Abstract
Porcine hemoglobin hydrolysate (PHH) was prepared with 6 different proteases (flavourzyme, papain, A.S.1398, alcalase, pepsin and trypsin). There was no correlation between extent of hydrolysis and antioxidant activity (p > 0.05). The peptic hydrolysate prepared at 60 min possessed the strongest antioxidant potential (67.0 ± 1.84%) among different hydrolysates, which was fractionated into 4 major types by ultrafiltration membranes with different molecular weight cut-off (MWCO), PHH-I (Mw > 10 kDa), PHH-II (Mw = 5–10 kDa), PHH-III (Mw = 3–5 kDa) and PHH-IV (Mw < 3 kDa). PHH-IV possessed higher inhibitory effects of lipid peroxidation and scavenging effects on superoxide radical compared with larger MW fractions. Four fractions possessed the scavenging effects on 1, 1-diphenyl-2-picrylhy-drazyl (DPPH) and hydroxyl radicals in the order PHH-IV > PHH-I > PHH-III > PHH-II. MW of the 2 major peptide fractions from PHH-IV was located at 2476 Da (F1) and 1042 Da (F2), respectively. PHH-IV could be utilized to develop physiologically functional foods or therapy drugs.
Keywords: Porcine hemoglobin hydrolysate, Pepsin, Enzymatic hydrolysis, Antioxidant, Free radical scavenging activity, Molecular weight distribution
Introduction
Free radical-mediated reactive oxygen species (ROS) and lipid peroxidation (LPO) have gained considerable attention nowadays (Athukorala et al. 2006; Manso et al. 2008; Seema et al. 2008; Nasirullah et al. 2009). ROS were thought to create oxidative stress thereby causing various degenerative diseases, such as atherosclerosis, rheumatoid arthritis, diabetes mellitus, and Alzheimer’s diseases (Butterfield et al. 2002). Autoxidation occurring in food materials are also induced by ROS because LPO is kept on by a free radical mechanism (Je et al. 2007). LPO contributes to the subsequent development of unpleasant off-flavours, dark colors, poor texture and may also generate potentially toxic end products (Thiansilakul et al. 2007). Recently, some protein hydrolysates have been reported to exhibit antioxidant activity (Je et al. 2005; Sakanaka and Tachibana 2006; Klompong et al. 2007). In the past several years, many researches reported that proteinases can affect the antioxidative activity of protein hydrolysate (Jao and Ko 2002; Saiga et al. 2003). Thiansilakul et al. (2007) found that round scad muscle hydrolysates produced with flavourzyme exhibited a higher DPPH radical scavenging activity and reducing power, but a lower Fe2+ chelating ability than alcalase derived hydrolysates. Je et al. (2007) reported that the hydrolysate from tuna backbone protein exhibited the highest inhibitory effects on LPO than those prepared by other proteases (alcalase, flavourzyme, neutrase, papain and trypsin). The specificities of proteases contribute to the length and amino acid sequence of peptides, which may influence antioxidant activities of protein hydrolysates.
Porcine blood is an abundant by-product in the slaughtering process in China with an estimated amount of one million tons per year and the availability is less than 10%. Except some porcine blood utilized as “blood tofu” for human consumption and blood powder for animal feed, quite a few portion is discharged as sewage, which causes severe environmental problems. Blood protein is nutritional and functional, and it includes two main components, plasma protein and hemoglobin. The proportion of hemoglobin in blood protein is 60 ~ 70%. One approach for the effective application of hemoglobin is enzymatic hydrolysis, which is widely used in food industry to prepare hydrolysate with opioid activity (Nyberg et al. 1997), bradykinin-potentiating activity (Lignot et al. 1999), antibacterial activity (Nedjar-Arroume et al. 2006) and angiotensin I-converting enzyme inhibition activity (Yu et al. 2006) for nutritional therapy. Recently, a study on the antioxidant activity of PHH produced using alcalase and flavourzyme has been performed (Chang et al. 2007). Nevertheless, reports on the antioxidant activity of PHH prepared with other proteases are still scarce. An ultrafiltration (UF) membrane system is one of the methods to obtain hydrolysate with the desired molecular size and good functional properties. In the present study, the antioxidant activity of PHH obtained with six different proteases and of the fractionated hydrolysates prepared using UF membranes were investigated by various assays, including the ability to inhibit the autoxidation of linoleic acid and the scavenging effects on DPPH/superoxide/hydroxyl radicals. In addition, molecular weight distribution of the peptide fraction with the highest antioxidant activity was also evaluated.
Materials and methods
Fresh blood of porcine species was obtained from Shunxin Agricultural Co. Ltd. (Shun-Yi District, Beijing). The porcine blood was collected as the animal was bled and sodium citrate (3 g/l blood) was quickly added to prevent clotting and kept blow 4 °C to avoid bacterial proliferation. The UF membrane reactor system for the fractionation of hydrolysate, based on molecular weights, was from XuBang Membrane Equipment Co., Ltd, Beijing, China. Proteases used for enzymatic hydrolysis were as follows: Trypsin and pepsin were from Amresco (Solon, OH, USA). Alcalase and flavorzyme were from Novozymes (Novo Nordisk, Bagsvaerd, Denmark). The crude protease A.S.1398 was from Donghua Qiangsheng Biotechnology Co., Ltd, Beijing, China and papain was from Javely Biological Products Co., Ltd, Nanning, China. 2, 4, 6-Trinitrobenzenesulphonic acid (TNBS, P2297), 2-deoxy-D-ribose (D5899), linoleic acid (L1376), DPPH (D9132) and ascorbic acid (A7506) were purchased from Sigma–Aldrich (St. Louis, MO, USA). α-Tocopherol was the product of Amresco (Solon, OH, USA). Vitamin B12 (Mw 1355 Da), L-glutathione oxidized (Mw 612 Da) and L-tyrosine (Mw 181 Da) were purchased from BioDee BioTech Co., Ltd, Beijing, China. All reagents used were of analytical grade.
Preparation of hemoglobin sample
The blood was centrifuged at 9000 × g and 4 °C for 10 min using a high speed refrigerated centrifuge (Model TGL-16A, Changsha PINGFAN Instrument and Meter Co., Ltd., Changsha, China), and the blood cells were obtained. The blood cells were then added with an equal volume of water to burst, and centrifuged at 4000 × g and 4 °C to remove the stroma and obtain the hemoglobin. The hemoglobin was lyophilized using a FD-1PF freeze dryer (Beijing DETIANYOU Technology Development Co., Ltd., Beijing, China) to dry powder and stored at 4 °C until use. Crude protein of the hemoglobin sample was assayed using the method of AOAC (2005).
Preparation of PHH
Porcine hemoglobin (PH) was separately hydrolyzed with 6 different enzymes. The characteristics and optimal reaction conditions of 6 enzymes are shown in Table 1. Five g of dried PH were dissolved in 100 ml distilled water at room temperature (25 °C). The PH solutions were incubated with each protease at an optimal temperature for different times with stirring and then heated at 100 °C for 10 min to inactivate the enzyme. During hydrolysis, the pH of the solution was adjusted to its optimal value for each protease using 1 M NaOH or 1 M HCl. After treatment, all hydrolysates obtained at different times were centrifuged at 4200 × g and 4 °C for 10 min to remove the precipitates (low degraded hemoglobin and heme). All hydrolysates were lyophilized for 96 h at −60 °C and 0.04 mbar and stored at −20 °C. All hydrolysates powders were used for preliminary screening of antioxidant activity.
Table 1.
Characteristics and optimal reaction conditions of six proteases
| Proteases | Source | Activity, U/g | Optimal conditions | |||
|---|---|---|---|---|---|---|
| Temperature °C | pH | Time h | E/S w/w | |||
| Flavourzyme | Aspergillus oryzae | 20816 | 45 | 6.5 | 8 | 0.5% |
| Papain | Carica papaya | 36444 | 37 | 6.5 | 8 | 0.3% |
| A.S.1398 | Bacillus subtilis | 44660 | 45 | 7.0 | 8 | 0.2% |
| Alcalase | Bacillus licheniformis | 49355 | 55 | 8.0 | 8 | 0.2% |
| Pepsin | Porcine stomach mucosa | 6907 | 37 | 2.0 | 8 | 1.6% |
| Trypsin | Porcine pancreas | 45180 | 45 | 7.5 | 8 | 0.2% |
E/S: Enzyme to substrate ratio
Degree of hydrolysis (DH)
The cleavage of peptide bonds during hydrolysis was quantified by the TNBS method (Adler-Nissen 1979). After hydrolysis, test samples diluted with deionized water (0.25 ml) were mixed with 2 ml of sodium phosphate buffer (0.2 M, pH 8.2). TNBS reagent (2 ml) was then added to each tube followed by mixing and incubation at 50 °C for 60 min in a covered water bath to exclude light. After incubation, reaction was quenched by adding 0.1 M HCl (4.0 ml) to each tube. Samples were then allowed to cool at room temperature for 30 min before the absorbance were measured at 340 nm (Model UV-2600A, UNICO, Shanghai, China). L-Leucine (0–2.0 mM) was used to generate a standard curve and free amino groups were expressed in terms of L-leucine. DH was calculated using the following formula:
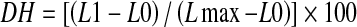 |
where, L1 is the amount of free amino groups released after hydrolysis, L0 is the amount of free amino groups in the original PH, and Lmax is total amount of free amino groups in the original PH obtained after acid hydrolysis (6 M HCl at 100 °C for 24 h).
Separation of active peptide fractions
PHH with the highest antioxidative activity was fractionated through three different UF membranes having a range of MWCO of 10, 5, and 3 kDa, respectively. Fractionates were designed as follows: PHH-I with MW distribution > 10 kDa, PHH-II with MW distribution of 5–10 kDa, PHH-III with MW distribution of 3–5 kDa and PHH-IV with MW distribution < 3 kDa. All peptide fractions recovered were lyophilized in a freeze drier and stored at −20 °C.
Antioxidant activity in linoleic acid model system
The antioxidant activity in a linoleic acid model system was determined according to the method of Saha et al. (2004) with some modifications. PHH was dissolved in 2.5 ml of 50 mM phosphate buffer (pH 7.0) (5 mg/ml) and added into a 99.5% ethanol (2.5 ml) and 99% linoleic acid (32.5 μl) mixture. The final volume was adjusted to 6.25 ml with distilled water. The same mixture without sample was used as control. The mixed solution in sealed screw cap conical tube was incubated at 40 °C in darkness and air-circulating conditions. At 24-h intervals, aliquots of the reaction mixtures were taken for oxidation determination using the ferric thiocyanate (FTC) method (Mitsuda et al. 1996). An aliquot (0.1 ml) of the reaction mixture was mixed with 75% ethanol (4.7 ml) followed by the addition of 30% ammonium thiocyanate (0.1 ml) and 20 mM ferrous chloride solution (0.1 ml) in 3.5% HCl. After 3 min, extent of colour development was measured at 500 nm. α-tocopherol and butylated hydroxytoluene (BHT) were used as positive controls. α-Tocopherol was diluted to 0.05 and 0.1 mg/ml and BHT was diluted to 0.1 mg/ml for further uses.
DPPH radical scavenging activity
The DPPH radical scavenging activity of PHH was measured according to the procedure described by Shimada et al. (1992). Test samples in 1.5 ml of water were mixed with 1.5 ml of 1 mM DPPH ethanol solution. The sample concentration (1.5 mg/ml) was applied for peptide fractions. This mixture was shaken and kept at 25 °C for 30 min and then the absorbance of the mixture was measured at 517 nm against a blank.
Superoxide radical scavenging activity (SRSA)
The SRSA was determined by measuring the inhibition of the auto-oxidation of pyrogallol using a slightly modified method of Marklund and Marklund (1974). A sample solution (0.3 ml) (5 mg/ml) and 2.61 ml of 50 mM phosphate buffer (pH 8.2) were added into freshly prepared 90 μl of 3 mM pyrogallol (dissolved in 10 mM HCl). The reaction mixture was added 100 μl of 0.2 M ascorbic acid immediately after incubation for 4 min and the absorbance was measured at 325 nm against a blank.
Hydroxyl radical scavenging activity (HRSA)
The HRSA was measured by the deoxyribose method (Siddhuraju and Becker 2007) with some modifications. The reactions were performed in 0.1 M, phosphate buffer (pH 7.4), containing 10 mM deoxyribose, 10 mM FeSO4·7H2O, 10 mM EDTA and the samples with 5 mg/ml. The reaction was activated by adding H2O2 to a concentration of 10 mM and the reaction mixture was incubated for 1 h at 37 °C in a water bath. After incubation, the colour was developed by adding 1 ml of 1% thiobarbituric acid (TBA) and 1 ml of 2.8% ice-cold trichloroacetic acid (TCA) and heated at 100 °C for 10 min. After cooling, the absorbance was measured at 532 nm against a blank.
Determination of molecular weight distribution
The PHH fraction with the highest antioxidant activity was analyzed for molecular weight distribution by gel filtration chromatography. The peptides were loaded onto Sephadex G-10 column (1.6 × 40 cm, Pharmacia, Sweden), eluted with deionized water at a flow rate of 0.5 ml/min, and 3-ml fractions were collected and monitored at 280 nm. A molecular weight calibration curve was obtained from the following standards: Vitamin B12 (1,355 Da), L-glutathione oxidized (612 Da) and L-tyrosine (181 Da).
Statistical analysis
Experimental results were mean ± SD of three measurements. The values p < 0.05 were regarded as significant. Statistical analysis of the data was performed by Student t test with the Statistical Analysis System software (SAS Institute, 1999).
Results and discussion
Preparation of protein hydrolysates and their antioxidant activity
The crude protein content of the PH powder prepared was 92%, which indicated that PH is very suitable as a protein source for high-valued functional products. The antioxidant activity of the PHH was investigated in DPPH scavenging system and compared with that of PH. This assay can be used for the primary characterization of the scavenging potential of compounds (Krings and Berger 2001; Ramakrishna et al. 2008). It is well accepted that the DPPH radical scavenging by antioxidants is attributable to their hydrogen donating ability (Chen and Ho 1995). Therefore, DPPH is often used as a substrate to evaluate the antioxidative activity of an antioxidant.
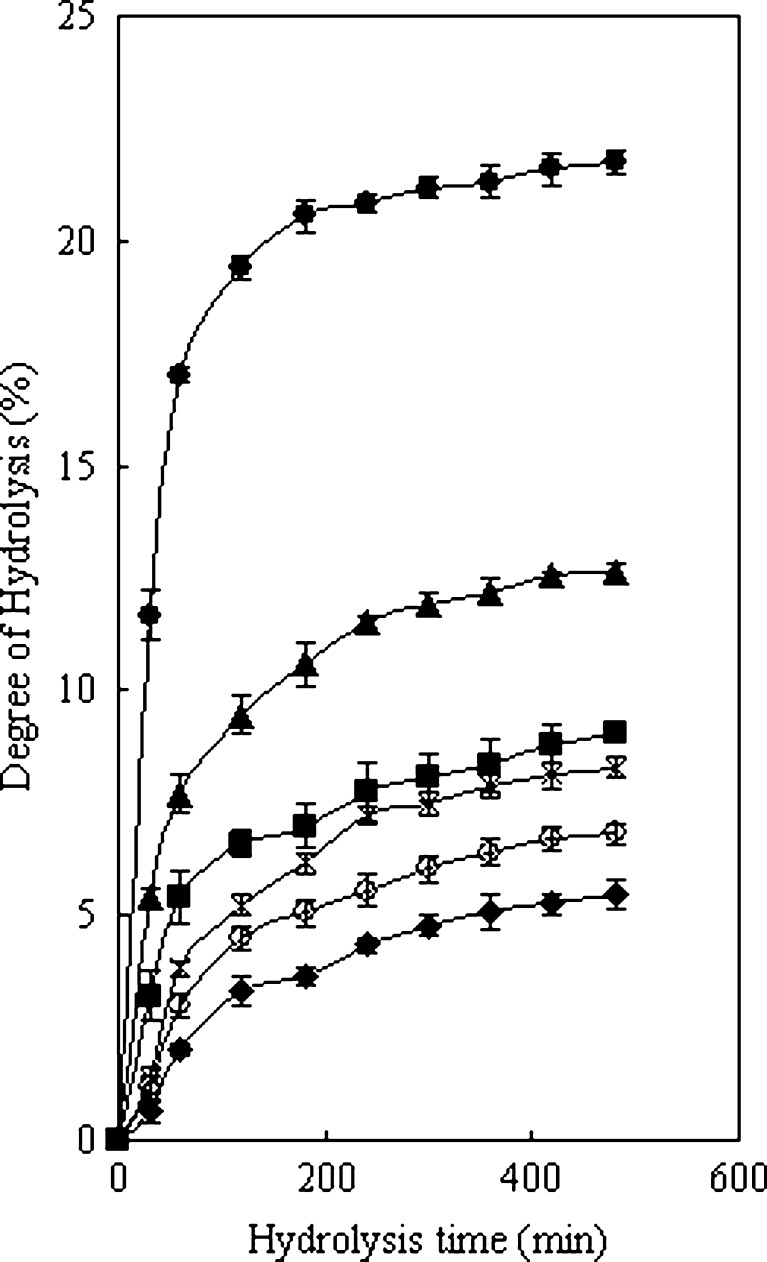
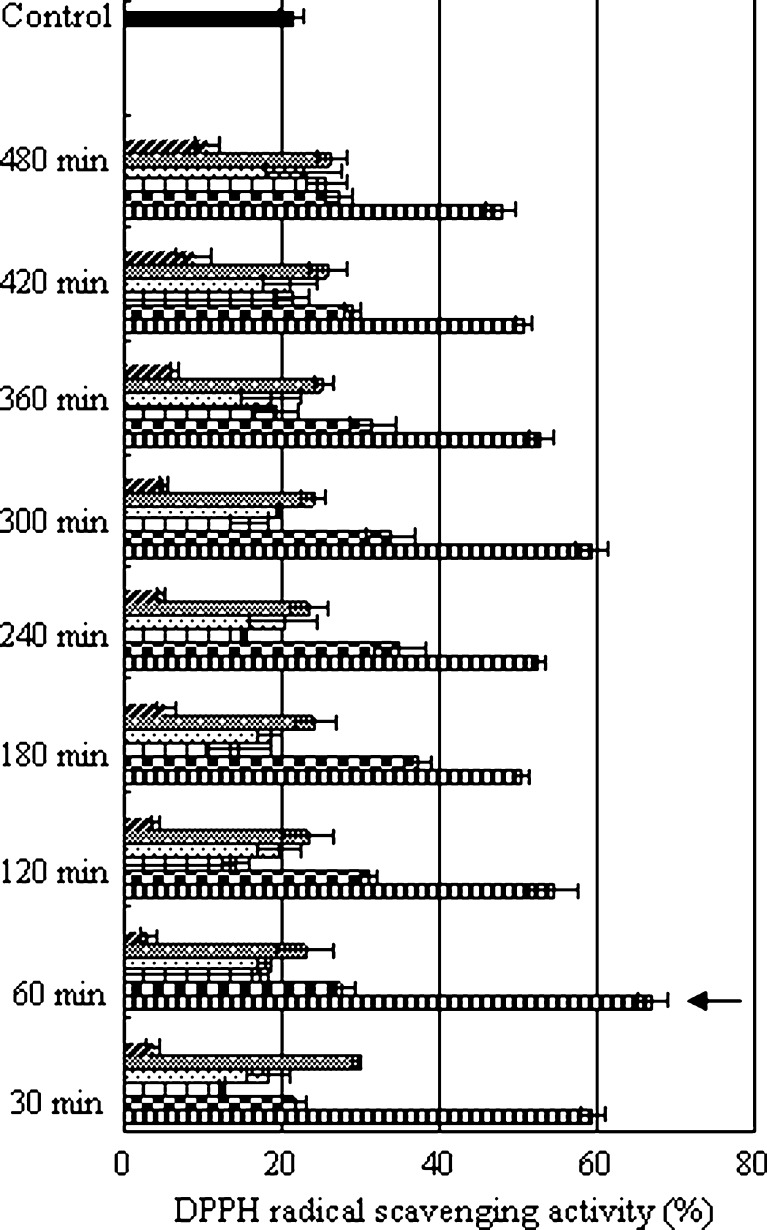
Six different proteases, alcalase, A.S.1398, papain, flavourzyme, pepsin and trypsin, originated from different sources and with distinct substrate specificities, were used for the hydrolysis of PH. DH and antioxidant activity of the hydrolysates were monitored for a 6-h period for each protease (Fig. 1). The DH obtained increased with increasing hydrolysis time. The hydrolysis of PH with proteases proceeded at a high rate during the initial 60 min and thereafter slowed down. However, there was no correlation (p > 0.05) between hydrolysis time and antioxidant activity. The result was also found in the process of alcalase-hydrolyzed silver carp protein (Dong et al. 2008), Protease P-hydrolyzed sericin (Wu et al. 2008) and five proteases hydrolyzed rice bran protein (Adebiyi et al. 2007). This result indicates that the antioxidant activity of the hydrolysates is inherent to their characteristics amino acid sequences of peptides depending on protease specificities. Alcalase showed the highest DH values, followed by pepsin (Fig. 1). However, among the hydrolysates, pepsin-derived PHH prepared at 60 min showed the strongest antioxidant activity (67.0 ± 1.84%), which was significantly higher than that of the same dose of PH (21.2 ± 1.61%) (p < 0.01) (Fig. 2). Sakanaka et al. (2004) also indicated that egg-yolk protein hydrolysate exhibited strong antioxidative activity in comparison with the native protein. It suggested that the disruption of native structure by enzymatic hydrolysis might result in unfolding, thereby exposing active amino acid residues and patches, which were capable of reacting with oxidants. The antioxidant activities of PHH in other antioxidant assays (e.g. inhibition of linoleic acid autoxidation) with various concentrations were also evaluated and the results also showed that the peptic hydrolysate exhibited the excellent antioxidant activity (data not shown). Pepsin preferably digests peptide bonds by cleaving after the N-terminal of aromatic amino acids such as Phe, Trp and Tyr. The phenyl groups of the residues at peptide ends were likely to scavenging the free radical to prevent DNA damages. Aubes-Dufau et al. (1996) found that the highest concentrations of peptides composing mainly of hydrophobic amino acid residues were reached in pepsin-derived bovine hemoglobin hydrolysates with 8 ~ 16% DH. The DH was 7.7% at 60 min in this work, which was very close to 8%. Beyond 60 min, most of the antioxidant peptides were possibly get hydrolyzed into lower antioxidant ones. The antioxidant activity of PHH prepared with some proteases (e.g. flavourzyme, which is the endo- and exopeptidases enzyme mixture that can produce both amino acid and peptides) was lower than PH irrespective of the hydrolysis time.
Fig. 1.
Hydrolysis process of porcine hemoglobin (PH). Six proteases: (♦)Trypsin, (○)Flavorzyme, (■)Papain, (×)A.S.1398, (●)Alcalase, (▲)Pepsin. The values are means ± SD of triplicate measurements
Fig. 2.
Comparison on DPPH scavenging effects of porcine hemoglobin hydrolysate prepared using six proteases. Six proteases: ( )Flavorzyme, (
)Flavorzyme, ( )Trypsin, (
)Trypsin, ( )Papain, (
)Papain, ( )A.S.1398, (
)A.S.1398, ( )Alcalase, (
)Alcalase, ( )Pepsin. Native porcine hemoglobin (■) was adopted as control. The hydrolysate with the highest DPPH radical scavenging activity was indicated by an arrow. The values are means ± SD of triplicate measurements
)Pepsin. Native porcine hemoglobin (■) was adopted as control. The hydrolysate with the highest DPPH radical scavenging activity was indicated by an arrow. The values are means ± SD of triplicate measurements
In order to obtain a hydrolysate or peptide fraction with both the desired molecular size and nutritional properties, the PHH was further separated by using three kinds of UF membranes (10-, 5-, and 3-kDa MWCO membranes) according to molecular size and four kinds of permeates (PHH-I, PHH-II, PHH-III and PHH-IV) were obtained.
Measurement of UF fractions on inhibition of linoleic acid autoxidation
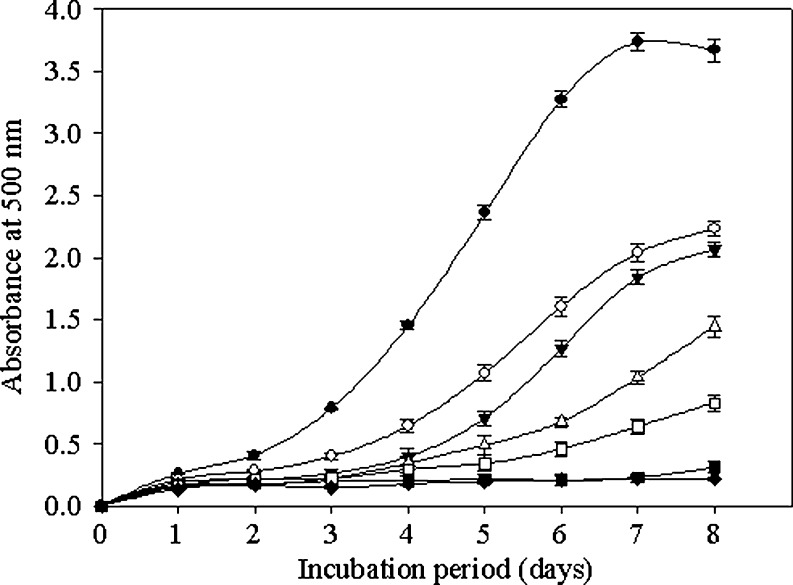
Several toxic by-products of LPO can damage biomolecules including DNA, although these biomolecules are away from the site of their generation (Box and Maccubbin 1997). PHH-IV, which is 3-kDa permeate, had a higher antioxidant activity than the other fractions (Fig. 3). The auto-oxidation of linoleic acid of control was accompanied by a rapid increase of peroxide value at day 1 of testing, reached maximum levels on day 7 and dropped on day 8. PHH fractions showed strong inhibitory effects, as compared to the control (p < 0.05), and significantly prolonged the induction period of auto-oxidation of linoleic acid. From the FTC results, significant differences (p < 0.05) in inhibitory effects were found among four fractions in the order of PHH-IV > PHH-III > PHH-II > PHH-I. The inhibitory effect by PHH-IV was found to be 93.80 ± 0.97%, which was significantly higher than the effect of 0.1 mg/ml of a lipid-soluble natural antioxidant α-tocopherol (p < 0.01) and equivalent to a commonly used synthetic antioxidant BHT (0.1 mg/ml) (p > 0.05). This result showed that higher antioxidant activities by PHH-IV are thought to be due to the low molecular weight as it can be easily adsorbed or loosely localize at oil/water interface where oxidation takes places and thereby reduce radical-mediated LPO. The inhibitory effect by PHH-IV was similar to the trypsin-derived peptides from the hydrolysate of hoki skin gelatin (Mendis et al. 2005). The differences may be attributed to differences in type and nature of protein and hydrolysis pattern of protease.
Fig. 3.
Antioxidative activity measured in a linoleic acid model system in the presence of peptide fractions. Higher UV absorbance at 500 nm represents higher lipid peroxidation: (▲) control, (○) PHH-I, (▼) PHH-II, (△) PHH-III, (■) PHH-IV, (□)α-tocopherol, (♦) BHT. The values are means ± SD of triplicate measurements
DPPH radical scavenging activity of UF fractions
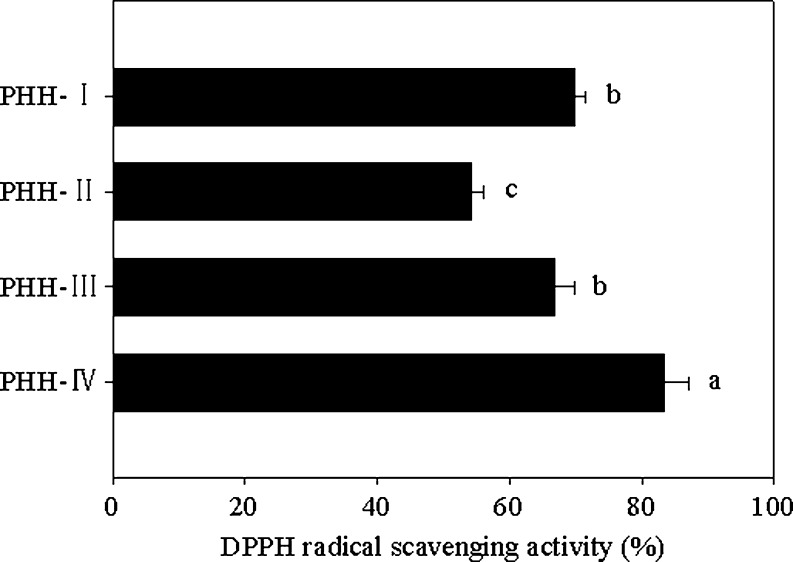
DPPH is a stable free radical with a characteristic absorption, which decreases significantly on exposure to proton radical scavengers (Tung et al. 2007; Singh et al. 2009). PHH-IV shows strong scavenging activity (83.4 ± 3.83%) on DPPH radicals, which was significantly higher than that of other fractions (p < 0.05) (Fig. 4). The scavenging activity of PHH-IV was similar to that of peptides isolated from alcalase-derived hydrolysate of alfalfa leaf protein (Xie et al. 2008) and closer to that of egg-yolk protein hydrolysates prepared by proteinase from Bacillus sp. (Sakanaka and Tachibana 2006). Other peptide fractions also showed strong DPPH radical scavenging activities; PHH-I showed higher scavenging activity (69.9 ± 1.67%) than PHH-II and PHH-III but no significant difference was found between PHH-I and PHH-III (p > 0.05). The order of DPPH scavenging activity of peptide fractions from PHH was similar to that of chickpea protein peptide fractions (Li et al. 2008). It was evident that PHH-IV did show a strong hydrogen donating ability to act as a peroxyl radical scavenger, thereby terminating the oxidation chain reaction of fat.
Fig. 4.
DPPH radical scavenging activity of peptide fractions. The values are means ± SD of triplicate measurements. Values with different letters are significantly different (P < 0.05)
SRSA of UF fractions
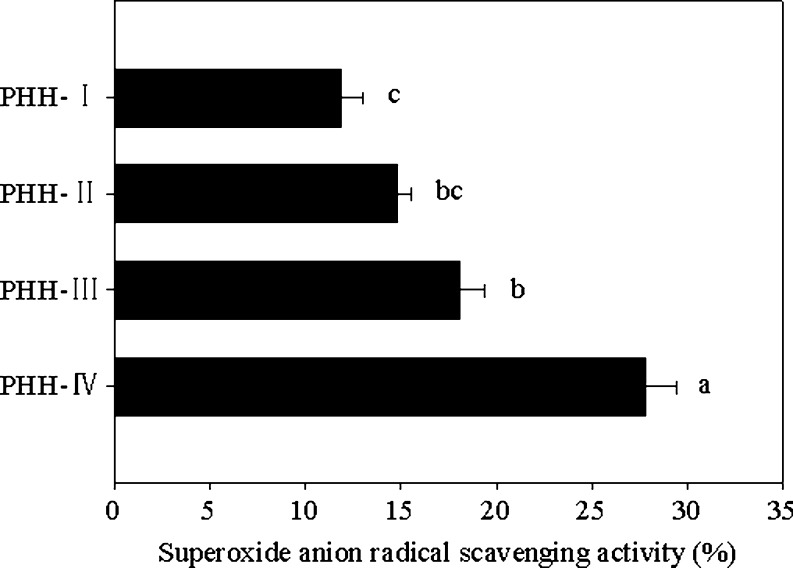
Superoxide radical is known to be very harmful to cellular components as a precursor of the more reactive oxygen species, which contribute to tissue damage and cause various diseases, and thus study of the scavenging activity of this radical is important (Kanatt et al. 2007). Superoxide anion scavenging of different PHH fractions is presented in Fig. 5. All samples treated in this experiment showed considerable scavenging abilities over superoxide anion. The PHH-IV showed significant stronger superoxide anion scavenging activity (27.8 ± 1.64%) than other peptide fractions (p < 0.05), which was similar to that of antioxidant peptides isolated from hoki (Johnius belengerii) frame protein (Kim et al. 2007). The other fractions when used at the same concentration also showed superoxide anion scavenging activities in the order PHH-III > PHH-II > PHH-I. This result implied that PHH-IV is a strong superoxide scavenger and its capacity to scavenge superoxide may contribute to its antioxidant activity.
Fig. 5.
Superoxide anion radical scavenging activity of peptide fractions. The values are means ± SD of triplicate measurements. Values with different letters are significantly different (P < 0.05)
HRSA of UF fractions
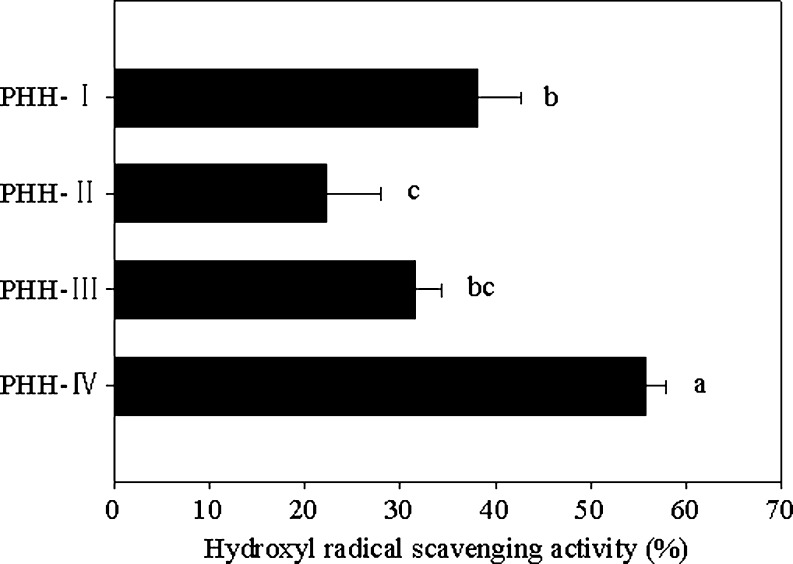
The chemical activity of hydroxyl radical is the strongest among ROS. It easily induces severe damage to DNA leading to carcinogenesis, mutagenesis and cytotoxicity (Ardestani and Yazdanparast 2007). Therefore, the removal of hydroxyl radical is probably one of the most effective defenses of a living body against various diseases. Hydrolysates from various protein sources, such as Alaska pollack frame (Je et al. 2005) and conger eel muscle (Ranathunga et al. 2006) have been reported to have good hydroxyl radical scavenging activity. Figure 6 shows that PHH-IV had strong hydroxyl radical scavenging activity, which could be attributed to the combined effects of donation of hydrogen atoms and scavenging of active oxygen. PHH-IV led to the highest hydroxyl radical scavenging activity (55.8 ± 2.15%), which was significantly higher than that of other fractions (p < 0.05). At the same dose, PHH-I showed higher scavenging activity (38.0 ± 4.60%) than PHH-II and PHH-III but no significant difference was found between PHH-I and PHH-III (p > 0.05). Moreover, no significant difference (p > 0.05) in scavenging activity was noticeable between PHH-II and PHH-III. The scavenging activities of peptide fractions on hydroxyl radical and DPPH radical followed the same order, which could be concluded that PHH-IV possessed the strongest donating hydrogen ability, followed by PHH-I, PHH-III and PHH-II. This result suggested that PHH-IV can be considered as a scavenger of hydroxyl radicals to exert protection against oxidative damage.
Fig. 6.
Hydroxyl radical scavenging activity of peptide fractions. The values are means ± SD of triplicate measurements. Values with different letters are significantly different (P < 0.05)
Molecular weight distribution
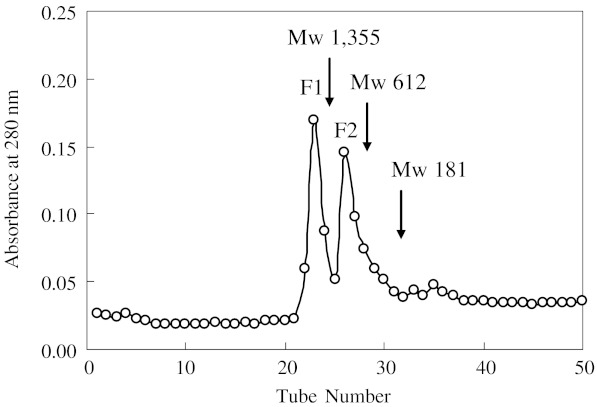
Considering that PHH-IV was found to possess the highest antioxidant activity, this fraction was analyzed for molecular weight distribution (Fig. 7). The chromatographic data indicated that PHH-IV was composed of two major fractions, named F1 and F2, respectively. Molecular weight of the two fractions was determined as 2476 Da (F1) and 1042 Da (F2). Moure et al. (2006) had reported that the antioxidant activity of hydrolysates is dependent on their molecular weight distribution. In this study, results revealed that the peptide fraction with molecular weight ranging from 180 to 3000 Da was probably associated with higher antioxidant activity. These findings are in agreement with other studies (Jeon et al. 2000; Kim et al. 2007) and support the fact that functional properties of antioxidant peptides are highly influenced by properties such as molecular mass.
Fig. 7.
Molecular weight distribution of PHH-IV with the highest antioxidant activity. Molecular weight for each peak: 2476 Da (F1) and 1042 Da (F2)
Conclusion
Among 6 proteases, pepsin was more effective than other proteases in preparing PHH with antioxidant activity. UF membrane technology was an efficient and ecological process for the fractionation of antioxidative peptides from PHH. We separated peptic PHH with different MW distributions using UF system and among these PHH-IV exhibited the highest antioxidant activity, including the ability to inhibit the autoxidation of linoleic acid and the scavenging effect on DPPH, superoxide and hydroxyl radicals. PHH-IV possessed the strongest hydrogen donating ability, followed by PHH-I, PHH-III and PHH-II. PHH-IV was composed of two major peptide fractions, which were located at 2476 Da (F1) and 1042 Da (F2). PHH-IV would be expected to protect against oxidative damage in living systems in relation to aging and carcinogenesis. Therefore, it could be used as a promising natural antioxidant for application in food, cosmetic and medicine industries.
Acknowledgment
This work was supported financially by National Science Technology Minister of China (award nr 2008GA741002 and nr 2006BAD05A16) and Department of Science and Technology of Zhejiang Province of China (award nr 2006 C12082).
References
- Adebiyi AP, Adebiyi AO, Ogawa T, Muramoto K. Purification and characterisation of antioxidative peptides from unfractionated rice bran protein hydrolysates. Int J Food Sci Technol. 2007;43:35–43. [Google Scholar]
- Adler-Nissen J. Determination of the degree of hydroysis of food protein hydrolysates by trinitrobenzensulfonic acid. J Agric Food Chem. 1979;27:1256–1262. doi: 10.1021/jf60226a042. [DOI] [PubMed] [Google Scholar]
- Official methods of analysis. 16. Washington DC: Association of Official Analytical Chemists; 2005. [Google Scholar]
- Ardestani A, Yazdanparast R. Antioxidant and free radical scavenging potential of Achillea santolina extracts. Food Chem. 2007;104:21–29. doi: 10.1016/j.foodchem.2006.10.066. [DOI] [Google Scholar]
- Athukorala Y, Kim KN, Jeon YJ. Antiproliferative and antioxidant properties of an enzymatic hydrolysate from brown alga, Ecklonia cava. Food Chem Toxicol. 2006;44:1065–1074. doi: 10.1016/j.fct.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Aubes-Dufau I, Capdevielle J, Seris JL, Combes D. Bitter peptide from hemoglobin hydrolysate: isolation and characterization. FEBS Lett. 1996;364:115–119. doi: 10.1016/0014-5793(95)00361-C. [DOI] [PubMed] [Google Scholar]
- Box HC, Maccubbin AE. Lipid peroxidation and DNA damage. Nutrition. 1997;13:920–921. doi: 10.1016/S0899-9007(97)00260-8. [DOI] [PubMed] [Google Scholar]
- ButterField DA, Castegna A, Pocernich CB, Drake J, Scapagnini G, Calabrese V. Nutritional approaches to combat oxidative stress in Alzheimer’s diseases. J Nutr Biochem. 2002;13:444–461. doi: 10.1016/S0955-2863(02)00205-X. [DOI] [PubMed] [Google Scholar]
- Chang CY, Wu KC, Chiang SH. Antioxidant properties and protein compositions of porcine haemoglobin hydrolysates. Food Chem. 2007;100:1537–1543. doi: 10.1016/j.foodchem.2005.12.019. [DOI] [Google Scholar]
- Chen CW, Ho CT. Antioxidant properties of polyphenols extracted from green and black tea. J Food Lipids. 1995;2:35–46. doi: 10.1111/j.1745-4522.1995.tb00028.x. [DOI] [Google Scholar]
- Dong S, Zeng M, Wang D, Liu Z, Zhao Y, Yang H. Antioxidant and biochemical properties of protein hydrolysates prepared from silver carp (Hypophthalmichthys molitrix) Food Chem. 2008;107:1485–1493. doi: 10.1016/j.foodchem.2007.10.011. [DOI] [Google Scholar]
- Jao CL, Ko WC. 1, 1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging by protein hydrolysates from tuna cooking juice. Fishery Sci. 2002;68:430–435. doi: 10.1046/j.1444-2906.2002.00442.x. [DOI] [Google Scholar]
- Je JY, Park PJ, Kim SK. Antioxidant activity of a peptide isolated from Alaska pollack (Theragra chalcogramma) frame protein hydrolysate. Food Res Int. 2005;38:45–50. doi: 10.1016/j.foodres.2004.07.005. [DOI] [Google Scholar]
- Je JY, Qian ZJ, Byun HG, Kim SK. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 2007;42:840–846. doi: 10.1016/j.procbio.2007.02.006. [DOI] [Google Scholar]
- Jeon YJ, Byun HG, Kim SK. Improvement of functional properties of cod frame protein hydrolysates using ultrafiltration membranes. Process Biochem. 2000;35:471–478. doi: 10.1016/S0032-9592(99)00098-9. [DOI] [Google Scholar]
- Kanatt SR, Chander R, Sharma A. Antioxidant potential of mint (Mentha spicata L.) in radiation-processed lamb meat. Food Chem. 2007;100:451–458. doi: 10.1016/j.foodchem.2005.09.066. [DOI] [Google Scholar]
- Kim SY, Je JY, Kim SK. Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion. J Nutr Biochem. 2007;18:31–38. doi: 10.1016/j.jnutbio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Klompong V, Benjakul S, Kantachote D, Shahidi F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007;102:1317–1327. doi: 10.1016/j.foodchem.2006.07.016. [DOI] [Google Scholar]
- Krings U, Berger RG. Antioxidant activity of some roasted foods. Food Chem. 2001;72:223–229. doi: 10.1016/S0308-8146(00)00226-0. [DOI] [Google Scholar]
- Li YH, Jiang B, Zhang T, Mu WM, Liu J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH) Food Chem. 2008;106:444–450. doi: 10.1016/j.foodchem.2007.04.067. [DOI] [Google Scholar]
- Lignot B, Froidevaux R, Nedjar-Arroume N, Guillochon D. Solvent effect on kinetics of appearance of neokyotorphin VVh4 and a bradykinin-potentiating peptide in the course of peptic hydrolysis of bovine haemoglobin. Biotechnol Appl Biochem. 1999;30:201–207. [PubMed] [Google Scholar]
- Manso MA, Miguel M, Even J, Hernández R, Aleixandre A, López-Fandińo R. Effect of the long-term intake of an egg white hydrolysate on the oxidative status and blood lipid profile of spontaneously hypertensive rats. Food Chem. 2008;109:361–367. doi: 10.1016/j.foodchem.2007.12.049. [DOI] [PubMed] [Google Scholar]
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Mendis E, Rajapakse N, Kim SK. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J Agric Food Chem. 2005;53:581–587. doi: 10.1021/jf048877v. [DOI] [PubMed] [Google Scholar]
- Mitsuda H, Yasumoto K, Iwami K. Antioxidative action of indole compounds during the autoxidation of linoleic acid. J Japan Soc Nutr Food Sci. 1996;19:210–214. [Google Scholar]
- Moure A, Domínguez H, Parajó JC. Antioxidant properties of ultrafiltration-recovered soy protein fractions from industrial effluents and their hydrolysates. Process Biochem. 2006;41:447–456. doi: 10.1016/j.procbio.2005.07.014. [DOI] [Google Scholar]
- Nasirullah, Jeyarani T, Rakshitha D. Isolation and antioxidant efficacy of nutraceutical concentrates from sesame and flax seed oils. J Food Sci Technol. 2009;46:66–69. [Google Scholar]
- Nedjar-Arroume N, Dubois-Delval V, Miloudi K, Daoud R, Krier F, Kouach M, Briand G, Guillochon D. Isolation and characterization of four antibacterial peptides from bovine hemoglobin. Peptides. 2006;27:2082–2089. doi: 10.1016/j.peptides.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Nyberg F, Sanderson K, Glämsta E. The hemorphins: a new class of opioid peptides derived from the blood protein haemoglobin. Biopolymers. 1997;43:147–156. doi: 10.1002/(SICI)1097-0282(1997)43:2<147::AID-BIP8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Ramakrishna BV, Jayaprakasha GK, Jena BS, Singh RP. Antioxidant activities of roselle (Hibiscus sabdariffa) calyces and fruit extracts. J Food Sci Technol. 2008;45:223–227. [Google Scholar]
- Ranathunga S, Rajapakse N, Kim SK. Purification and characterization of antioxidative peptide derived from muscle of conger eel (Conger myriaster) Eur Food Res Technol. 2006;222:310–315. doi: 10.1007/s00217-005-0079-x. [DOI] [Google Scholar]
- Saha K, Lajis NH, Israf DA, Hamzah AS, Khozirah S, Khamis S, Syahida A. Evaluation of antioxidant and nitric oxide inhibitory activities of selected Malaysian medicinal plants. J Ethnopharmacol. 2004;92:263–267. doi: 10.1016/j.jep.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Saiga A, Tanabe S, Nishimura T. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J Agric Food Chem. 2003;51:366–367. doi: 10.1021/jf021156g. [DOI] [PubMed] [Google Scholar]
- Sakanaka S, Tachibana Y. Active oxygen scavenging activity of egg-yolk protein hydrolysates and their effects on lipid oxidation in beef and tuna homogenates. Food Chem. 2006;95:243–249. doi: 10.1016/j.foodchem.2004.11.056. [DOI] [Google Scholar]
- Sakanaka S, Tachibana Y, Ishihara N, Juneja LR. Antioxidant activity of egg yolk protein hydrolysates in a linoleic acid oxidation system. Food Chem. 2004;86:99–103. doi: 10.1016/j.foodchem.2003.08.014. [DOI] [Google Scholar]
- Seema MN, Jayaprakasha GK, Singh RP. Antioxidant activity of custard apple (Annona squamosa) peel and seed extracts. J Food Sci Technol. 2008;45:349–352. [Google Scholar]
- Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the antioxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40:945–948. doi: 10.1021/jf00018a005. [DOI] [Google Scholar]
- Siddhuraju P, Becker K. The antioxidant and free radical scavenging activities of processed cowpea (Vigna unguiculata L. Walp.) seed extracts. Food Chem. 2007;101:10–19. doi: 10.1016/j.foodchem.2006.01.004. [DOI] [Google Scholar]
- Singh UAK, Singh S, Rai M. Total phenolics content and free radical scavenging activity of brassica vegetables. J Food Sci Technol. 2009;46:595–597. [Google Scholar]
- Thiansilakul Y, Benjakul S, Shahidi F. Antioxidative activity of protein hydrolysate from round scad muscle using Alcalase and Flavourzyme. J Food Biochem. 2007;31:266–287. doi: 10.1111/j.1745-4514.2007.00111.x. [DOI] [Google Scholar]
- Tung YT, Wu JH, Kuo YH, Chang ST. Antioxidant activities of natural phenolic compounds from Acacia confusa bark. Bioresource Technol. 2007;98:1120–1123. doi: 10.1016/j.biortech.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Wu JH, Wang Z, Xu SY. Enzymatic production of bioactive peptides from sericin recovered from silk industry wastewater. Process Biochem. 2008;43:480–487. doi: 10.1016/j.procbio.2007.11.018. [DOI] [Google Scholar]
- Xie ZJ, Huang JR, Xu XM, Jin ZY. Antioxidant activity of peptides isolated from alfalfa leaf protein hydrolysate. Food Chem. 2008;111:370–376. doi: 10.1016/j.foodchem.2008.03.078. [DOI] [PubMed] [Google Scholar]
- Yu YK, Hu JE, Miyaguchi Y, Bai XF, Du YG, Lin BC. Isolation and characterization of angiotensin I-converting enzyme inhibitory peptides derived from porcine hemoglobin. Peptides. 2006;27:2950–2956. doi: 10.1016/j.peptides.2006.05.025. [DOI] [PubMed] [Google Scholar]