Abstract
The cholesterol-lowering properties of Auricularia auricula are commonly attributed to the presence of polysaccharides based on previous research. The present study was designed to investigate the effects of ethanol extract of A. auricula (AAE) on hypercholesterolemia in ICR mice. AAE contained more than 16% (g/g) polyphenolic compounds, excluding other interfering factors such as polysaccharides, water-soluble fibre and protein. Thirty-six mice were randomly assigned to three groups (n = 12). The experimental group was fed cholesterol-enriched diet (CED) with oral administration of AAE (150 mg/kg/d b.w.) for 8-week, normal control group and CED control group received either a regular diet (RD) or CED along with oral administration of equal volume distilled water. Serum lipid profiles and antioxidant status were measured in addition to fecal neutral cholesterol and bile acids. AAE showed a remarkable hypocholesterolemic effect, improving antioxidant status, decreasing the level of total cholesterol and atherosclerosis index, increasing the level of high-density lipoprotein cholesterol and fecal excretion of bile acids. No apparent effects on serum triglycerides, low-density lipoprotein cholesterol, fecal excretion of neutral cholesterol and feeding efficiency were observed among all groups. These results indicated that A. auricula functional components, which prevented hypercholesterolemia contained polyphenolic compounds, in addition to polysaccharides.
Keywords: Auricularia auricula, Hypercholesterolemia, Polyphenolic compounds, HMG-CoA reductase, Antioxidant status
Introduction
Auricularia auricula is a precious macro-fungus and its fruiting bodies have been widely used in Chinese cuisine and are known for their pharmaceutical effects in Chinese traditional medicine. In recent years, A. auricula was reported to have many biological activities, including anticoagulant activity (Yoon et al. 2003), antitumor activity (Mizuno et al. 1995), hypoglycemia (Takeuchi et al. 2004) and hypocholesterolemia (Cheung 1996). Up to now, considerable attention of A. auricula has been focused on dietary fiber and polysaccharide components, mainly extracted from its fruiting body. Previous studies have also shown that A. auricula polysaccharides have similar biological activities as A. auricula. Many nutritionists probed into A. auricula polysaccharide components (Aletor 1995), conformational change of A. auricula polysaccharides (Zhang et al. 1995a), molecular weights of A. auricula polysaccharides (Zhang et al. 1995b), improvement of production of A. auricula polysaccharides (Wu et al. 2006) and impact on lipid metabolism (Chen et al. 2008). Most recently, some health-promoting diet formulae have been developed using A. auricula as the major ingredient with combination of other nutritional herbal foods (Luo et al. 2009a, b). In fact, the fruiting bodies of A. auricula contain not only polysaccharides, but also abundant alkaloid, thiamin, riboflavin, ascorbic acid, vitamin D2 and minerals. Acharya et al. (2004) reported that A. auricula ethanol extracts possessed the antioxidant and nitric oxide synthase activation properties. Methanol extract of A. auricula could inhibit lipid peroxidation and decrease liver damage in benzo(α)pyrene-treated mice (Chang et al. 1998). However, there is no further investigation on the effect of these substances of A. auricula on lipid metabolism.
Therefore, the present study was designed to investigate the hypocholesterolemic effects of A. auricula ethanol extract (AAE), we investigated effects of AAE on serum and hepatic lipid profiles, HMG-CoA reductase activity, antioxidant status in addition to fecal excretion of neutral cholesterol and bile acids in ICR mice. The results of this study will advance knowledge of cardiovascular benefits of A. auricula to better understand their application in the prevention/treatment of cardiovascular disease using functional foods and nutraceuticals.
Materials and methods
Plant material and preparation of AAE
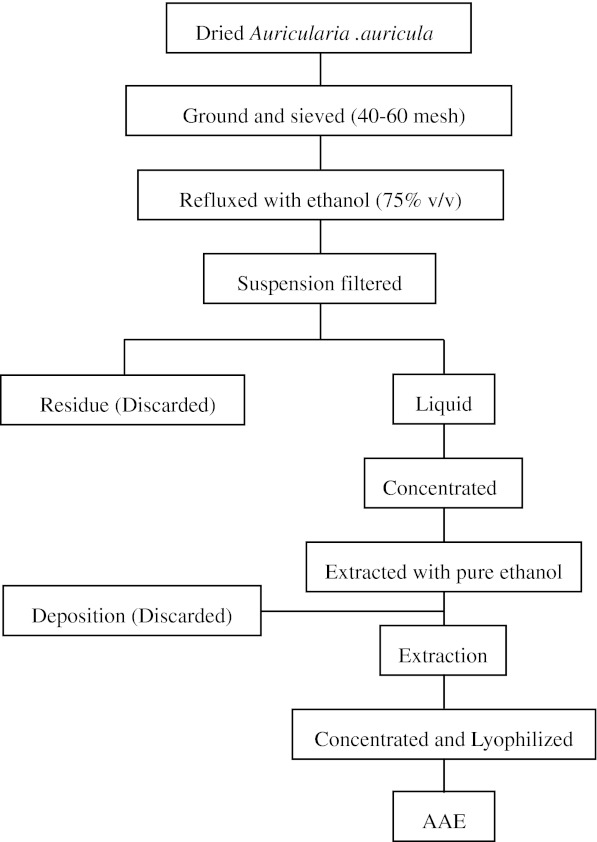
The sample of dried A. auricula fruiting bodies were cultured in the Daxinganling region, Heilongjiang province, China, and it was authenticated by Dr. B. Zhao, Department of Plant Sciences, China Agricultural University. The procedure for Auricularia auricula ethanol extracts was carried out as shown in Fig. 1. The sample of dried A. auricula fruiting bodies was ground and sieved through screens (40–60 mesh). Dried-powder of A. auricula contained (g/100 g dry weight): carbohydrates 82 (including 54 g total dietary fibres), crude protein 8, fat 1.5 and ash 8.5. This dried powder (200 g) was suspended in 6 l of ethanol (75%, v/v) and refluxed for 2 h at 80 °C. This procedure was repeated thrice and the suspension was filtered through Whatman No. 2 paper to remove the ethanol-insoluble materials such as fiber and polysaccharide compounds. This liquid was concentrated in a rotary evaporator under reduced pressure, and concentrated solution was extracted by pure ethanol for 24 h at 4 °C (proportion of concentrated solution to ethanol was 1:4). The solution of ethanol was filtered through Whatman No. 2 paper to remove deposition such as water-soluble fibre, polysaccharide, and protein components. This procedure was repeated four times and the ethanol extract was concentrated in a rotary evaporator under reduced pressure and then lyophilized. This preparation is named A. auricula ethanol extract (AAE). The total phenol content of AAE was determined using the Folin Ciocalteu method (Zhang et al. 2008). The total phenol content was 183.7 ± 15.6 mg/g, expressed as gallic acid equivalent.
Fig. 1.
Flow process chart about preparation of Auricularia auricular ethanol extract (AAE)
Animals and treatment
Four-week-old male ICR mice (Experimental Animal Center of Beijing, China) were acclimatized for 1 week before being randomly assigned into the experimental groups. The animals were housed in plastic cages (6 mice/cage) in a room with a 12 h light–dark cycle (8 am–8 pm), temperature of 23 ± 2 °C and a humidity of 55 ± 5%. During the acclimatization period, each animal was raised on regular diet and water ad libitum. At five-week old, the ICR mice were randomly divided into three groups (n = 12), the first group (EG) was experimental group fed cholesterol-enriched diet (CED) and orally administered 150 mg/kg/d b w of A. auricula ethanol extract; the second group (RD) was fed regular diet; the third group (CEDC) was CED control group which were only fed CED; meanwhile RD and CEDC were fed along with oral administration of equal volume of distilled water. At the end of the experimental period (8th week), the fecal samples of each group were collected, lyophilized and stored under ambient temperature prior to use. After blooding completely, the animals were sacrificed by cervical dislocation. The livers were excised, weighed and stored at −80 °C until analysis. Composition of animal diets is listed in Table 1 (Experiment Animal Center of Beijing, China). Body weights and daily food intake were measured every 2 days and at every other week blood samples were collected for analysis. The experimental protocols were conducted according to the Guide for the Care and Use of Laboratory Animals established by China Agricultural University, Beijing, China.
Table 1.
Chemical composition of fed diets (g/100 g d.m.)
| Ingredients | RD | CED |
|---|---|---|
| Corn starch | 59 | 38 |
| Casein | 21.1 | 21.1 |
| Cellulose | 4.9 | 4.9 |
| Fat | 4.2 | 4.2 |
| Minerals | 10.8 | 10.8 |
| Yolk powder | – | 10 |
| Cholesterol | – | 1 |
| Lard | – | 10 |
RD Regular diet, CED Cholesterol enriched diet
Blood sampling
On weeks 2, 4, 6 and 8 of the experimental period, blood samples were taken from the orbital venous plexus of ICR mice using a capillary tube without anesthesia, after a 16 h fast. The blood samples were placed in a plastic tube and incubated at 37 °C for 15 min, then centrifuged for 8 min at 4,000 rpm. The serum samples were stored at −20 °C until analysis.
Serum and hepatic lipids
Serum lipid profile, including total cholesterol (TC), low density lipoprotein-cholesterol (LDL-C), high density lipoprotein-cholesterol (HDL-C) and triglyceride (TG) was assayed individually using the enzyme kits (Beijing Zhongsheng Hightech Bioengineering Company, Beijing, China) on Alcyon 300 auto-analyzer (Abbott Laboratories Ltd., US). The atherogenic index (AI) was calculated as: (TC−(HDL-C))/HDL-C.
Hepatic lipids were extracted from 300 mg of the liver of ICR mice with chloroform: methanol (2:1, v/v), according to method of Folch et al. (1957). After extraction, 1 ml extraction was dried under a nitrogen stream. The dried lipid residues were dissolved in 500 μl distilled water. Triton X-100 was added to the dissolved lipid solution to produce final concentration of 5 g/L. The hepatic TC and TG were analyzed with the same enzyme kit as used for the serum analysis.
Hepatic antioxidant status determination
The total antioxidant capacity (TAC), superoxide dismutase (SOD) activity and malondialdehyde (MDA) concentration were determined in liver homogenates with commercial kits obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). TAC was measured by the reaction of phenanthroline and Fe2+ using a spectrophotometer at 520 nm, a TAC unit is defined as the amount of antioxidants required to make absorbance increase by 0.01 in 1 ml serum. For SOD measurement, superoxide radicals were generated by NADPH and phenazine methosulfate PMS under non-acidic conditions, which reduce (NBT) nitroblue tetrazolium salt and form a blue coloured formazon, which can be measured at 560 nm. Free radical damage was determined by specially measuring MDA. MDA formed as an end product of lipid peroxidation was treated with thiobarbituric acid to generate a colored product that was measured at 532 nm.
Hepatic 3-hydroxy-3-methylglutary CoA (HMG-CoA) reductase activity
The activity of HMG-CoA reductase in mice was determined by the method of Edwards et al. (1979). The isolated hepatic microsomes were mixed with an equal volume of 50% glycerol in phosphate buffer (0.1M sucrose, 0.05M KCl, 0.04M potasium phosphate, 0.03M potassium EDTA and 10 mM DTT; pH 7.2). The suspension was homogenized and incubated at 37 °C for 60 min to solubilize HMG-CoA reductase. The activity of the solubilized HMG-CoA reductase was obtained by assaying the enzyme in phosphate buffer (0.2M KCl, 0.16M potassium phosphate, 0.004M EDTA, and 0.01M DTT, pH 6.8) together with 0.2 mM NADPH and 0.1 mM RS-HMG-CoA and determined at 37 °C using a spectrophotometer (GBC scientific equipment Pty Ltd, Australia). The results were expressed as nmol mevalonate synthesized per min per mg protein.
Determination of fecal neutral cholesterol and bile acids
The fecal neutral cholesterol was extracted and analyzed as per the method of Martensson (AOAC 2000) with slight modification. Fecal samples (0.2 g) were homogenized with 2 ml of a mixture of 10 mol/l NaOH and 96% ethanol (1:2, v/v) and kept at 70 °C for 45 min. The samples were allowed to cool to room temperature followed by centrifugation at 1,000 × g for 5 min. The supernatant was removed and extracted with n-hexane thrice. The combined hexane phases were washed with 70% ethanol until neutral for neutral cholesterol analysis; and the lower phases were acidified with HCl and extracted with chloroform:methanol (2:1, v/v) for bile acids analysis. The cholesterol and coprostanol were analyzed using a Shimadzu GC-14 gas chromatography, Japan with a flame ionization detector and a 30 m × 0.25 mm DM-5 capillary column maintained at 290 °C using nitrogen gas as carrier. The initial column temperature was 220 °C and was increased to 300 °C at a rate of 2 °C/min. The final temperature was held for 10 min. Column flow rate was 1.5 ml/min. Pure cholesterol and coprostanol were used as standards (Sigma-Aldrich, St. Louis, MO). Total neutral cholesterol was expressed as percent of total fecal cholesterol plus coprostanol. Fecal acidic steroids were determined by an enzyme kit (Total Bile Acid Kit, Wako, Japan).
Statistical analysis
Data were presented as mean ± SD for 12 ICR mice per group. Statistical analysis was performed using one-way analysis of variance followed by Duncan’s multiple range tests. P-values of less than 0.05 were considered to be statisticaly significant. Analysis was performed with SPSS 10.0.
Results and discussion
Body weight, food intake, and feeding efficiency
As shown in Table 2, after the 8-week experimental period, the final body weights of the EG and CEDC (39 ± 1.6 and 41 ± 2.4 g, respectively) were not significantly different from that of RD (39 ± 2.2 g), and the levels of food intake of the EG and CEDC (2.1 ± 0.7 and 2.1 ± 0.3 g, respectively) were not significantly different from that of RD (2.2 ± 0.1). The levels of feeding efficiency of the EG and CEDC were (14.2 ± 0.5 and 15.2 ± 0.4%, respectively) also not significantly different from that of RD (13.6 ± 0.6%).
Table 2.
Changes in body weight and food intake of mice during 8-week experimental period
| RD | CEDC | EG | |
|---|---|---|---|
| Initial body wt, g | 20 ± 1.2a | 20 ± 1.2a | 20 ± 1.2a |
| Final body wt, g | 39 ± 2.2a | 41 ± 2.4a | 39 ± 1.6a |
| Weight gain, g/day | 0.3 ± 0.1a | 0.3 ± 0.2a | 0.3 ± 0.1a |
| Food intake, g/day | 2.2 ± 0.1a | 2.1 ± 0.3a | 2.1 ± 0.7a |
| Feeding efficiency,% | 13.6 ± 0.6a | 15.2 ± 0.4a | 14.2 ± 0.5a |
n = 12 mice per group; Values in a row with different superscript letters differ significantly (p < 0.05). Feeding efficiency: (daily weight gain/daily food intake) × 100; RD: Regular diet control group; CEDC: Cholesterol-enriched diet control group; EG: Cholesterol-enriched diet + oral administration of Auricularia auricula ethanol extract experimental group
Serum lipids and atherogenic index profiles
As shown in Table 3, the serum concentrations of total cholesterol increased significantly (p < 0.05) after feeding CED. The administration of AAE (150 mg/kg/d bw) significantly attenuated the increase of serum TC (p < 0.05) during initial 6-week, however, its level was elevated gradually and close to the CEDC level at 8th week. Oral administration of AAE gradually increased the level of HDL-C since 4th week significantly (p < 0.05), and the difference became greater over time. Nevertheless, the consumption of AAE had no significant effect on serum TG and had a tendency to regulate the levels of TG. In addition, the administration of AAE had no effect on serum levels of LDL-C in ICR mice fed CED. Additionally, the oral AAE administration decreased AI in EG group significantly (p < 0.05), compared with that in CEDC group.
Table 3.
Effect of Auricularia auricula ethanol extract on serum lipids in mice during 8-week experimental period
| Experimental period | Serum lipids mmol/L | ||
|---|---|---|---|
| RD | CEDC | EG | |
| TG | |||
| 2 | 0.21 ± 0.03a | 0.16 ± 0.02b | 0.16 ± 0.02b |
| 4 | 0.22 ± 0.05a | 0.15 ± 0.04b | 0.16 ± 0.04b |
| 6 | 0.21 ± 0.06a | 0.16 ± 0.05b | 0.18 ± 0.07c |
| 8 | 0.23 ± 0.04a | 0.17 ± 0.03b | 0.21 ± 0.04c |
| TC | |||
| 2 | 0.70 ± 0.03a | 1.58 ± 0.08b | 1.34 ± 0.02c |
| 4 | 0.71 ± 0.04a | 1.62 ± 0.07b | 1.45 ± 0.04c |
| 6 | 0.69 ± 0.08a | 1.64 ± 0.06b | 1.54 ± 0.03c |
| 8 | 0.72 ± 0.06a | 1.68 ± 0.09b | 1.63 ± 0.06b |
| HDL-C | |||
| 2 | 0.25 ± 0.03a | 0.37 ± 0.04b | 0.39 ± 0.03b |
| 4 | 0.26 ± 0.04a | 0.36 ± 0.08b | 0.41 ± 0.08c |
| 6 | 0.25 ± 0.06a | 0.36 ± 0.04b | 0.45 ± 0.07c |
| 8 | 0.26 ± 0.04a | 0.38 ± 0.06b | 0.48 ± 0.09c |
| LDL-C | |||
| 2 | 0.03 ± 0.01a | 0.06 ± 0.01b | 0.05 ± 0.01b |
| 4 | 0.03 ± 0.02a | 0.06 ± 0.02b | 0.06 ± 0.01b |
| 6 | 0.02 ± 0.01a | 0.05 ± 0.02b | 0.05 ± 0.02b |
| 8 | 0.02 ± 0.01a | 0.05 ± 0.01b | 0.04 ± 0.02b |
| AI | |||
| 2 | 1.80 ± 0.04a | 3.27 ± 0.08b | 2.44 ± 0.05c |
| 4 | 1.73 ± 0.05a | 3.50 ± 0.10b | 2.52 ± 0.04c |
| 6 | 1.76 ± 0.04a | 3.56 ± 0.09b | 2.42 ± 0.06c |
| 8 | 1.76 ± 0.03a | 3.42 ± 0.11b | 2.40 ± 0.08c |
n = 12 mice/group; Values in a row with different superscript letters differ significantly (p < 0.05). AI: atherosclerotic index; RD: CEDC, EG: See Table 2
Hepatic lipid profiles
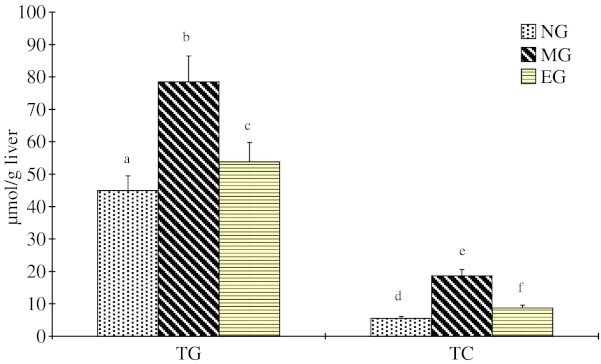
Hepatic TC and TG levels were significantly (p < 0.05) elevated by CED (Fig. 2). Expectedly, the consumption of AAE in EG group significantly (p < 0.05) decreased TC and TG content in the whole liver by 113.80% and 45.86%, respectively.
Fig. 2.
Effect of Auricularia auricular ethanol extract on hepatic lipids profile in hyperlipidemia mice n = 12 mice/group. Values not sharing the same letter within TG or TC are significantly different (p < 0.05); RD; CEDC; EG: As in Table 2
Hepatic antioxidant status
Cholesterol-enriched diet significantly reduced the levels of TAC, SOD and elevated MDA concentration (p < 0.05) in CEDC (Table 4). Oral administration of AAE significantly improved the hepatic antioxidant status through elevating TAC and SOD levels with lowering MDA concentration (p < 0.05).
Table 4.
Effect of Auricularia auricula ethanol extract on hepatic antioxidant status of CED-fed mice after 8 weeks
| TAC, U/mg pro | SOD, U/mg pro | MDA, nmol/mg pro | |
|---|---|---|---|
| RD | 1.91 ± 0.26a | 175.09 ± 10.53a | 0.75 ± 0.08a |
| CEDC | 1.03 ± 0.17b | 91.22 ± 8.27b | 1.54 ± 0.09b |
| EG | 1.75 ± 1.62c | 138.32 ± 12.27c | 0.88 ± 0.06a |
n = 12 mice/group; Values in a column with different superscript letters differ significantly (p < 0.05). RD: CEDC: EG: As in Table 2
Hepatic HMG-CoA reductase activity
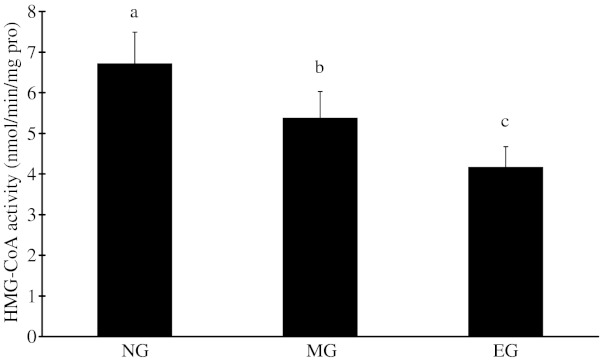
Hepatic HMG-CoA reductase activity was significantly decreased in CEDC compared with RD (p < 0.05), oral administration of AAE significantly decreased HMG-CoA reductase activity compared with CEDC (p < 0.05) (Fig. 3).
Fig. 3.
Effect of Auricularia auricular ethanol extract on HMG-CoA activity on hyperlipidemia mice n = 12 mice/group. Values not having the same letter are significantly different (p < 0.05); RD; CEDC; As in Table 2
Fecal excretion of neutral cholesterol and bile acids
The daily fecal dry mass showed no significant differences among groups (Table 5). The fecal excretion of neutral steroids and bile acids were increased in CEDC compared with RD. However, oral administration of AAE could promote fecal bile acids output significantly by 4.5-fold, compared with CEDC (p < 0.05) but no significant effect was observed on fecal excretion of neutral steroids.
Table 5.
Effect of Auricularia auricula ethanol extract on fecal neutral sterol and total bile acids concentrations of CED-fed mice
| Neutral steroids | RD | CEDC | EG |
|---|---|---|---|
| Cholesterol, mg/d | 0.20 ± 0.03a | 0.97 ± 0.05b | 0.95 ± 0.04b |
| Coprostanol, mg/d | 0.52 ± 0.07a | 1.30 ± 0.25b | 1.35 ± 0.11b |
| Total, mg/d | 0.72 ± 0.06a | 2.27 ± 0.26b | 2.30 ± 0.21b |
| Total bile acids, mg/d | 0.48 ± 0.03a | 1.51 ± 0.10b | 6.97 ± 0.12c |
| Dry feces weight (g/mouse/d) | 0.45 ± 0.03b | 0.43 ± 0.02b | 0.46 ± 0.01b |
n = 12 mice/group; Values in a row with different superscript letters differ significantly (p < 0.05) RD: CEDC: EG: As in Table 2
Recent studies in animals and human subjects have shown a significant hypocholesterolemic effect of dietary A. auricula. In this study, we further determined the effects of A. auricula ethanol extract on the serum lipid profiles, hepatic lipid profiles, antioxidant status and fecal excretion of neutral cholesterol and bile acids in ICR mice fed with CED. The AAE made with this purification process contained more than 16% (g/g) A. auricula polyphenolic compounds, excluding other interfering components such as polysaccharides, water-soluble fibre and protein components.
Polyphenolic compounds have been reported to exert an inhibitory effect on food intake and growth by decreasing protein digestibility (Tebib et al. 1994). In this experiment, administration of AAE (150 mg/kg/d b. w.) to the CED-fed ICR mice showed no significant difference on levels of weight gain, food intake and feeding efficiency. This observation is in agreement with other investigators, who found no effect in food intake and weight gain of dietary fiber and polyphenols, such as catechin, tannic acid or condensed tannins (Bravo et al. 1994a, b).
High serum TC and LDL-C are the main risk factors in the pathogenesis of coronary heart disease. The results of the present investigation have shown that a CED intake led to an increase of cholesterol content both in serum and liver. Somewhat different from most studies (Wei et al. 2003; Zhao et al. 2006), serum HDL-C levels were increased significantly in CEDC compared with RD. This HDL-C elevating effect in hyperlipidemia ICR mice was also reported by Lee et al. (2006). It might be due to cholesterol-enriched diet causing stress reaction in ICR mice, which is associated with high levels of serum cholesterol, including LDL-C and HDL-C. Oral administration of AAE (150 mg/kg/d bw) had a distinct cholesterol-lowering effect by decreasing the levels of serum TC, hepatic TC and TG, accordingly reduced the risk of CHD. Similar cholesterol-lowering results had been previously reported in many polyphenolic compounds from different plant foods (Auger et al. 2005; Suzuki et al. 2005). AI has direct correlation with the cardiovascular disease. AAE was able to decrease AI significantly, contributing to the lower risk in pathology of hyperlipidemia and atherosclerosis.
The rise of cholesterol in liver and serum may be due to increased uptake of exogenous cholesterol and subsequent deposition and decreased cholesterol catabolism of cholesterol to bile acids (Jaganathan et al. 1974). To elucidate the underlying mechanism of the cholesterol-lowering action of AAE, we investigated the hepatic HMG-CoA reductase activity and fecal cholesterol and bile acids excretion. HMG-CoA reductase is the rate-limiting enzyme in the cholesterol biosynthetic pathway. The inhibition of HMG-CoA reductase decreases cholesterol synthesis and is very effective in lowering plasma cholesterol (Goldstein and Brown 1990). High cholesterol diet itself inhibited HMG-CoA reductase activity in our study for the feedback regulation of cholesterol, which was also illustrated by Hayashi et al. (2004). Previous studies demonstrated that polyphenols extract from virgin olive oils (Benkhalti et al. 2002) and naringenin (Lee et al. 2003), a kind of phenolic compound, decreased liver microsome HMG-CoA reductase activity. Similarly, AAE significantly reduced the activity of HMG-CoA reductase compared with CEDC, suggesting the hypocholesterolemic effects of AAE phenolic compounds through suppressing the rate of cholesterol synthesis. Cholesterol can be excreted into the bile directly or after conversion to bile acids. Bile acid synthesis occurs exclusively in the liver, and cholesterol 7α-hydroxylase is the first rate-limiting enzyme in this pathway. EG significantly increased the fecal excretion of bile acids compared with CEDC; we conjectured the hypocholesterolemic effect of AAE phenolic compounds might be due to the accelerated rate of catabolism of cholesterol to bile acids and also be related to enhance the activity of cholesterol 7α-hydroxylase, which would be another mechanism of the hypocholesterolemic effect of AAE. Del Bas et al. (2005) also reported that polyphenols in red wine induce cholesterol 7α-hydroxylase expression and increase of cholesterol elimination via bile acids. The present results are consistent with above research. Further, A. auricula was previously reported with the ability to increase the fecal excretion of neutral steroids and bile acids in rats (Cheung 1996). The author considered that β-glucans and glucuronoxylomannan of A. auricula interfered with the absorption of cholesterol from the digestive tract of the animal, so increased the fecal neutral steroids output, but he doubted that some other components in A. auricula having the responsibility for increasing the fecal bile acids output. In the present study, we found AAE had no obvious effect on fecal excretion of neutral cholesterol but significantly increased fecal excretion of bile acids (Table 5). Therefore, it was assumed that polyphenolic compounds were able to increase fecal excretion of bile acids, but the active compounds and the underlying mechanism require further analysis.
Furthermore, it is well reported that the cholesterol-enriched diet would appear to induce free radical production, followed by oxidative stress and hypercholesterolemia (Tarladgis et al. 1964), such as decreasing the activities of catalase and SOD and thereby elevating the lipid peroxide contents, resulting in the production of toxic intermediates. In the present study, administration of AAE (150 mg/kg/d bw) could significantly lower the levels of lipid peroxides, MDA, and enhanced the activities of the hepatic SOD and TAC (Table 4). Further research is in progress, aimed at characterizing the polyphenolic compounds and isolation of the active compounds.
Conclusion
Ethanol extract of A. auricula is rich in polyphenolic compounds and thus possesses potent hypocholesterolemic effects. Through the in vivo study, it was found that consumption of AAE could significantly improve the antioxidant status and lipids profile, inhibit cholesterol synthesis in liver and elevate fecal bile acids excretion. However, the study on the polyphenolic compounds in A. auricula is very limited. Further study on AAE is being carried out to elucidate the specific compounds contributing to hypolipidemic activity and to explore other nutritional properties of A. auricula, such as anti-diabetes and anti-atherosclerosis.
Acknowledgment
Authors thank Fei Guo and Shuang Yan for their excellent technical assistance. This work was supported by Development of Science and Technology of the Daxing’an Mountain range, Heilong Jiang province, China.
Contributor Information
Yang-Chao Luo, Email: luo142@gmail.com.
Bao-Ping Ji, Email: zn_jibp@163.com.
Bo Li, Email: libo@cau.edu.cn.
References
- Acharya K, Samui K, Rai M, Dutta B, Acharya R. Antioxidant and nitric oxide synthase activation properties of Auricularia auricula. Indian J Exp Biol. 2004;42:538–540. [PubMed] [Google Scholar]
- Aletor VA. Compositional studies on edible tropical species of mushrooms. Food Chem. 1995;54:265–268. doi: 10.1016/0308-8146(95)00044-J. [DOI] [Google Scholar]
- Official methods of analysis. 17. Gainthersburg: Association of Official Analytical Chemists; 2000. [Google Scholar]
- Auger C, Teissedre PL, Gerain P, Lequeux N, Bornet A, Serisier S, Besancon P, Caoriccio B, Criatol JP, Rouanet JM. Dietary wine phenolics catechin, quercetin, and resveratrol efficiently protect hypercholesterolemic hamsters against aortic fatty streak accumulation. J Agric Food Chem. 2005;53:2015–2021. doi: 10.1021/jf048177q. [DOI] [PubMed] [Google Scholar]
- Benkhalti F, Prost J, Paz E, Perez-Jimenez F, El-Modafar C, El-Boustani E. Effects of feeding virgin olive oil or their polyphenols on lipid of rat liver. Nutr Res. 2002;22:1067–1075. doi: 10.1016/S0271-5317(02)00400-1. [DOI] [Google Scholar]
- Bravo L, Abia R, Eastwood M, Saura-Calixto F. Degradation of polyphenols (catechin and tannin acid) in the rat intestinal tract. Effect on colonic fermentation and faecal output. Br J Nutr. 1994;71:933–946. doi: 10.1079/BJN19940197. [DOI] [PubMed] [Google Scholar]
- Bravo L, Abia R, Saura-Calixto F. Polyphenols as dietary fiber associated compounds.Comparative study on in vivo and in vitro properties. J Agric Food Chem. 1994;42:1481–1487. doi: 10.1021/jf00043a017. [DOI] [Google Scholar]
- Chang JS, Kim HJ, Bae JT, Park SH, Kim SE, Kim OM. Inhibition effects of Auricularia auricula-judae methanol extract on lipid peroxidation and liver damage in benzo(α)pyrene-treated mice. J Korean Soc Food Sci Nutr. 1998;27:712–717. [Google Scholar]
- Chen G, Luo YC, Ji BP, Li B, Guo Y, Li Y, Su W, Xiao ZL. Effect of polysaccharide from Auricularia auricula on blood lipid metabolism and lipoprotein lipase activity of ICR mice fed a cholesterol-enriched diet. J Food Sci. 2008;73:H103–H108. doi: 10.1111/j.1750-3841.2008.00821.x. [DOI] [PubMed] [Google Scholar]
- Cheung PCK. The hypocholesterolemic effect of two edible mushrooms: Auricularia auricula (tree-ear) and Tremella fuciformis (white jelly-leaf) in hypercholesterolemic rats. Nutr Res. 1996;16:1721–1725. doi: 10.1016/0271-5317(96)00191-1. [DOI] [Google Scholar]
- Del Bas JM, Fernandez-Larrea J, Blay M, Ardevol A, Salvado MJ, Arola L, Blade C. Grape seed procyanidins improve atherosclerotic risk index and induce liver CYP7A1 and SHP expression in healthy rats. FASEB J. 2005;19:479–481. doi: 10.1096/fj.04-3095fje. [DOI] [PubMed] [Google Scholar]
- Edwards PA, Lemongello D, Fogelman AM. Improved methods for the solubilization and assay of hepatic 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Lipid Res. 1979;20:40–46. [PubMed] [Google Scholar]
- Folch J, Lees M, Sloan-Stanley GH. A simple method for isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Rani JA, Fukatsu A, Matsui-Hirai H, Osawa M, Miyazaki A. A new HMG-CoA reductase inhibitor, pitavastatin remarkably retards the progression of high cholesterol induced atherosclerosis in rabbits. Atherosclerosis. 2004;176:255–263. doi: 10.1016/j.atherosclerosis.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Jaganathan SN, Conor WE, Baker W, Bhattacharya AK. The turnover of cholesterol in human atherosclerotic arteries. J Clin Invest. 1974;54:366–370. doi: 10.1172/JCI107772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Moon SS, Lee SE, Bok SH, Jeong TS, Park YB, Choi MS. Naringenin 7-O-cetyl ether as inhibitor of HMG-CoA reductase and modulator of plasma and hepatic lipids in high cholesterol-fed rats. Bioorg Med Chem. 2003;11:393–398. doi: 10.1016/S0968-0896(02)00441-8. [DOI] [PubMed] [Google Scholar]
- Lee HS, Ahn HC, Ku SK. Hypolipemic effect of water extracts of Picrorrhiza rhizoma in PX-407 induced hyperlipemic ICR mouse model with hepatoprotective effects: a prevention study. J Ethnopharmacol. 2006;105:380–386. doi: 10.1016/j.jep.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Luo YC, Chen G, Li B, Ji BP, Guo Y, Tian F. Evaluation of antioxidative and hypolipidemic properties of a novel functional diet formulation of Auricularia auricula and hawthorn. Innov Food Sci Emerg Technol. 2009;10:215–221. doi: 10.1016/j.ifset.2008.06.004. [DOI] [Google Scholar]
- Luo YC, Chen G, Li B, BP JI, Xiao ZL, Guo Y, Tian F. Dietary intervention with AHP, a functional formula diet, improves both serum and hepatic lipids profile in dyslipidemia mice. J Food Sci. 2009;74:H189–H195. doi: 10.1111/j.1750-3841.2009.01241.x. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Saito H, Nishitoba T, Kawagishi H. Antitumor active substances from mushrooms. Food Rev Int. 1995;11:23–61. doi: 10.1080/87559129509541018. [DOI] [Google Scholar]
- Suzuki Y, Kobayashi M, Unno T, Nozawa A, Sagesaka YM, Kakuda T. Hypolipidemic effect of tea catechins with a galloyl moiety in hamsters fed a high fat diet. J Jap Soc Food Sci Technol. 2005;52:167–171. doi: 10.3136/nskkk.52.167. [DOI] [Google Scholar]
- Takeuchi H, He P, Mooi L. Reductive effect of hot-water extracts from woody ear (Auricularia auricula-judae Quel) on food intake and blood glucose concentration in genetically diabetic KK-AY mice. J Nutr Sci Vitaminol. 2004;50:300–304. doi: 10.3177/jnsv.50.300. [DOI] [PubMed] [Google Scholar]
- Tarladgis BG, Pearson AM, Duran LR. Chemistry of the 2-thiobarbituric acid test for determination of oxidative rancidity in foods. J Sci Food Agric. 1964;15:602–607. doi: 10.1002/jsfa.2740150904. [DOI] [Google Scholar]
- Tebib K, Bitri L, Besancon P, Rouanet JM. Polymeric grape seed tannins prevent plasma cholesterol changes in high-cholesterol-fed rats. Food Chem. 1994;49:403–406. doi: 10.1016/0308-8146(94)90012-4. [DOI] [Google Scholar]
- Wei W, Li CL, Wang YY, Su HD, Zhu JS, Kritchevsky D. Hypolipidemic and anti-atherogenic effects of long-term Cholestin (Monascus purpureus-fermented rice, red yeast rice) in cholesterol fed rabbits. J Nutr Biochem. 2003;14:314–318. doi: 10.1016/S0955-2863(03)00051-2. [DOI] [PubMed] [Google Scholar]
- Wu J, Ding ZY, Zhang KC. Improvement of exopolysaccharide production by macro-fungus Auricularia auricual in submerged culture. Enz Microb Technol. 2006;39:743–749. doi: 10.1016/j.enzmictec.2005.12.012. [DOI] [Google Scholar]
- Yoon SJ, Yu MA, Pyun YR, Hwang JK, Chu DC. Nontoxic mushroom Auricularia auricula contains a polysaccharide with anticoagulant activity mediated by antithrombin. Throm Res. 2003;112:151–158. doi: 10.1016/j.thromres.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Zhang LN, Yang LQ, Chen JH. Conformational change of the β-D-glucan of Auricularia auricula-judae in water-dimethyl sulfoxide mixtures. Carbohyd Res. 1995;276:443–447. doi: 10.1016/0008-6215(95)00185-V. [DOI] [Google Scholar]
- Zhang LN, Yang LQ, Ding Q, Chen XF. Studies on molecular weights of polysaccharides of Auricularia auricula-judae. Carbohyd Res. 1995;270:1–10. doi: 10.1016/0008-6215(94)00008-4. [DOI] [Google Scholar]
- Zhang GZ, Ji BP, Li B, Tian F, Chen G, Ji FD, Zhang HJ, Yang ZW, Zhao L. Effects of processing and storage condition on phenolic concentration and antioxidant activities of apple and apple juices. J Food Sci Technol. 2008;45:339–343. [Google Scholar]
- Zhao HL, Cho KH, Ha YW, Jeong TS, Lee WS, Kim YS. Cholesterol-lowering effect of platcodin D in hypercholesterolemic ICR mice. Eur J Pharmacol. 2006;537:166–173. doi: 10.1016/j.ejphar.2006.03.032. [DOI] [PubMed] [Google Scholar]