Abstract
This study was undertaken to evaluate the behaviour of vegetative cells and spores of four potent native toxigenic food isolates of Bacillus cereus as affected by selected time-temperature combinations used in processing of Indian traditional foods. The vegetative cells of B. cereus when subjected to sublethal heat treatments, individually, in different heating menstra showed a sigmoidal inactivation pattern, with D-values in the range of 3.45 min at 60 °C to 10.6 min at 56 °C in saline. Accordingly, the z-values recorded across the heating menstra ranged from 9.3 °C in culture broth to 24 °C in whole milk. Similarly, the inactivation pattern for spores for the same isolates was curvilinear with D-values ranging from 4.4 min at 95 °C in whole milk to 19.45 min at 85 °C in saline. The z-values for spores ranged from 16.6 °C in saline to 38.4 °C in whole milk. The thermal inactivation pattern observed for vegetative cells and spores indicate that the death rate was not constant during the process of heat treatment.
Keywords: Bacillus cereus, Spores, Vegetative cells, Heating menstra, D-values, z values
Introduction
Consumer preferences are on an increase towards traditional foods, which are heat processed by minimal time-temperature combinations. Besides, several new brands of food products (ready-to-eat and ready-to-cook) have arrived on the market shelves, which are mostly semi-processed and needs additional one or two steps of heat processing of varying degrees prior to consumption by human population. These aspects of consumer preferences should view microbial safety of foods with serious concern. In most of the unit operations of optimum thermal processing of foods, the risk involved due to the occurrence of opportunistic foodborne pathogen, Bacillus cereus attains greater significance in view of the existence of both, vegetative and spore phases. The occurrence of B. cereus as a pre- and post-processing contaminant in a wide range of foods and its ability to cause two different types of foodborne illnesses in humans namely, diarrhoea and emesis are very well established (Ehling-Schulz et al. 2004; Schoeni and Wong 2005, Roy et al. 2007; Ouoba et al. 2008).
In the food chain of Indian traditional foods, there occur intermittent sub-lethal heating processes of either the ingredients and/or final products, as majority of traditional foods are consumed in hot and/or warm conditions. Although pasteurization is known to inactivate the vegetative cells, spores of B. cereus are known to be highly heat resistant and are usually unaffected by physical, chemical and biological factors (Gaillard et al. 1998; González et al. 1999). In general, investigations have addressed these aspects relating to either vegetative cells or spores and not both the phases together of one and the same species, except for a not very recent study (Byrne et al. 2006).
The profile of heat resistance in a bacterial species is a complex phenomenon which is dependent on the physiological status of specific species, heating medium, intrinsic and extrinsic factors. In these circumstances, D-values (decimal reduction time) is defined as the time in minutes required to reduce the viable cell population by 90% is being considered as a basic parameter to assess the thermal resistance of the organism. It assumes significance for quantitative expression and comparison of the heat resistance of the organism involved.
The objective of this study was to gain knowledge about the behaviour of both vegetative cells and spores of three potent toxigenic native food isolates of B. cereus under probable time-temperature combinations of heat processing encountered in the food chain of Indian traditional foods.
Materials and methods
All dehydrated media and other bacterial reagents and stains used in this study were procured from HiMedia Laboratories, Mumbai, India. The water used in the experimental trials was Milli-Q water (A10 Elix 3, Millipore Corporation, Billerica, USA).
Bacterial cultures
The cultures included three potent toxigenic native food isolates of B. cereus (CFR 1521, CFR 1532 and CFR 1534) selected from the departmental collection of bacterial cultures that have been isolated from a range of Indian traditional fast foods collected from local markets. In addition, strain of B. cereus F 4810 obtained through the courtesy of JH Kramer, Central Public Health Laboratory, London, United Kingdom served as the reference culture. The cultures were individually maintained at 6 °C on Brain Heart Infusion (BHI) agar slants and the cultures were propagated in BHI broth for 18 h at 37 °C prior to use in experimental trials.
Preparation of vegetative cells
A loopful of test cultures from individual active agar slants were inoculated into aliquots of 10 ml of BHI broth and incubated for 14 h at 37 °C in an orbital shaker incubator (Alpha Scientific Co., Bangalore, India) at 70 rpm. A smear of loopful of individual culture broth was observed under microscope for the presence of spore and/or spore-former. Cell suspensions of cultures were individually prepared by centrifugation at 8,000 rpm (Superspin R-V/FM, Plasto Crafts, Mumbai, India) for 20 min at 4 °C followed by washing harvested cells twice with 0.85% saline. The resultant individual cell suspension with a number of 9.3 log10 cfu/ml was resuspended in sterile 10 ml aliquots of 0.85% saline and stored at 6 °C in sterile screw-capped tubes until further use. The cell titres were enumerated by surface plating of serial dilutions of the initial cell suspensions on pre-poured BHI agar plates and incubated for 24 h at 37 °C.
Preparation of spore suspension
Spore suspensions of individual isolate of B. cereus was prepared in accordance with the procedure of Novak et al. (2005). A loopful of culture from individual active agar slant was inoculated into 10 ml of BHI broth and incubated for 18 h at 37 °C under static condition. The culture broths were diluted in 0.85% saline and surface plated on pre-poured BHI agar plates containing 3 mg MgSO4/ml. The plates were incubated for 72 h at 37 °C and subsequently smears of the colonies were observed under microscope for more than 90% sporulation. Spores were harvested from multiple plates by gentle scrapping with sterile L-shaped spreaders, pooling, washing in 0.85% saline and resuspending in 10 ml aliquots of saline with a concentration of 9.3 log spores/ml. Ethanol was added at a concentration of 20% v/v and spore suspensions were stored at 6 °C in sterile screw-capped tubes until further use. The spore titres were determined as described previously for cell titres of vegetative cells.
Heating menstra
The heating menstra used in this study were saline, BHI broth, skim milk and whole milk. Physiological saline (0.85% NaCl) served as a control medium without any complex nutrient ingredients. BHI broth was used as a representative of routinely used laboratory complex microbiological general purpose medium. Skim milk was prepared by reconstituting commercially available skim milk powder at 10% level in water. Pasteurized whole milk with 3% fat was procured from a local commercial dairy. Both skim and whole milk samples served as the heating media with food constituents. All the 4 heating media were prepared individually, dispensed in requisite quantities in test tubes of appropriate dimension and sterilized. Saline and BHI broth (pH level of 6.8) were autoclaved at 121 °C for 15 min, while skim and whole milks (pH level of 6.6) for 10 min.
Thermal inactivation and enumeration of survivors
Individual pre-sterilized heating menstra were taken in aliquots of 5 ml in test tubes of 15 x 125 mm and placed initially at pre-selected temperatures in a thermostatically controlled water bath (Julabo SW 22, Labortechnik GMBH, Seelbach, Germany), so that the menstra attains the defined temperature to be used in this experimental trials. These tempered aliquots of individual heating menstra were inoculated with vegetative cells of individual bacterial test cultures of B. cereus at 8.3 log cfu/ml. The inoculated tubes were subjected to temperatures of 56, 58 and 60 °C, respectively, for specific durations of 3, 6, 9, 12, 15 and 18 min. After the specific time-temperature periods were completed, tubes were removed and immediately cooled in ice-water bath. The experimental samples were appropriately diluted in 0.85% saline and surface plated on pre-poured plates of BHI agar and incubated aerobically for 24 h at 37 °C. Colonies formed in the incubated plates were enumerated and recorded in log10 CFU/ml. Similar heat treatment and enumeration procedures as described for vegetative cells were performed in four individual heating menstra for spores of individual test cultures of B. cereus at pre-selected temperatures of 85, 90 and 95 °C, respectively, for 5, 10, 15, 20, 25 and 30 min. The results recorded were the average of two experimental trials.
Determination of thermal resistance and statistical analysis
The D-values were determined by separately plotting the log10 number of survivors against time at each temperature using Microsoft excel 2003 software (Microsoft Corporation, Redmond, USA). Only the values in straight portion of the curve were considered for calculation. The line-of-best fit for survivor plots were determined by regression analysis (Ostle and Malone 1988). A regression equation of the type 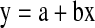 was derived, where, b is the slope of the best straight line that when inverted and changed from negative to positive, gives the D-value for a specific temperature. The average of D-values from 2 sets of experiments is used for calculating z-values. The z-values were computed by plotting the log10D-values against respective temperatures as a semi-logarithmic plot and calculated the negative inverse of the slope of the thermal death time curve (z value = −1/slope).
was derived, where, b is the slope of the best straight line that when inverted and changed from negative to positive, gives the D-value for a specific temperature. The average of D-values from 2 sets of experiments is used for calculating z-values. The z-values were computed by plotting the log10D-values against respective temperatures as a semi-logarithmic plot and calculated the negative inverse of the slope of the thermal death time curve (z value = −1/slope).
The significant difference in D-values among the different strains tested was performed by Duncan’s New Multiple Range Test. One-way-analysis of variance of mean D-values of the cultures across heating media and temperatures as well as that with respect to cultures and temperatures was performed using Origin Pro 6.1 software (Origin Lab Corporation, Northampton MA, USA).
Results and discussion
Thermal inactivation profile of vegetative cells
Decimal reduction times of vegetative cells of the individual cultures of B. cereus in selected heating menstra and their corresponding 12 D-values are presented in Table 1. The inactivation pattern for vegetative cells was sigmoidal with R2 (correlation coefficient) of 0.95. As the pattern was almost same in all the 4 cultures studied for all the different heating menstra at varied temperatures, the sigmoidal curve for only one representative culture of B. cereus CFR 1534 is shown in Fig. 1a. The D-values for four strains of vegetative cells across the different menstra ranged from the lowest of 3.45 min at 60 °C to the highest of 10.6 min at 56 °C in saline. The findings of present study with native food isolates of B. cereus known to occur in a wide variety of traditional foods gains significance in the background of not many reports available with respect to the thermal inactivation of vegetative cells of B. cereus. In a similar experimental approach with almost different types of heating menstrum (pork luncheon roll), the values recorded were from a lowest of 1 min at 60 °C to a highest of 6.4 min at 55 °C (Byrne et al. 2006).
Table 1.
Decimal reduction time* (min) (D-values) of vegetative cells of B. cereus cultures
| Culture | Heating menstra | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saline | BHI broth | Skim milk | Whole milk | |||||||||
| 56 °C | 58 °C | 60 °C | 56 °C | 58 °C | 60 °C | 56 °C | 58 °C | 60 °C | 56 °C | 58 °C | 60 °C | |
| B. cereus CFR 1521 | 7.40a | 3.65a | 3.50a | 10.55a | 3.50a | 4.05a | 7.60a | 6.90a | 3.70a | 7.65a | 5.45a | 3.60a |
| (88.8) | (44.4) | (42.0)+ | (127.2) | (42.0) | (49.2) | (91.2) | (82.8) | (44.4) | (92.4) | (66.0) | (43.2) | |
| B. cereus CFR 1532 | 7.20a | 4.85b | 3.45a | 6.20b | 4.25ab | 3.50a | 5.30b | 4.75b | 3.45a | 5.15b | 4.10a | 3.55a |
| (86.4) | (58.8) | (42.0) | (74.4) | (51.6) | (42.0) | (63.6) | (57.6) | (42.0) | (62.4) | (49.2) | (43.2) | |
| B. cereus CFR 1534 | 10.60b | 4.90b | 4.10a | 8.05ab | 5.80c | 4.15a | 7.15a | 5.45b | 3.90a | 6.50c | 4.75a | 4.15a |
| (127.2) | (58.8) | (49.2) | (97.2) | (69.6) | (50.4) | (86.4) | (66.0) | (46.8) | (78.0) | (57.6) | (50.4) | |
| B. cereus F 4810 | 7.70a | 5.45c | 3.90a | 7.00b | 5.25bc | 3.65a | 7.65a | 4.65b | 4.25a | 6.50c | 4.90a | 4.15a |
| (97.2) | (66.0) | (46.8) | (84.0) | (63.6) | (44.4) | (92.4) | (56.4) | (51.6) | (78.0) | (58.8) | (50.4) | |
| SEM | ±0.43 | ±0.12 | ±0.12 | ±0.67 | ±0.30 | ±0.39 | ±0.32 | ±0.26 | ±0.21 | ±0.16 | ±0.45 | ±0.22 |
a-cMeans in the same column followed by different superscripts differ significantly (p < 0.05)
*D-values are the means of two experimental trials with each performed in duplicates
+Figures in parentheses indicate the 12D (commercial sterility) values
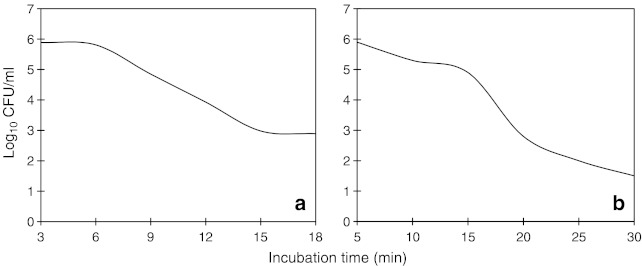
Fig. 1.
Survivor curves for vegetative cells of B. cereus CFR 1534 in skim milk at 60 °C showing the sigmoidal curve (a) and for spores of B. cereus CFR 1534 in skim milk at 95 °C showing the curvilinear curve (b)
The D-values observed in the present study appear to be quite different from those reported by earlier researchers. This variation could be attributed to the genotypic characteristics of bacterial cultures and basic experimental design used in the respective studies. The predominant influencing factor appears to arise from biochemical and physical constituents of heating menstra used in experimental studies like levels of carbohydrates, fat and protein in medium, which could enhance thermal resistance (Ababouch et al. 1995; Oteiza et al. 2003) and so also higher acidic environment (low pH levels) has a similar effect (Casadei et al. 2001). Usually, differences observed have been due to the effect of combination of these influencing factors. In the present study, all the 4 heating media had no marked differential effect on thermal resistance pattern of vegetative cells. The mean D-values for saline, BHI broth, skim milk and whole milk were 5.6, 5.5, 5.4 and 5.1 min, respectively and were statistically at par.
The mean D-values at 56, 58 and 60 °C across the cultures and heating media were 7.4, 4.9 and 3.8, respectively and were significant (p ≤ 0.05) and thus indicating the sensitivity range of cultures to temperature effect. Further, the phenomenon appears to be more pronounced in the transition from 58 to 60 °C, suggesting that 56 °C is too low a temperature to affect any inactivation of vegetative cells. The magnitude of decrease in D-values from D58 to D60 being low appears to be crucial, since a further increase in temperature could have completely inactivated all the vegetative cells. A similarity to this could be evidenced in the study with vegetative cells of Clostridium perfringens (an anaerobic spore former), wherein inactivation of cells occurred above 60 °C. However, presence of salts and/or other food constituents in the medium could alter the heat resistance pattern (Doyle 2002).
The findings observed with respect to thermal inactivation pattern of cultures studied become relevant when the projected commercial sterility values are presented and discussed. The 12 D-values, also termed as commercial sterility values for vegetative cells of B. cereus cultures ranged from a lowest of 42 min at 58 and 60 °C to a highest of 127.2 min at 56 °C (Table 1). The commercial sterility, defined as the condition in which no viable organism that exists can multiply under the storage conditions of foods. The 12 D-values for vegetative cells at a temperature of 60 °C across all the cultures and heating menstra were in the range of 42 to 51.6 min. This comes in close agreement with the general time-temperature combination of 62.8 °C for 30 min for the commercial low temperature long time holding method of pasteurization for fluid milk.
The z-values of vegetative cells of B. cereus cultures (Table 2) ranged from 9.3 °C for B. cereus CFR 1521 in BHI broth to 24 °C for B. cereus CFR 1532 in whole milk. The average z values across the heating menstra recorded for B. cereus were 12 °C for CFR 1521, 18.5 °C for CFR 1532, 15.6 °C for CFR 1534 and 16.3 °C for F 4810. These values for different menstra tested for each of the cultures were not significantly different, thus showing that the heating media did not influence as a singular effect on heat inactivation pattern of vegetative cells. Nevertheless, a progressive increase in z values was observed for most of the cultures in the ascending order of saline, BHI broth, skim milk and whole milk. The z values recorded in the present study appear to be quite significant, as the heating menstra were not rich in concentration of major food constituents. Even in a study with pork luncheon roll, the z value recorded for vegetative cells of B. cereus was 6.6 °C (Byrne et al. 2006).
Table 2.
z values for vegetative cells and spores of B. cereus cultures in different heating menstra
| Culture | Heating menstra | |||
|---|---|---|---|---|
| Saline | BHI broth | Skim milk | Whole milk | |
| (Z value in °C) | ||||
| Vegetative cells | ||||
| B. cereus CFR 1521 | 12.1 | 9.3 | 12.5 | 14.2 |
| B. cereus CFR 1532 | 12.5 | 16.1 | 21.2 | 24.0 |
| B. cereus CFR 1534 | 10.3 | 14.3 | 16.7 | 21.0 |
| B. cereus F 4810 | 13.8 | 14.3 | 16.0 | 21.0 |
| Spores | ||||
| B. cereus CFR 1521 | 25.0 | 25.0 | 33.3 | 33.3 |
| B. cereus CFR 1532 | 20.0 | 25.0 | 25.0 | 33.3 |
| B. cereus CFR 1534 | 16.6 | 20.0 | 25.0 | 38.4 |
| B. cereus F 4810 | 16.7 | 26.3 | 29.4 | 33.3 |
The thermal death curve for the vegetative cells in the present study was non-linear (Fig. 1a), indicating that the inactivation rate was not constant, but rather exhibited a sigmoidal shape with shoulder and tailing pattern (Xiong et al. 1999). It is possible that the shoulder response could be due to clumping of organisms in the suspension. It is well known, that vegetative cells of B. cereus do not survive routine cooking temperatures, their study relating to thermal inactivation pattern holds significance due to the production of diarrhoeal (heat stable) and emetic toxins by these cultures during their vegetative phase. Besides, the ubiquitous presence of B. cereus, its ability to contaminate a wide range of foods as pre- and post-processing contaminant and the increase in demand for minimally processed foods underscores the relevance of thermal inactivation of vegetative cells of B. cereus, which was hitherto not given due importance.
Thermal inactivation profile of spores
The D-values recorded for spores of B. cereus cultures studied and the corresponding derived 12 D-values in different heating menstra are presented in Table 3. All the observations recorded were with R2 of 0.95 and the inactivation pattern was curvelinear (Fig. 1b). The D-values of spores ranged from 4.4 min at 95 °C to 19.45 min at 85 °C. These values were in close agreement with those reported in an earlier study undertaken with a not similar heating menstrum (pork luncheon roll), wherein the values were 29.5, 10.1 and 2.0 min at 85, 90 and 95 °C respectively (Byrne et al. 2006).
Table 3.
Decimal reduction time* (min) (D-values) of spores of B. cereus cultures
| Culture | Heating menstra | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saline | BHI broth | Skim milk | Whole milk | |||||||||
| 85 °C | 90 °C | 95 °C | 85 °C | 90 °C | 95 °C | 85 °C | 90 °C | 95 °C | 85 °C | 90 °C | 95 °C | |
| B. cereus CFR 1521 | 13.40a | 6.30a | 5.45a | 12.50ab | 7.80a | 5.50a | 11.95a | 6.20a | 5.65a | 9.25a | 7.50a | 4.40a |
| (160.8) | (75.6) | (66.0)+ | (150.0) | (93.6) | (62.4) | (144.0) | (74.4) | (68.4) | (111.6) | (90.0) | (52.8) | |
| B. cereus CFR 1532 | 14.30a | 6.80a | 5.10a | 12.15b | 9.55a | 4.80a | 15.45a | 12.50b | 7.05a | 9.55a | 6.90a | 4.75a |
| (171.6) | (81.6) | (61.2) | (145.2) | (114.0) | (57.6) | (186.0) | (150.0) | (84.0) | (115.2) | (81.6) | (57.6) | |
| B. cereus CFR 1534 | 19.45b | 7.90a | 5.90a | 14.30c | 8.30a | 5.35a | 16.45a | 7.70a | 6.10a | 9.15a | 8.75a | 4.95a |
| (234.0) | (94.8) | (70.8) | (171.6) | (99.6) | (63.6) | (198.0) | (92.4) | (73.2) | (110.4) | (105.6) | (60.0) | |
| B. cereus F 4810 | 19.10b | 6.35a | 5.35a | 13.25a | 7.95a | 5.50a | 12.15a | 7.90a | 5.80a | 10.00a | 7.25a | 5.30a |
| (229.2) | (76.8) | (64.8) | (159.6) | (96.0) | (67.2) | (146.4) | (94.8) | (69.6) | (120.0) | (87.6) | (69.6) | |
| SEM | ±1.00 | ±0.40 | ±0.31 | ±0.21 | ±0.35 | ±0.18 | ±1.26 | ±0.80 | ±0.53 | ±0.82 | ±0.47 | ±0.59 |
a-cMeans in the same column followed by different superscripts differ significantly (p < 0.05)
*D-values are the means of two experimental trials, with each performed in duplicates
+Figures in parentheses indicate the 12D (commercial sterility) values
The thermal inactivation pattern of spores of B. cereus cultures observed in most of the earlier studies have revealed that D-values differ very much depending primarily on the strains used and to a lesser extent the extrinsic and intrinsic factors. Earlier studies have reported varying D-values for ATCC strains of B. cereus 4342, 7004 and 9818 in buffers and substrates similar to culture broth and those containing food constituents (Mazas et al. 1995, 1999a; González et al. 1999; Montville et al. 2005; Moussa-Boudjemma et al. 2006). A similar situation was also reported for a strain of Bacillus anthracis Sterne (Novak et al. 2005). The D-values recorded in the present study for three cultures of B. cereus were almost closer to those values reported earlier for the strain of B. cereus ATCC 4342 and 7004 when subjected to heat treatment in buffers of pH 7.0 and milk medium at 90 °C (Montville et al. 2005; Moussa-Boudjemma et al. 2006) and 95 °C (Mazas et al. 1999a). In a study with spores of B. cereus ATCC 7004 (Mazas et al. 1999b), the D-values recorded at 92 and 96 °C in saline and milk medium were quite lower to those observed in this study. As B. anthracis was in the same cluster as that of B. cereus, a similarity was observed in an earlier study with spores of B. anthracis Sterne (Novak et al. 2005), wherein the D-values recorded at 100 °C in skim milk was almost near to those recorded in the present study. In contrast to the above, studies with different cultures like B. anthracis in whole milk at 100 °C (Xu et al. 2006) and a strain of B. cereus ATCC 9818 at 100 °C in distilled water, BHI broth and skim milk (Novak et al. 2005) revealed higher D-values (14.4 to 35.7 min) in comparison to those recorded in this study with three native food isolates of B. cereus.
The wide variations observed in the thermal inactivation pattern of spores of B. cereus could be attributed to one or more of the following (1) characteristics of strains of B. cereus with environmentally induced resistance due to dipicolinic acid content, degree of hydration and mineral content (Marquis and Shin 1994; Palop et al. 1996; Mazas et al. 1999b; Melly et al. 2002; Baweja et al. 2008), (2) temperatures which have induced spore formation as well as other conditions prevailing during sporulation (Condon et al. 1992; Baweja et al. 2008) and (3) heterogeneity in the cluster of spores with respect to germination and/or survival (Byrne et al. 2006).
Similar to the behavioural pattern of vegetative cells, even the spores of B. cereus cultures used in the present study showed certain differences in their thermal resistance. The isolate of B. cereus CFR 1521 exhibited a lowest mean D-value of 8 min, whereas the potent toxigenic isolate of B. cereus CFR 1534 had a mean D-value of 9.5 min. Further, mean D-values obtained for the spores in different heating menstra like saline, BHI broth and skim milk were 9.6, 8.9 and 9.6 min, respectively. The lowest of 7.3 min was observed with whole milk, which indicates that spores present in this medium were more sensitive to heat treatment. The mean D-values in the heating menstra tested for each of the isolates were not significantly different, wherein it could be said that medium composition of heating menstra had no marked effect on the inactivation profile of the cultures studied.
The survival curves obtained for spores of isolates of B. cereus tested in the present study showed a curvilinear pattern with shoulder (lag phase) followed by a linear declining pattern, an observation reported in one of the earlier studies (Kamau et al. 1990). The shoulder effect could be due to clumping of spores, which could result in an increase of thermal resistance. Hence, all spores in the clumps need to be inactivated prior to the destruction of colony forming ability of the clump (Adams and Moss 1997; Furukawa et al. 2005). The effect could also be due to the heterogeneous sub-populations of spores (dormant, germinated and inactivated) differing in their physiological state during heat treatment and become resistant within a highly extreme resistant sub-population (Peleg and Cole 1998). The lag phase in spore inactivation pattern revealed that there was a small decrease in the concentration of spores and the linear decline phase show that specific death rate was constant. This follows the first order kinetics and goes to indicate that as the microbial population is subjected to heat treatment, the spores get inactivated at a constant rate. The absence of any drastic decrease in the initial phase of inactivation rules out the possibility of vegetative cells being present in the spore suspension used in this study.
The z-values of the spores of isolates of B. cereus ranged from 16.6 to 38.4 °C (Table 2). The average z-values (°C) for individual isolates across the menstra were 29.2 ± 4.8 for B. cereus CFR 1521, 25.8 ± 5.5 for CFR 1532, 25 ± 9.5 for CFR 1534 and 26.4 ± 7.0 for B. cereus F 4810. The z-values recorded in the study can be compared to an earlier study on spores of B. anthracis Sterne and B. cereus ATCC 9818 in similar heating media (distilled water, BHI broth and skim milk) with z-values ranging from 20.4 to 36.5 °C (Novak et al. 2005).
Earlier reports have shown that high level of fat (>20%) provides a protective effect for bacterial pathogens subjected to heat treatment (Fain et al. 1991; Murphy et al. 2004). This protective effect could be a consequence of reduced water activity in milk medium (Senhaji and Loncin 1977). In the present study, the fat level in whole milk was 3% and negligible in skim milk, wherein there may not be very significant protective effect of lipids for the spores of B. cereus. It is well established, that pasteurization process kills vegetative cells. However, spores, which are formed from the same vegetative cells, achieve resistance to heat processing. This resistance is invariably attributed to a metabolically inactive complex of nucleic acids and calcium dipicolinic acid formed under a set of prevailing unfavourable conditions (Murrel 1988; Nicholson et al. 2000; Baweja et al. 2008).
A few of the earlier studies have shown that pH of heating menstra has an influence on thermal resistance of the organism. Mazas et al. (1998) observed in their studies that acidification from pH 7 to 4 resulted in a decrease of D-values in the spores of B. cereus strains. Similarly, studies by Montville et al. (2005) showed that a higher resistance occurred in B. cereus spores at pH 7 in milk rather than in apple juice or buffer of pH 4.5. In the present study, although variations in D-values were observed among the media used, the same appear to be less influenced by minor difference in pH levels and more so by the constituents of heating menstra and specificity of cultures used in the study.
Conclusion
The quantitative determination of heat resistance in terms of D- and z-values was significant in giving an insight about the thermal inactivation pattern of native food isolates of B. cereus and provided baseline data to formulate time-temperature combinations, both for spores and vegetative cells of the same culture, an aspect not being addressed in most of the earlier investigations. This understanding of thermal resistance of spores and vegetative cells would help in modelling the combined effects for inactivation and assessing the benefits of optimum time-temperature combined unit operations to achieve microbiologically safe food for human consumption in the food chain of traditional foods.
Acknowledgement
Authors are thankful to Prakash V, Director, CFTRI, Mysore, India for providing the facilities and interest in present work. First author is grateful to Council of Scientific and Industrial Research, New Delhi, India for awarding the Senior Research Fellowship.
References
- Ababouch LH, Grimit L, Eddafry R. Thermal inactivation kinetics of Bacillus subtilis spores suspended in buffer and in oils. J Appl Bacteriol. 1995;78:669–676. doi: 10.1111/j.1365-2672.1995.tb03114.x. [DOI] [PubMed] [Google Scholar]
- Adams MR, Moss MO. Food microbiology. London: The Royal Society of Chemistry; 1997. [Google Scholar]
- Baweja RB, Zaman MS, Mattoo AR, Sharma K, Tripathi V, Aggarwal A, Dubey GP, Kurupati RK, Ganguli M, Chaudhury NK, Sen S, Das TK, Gade WN, Singh Y. Properties of Bacillus anthracis spores prepared under various environmental conditions. Arch Microbiol. 2008;189:71–79. doi: 10.1007/s00203-007-0295-9. [DOI] [PubMed] [Google Scholar]
- Byrne B, Dunne G, Bolton DJ. Thermal inactivation of Bacillus cereus and Clostridium perfringens vegetative cells and spores in pork luncheon roll. Food Microbiol. 2006;23:803–808. doi: 10.1016/j.fm.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Casadei MA, Ingram R, Hitchings E, Gaze JAE. Heat resistance of Bacillus cereus, Salmonella typhimurium and Lactobacillus delbreuckii in relation to pH and ethanol. Int J Food Microbiol. 2001;63:125–134. doi: 10.1016/S0168-1605(00)00465-7. [DOI] [PubMed] [Google Scholar]
- Condon S, Bayarte M, Sala EJ. Influence of the sporulation temperature upon heat resistance of Bacillus subtilis. J Appl Bacteriol. 1992;73:251–256. doi: 10.1111/j.1365-2672.1992.tb02985.x. [DOI] [PubMed] [Google Scholar]
- Doyle E (2002) Survival and growth of Clostridium perfringens during the cooling step of thermal processing of meat products. Food Research Institute Briefings, University of Wisconsin, USA
- Ehling-Schulz M, Fricker M, Scherer S. Bacillus cereus, the causative agent of an emetic type of foodborne illness. Mol Nutr Food Res. 2004;48:479–487. doi: 10.1002/mnfr.200400055. [DOI] [PubMed] [Google Scholar]
- Fain AR, Line JE, Moran AB, Martin LM, Lechovich RV, Kerosella JM, Brown WL. Lethality of heat to Listeria monocytogenes Scott A: D-value and z-value determinations in ground beef and turkey. J Food Prot. 1991;54:756–761. doi: 10.4315/0362-028X-54.10.756. [DOI] [PubMed] [Google Scholar]
- Furukawa S, Narisawa N, Watanabe T, Kawarai T, Myozen K, Okazaki S, Ogihara H, Yamasaki M. Formation of the spore clumps during heat treatment increases the heat resistance of bacterial spores. Int J Food Microbiol. 2005;102:107–111. doi: 10.1016/j.ijfoodmicro.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Gaillard S, Leguerinel I, Mafart P. Model for combined effects of temperature, pH and water activity on thermal inactivation of Bacillus cereus spores. J Food Sci. 1998;63:887–889. doi: 10.1111/j.1365-2621.1998.tb17920.x. [DOI] [Google Scholar]
- González I, López M, Martínez S, Bernardo A, González J. Thermal inactivation of Bacillus cereus spores formed at different temperatures. Int J Food Microbiol. 1999;51:81–84. doi: 10.1016/S0168-1605(99)00109-9. [DOI] [PubMed] [Google Scholar]
- Kamau DN, Doores S, Pruitt KM. Enhanced thermal destruction of Listeria monocytogenes and Staphylococcus aureus by the lactoperoxidase system. Appl Environ Microbiol. 1990;56:2711–2716. doi: 10.1128/aem.56.9.2711-2716.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis RE, Shin SY. Mineralization and responses of bacterial spores to heat and oxidative agents. FEMS Microbiol Rev. 1994;14:375–379. doi: 10.1111/j.1574-6976.1994.tb00111.x. [DOI] [PubMed] [Google Scholar]
- Mazas M, González I, López M, González J, Martin R. Effects of sporulation media and strain on thermal resistance of Bacillus cereus spores. Int J Food Sci Technol. 1995;30:71–78. [Google Scholar]
- Mazas M, López M, González I, González J, Bernardo A, Martin R. Effects of the heating medium pH on heat resistance of Bacillus cereus spores. J Food Saf. 1998;18:25–36. doi: 10.1111/j.1745-4565.1998.tb00199.x. [DOI] [Google Scholar]
- Mazas M, López M, Martínez S, Bernardo A, Martin R. Heat resistance of Bacillus cereus spores: effects of milk constituents and stabilizing additives. J Food Prot. 1999;62:410–413. doi: 10.4315/0362-028x-62.4.410. [DOI] [PubMed] [Google Scholar]
- Mazas M, Martínez S, López M, Alvarez AB, Martin R. Thermal inactivation of Bacillus cereus spores affected by the solutes used to control water activity of the heating medium. Int J Food Microbiol. 1999;53:61–67. doi: 10.1016/S0168-1605(99)00145-2. [DOI] [PubMed] [Google Scholar]
- Melly E, Genest PC, Gilmore ME, Little S, Popham DL, Dirks A, Setlow P. Analysis of the properties of spores of Bacillus subtilis prepared at different temperatures. J Appl Microbiol. 2002;92:1105–1115. doi: 10.1046/j.1365-2672.2002.01644.x. [DOI] [PubMed] [Google Scholar]
- Montville TJ, Dengrove R, Siano TD, Bonnet M, Schaffner DW. Thermal resistance of spores from virulent strains of Bacilius anthracis and potential surrogates. J Food Prot. 2005;68:2362–2366. doi: 10.4315/0362-028x-68.11.2362. [DOI] [PubMed] [Google Scholar]
- Moussa-Boudjemma B, González J, López M. Heat resistance of Bacillus cereus spores in carrot extract acidified with different acidulants. Food Control. 2006;17:819–824. doi: 10.1016/j.foodcont.2005.05.009. [DOI] [Google Scholar]
- Murphy RY, Osaili T, Duncan LK, Marcy JA. Thermal inactivation of Salmonella and Listeria monocytogenes in ground chicken thigh/leg meat and skin. Poultry Sci. 2004;83:1218–1225. doi: 10.1093/ps/83.7.1218. [DOI] [PubMed] [Google Scholar]
- Murrel WG. Bacterial spores —nature’s ultimate survival package. In: Murrel WG, Kennedy IR, editors. Microbiology in action. New York: Wiley; 1988. pp. 311–346. [Google Scholar]
- Nicholson WL, Munakata N, Horneck G, Melosh HJ, Setlow P. Resistance of Bacillus endospores to extreme terrestrial and extra-terrestrial environments. Microbiol Mol Biol Rev. 2000;64:548–572. doi: 10.1128/MMBR.64.3.548-572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak JS, Call J, Tomasula P, Luchansky J. An assessment of pasteurization treatment of water, media and milk with respect to Bacillus spores. J Food Prot. 2005;68:751–757. doi: 10.4315/0362-028x-68.4.751. [DOI] [PubMed] [Google Scholar]
- Ostle B, Malone LC. Statistics in research: basic concepts and techniques for research workers. 4. Iowa: Iowa State Press; 1988. [Google Scholar]
- Oteiza JM, Giannuzzi L, Califano AN. Thermal inactivation of Escherichia coli O157:H7 and Escherichia coli isolated from moricella as affected by composition of the product. Food Res Int. 2003;36:703–712. doi: 10.1016/S0963-9969(03)00050-4. [DOI] [Google Scholar]
- Ouoba LI, Thorsen L, Varnam AH. Enterotoxins and emetic toxins production by Bacillus cereus and other species of Bacillus isolated from Soumbala and Bikalga, African alkaline fermented food condiments. Int J Food Microbiol. 2008;124:224–230. doi: 10.1016/j.ijfoodmicro.2008.03.026. [DOI] [PubMed] [Google Scholar]
- Palop A, Raso J, Condon S, Sala EJ. Heat resistance of Bacillus subtilis and Bacillus coagulans: effect of sporulation temperature in foods with various acidulants. J Food Prot. 1996;59:487–492. doi: 10.4315/0362-028X-59.5.487. [DOI] [PubMed] [Google Scholar]
- Peleg M, Cole MB. Reinterpretation of microbial survival curves. Crit Rev Food Sci Nutr. 1998;38:353–380. doi: 10.1080/10408699891274246. [DOI] [PubMed] [Google Scholar]
- Roy A, Moktan B, Sarkar PK. Characteristics of Bacillus cereus isolates from legume-based Indian fermented foods. Food Control. 2007;18:1555–1564. doi: 10.1016/j.foodcont.2006.12.006. [DOI] [Google Scholar]
- Schoeni JL, Wong ACL. Bacillus cereus food poisoning and its toxins. J Food Prot. 2005;68:636–648. doi: 10.4315/0362-028x-68.3.636. [DOI] [PubMed] [Google Scholar]
- Senhaji AF, Loncin M. The protective effect of fat on the heat resistance of bacteria. J Food Technol. 1977;12:203–216. doi: 10.1111/j.1365-2621.1977.tb00102.x. [DOI] [Google Scholar]
- Xiong R, Xie G, Edmondson AE, Sheard MA. A mathematical model for bacterial inactivation. Int J Food Microbiol. 1999;46:45–55. doi: 10.1016/S0168-1605(98)00172-X. [DOI] [PubMed] [Google Scholar]
- Xu S, Labuza TP, Diez-Gonzalez F. Thermal inactivation of Bacillus anthracis spores in cow’s milk. Appl Environ Microbiol. 2006;72:4479–4483. doi: 10.1128/AEM.00096-06. [DOI] [PMC free article] [PubMed] [Google Scholar]