antioxidant capacity measured by four methods. Food Chem. 2009;112:721–726. doi: 10.1016/j.foodchem.2008.06.009. [DOI] [Google Scholar]
- Cano-Chauca M, Stringheta PC, Ramos AM, Cal-Vidal J. Effect of carriers on the microstructure of mango powder obtained by spray drying and its functional characterization. Innov Food Sci Emerg Tech. 2005;6:420–428. doi: 10.1016/j.ifset.2005.05.003. [DOI] [Google Scholar]
- Chegini GR, Ghobadian B. Spray dryer parameters for fruit juice drying. World J Agric Sci. 2007;3(2):230–236. [Google Scholar]
- Eastman JE, Moore CO (1984) Cold water soluble granular starch for gelled food composition. US Patent 4 465 702
- Ersus S, Yurdagel U. Microencapsulation of anthocyanin pigments of black carrot (Daucus Carota L.) by spray drier. J Food Eng. 2007;80:805–812. doi: 10.1016/j.jfoodeng.2006.07.009. [DOI] [Google Scholar]
- Gil MI, Tomás-Barberan FA, Hess-Pierce B, Holcroft DM. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- Gokoglu N, Topuz SK, Yerlikaya P. Effects of pomegranate sauce on quality of marinated anchovy during refrigerated storage. LWT Food Sci Tech. 2009;42:113–118. doi: 10.1016/j.lwt.2008.04.007. [DOI] [Google Scholar]
- Goula AM, Adamopoulos KG. Spray drying of tomato pulp: effect of feed concentration. Drying Tech. 2004;22(10):2309–2330. doi: 10.1081/DRT-200040007. [DOI] [Google Scholar]
- Goula AM, Karapantsios TD, Achilias DS, Adamopoulos KG. Water sorption isotherms and glass transition temperature of spray dried tomato pulp. J Food Eng. 2008;85:73–83. doi: 10.1016/j.jfoodeng.2007.07.015. [DOI] [Google Scholar]
- Hertog MGL, Sweetnam PM, Fehily AM, Elwood PC, Kronhout D. Antioxidant flavonols and ischaemic heart disease in a welsh population of men. Caerphilly Study Am J Clin Nutr. 1997;65:1489–1494. doi: 10.1093/ajcn/65.5.1489. [DOI] [PubMed] [Google Scholar]
- Hong MY, Seeram NP, Heber H. Pomegranate polyphenols down-regulate expression of androgen-synthesizing genes in human prostate cancer cells overexpressing the androgen receptor. J Nutr Biochem. 2008;19:848–855. doi: 10.1016/j.jnutbio.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson K. Powder flow properties. In: Onwulata C, editor. Encapsulated and powdered foods. Boca Raton: Taylor and Francis; 2005. pp. 331–361. [Google Scholar]
- Kulkarni PA, Aradhya SM. Chemical changes and antioxidant activity in pomegranate arils during fruit development. Food Chem. 2005;87:319–324. doi: 10.1016/j.foodchem.2004.09.029. [DOI] [Google Scholar]
- Lako J, Trenerry VC, Wahlqvist M, Wattanapenpaiboon N, Sotheeswaran S, Premier R. Phytochemical flavonols, carotenoids and the antioxidant properties of a wide selection of Fijian fruit, vegetables and other readily available foods. Food Chem. 2007;101:1727–1741. doi: 10.1016/j.foodchem.2006.01.031. [DOI] [Google Scholar]
- Laleh GH, Frydoonfar H, Heidary R, Jameei R. The effect of light, temperature, ph and species on stability of anthocyanin pigments in four berberís species. Pak J Nutr. 2006;5(1):90–92. doi: 10.3923/pjn.2006.90.92. [DOI] [Google Scholar]
- Magerramov MA, Abdulagatov AL, Azizov ND, Abdulagatov IM. Effect of temperature, concentration, and pressure on the viscosity of pomegranate and pear juice concentrates. J Food Eng. 2007;80:476–489. doi: 10.1016/j.jfoodeng.2006.05.030. [DOI] [Google Scholar]
- Maskan M. Production of pomegranate (Punica Granatum L.) juice concentrate by various heating methods: colour degradation and kinetics. J Food Eng. 2006;72:218–224. doi: 10.1016/j.jfoodeng.2004.11.012. [DOI] [Google Scholar]
- Miao S, Roos YH. Isothermal study of nonenzymatic browning kinetics in spray-dried and freeze-dried systems at different relative vapor pressure environments. Innov Food Sci Emerg Tech. 2006;7:182–194. doi: 10.1016/j.ifset.2005.11.001. [DOI] [Google Scholar]
- Mirsaeedghazi H, Emam-Djomeh Z, Mousavi SM. Concentration of pomegranate juice by membrane processing: membrane fouling and changes in juice properties. J Food Sci Technol. 2009;46(6):538–542. [Google Scholar]
- Negi PS, Jayapraprakasha GK, Jena BS. Antioxidant and antimutagenic activities of pomegranate peel extract. Food Chem. 2005;80:393–397. doi: 10.1016/S0308-8146(02)00279-0. [DOI] [Google Scholar]
- Quek SY, Chok NK, Swedlund P. The physicochemical properties of spray dried watermelon powders. Chem Eng Proc. 2007;46:386–392. doi: 10.1016/j.cep.2006.06.020. [DOI] [Google Scholar]
- Sagar VR, Suresh Kumar P. Recent advances in drying and dehydration of fruits and vegetables: a review. J Food Sci Technol. 2010;47(1):15–26. doi: 10.1007/s13197-010-0010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schebor C, Buera MDP, Karel M, Chirife J. Color formation due to non enzymatic browning in amorphous, glassy, anhydrous, model systems. Food Chem. 1999;65:427–432. doi: 10.1016/S0308-8146(98)00041-7. [DOI] [Google Scholar]
- Shrestha AK, Howes T, Adhikari BP, Bhandari BR. Water sorption and glass transition properties of spray dried lactose hydrolyzed skim milk powder. LWT Food Sci Tech. 2007;40:1593–1600. doi: 10.1016/j.lwt.2006.11.003. [DOI] [Google Scholar]
- Silva MA, Sobral PJA, Kieckbusch TG. State diagrams of freeze-driedcamu–camu (Myrciariadubia (HBK)McVaugh) pulp with and without maltodextrin addition. J Food Eng. 2006;77(3):426–432. doi: 10.1016/j.jfoodeng.2005.07.009. [DOI] [Google Scholar]
- Sumner MD, Elliott-Eller M, Weidner G, Daubenmier JJ, Chew MH, Marlin R, Raisin CJ, Ornish D. Effects of pomegranate juice consumption on myoca rdial perfusion in patients with coronary heart disease. Am J Cardiol. 2005;96:810–814. doi: 10.1016/j.amjcard.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Tonon RV, Baroni AF, Brabet C, Gibert O, Pallet D, Hubinger MD. Water sorption and glass transition temperature of spray dried Açai (Euterpe Oleracea Mart.) juice. J Food Eng. 2009;94:215–221. doi: 10.1016/j.jfoodeng.2009.03.009. [DOI] [Google Scholar]
- Truong V, Bhandari BR, Howes T. Part1: moisture and glass optimization of co-current spray drying process of sugar rich foods: transition temperature profile during drying. J Food Eng. 2005;71:55–65. doi: 10.1016/j.jfoodeng.2004.10.017. [DOI] [Google Scholar]
- Turk M, Sonmez M, Aydin M, Yuce A, Gur S, Yuksel M, Aksu EH, Aksoy H. Effects of pomegranate juice consumption on sperm quality, spermatogenic cell density, antioxidant activity and testosterone level in male rats. Clin Nutr. 2008;27:289–296. doi: 10.1016/j.clnu.2007.12.006. [DOI] [PubMed] [Google Scholar]