Abstract
Vacuum packaged beef strip-loins (fresh and aged) were repackaged on polystyrene trays and over-wrapped with food grade cling film for the storage study. Several volatile compounds such as 3-methyl-1-butanol, 2,3-butanedione, 2-butanone, 3-hydroxy-2-butanone, acetic acid and a few hydrocarbons were detected in the headspace of these tray packaged fresh and aged beef strip loins both in the control and Salmonella typhimurium inoculated samples, in varying concentrations. These compounds were identified using manual headspace solid-phase microextraction (HS-SPME) in combination with gas chromatography/mass spectrometry (GC-MS) over a storage period of 4 days and samples were incubated at 20°C. No naturally occurring Salmonella was present in the control samples. Hexanal (r = 0.99), carbon dioxide (r = 0.98), 3-hydroxy-2-butanone (r = 0.93) and 2-methyl propane (r = 0.95) showed positive correlations with Salmonella population for fresh beef samples. In aged beef samples, 3-methyl-1-butanol (r = 0.99), 3-hydroxy-2-butanone (r = 0.98), carbon dioxide (r = 0.98) and acetic acid (r = 0.86) showed similar trends. In fresh beef samples, F values were significant at p < 0.05 for 3-hydroxy-2-butanone and for carbon dioxide with storage time for fresh beef samples; they were significant for 3-hydroxy-2-butanone, acetic acid and carbon dioxide for aged beef samples.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-010-0138-6) contains supplementary material, which is available to authorized users.
Keywords: Over wrapped beef; Aged, fresh, Salmonella; HS-SPME/GC-MS; Volatiles
Introduction
Salmonella typhimurium in beef has caused frequent food poisoning. Controlling outbreaks of Salmonella is an important task for food regulators, restaurants and the food industry in general. The Salmonella family includes over 2,300 serotypes of bacteria, but two types, Salmonella enteritidis and Salmonella typhimurium are responsible for half of human infections. Some foodborne outbreaks of Salmonella in the US have been traced to a range of food products including dairy, poultry, fresh fruit and vegetables and meat products. Raw meat has been implicated as an important source of Salmonella.
In recent years, a general trend in food microbiology has been towards the replacement of cumbersome and time-consuming microbiological analyses for monitoring microbial contamination of meat and other food products with rapid real time technologies. The correlation between microbial growth and the development of chemical changes has been recognized as the means of revealing specific substrates and/or end products that could be useful for assessing meat quality (Dainty 1996; Tsigarida and Nychas 2001). Thus, the quality of beef can be tested by analyzing the gaseous metabolites of the associated bacteria.
During the last few decades, gas chromatography-mass spectrometry (GC-MS) has become the first choice for analysis of volatile compounds in meat due to its high performances in the separation and identification of complicated and similar compounds (Cadwallader and MacLeod 1998). Because raw, uncooked meat has little aroma as reported by Shahidi (1998), it is essential to select a suitable sampling method. Manual headspace-solid phase microextraction (HS-SPME) in combination with gas chromatography-mass spectrometry (GC-MS), which is reportedly a simple, rapid and sensitive method, was used for the identification of the volatile compounds in this study. HS-SPME is a popular method of sampling and preconcentration of volatiles and semi-volatiles, which is being routinely used in combination with GC-MS. It is an inexpensive, solvent-free, and reliable technique with excellent sensitivity and good selectivity (Pinho et al. 2002). Although SPME has maximum sensitivity at the equilibrium point, full equilibrium is not necessary for the purpose of identification of volatiles and for quantification of the same, because of the linear relationship between the amount of analyte absorbed by the SPME fiber and its initial concentration in the sample matrix under non-equilibrium conditions (Shang et al. 2002). The use of SPME in conjunction with GC-MS has been reported for the extraction of organic compounds from several matrices such as Salmonella-infected beef (Ogihara et al. 2000), cooked beef (Machiels and Istasse 2003), cured hams (Gianelli et al. 2002; Luna et al. 2006), pork (Elmore et al. 2000; Ramírez et al. 2004), cooked turkey (Brunton et al. 2002), chicken myofibrils (Goodridge et al. 2003) and fermented sausages (Marco et al. 2004).
SPME has also proven to be useful for the detection of contamination of food by microbial pathogens. It has been reported to be used for the qualitative study of volatile metabolites by Salmonella typhimurium and Escherichia coli O157:H7 on selective agar medium at 35°C for 24 h. Salmonella specific volatile organic compounds (VOCs) grown on TSY were 3-methyl-1-butanol, dimethyl sulphide, 2-undecenol, 2-pentadecanol and 1-octanol (Senecal et al. 2002). Studies were also reported for the profiles of volatile compounds from eight strains of Salmonella in Trypticase Soya Broth (TSB) and for Salmonella enteritidis in beef incubated at 37°C for 24 h (Ogihara et al. 2000). However, the volatile compounds emitted in these studies were not identified.
The overall objective of our project was to build portable hand-held sensors (electronic noses) to enable consumers to identify whether packaged beef purchased from retail stores are safe for consumption. In an attempt to design these sensors, investigations were needed to be carried out to identify the headspaces gases (VOCs) produced by Salmonella in these packaged beef. The packaged beef could have several microflora, besides Salmonella; therefore, identification of the spectrum of volatile compounds in the headspace of meat trays specifically due to Salmonella would be difficult. Hence, preliminary investigations were first carried out in our laboratory to identify headspace gases associated with Salmonella contamination of sterile beef in 20-ml headspace vials using HS-SPME/GC-MS (Bhattacharjee et al. 2010). An array of volatile compounds was detected in the headspace of sterile (fresh strip loins) both in control and inoculated samples. The samples were inoculated with Salmonella typhimurium and both control and inoculated samples were stored at 20°C in 20-ml headspace vials covered with food grade cling film. The study was conducted for 4 days and the volatiles in the headspace were analyzed each day using HS-SPME in combination with GC-MS. Acetic acid, ethanol, carbon dioxide and 3-hydroxy-2-butanone were the most important compounds detected in the study. Further investigations were therefore designed to observe whether similar volatiles could also be detected in commercially available packaged beef when stored at similar conditions, mimicking the real-world conditions.
This study investigated the volatile compounds (gaseous metabolites) produced by Salmonella typhimurium in the headspace of the repackaged beef samples (aged and fresh) when stored at 20°C for 4 days; using HS-SPME in combination with GC-MS. Detection of volatile compounds in the headspace of tray samples would provide us information regarding the status of the beef samples prior to consumption if it had been contaminated with Salmonella.
The purpose of conducting the studies at 20°C was to model the behavior of the pathogen, Salmonella typhimurium in relation to the changes that occur in the meat over a passage of time. From slaughter to consumption, it is possible that the temperature of the meat may rise up to 20°C making conditions amenable for growth of pathogens. Our studies would enable us to assess optimum conditions for designing sensors to detect these changes.
Materials and methods
Beef strip loins (M. longissimus dorsi) from commercial slaughter houses, red colored polystyrene base trays (Styrofoam trays, 14.5 cm × 20.3 cm × 1.2 cm), oxygen permeable food grade cling wrapping film (Foodservice 912 film; M/s Reynolds Foods Service Packaging, Lincolnshire, IL, USA) were used for the study. All media and chemicals used for microbiological analyses were of analytical grade.
In the current investigation, vacuum packaged beef strip-loins (both from freshly slaughtered animals and those aged for 2–3 weeks at 4°C) were procured from slaughter houses, and subsequently repackaged in polystyrene (Styrofoam) trays and over wrapped with food grade cling film, mimicking typical real-world conditions.
The aging time of procured meat varied from 2–3 weeks. Discrepancies in sample history arose because it was not practically feasible to procure samples of identical age during experimental trials or to obtain exact information about the date of slaughter of the animal. This randomness in the sample history is desirable and made our study realistic.
Sample preparation
Fresh beef was obtained from strip-loins of a freshly slaughtered animal from commercial slaughter houses. The beef was packaged under vacuum and transported to the laboratory at chilled temperatures. Aged beef was also procured from commercial slaughter houses wherein the beef obtained post-slaughtering was vacuum packaged and allowed to age for 2–3 weeks under commercial refrigerated conditions (typically 4°C), prior to delivery to our laboratory.
During experimentation, the vacuum wrap was removed from the strip-loins, and the fat along with the connective tissues was trimmed from the meat, and cut into pieces of 1–1.5 cm thickness and weighed (~50 g and 100 g pieces). The experiments with fresh meat were staggered for a very long period. Variation in real-life samples, procured by consumers from retail stores could be reasonably high which if discounted, could render our studies unrealistic. Therefore, samples chosen for our study were procured from slaughter houses at different time periods to account for sample variation and obtain robust pathogen-indicator compounds. Five trials were planned to obtain a complete data set for statistical analyses.
Salmonella inoculum preparation
An avirulent strain of Salmonella typhimurium (laboratory stock strain), was made resistant to nalidixic acid (50 μg/ml; M/s Sigma-Aldrich, St. Louis, MO) and streptomycin sulphate (1000 μg/ml, Sigma-Aldrich) using the method described by Blackburn and Davies (1994). This resistant strain was stored at −80°C in brain heart infusion broth (BHI, M/s DIFCO Labs, Detroit, MI) with 20% glycerol and was used to allow us to track, identify and select for our inoculated strain without interference from the natural background flora of the meat. Prior to inoculation of beef samples, the mutant strain was incubated at 35°C overnight in Brain Heart Infusion (BHI) broth (M/s DIFCO Labs, Detroit, MI, USA). Following incubation, the optical density (OD) of the Salmonella culture was measured at 600 nm using a Smartspec 300 Spectrophotometer (Bio-Rad, Hercules, CA, USA). The OD was measured to obtain same inoculum level for all experiments so as to ensure consistent inoculation levels of Salmonella in all samples. Then the culture was serially diluted in Maximum Recovery Diluent (MRD; M/s Oxoid Ltd, Basingstoke, Hampshire, England) and adjusted to yield an inoculum containing approximately 100,000 colony-forming units (cfu)/ml of Salmonella. Fresh inoculum was prepared for each experimental trial.
Inoculation of beef samples with Salmonella
The samples to be spiked, (i.e., inoculated with Salmonella) were all transferred using sterile forceps into a sterile plastic bag containing the inoculum in 500 ml of MRD solution. The meat samples (both aged and fresh beef) were inoculated with approximately 103–104 cfu of Salmonella per gram of meat. The samples were mixed well for about a minute inside the sterile plastic bag to ensure homogeneity of the inoculated microflora. The spiked samples were then placed on the Styrofoam trays. Fifty gram pieces of meat samples were used for microbiological analysis and 100 g pieces were used for SPME/GC-MS analysis. The control samples were also immersed in MRD (without any culture added to it) and were placed in respective pre-labeled trays. The trays were immediately wrapped with oxygen-permeable food grade cling film, mimicking typical retail packages.
A temperature of 20°C was chosen for our study for the following reasons. The overall objective of this work is to develop hand-held sensors for Salmonella detection for consumer use and consumers are more likely to leave the meat at room temperature conditions (around 20°C) before consumption. This may happen during transport of the meat from the store or during preparation. Moreover, a temperature higher than 20°C would generate ‘warmed-over flavor’ compounds in the headspace of the meat samples consequent to biochemical reactions in the meat samples (Kerler and Grosch 1996) and these artifacts would complicate our analysis; while a temperature below 20°C would slow down growth of Salmonella considerably and presumably, a longer storage period for study would be necessary. Moreover, our choice of this temperature is dictated chiefly by consumer behavior as stated earlier. Room temperature was purposely not chosen for storage study since it is subjected to fluctuation; a temperature-controlled incubator was used instead. Both control and spiked samples were removed from the incubator each day (for 4 days) and were subjected to microbiological analysis and for analysis of volatile compounds.
Microbiological analysis of beef samples
On each sampling day, approximately 10 g of the meat sample (control or spiked) was aseptically removed from the trays. Meat pieces were removed from different areas of the tray-packed sample and pooled to provide a 10 g homogeneous mass which was subsequently transferred to a sterile stomacher bag (VWR Scientific, West Chester, PA, USA). The sample was homogenized in the stomacher (Masticator from IUL, Cincinnati, OH) with 90 ml of MRD for 90 s and subsequently serially diluted in 9 ml of MRD. The homogenate or the serial dilutions were plated out; total plate counts were determined on plate count agar (PCA) after incubation at 25°C for 72 h, while Salmonella typhimurium were enumerated on mannitol lysine crystal violet brilliant green (MLCB) agar containing nalidixic acid (50 μg/ml) and streptomycin sulphate (1,000 μg/ml) after incubation at 35°C for 48 h. The bacterial populations were enumerated and calculated as log10 (cfu/g) of the beef sample. To evaluate the number of naturally occurring Salmonella in the control meat samples and to confirm that the meat was Salmonella negative at the onset of experimentation, samples of the meat were prepared as described above and plated out on MLCB without nalidixic acid and streptomycin sulphate and incubated under similar conditions.
Pseudomonas spp was enumerated on Pseudomonas Agar CM 559 (M/s Oxoid Ltd, Basingstoke, Hampshire, England) after incubation at 25°C for 48 h; Brocothrix spp on STAA Agar CM881 (M/s Oxoid) after incubation at 25°C for 48 h; Enterobacteriaceae spp on Violet Red Bile Glucose Agar (VRBG) CM (M/s Oxoid) by incubating at 37°C for 24 h and Lactobacillus spp on All Purpose Tween (APT) Agar 0654-17 (M/s Difco) after incubating anaerobically at 30°C for 72 h.
Previous work conducted in the laboratory had shown similar results when 10 g pieces were individually wrapped instead of obtaining the same from different portions of a single piece. A similar procedure for determining plate counts was used for both fresh and aged meat samples.
Isolation of volatiles by HS-SPME
The isolation of volatile compounds from the headspace of trays were carried out at room temperature (23 ± 2°C). Ideally SPME studies should have been conducted at the conditions of meat storage (i.e., at 20°C) but it was not feasible. A variation of ±2–3°C is realistic and does not diminute our objectives. It is known that increased extraction temperatures would increase the efficiency of extraction by the SPME fiber, but an extraction temperature above room temperature was not used for our study since commercially available beef samples, before consumption, are not likely to be kept at temperatures higher than room temperature. A 75-μm Carboxen-Polydimethylsiloxane (CAR-PDMS) fiber (M/s Supelco, Bellefonte, PA, USA) was used for SPME. Among several types of fibers tested, this fiber is reported to give the highest response for VOC from Salmonella contaminated beef (Machiels and Istasse 2003; Ogihara et al. 2000) and therefore this fiber was chosen for our study. The fiber was pre-conditioned in the injector port of the gas chromatograph at 270°C under helium flow for 1.5 h prior to use. The O-ring was adjusted and kept fixed at a certain position to enable equal depth of penetration in all beef samples analyzed. The pre-conditioned fiber was then inserted into the headspace of the Styrofoam trays (containing the beef samples) from the side of the tray, the wire was released to the pre-fixed position and the tip of the wire was kept 5–6 cm away from the meat sample. Care was taken to ensure that at no point of time during preconcentration, the fiber tip touched the meat sample. Sampling of gaseous volatiles was carried out for 30 min at a constant depth. Subsequently the SPME fiber was withdrawn into the manual holder, pulled out from the tray and inserted into the GC injector port, where the fiber was released again to the prefixed position and allowed to remain in the injector port until the end of the GC-MS run. After completion of the run, the fiber was kept in the injector port for an additional 10 min to desorb the volatile compounds and also to clean the fiber for successive analyses. A blank run was conducted for each fiber in between the runs to reduce memory effects of the fibers. Four control and four spiked samples were run on each sampling day for the 4-day storage period. Five such experimental trials were conducted for both fresh and aged beef samples separately.
Analysis of volatiles by GC-MS
A Shimadzu GC-17A gas chromatograph coupled to a GC-MS/QP5000 mass spectrometer (Columbia, MA, USA) was used for the analysis of the volatiles entrapped in the SPME fiber. The gas chromatograph was operated in the splitless mode throughout the run with the mass spectrometer in electron ionization (EI) mode (70 eV). Shimadzu GCMS solution software (version 2.01) was used for instrument control as well as for data acquisition and processing.
GC separations were performed on an XTi-5 fused silica capillary column (30 m × 0.25 mm; M/s Restek, Bellefonte, PA, USA), coated with 0.25 μm thickness of 5% diphenyl/95% dimethylpolysiloxane stationary phase. A narrow inlet SPME liner (0.75 mm i.d., M/s Supelco, Bellefonte, PA, USA) specifically for SPME fiber was used in the GC injector port in order to obtain the narrow peaks for the early-eluting compounds. Helium was used as the carrier gas at the constant flow rate of 1.0 ml/min. Thermal desorption of the compounds from the SPME fiber was carried out in the injector port at 270°C in splitless mode so that the benefit of the preconcentration (of very dilute volatiles and of those present at concentration below 1 ppm) by SPME was not wasted. The following GC oven temperature programming was applied: initially the column was held at 35°C for 2 min, and then it was programmed from 35 to 75°C at 5°C/min and further heated to 95°C at 2°C/min. Finally, the temperature was increased to 220°C at a rate of 10°C/min and maintained at 220°C for 2.5 min. The injector and GC-MS interface (detector) were kept at 270°C and 260°C respectively. The mass spectra were collected over the range of 35 to 350 m/z in the total ion monitoring (TIM) mode, with scan intervals being 0.3 s.
Preliminary identification of the volatile compounds was carried out using the NIST07 library of mass spectra. For some of the VOCs, identification was established by comparison of their retention times and spectra with those of authentic external standard compounds. These compounds have been indicated in the results and discussion section. The compounds with their experiment dates, types, retention times, peak areas and the similarity indices (SI) obtained from the NIST07 library were saved into a relational compound database. An additional computer program was developed in-house to process the acquired chromatograph data for further analysis and interpretation. One of the outputs of this program was ‘individual compound table’ which was used for further statistical analysis.
Both the control and spiked samples were analyzed using SPME-GC-MS on each sampling day. The relative concentrations (in terms of peak area responses) of the volatile compounds were monitored throughout the storage period to identify the compounds whose concentration increased with the growth of Salmonella. These compounds could serve as potential indicators of Salmonella contamination of beef samples.
It was hypothesized that the Styrofoam trays used in the experiment could contribute some artifacts to the peak profile of the volatile compounds. Therefore, parallel to these experiments, HS-SPME/GC-MS of empty Styrofoam trays (wrapped in food grade cling film) were also carried out for a few trays throughout the entire storage period, to identify these artifacts.
Statistical analysis
A total of five experimental trials were conducted for fresh and aged beef individually. In each experiment, average values of four samples were analyzed on each day for both control and spiked individually. A two-way analysis of variance (ANOVA), also called two-factor analysis of variance, was used to analyze the database. It is a general technique used to test the hypothesis that means among two or more groups are equal. ANOVA measures the effects of two factors simultaneously such as treatment and time and also analyzes whether there is an interaction between the two. This is conducted using a sum of squares decomposition (the total sum of squares for a dataset is a measure of the variability among all the data). The total observed variability is then partitioned into components based on different sources of variations—variability due to interaction of the two factors and variability within groups or cells (also known as error variability). Using F statistics, the three sources of variability (due to first factor, second factor and interaction) are compared with the error variability. In each test, the resulting p-value allows us to determine whether that specific effect is significant or not.
In the present study, the two-way ANOVA model considered day and treatment as the main effects and the day-by-treatment interaction was fit using the rescaled peak areas as the dependent variable. The means were compared using post hoc t-tests and a Bonferroni adjustment to control Type I error due to multiple testing (Westfall et al. 1999). This multiple testing operation is performed to control for occurrence of false positives that rise by virtue of performing many tests. This test would help keep the overall error rate lower. A default Type I error rate (alpha level) of 0.05 was used for all tests. A Pearson correlation test was also carried out to observe for competition between Salmonella and other microbes in both fresh and aged samples during the storage period.
Results and discussion
Microbiological analysis
Table 1 shows the counts of different microbial populations on fresh beef samples, over the 4-day storage period. In these trials with fresh beef, the initial level of Salmonella in the spiked samples were slightly above 2 log10 (cfu/g) on day zero which increased to around 6.8 log10 (cfu/g) by fourth day of storage. Table 2 shows the counts of different microbial populations on aged beef samples, over the 4-day storage period. For the trials with aged beef, the initial level of Salmonella in the spiked samples were slightly above 2 log10 (cfu/g) on day zero which increased to around 5 log10 (cfu/g) by fourth day of storage.
Table 1.
Counts of microbial population on fresh beef samples over a 4-day storage period
| Control | Spiked | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day# | Sala log10 | Psub log10 | Lacc log10 | Brod log10 | Ente log10 | PCf log10 | Sala log10 | Psub log10 | Lacc log10 | Brod log10 | Ente log10 | PCf log10 |
| The data below are the average of the data of all the five trials | ||||||||||||
| 0 | <0.7 | 2.6 | 3.5 | 1.8 | 1.6 | 4.1 | 2.5 | 2.3 | 3.2 | 1.8 | 2.5 | 4.0 |
| 1 | <0.7 | 5.4 | 6.5 | 2.6 | 4.1 | 6.7 | 4.3 | 5.6 | 6.6 | 4.2 | 4.5 | 6.9 |
| 2 | <0.7 | 7.9 | 8.0 | 3.2 | 6.5 | 8.4 | 5.6 | 8.1 | 8.3 | 5.3 | 7.4 | 8.9 |
| 3 | <0.7 | 8.5 | 9.0 | 3.9 | 8.2 | 9.2 | 6.8 | 9.0 | 9.1 | 6.3 | 8.7 | 9.6 |
| The data below are the standard deviation of the data of all the five trials | ||||||||||||
| 0 | ND | 0.15 | 0.29 | 0.05 | 0.06 | 0.10 | 0.14 | 0.09 | 0.12 | 0.07 | 0.09 | 0.05 |
| 1 | ND | 0.14 | 0.08 | 0.14 | 0.18 | 0.16 | 0.09 | 0.09 | 0.17 | 0.13 | 0.14 | 0.10 |
| 2 | ND | 0.19 | 0.08 | 0.10 | 0.20 | 0.02 | 0.25 | 0.10 | 0.22 | 0.18 | 0.39 | 0.11 |
| 3 | ND | 0.22 | 0.28 | 0.29 | 0.19 | 0.16 | 0.36 | 0.26 | 0.11 | 0.15 | 0.32 | 0.14 |
ND not detected
aSalmonella
bPseudomonas,
cLactic acid bacteria
dBrocothrix
eEnterobacteriaceae
fPlate count on general agar
Table 2.
Counts of microbial population on aged beef samples over a 4-day storage period
| Control | Spiked | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day# | Sala log10 | Psub log10 | Lacc log10 | Brod log10 | Ente log10 | PCf log10 | Sala log10 | Psub log10 | Lacc log10 | Brod log10 | Ente log10 | PCf log10 |
| The data below are the average of the data of all the five trials | ||||||||||||
| 0 | <1.7 | 3.6 | 5.5 | 2.0 | 2.3 | 5.5 | 2.4 | 3.3 | 5.0 | 2.0 | 2.7 | 5.1 |
| 1 | <1.7 | 6.0 | 7.4 | 2.4 | 4.5 | 7.7 | 3.3 | 5.9 | 7.5 | 3.2 | 4.5 | 7.7 |
| 2 | <1.7 | 7.6 | 8.7 | 2.8 | 6.9 | 8.8 | 4.4 | 7.4 | 8.6 | 3.7 | 6.3 | 8.6 |
| 3 | <1.7 | 8.3 | 9.4 | 3.4 | 8.1 | 9.2 | 5.0 | 8.3 | 9.2 | 5.4 | 8.2 | 9.2 |
| The data below are the standard deviation of the data of all the five trials | ||||||||||||
| 0 | N/A | 0.06 | 0.12 | 0.03 | 0.13 | 0.12 | 0.14 | 0.18 | 0.20 | 0.04 | 0.31 | 0.19 |
| 1 | N/A | 0.09 | 0.60 | 0.11 | 0.45 | 0.13 | 0.28 | 0.16 | 0.12 | 0.43 | 0.16 | 0.09 |
| 2 | N/A | 0.05 | 0.05 | 0.27 | 0.36 | 0.05 | 0.23 | 0.14 | 0.11 | 0.31 | 0.70 | 0.10 |
| 3 | N/A | 0.11 | 0.14 | 0.20 | 0.26 | 0.04 | 0.14 | 0.12 | 0.12 | 0.12 | 0.20 | 0.15 |
aSalmonella
bPseudomonas,
cLactic acid bacteria
dBrocothrix
eEnterobacteriaceae
fPlate count on general agar
For fresh and aged samples, no Salmonella was detected in the control samples (our minimum detection limits were <0.7 log10 (cfu/g) for fresh and <1.70 for aged). The data indicates that number of Salmonella colonies were less than 50 (the minimum detection limit for our plate count) on all days of storage which implied that there was no Salmonella in the control samples and no possibility of growth of the same in the control samples (fresh and aged). Thus no naturally occurring Salmonella was present in the beef samples prior to inoculation. The detection limit of Salmonella is higher in aged meat since it was not practically possible to detect contamination level lower than 1.7 log10 (cfu/g) in the same. This was principally because the meat was older and therefore the plate counts for spoilage microflora were higher. This condition made it difficult to differentiate Salmonella among the background microflora.
The conditions in the Styrofoam trays can be considered to be predominantly aerobic which favored the growth of Pseudomonas, Lactobacilli, Brocothrix and Enterobacteriaceae, besides Salmonella. Lactic acid bacteria predominated towards the end of the storage period. No growth of Salmonella was found in the control samples in all the experimental trials. The growth of Salmonella on aged beef has been comparatively slower compared to that in fresh beef samples. In the aged meat samples there was background microflora, chiefly Pseudomonas and lactic acid bacteria as is seen in Table 2. The latter produce lactic acid, thereby potentially reducing the pH of the meat which may have contributed to the inhibition of the test strain. Moreover, some strains of lactic acid bacteria are known to produce bacteriocins in the medium which are growth inhibitors of several bacterial species. It is known that Pseudomonads also tend to dominate the microbial consortium in aerobically stored meats; several other spoilage microflora could also outgrow the pathogens such as Salmonella (Stanbridge and Davies, 1998). In the fresh samples, there is comparatively lower count of lactic acid bacteria (Table 1). Therefore competition for Salmonella was considerably less. This observation was validated by performing Pearson’s correlations among the several microflora and fairly high correlation co-efficients (>0.8) was found for all microbes amongst themselves. Thus growth of competing microbes did not suppress growth of Salmonella in fresh beef samples. The behavior of the microbes in aged beef samples mirrored that of the fresh samples, although the level of correlations were lower by 0.1–0.2 units compared to the fresh samples. Only Brocothrix showed poor correlations with other microbes.
Analysis of volatiles by HS-SPME/GC-MS and statistical analysis of the obtained data: for empty trays
Analysis of empty Styrofoam trays wrapped with cling film showed the presence of butylated hydroxy toluene (BHT). Parafilms are reported to release BHT in the headspace of reaction flasks (Selby 2007), while cling films are known sources of 2-ethyl-1-hexanol. However, in our studies, 2-ethyl-1-hexanol was not detected in the empty trays.
For fresh beef
HS-SPME/GC-MS detected several volatile compounds in the headspace of beef samples (both fresh and aged) inoculated with Salmonella typhimurium over a 4-day storage period. Figure 1a shows the gas chromatogram of a Salmonella spiked fresh beef sample at 20°C and Table 3 shows the composition of the headspace volatiles of the same. Table 4 similarly lists the headspace volatiles in aged-beef samples (described later). Table 5 lists the compounds detected and the percentage of occurrence of each compound in the control and spiked samples (calculated as number of times each compound was detected in the samples divided by the total number of samples analyzed under control and spiked categories) for fresh beef samples Table 6. The mean peak areas of the compounds with % of occurrence ≥30% over the 4-day storage period have been listed in Table 7 for fresh spiked samples.
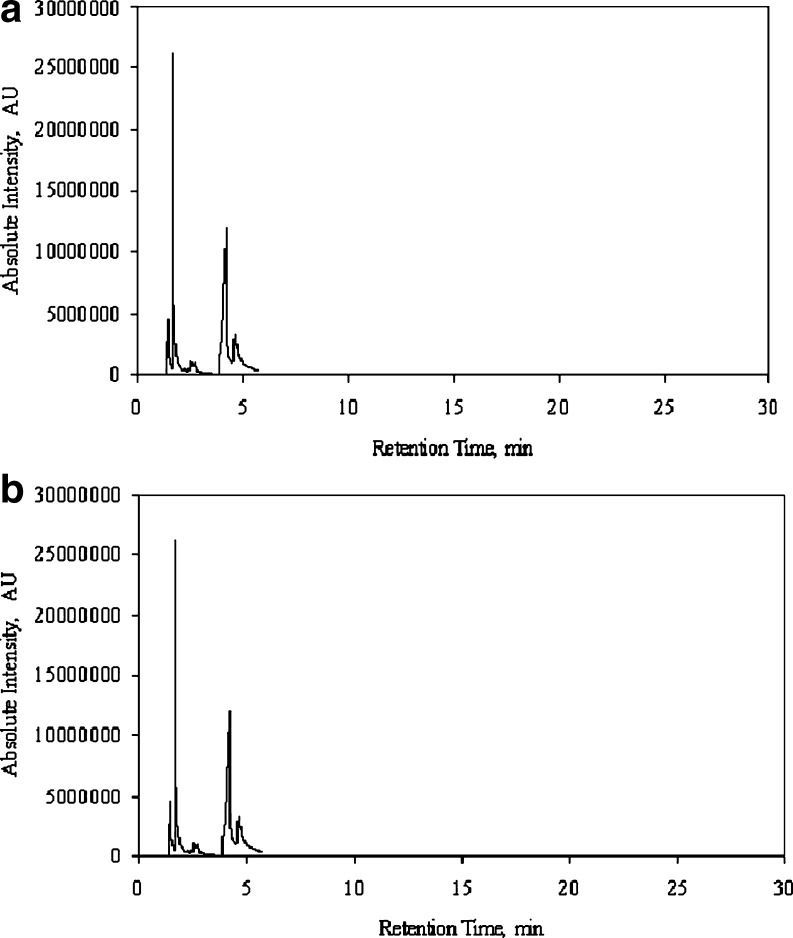
Fig. 1.
a A typical chromatographic profile (peak area of compounds vs. retention time) of a Salmonella spiked fresh beef sample stored in over-wrapped tray at 20°C (Refer to Table 3). b A typical chromatographic profile (peak area of compounds vs. retention time) of a Salmonella spiked aged beef sample stored in over-wrapped tray at 20°C (Refer to Table 4)
Table 3.
SPME-GC-MS analysis of headspace volatiles of a Salmonella spiked fresh beef sample at 20 °C (Fig. 1a)
| Retention time of peaks (min) | Tentative identification |
|---|---|
| 1.261 | Argon |
| 1.442 | Carbon dioxide |
| 1.543 | 2-methylpropane |
| 1.670 | 2-methylbutane |
| 2.203 | 2,3-butanedione |
| 2.467 | 2-butanone |
| 2.713 | Ethanoic acid (acetic acid) |
| 4.117 | 3-hydroxy-2-butanone |
| 6.433 | Hexanal |
| 11.808 | 2,2,5-trimethylhexane |
| 12.875 | 4,7-dimethylundecane |
| 14.550 | 3,7-dimethyldecane |
| 15.375 | 2,2,4-trimethylheptane |
| 20.342 | 2,2-dimethyl-3-hexanone |
| 29.117 | Dibutyl ester impurity |
| 29.616 | 2,6-di-tert-butyl-4-methyl phenol (butylated hydroxy toluene, BHT)a |
aVOC contributed by tray and/or cling film
Common names of the compounds are provided in parenthesis
Table 4.
SPME-GC-MS analysis of headspace volatiles of a Salmonella spiked aged beef sample at 20°C (Fig. 1b)
| Retention time of peaks (min) | Tentative identification |
|---|---|
| 1.451 | Carbon dioxide |
| 1.550 | 2-methylpropane |
| 1.680 | 2-methylbutane |
| 2.208 | 2,3-butanedione |
| 2.443 | 2-butanone |
| 2.572 | Ethanoic acid (acetic acid) |
| 4.213 | 3-hydroxy-2-butanone |
| 4.483 | 2-oxopropanal (pyruvaldehyde) |
| 4.649 | 3-methyl-1-butanol |
| 4.744 | 2-methyl-1-butanol |
| 15.395 | 3,7-dimethylnonane |
Common names of the compounds are provided in parenthesis
Table 5.
Compounds detected by SPME-GC-MS of control and spiked fresh beef samples
| Functional group/Compound | % of occurrence in five trials | |
|---|---|---|
| Control | Spiked | |
| Alcohol | ||
| 3-methyl-1-butanol | 20.78 | 19.74 |
| Hexanol | 10.39 | 9.21 |
| 2-ethyl-1-hexanol | 5.19 | 3.95 |
| 2-methyl-1-propanol | 10.39 | 3.95 |
| 3-butyn-1-ol | 5.19 | 3.95 |
| Butylated hydroxy toluene (BHT) | 42.86 | 46.05 |
| Aldehyde | ||
| 3-methyl-1-butanal | 9.09 | 3.95 |
| 3-methyl-1-pentanal | 7.79 | 5.26 |
| hexanal | 31.17 | 30.26 |
| Carboxylic Acid | ||
| Ethanoic acid (acetic acid) | 32.47 | 40.79 |
| Gas | ||
| Argon | 20.78 | 30.26 |
| Carbon dioxide | 64.94 | 64.47 |
| Hydrocarbon | ||
| 2-methylbutane | 77.92 | 78.95 |
| Trichloromethane (chloroform) | 18.18 | 21.05 |
| 3,7-dimethyldecane | 49.35 | 64.47 |
| 2,2,4-trimethylheptane | 38.96 | 50.00 |
| 3,3,4-trimethylheptane | 14.29 | 21.05 |
| 2,2,3-trimethylhexane | 6.49 | 6.58 |
| 2,2,5-trimethylhexane | 29.87 | 38.16 |
| 2-methylpropane | 53.25 | 53.95 |
| 5-methylnonane | 15.58 | 23.68 |
| 2,2,3,4-tetramethylpentane | 19.48 | 21.05 |
| 3-methylpentane | 12.99 | 13.16 |
| Styrene | 9.09 | 3.95 |
| 4,7-dimethylundecane | 12.99 | 22.37 |
| Ketone | ||
| 2,3-butanedione | 38.96 | 35.53 |
| 2-butanone | 24.68 | 25.00 |
| 3-methyl-2-butanone | 7.79 | 13.16 |
| 3-hydroxy-2-butanone | 90.91 | 98.68 |
| 2-pentanone | 6.49 | |
| 2,2-dimethyl-3-hexanone | 42.86 | 46.05 |
| 2-propanone (acetone) | 11.69 | 10.53 |
| Sulphurous Compounds | ||
| Dimethyldisulphide | 1.30 | 6.58 |
| Dimethylsulphide | 3.90 | 5.26 |
| Methanethiol | 7.79 | 6.58 |
| Impurity | ||
| Dibutyl ester impurity | 11.69 | 17.11 |
| Siloxane impurity | 2.60 | 9.21 |
| Impuritiesa | 90.91 | 100 |
| Unidentified hydrocarbon | 15.58 | 2.63 |
aFrom several sources such as GC column, injection port septum, laboratory atmosphere and meat trays
Common names of the compounds are provided in parenthesis
Table 6.
Compounds detected by SPME-GC-MS of control and spiked aged beef samples
| Functional group/Compound | % of occurrence in four trials | |
|---|---|---|
| Control | Spiked | |
| Alcohol | ||
| 3-methyl-1-butanol | 32.35 | 35.29 |
| 2-methyl-1-propanol | 13.24 | 11.76 |
| 2,6-di-tert-butyl-4-methyl phenol (butylated hydroxy toluene, BHT) | 11.76 | 13.24 |
| Aldehyde | ||
| 3-methyl-1-butanal | 4.41 | 2.94 |
| Hexanal | 11.76 | 7.35 |
| Carboxylic Acid | ||
| Ethanoic acid (acetic acid) | 69.12 | 57.35 |
| Gas | ||
| Argon | 14.71 | 13.24 |
| Carbon dioxide | 98.53 | 98.53 |
| Carbon disulphide | 11.76 | 2.94 |
| Hydrocarbon | ||
| 2-methylbutane | 97.06 | 100 |
| Trichloromethane (chloroform) | 4.41 | 8.82 |
| 3,7-dimethyldecane | 1.47 | 2.94 |
| 3,7-dimethylnonane | 45.59 | 60.29 |
| 2,2,4-trimethylheptane | 14.71 | 27.94 |
| 2,2,5-trimethylhexane | 17.65 | 23.53 |
| 2-methylpropane | 88.24 | 83.82 |
| 2,2,3,4-tetramethylpentane | 54.41 | 69.12 |
| Ketone | ||
| 2,3-butanedione | 54.41 | 51.47 |
| 2-butanone | 35.29 | 27.94 |
| 3-methyl-2-butanone | 1.47 | |
| 3-hydroxy-2-butanone | 100 | 97.06 |
| 2-propanone (acetone) | 17.65 | 13.24 |
| Sulphurous Compounds | ||
| Dimethyldisulphide | 7.35 | 7.35 |
| Dimethylsulphide | 14.71 | 11.76 |
| Methanethiol | 7.35 | 2.94 |
| Impurity | ||
| Siloxane impurity | 14.71 | 16.18 |
*From several sources such as GC column, injection port septum, laboratory atmosphere and meat trays
Common names of the compounds are provided in parenthesis
Table 7.
Mean peak areas of compounds with % of occurrence more than 30% in spiked fresh beef samples as a function of storage time
| Storage Time (days) | 3-hydroxy-2-butanone | 2,3-butanedione | 2,2-dimethyl-3-hexanone | acetic acid | 2-methylbutane | 2,6-di-tert-butyl-4-methyl phenol (butylated hydroxy toluene, BHT) | carbon dioxide | 3,7-dimethyldecane | 2,2,4-trimethylheptane | hexanal | 2,2,5-trimethylhexane | 2-methylpropane | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Spiked | Control | Spiked | Control | Spiked | Control | Spiked | Control | Spiked | Control | Spiked | Control | Spiked | Control | Spiked | Control | Spiked | Control | Spiked | Control | Spiked | Control | Spiked | |
| 0 | 1.37E + 06 | 1.49E + 06 | 1.02E + 06 | 1.21E + 05 | 1.40E + 05 | 4.89E + 05 | 3.29E + 06 | 1.29E + 08 | 9.35E + 07 | 6.06E + 05 | 5.28E + 05 | 5.19E + 06 | 4.05E + 06 | 6.40E + 05 | 1.24E + 06 | 5.57E + 05 | 9.38E + 05 | 8.02E + 04 | 1.65E + 05 | 3.25E + 05 | 5.06E + 05 | 3.83E + 07 | 3.00E + 07 | |
| 1 | 3.84E + 07 | 1.34E + 07 | 6.94E + 06 | 8.78E + 06 | 1.01E + 05 | 1.29E + 05 | 7.78E + 05 | 1.72E + 06 | 1.00E + 08 | 1.22E + 08 | 1.36E + 06 | 1.07E + 06 | 2.85E + 06 | 4.38E + 06 | 3.60E + 05 | 7.42E + 05 | 3.07E + 05 | 5.84E + 05 | 4.14E + 05 | 3.82E + 05 | 2.31E + 05 | 1.67E + 05 | 5.02E + 07 | 3.32E + 07 |
| 2 | 6.76E + 07 | 6.67E + 07 | 4.47E + 06 | 4.28E + 06 | 1.15E + 05 | 8.04E + 04 | 1.42E + 06 | 2.90E + 06 | 1.04E + 08 | 1.07E + 08 | 2.15E + 06 | 1.72E + 06 | 6.18E + 06 | 5.93E + 06 | 3.08E + 05 | 2.76E + 05 | 3.32E + 05 | 1.33E + 05 | 5.63E + 05 | 5.06E + 05 | 1.75E + 06 | 2.21E + 04 | 5.23E + 07 | 5.53E + 07 |
| 3 | 5.52E + 07 | 6.30E + 07 | 1.45E + 07 | 2.57E + 06 | 1.11E + 05 | 1.01E + 05 | 9.90E + 05 | 3.96E + 06 | 8.76E + 07 | 1.07E + 08 | 4.63E + 05 | 9.55E + 05 | 1.00E + 07 | 9.53E + 06 | 1.47E + 05 | 2.34E + 05 | 7.97E + 04 | 1.54E + 05 | 8.58E + 05 | 1.00E + 06 | 3.82E + 04 | 7.15E + 07 | 5.40E + 07 | |
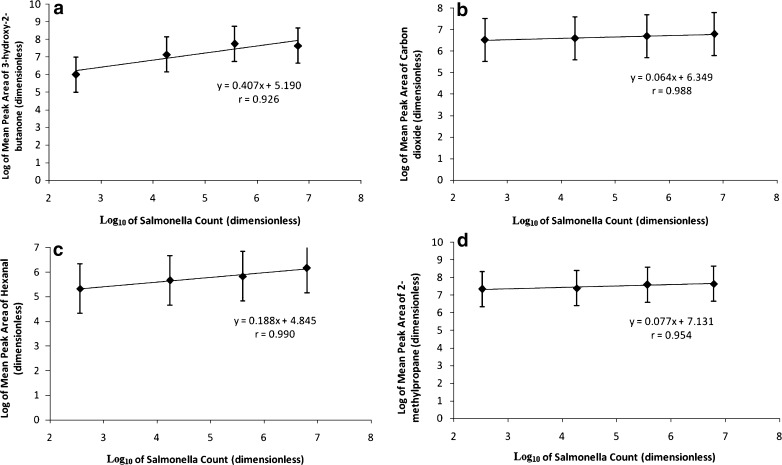
Linear correlations were obtained between the logarithm of average peak area and Salmonella count (log10 cfu/g) for all these compounds. The compounds which showed positive correlations with Salmonella in these fresh beef samples were 3-hydroxy-2-butanone (r = 0.926), carbon dioxide (r = 0.988), hexanal (r = 0.990) and 2-methylpropane (r = 0.954). These findings are summarized in Fig. 2 (a–d) for fresh beef samples. Although the correlation co-efficients for all these compounds have been found to be fairly high; from the slopes of these graphs, it was observed that the concentrations of these compounds (in terms of peak area responses) do not significantly change with the increase in the Salmonella population. Among all these compounds, the concentration of 3-hydroxy-2-butanone was found to change significantly with Salmonella count (as is evident from slope and intercept values), followed by that of 2,3-butanedione and hexanal. Significant changes were not observed for acetic acid and carbon dioxide.
Fig. 2.
a 3-hydroxy-2-butanone. b Carbon dioxide. c Hexanal. d 2-methlpropane. Plot of log Salmonella counts and log of peak area of VOCs in fresh beef samples. (Salmonella counts are in log 10 cfu/g of beef sample)
The F-tests (Fisher’s variance ratio) for the main effect of the sample source (peak areas was obtained from GC-MS data) were conducted for the compounds shown in Fig. 2 for the fresh samples. Among these, F values were significant at p < 0.05 for 3-hydroxy-2-butanone and for carbon dioxide with storage time (days). However, F values were not significant for type and type-day interaction for these compounds. The two-way interaction studies did not find any significant difference in the peak areas of these compounds between the control and spiked samples.
For aged beef
Figure 1b shows the gas chromatogram of a Salmonella spiked aged beef sample at 20°C and Table 4 gives the composition of the headspace volatiles of the same. Table 6 lists the compounds detected and the percentage of occurrence of each compound in the control and spiked samples for the aged beef samples. The mean peak areas of the compounds with % of occurrence ≥30% over the 4-day storage period have been listed in Table 8 for aged spiked samples.
Table 8.
Mean peak areas of compounds with % of occurrence more than 30% in spiked aged beef samples as a function of storage time
| Storage time (days) | 3-methyl-1-butanol | 2,3-butanedione | 2-butanone | 3-hydroxy-2-butanone | acetic acid | 2-methylbutane | carbon dioxide | 2-methylpropane | 3,7-dimethylnonane | 2,2,3,4-tetramethylpentane | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Spiked | Control | Spiked | Control | Spiked | Control | Spiked | Control | Spiked | Control | Spiked | Control | Spiked | Control | Spiked | Control | Spiked | Control | Spiked | |
| 0 | 3.55E + 05 | 1.52E + 05 | 5.29E + 04 | 2.61E + 06 | 2.43E + 06 | 4.93E + 05 | 4.43E + 05 | 7.06E + 07 | 6.34E + 07 | 4.51E + 06 | 3.29E + 06 | 2.20E + 06 | 1.26E + 07 | 2.05E + 06 | 4.00E + 06 | 2.35E + 06 | 3.02E + 06 | |||
| 1 | 3.45E + 05 | 2.40E + 05 | 1.51E + 06 | 1.60E + 06 | 1.86E + 06 | 8.90E + 05 | 1.00E + 07 | 7.91E + 06 | 2.16E + 06 | 2.32E + 06 | 9.82E + 07 | 1.10E + 08 | 3.77E + 06 | 4.11E + 06 | 2.05E + 06 | 1.05E + 07 | 1.24E + 06 | 1.53E + 06 | 1.15E + 06 | 1.54E + 06 |
| 2 | 3.96E + 06 | 4.93E + 06 | 1.14E + 06 | 1.29E + 06 | 9.51E + 05 | 4.37E + 05 | 3.05E + 07 | 3.91E + 07 | 8.36E + 06 | 5.71E + 06 | 1.02E + 08 | 9.01E + 07 | 1.05E + 07 | 9.56E + 06 | 2.35E + 06 | 2.94E + 06 | 7.93E + 05 | 5.04E + 05 | 7.41E + 05 | 5.47E + 05 |
| 3 | 1.30E + 07 | 1.47E + 07 | 2.24E + 06 | 5.94E + 05 | 2.43E + 06 | 6.42E + 05 | 6.84E + 07 | 4.17E + 07 | 1.12E + 07 | 4.68E + 06 | 9.24E + 07 | 8.07E + 07 | 1.28E + 07 | 1.76E + 07 | 3.33E + 06 | 2.99E + 06 | 4.29E + 05 | 3.50E + 05 | ||
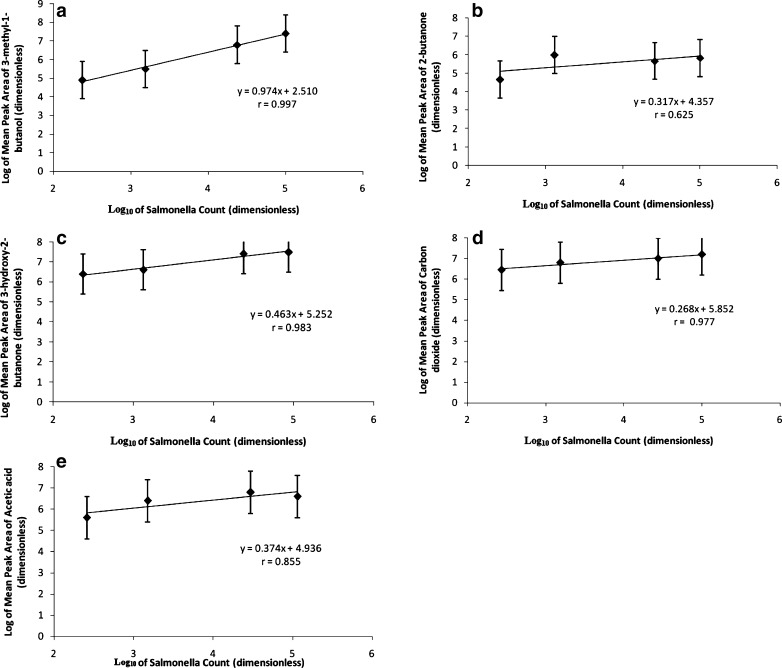
The compounds which showed positive correlations with Salmonella in these aged beef samples were 3-methyl-1-butanol (r = 0.997), 2-butanone (r = 0.625), 3-hydroxy-2-butanone (r = 0.983), carbon dioxide (r = 0.977) and acetic acid (r = 0.855). These findings are summarized in Fig. 3 (a–e) for aged samples. From the slopes of these graphs, it was observed that 3-methyl-1-butanol has the most significant change in peak area response with increasing Salmonella growth; followed by 3-hydroxy-2-butanone, acetic acid and 2-butanone. Carbon dioxide showed the least change in peak area response with growth of Salmonella.
Fig. 3.
a 3-methyl-1-butanol. b 2-butanone c 3-hydroxy-2-butanone d Carbon dioxide e Acetic acid. Plot of log salmonella counts and log of mean peak area of VOCs in aged beef samples. (Salmonella counts are in log10 cgu/g of beef sample)
Similar F-tests were also conducted for the compounds shown in Fig. 3 for the aged beef samples. Among these, F values were significant at p < 0.05 for 3-hydroxy-2-butanone, acetic acid and for carbon dioxide with storage time (days). However, F values were not significant for type and type-day interaction for these compounds. The two-way interaction studies however, did not show any significant difference in peak areas of these compounds between the control and spiked samples.
Comparison between aged and fresh beef
From Tables 7 and 8, it is observed that among all the compounds detected in fresh and aged beef samples alike, carbon dioxide, 2-methylbutane, 2-methylpropane, 2,3-butanedione, 3-hydroxy-2-butanone and acetic acid were found to be present in both fresh and aged spiked samples. On the other hand, 3,7-dimethyldecane, 2,2,5-trimethylhexane, 2,2,4-trimethylheptane, hexanal and 2,2-dimethyl-3-hexanone were found to occur at 30% level in only spiked fresh beef samples and 3-methyl-1-butanol, 2-butanone, 3,7-dimethylnonane and 2,2,3,4-tetramethylpentane were present in only aged beef samples.
These findings indicate that several compounds (volatile) that did not occur in the aged (packaged) beef samples occurred in the fresh beef samples. We hypothesize that along with these individual compounds, their associated functional groups might also provide additional information for development of sensors.
Conclusion
Solid phase microextraction and gas chromatography-mass spectrometry could detect several volatile compounds in the headspace of over-wrapped beef (fresh and aged) in Styrofoam trays, stored at 20°C for a period of 4 days; of which 3-hydroxy-2-butanone, carbon dioxide, hexanal and 2-methylpropane showed positive correlations with Salmonella in fresh beef samples; while 3-methyl-1-butanol (r = 0.997), 2-butanone (r = 0.646), 3-hydroxy-2-butanone (r = 0.977), carbon dioxide (r = 0.984) and acetic acid (r = 0.900) showed positive correlations with Salmonella in aged beef samples. This study qualitatively explored the profiles of volatile organic compounds present in packaged beef samples (contaminated and uncontaminated).
Future work is recommended for calibration and quantifications of these headspace gases. Further investigations to quantify the headspace gases in the retail meat packages are also recommended.
Electronic supplementary material
(DOC 757 kb)
Acknowledgment
The authors would like to express their profound gratitude to the United States Department of Agriculture-Cooperative State Research, Education and Extension Service (USDA-CSREES) for their financial support for this research.
This work was conducted at North Dakota State University, USA.
Footnotes
Classification: Muscle Foods
References
- Bhattacharjee P, Panigrahi S, Lin D, Logue CM, Sherwood JS, Doetkett C, Marchello M. Study of headspace gases associated with Salmonella contamination of sterile beef in vials using HS-SPME/GC-MS. TASABE. 2010;53:173–181. [Google Scholar]
- Blackburn CW, Davies AR. Development of antibiotic-resistant strains for the enumeration of foodborne pathogenic bacteria in stored foods. Int J Food Microbiol. 1994;24:125–136. doi: 10.1016/0168-1605(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Brunton NP, Cronin DA, Monahan FJ. Volatile components associated with freshly cooked and oxidized off-flavors in turkey breast meat. Flavour Frag J. 2002;17:327–334. doi: 10.1002/ffj.1087. [DOI] [Google Scholar]
- Cadwallader RK, MacLeod AJ (eds) (1998) Instrumental methods for analyzing the flavor of muscle foods. In: Flavor of meat, meat products and seafoods. Blackie Academic and Professional, London, 355–373
- Dainty RH. Chemical/biochemical detection of spoilage. Int J Food Microbiol. 1996;33:19–33. doi: 10.1016/0168-1605(96)01137-3. [DOI] [PubMed] [Google Scholar]
- Elmore JS, Mottram DS, Hierro E. Two-fiber solid-phase microextraction combined with gas chromatography-mass spectrometry for the analysis of volatile aroma compounds in cooked pork. J Chromatogr A. 2000;905:233–240. doi: 10.1016/S0021-9673(00)00990-0. [DOI] [PubMed] [Google Scholar]
- Gianelli MP, Flores M, Toldra F. Optimisation of solid phase microextraction (SPME) for the analysis of volatile compounds in dry-cured ham. J Sci Food Agric. 2002;82:1703–1709. doi: 10.1002/jsfa.1249. [DOI] [Google Scholar]
- Goodridge CF, Beaudry RM, Pestka JJ, Smith DM. Solid phase microextraction-gas chromatography for quantifying headspace hexanal above freeze-dried chicken myofibrils. J Agr Food Chem. 2003;51:4185–4190. doi: 10.1021/jf0260646. [DOI] [PubMed] [Google Scholar]
- Kerler J, Grosch W. Odorants contributing to warmed-over flavor (WOF) of refrigerated cooked beef. J Food Sci. 1996;61:1271–1274. doi: 10.1111/j.1365-2621.1996.tb10977.x. [DOI] [Google Scholar]
- Luna GR, Diego AL, García-González DL. A tentative characterization of white dry-cured hams from Teruel (Spain) by SPME-GC. Food Chem. 2006;97:621–630. doi: 10.1016/j.foodchem.2005.05.039. [DOI] [Google Scholar]
- Machiels D, Istasse L. Evaluation of two commercial solid-phase microextraction fibers for the analysis of target aroma compounds in cooked beef meat. Talanta. 2003;61:529–537. doi: 10.1016/S0039-9140(03)00319-9. [DOI] [PubMed] [Google Scholar]
- Marco A, Navarro JL, Flores M. Volatile compounds of dry fermented sausages as affected by solid phase microextraction (SPME) Food Chem. 2004;84:633–641. doi: 10.1016/S0308-8146(03)00288-7. [DOI] [Google Scholar]
- Ogihara H, Horimoto Y, Wang ZH, Skura BJ, Nakai S. Solid phase microextraction/gas chromatography of Salmonella-infected beef. J Agr Food Chem. 2000;48:2253–2259. doi: 10.1021/jf991201t. [DOI] [PubMed] [Google Scholar]
- Pinho O, Ferreira IMPLVO, Ferreira MA. Solid phase microextraction in combination with GC-MS for quantification of the major volatile free fatty acids in ewe cheese. Anal Chem. 2002;74:5199–5204. doi: 10.1021/ac020296m. [DOI] [PubMed] [Google Scholar]
- Ramírez MR, Estévez M, Morcuende D, Cava R. Effect of the type of frying culinary fat on volatile compounds isolated in fried pork loin chops by using SPME-GC-MS. J Agr Food Chem. 2004;52:7637–7643. doi: 10.1021/jf049207s. [DOI] [PubMed] [Google Scholar]
- Selby C (2007) Parafilm. Available at http://plant-tc.cfans.umn.edu/listserv/1996/log9612/msg00139.html. Accessed 27 December 2007
- Senecal AG, Magnone J, Yeomans W, Powers EM. Rapid detection of pathogenic bacteria by volatile organic compound (VOC) analysis. P SPIE. 2002;4575:121–131. doi: 10.1117/12.456915. [DOI] [Google Scholar]
- Shahidi F (ed) (1998) Flavour of muscle foods: an overview. In: Flavor of meat, meat products and seafoods. Blackie Academic and Professional, London, 1–14
- Shang C, Deng C, Zhang X, Chen Z, Hu Y. Headspace solid-phase microextraction and gas chromatography-mass spectrometry analysis of free volatile compounds in mango. Chromatographia. 2002;55:737–741. doi: 10.1007/BF02491790. [DOI] [Google Scholar]
- Stanbridge LH, Davies AR. The microbiology of chill-stored meat. In: Davies A, Board R, editors. The microbiology of meat and poultry. London: Blackie Academic and Professional; 1998. p. 175. [Google Scholar]
- Tsigarida E, Nychas GJE. Ecophysiological attributes of a Lactobacillus sp. and a Pseudomonas sp. on sterile beef fillets in relation to storage temperature and film permeability. J Appl Microbiol. 2001;90:696–705. doi: 10.1046/j.1365-2672.2001.01292.x. [DOI] [PubMed] [Google Scholar]
- Westfall PH, Tobias RD, Rom D, Wolfinger RD, Hochberg Y (1999) Multiple comparisons and multiple tests using the SAS system, SAS institute Inc., p 416
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 757 kb)